Abstract
Aim:
Therapeutic targeting of BRAF alterations in primary brain tumor patients has demonstrated clinical activity in case reports and early trials; however, there is limited high-level evidence of the efficacy.
Patients & results:
Targeting BRAF V600E mutations with concurrent dabrafenib and trametinib in anaplastic pleomorphic xanthoastrocytoma resulted in a transient radiographic and clinical response and no therapeutic benefit in a patient with an epithelioid glioblastoma.
Conclusion:
BRAF/MEK inhibition did not produce a durable treatment effect in glioblastoma or pleomorphic xanthoastrocytoma with BRAF V600E alterations. Heterogenicity of related cases in the literature makes an evaluation of efficacy BRAF targeting therapies in gliomas difficult and requires additional investigation.
Keywords: : BRAF inhibitor, BRAFV600E mutation, dabrafenib, glioblastoma, glioma, MEK inhibitor, pleomorphic xanthoastrocytoma, targeted therapy, trametinib
Next-generation sequencing of cancers has become commonly used to identify potentially targetable alterations [1]. BRAF is a gene that has roles in cellular proliferation, apoptosis, angiogenesis, migration and differentiation via the RAS/RAF/MEK/ERK kinase pathway. When mutated in cancers, it acts as an oncogene, leading to uncontrolled proliferation [2]. Targeting this pathway with BRAF and MEK inhibitors has been a successful approach to treat some cancers, including BRAF V600E mutant anaplastic thyroid cancer [3], metastatic non-small-cell lung cancer [4] and melanoma [5]. Among primary brain tumor subtypes, BRAF V600E mutations are found in up to two-thirds of pleomorphic xanthoastrocytomas, up to a half of gangliogliomas and in nearly a tenth of pilocytic astrocytomas [6]. The fusion oncogene KIAA1549-BRAF has also been identified in up to 66% of pilocytic astrocytomas and pediatric low-grade diffuse astrocytomas but at lower rates in higher grade gliomas [7–9]. There have not been any randomized trials in adults using BRAF-targeting therapies in gliomas and the literature is limited to one early open basket trial and approximately 28 case reports [10,11]. However, these case reports are likely biased toward unusually positive results. Here, we describe cases of anaplastic pleomorphic xanthoastrocytoma (PXA) and epithelioid glioblastoma with BRAF V600E mutations treated with trametinib and dabrafenib with variable outcomes.
Case series
Case 1
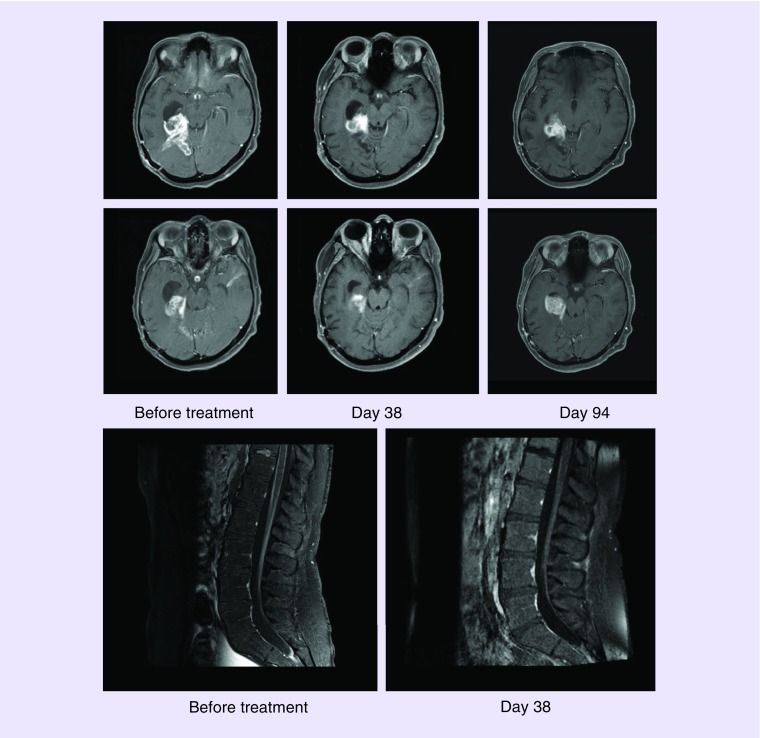
A 23-year-old woman presented with headaches and, after a near-total resection, was found to have a right temporal-parietal lobe mass diagnosed initially as a glioblastoma, IDH-wild type. She received standard therapy of concurrent radiation and temozolomide. Next-generation sequencing using DNA isolated from formalin-fixed paraffin-embedded tumor tissue identified a BRAF V600E alteration (with calculated mean allele frequency of 71%) and homozygous deletion of CDKN2A/B. Re-evaluation of the tissue slides revised the diagnosis to an anaplastic PXA. A total of 161 days after diagnosis, she was admitted to the hospital for a clinical decline and was found to have radiographic progression, including a mass in the right temporal horn of the lateral ventricle and marked diffuse enhancement of cauda equina and distal spinal cord meninges consistent with leptomeningeal metastasis. She was started on dabrafenib 150 mg twice daily and trametinib 2 mg by mouth daily, which she tolerated well. Follow-up imaging 38 days later showed a decreased size of the right temporal lesion from 5.2 × 3 cm to 2.3 × 2.2 cm and markedly decreased enhancement of the cauda equina and conus (Figure 1). Clinically, she had lessened back pain and improved energy levels. Durable response to targeted therapy was not seen and, 94 days after starting targeting therapy, she had radiographic and clinical decline. She was transitioned to monotherapy with bevacizumab dosed at 10 mg per kg every 2 weeks intravenously and transitioned to hospice. Her overall survival from tissue diagnosis was 292 days and from the start of concurrent dabrafenib and trametinib was 131 days.
Figure 1. . Gadolinium-enhanced T1 sequences magnetic resonance imaging from case 2.
(A) Right temporal anaplastic pleomorphic xanthoastrocytoma with BRAF V600E mutation before, 38 and 94 days after initiating dabrafenib at 150 mg twice a day and trametinib 2 mg daily. Partial treatment response is seen at day 38 of treatment followed by reoccurrence at day 94 of treatment. (B) Diffuse enhancement of the cauda equina before (left) and radiographic improvement (right) 38 days after concurrent treatment with dabrafenib and trametinib. Recurrence in the spine occurred on day 94 (not shown).
Case 2
A 47-year-old man presented with auditory and visual hallucinations, followed by a generalized seizure. After gross-total resection, he was found to have a right temporal lobe mass diagnosed as anaplastic astrocytoma, IDH-wild type, with molecular features of glioblastoma grade IV based on EGFR amplification, O-6-methylguanine-DNA methyltransferase (MGMT) promotor non-methylated. He received concurrent radiation and temozolomide without adjuvant temozolomide. He had reoccurrence 2 years later and was treated with two additional cycles of temozolomide before stopping due to continued progression. He was placed on infusions of bevacizumab dosed at 10 mg per kg every 2 weeks intravenously for 8 months until radiographic progression, including a new extracranial extension of tumor in the right parotid gland and lymph nodes. Subtotal resection of the tumor was consistent with epithelioid glioblastoma. Next-generation sequencing using DNA isolated from formalin-fixed paraffin-embedded tumor tissue demonstrated mutations in BRAF V600E (with calculated mean allele frequency of 57%), EGFR T263P, amplification of CDK6, point mutation of TERT (promoter-146C>T), androgen receptor (G461_G473del) and homozygous deletion of CDKN2A/B. The patient underwent a further 2 months of treatment with bevacizumab and additional radiation to his neck, parotid gland and intracranial mass for a total of 6000 cGy in 30 fractions. Further progression was seen 3.5 months later and included a new lesion in the left lateral ventricle. He was started on oral dabrafenib 150 mg twice daily and trametinib 2 mg daily, which he tolerated well. Only 43 days later, he demonstrated further clinical and radiographic progression and was transitioned to hospice care. His overall survival from initial tissue diagnosis was 3.3 years and from his second surgery was 240 days.
Discussion
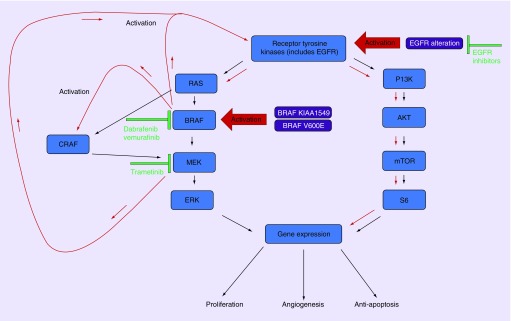
Both cases had BRAF V600E alterations targeted by dabrafenib and trametinib without durable response. Patient one, with an anaplastic PXA, had a transient response followed by rapid progression. The patient with epithelioid glioblastoma in case 2 had no benefit. Notably, the patient with epithelioid glioblastoma also had a gain of function mutation of EGFR (T263P). Colon cancers frequently have high expression of EGFR and further activation of EGFR has been seen in BRAF V600E mutant colon cancers treated with BRAF inhibitors and subsequent treatment resistance [12]. Conversely, EGFR expression in melanoma tends to be low and has been suggested to be a contributing reason why there has been an 80% response rate to BRAF inhibition in melanoma versus 5% in colon cancer [13,14]. This observation has also been seen in preclinical studies, showing that EGFR amplification in glioma cell lines leads to resistance to BRAF inhibition and that blocking EGFR may be a means to overcome resistance [15]. This resistance could be mediated in part to adaptive feedback reactivation of MAPK signaling, often mediated by EGFR which can, in turn, lead to activation of other RAF kinases, such as CRAF, which are resistant to BRAF inhibitors (Figure 2) [16,17]. MEK inhibitors are also known to enhance epidermal growth factor-induced AKT activation [18,19]. Studies in prostate cancer have shown that androgen receptor signaling also activates the RAS/MAPK pathway [20]. However, it is unclear to what degree the androgen receptor alteration in case 2 contributed to resistance to BRAK/MEK inhibition and there is insufficient data to support using androgen deprivation in primary brain tumor patients. Even without EGFR amplification, BRAF/MEK inhibition results in a multitude of poorly understood resistance mechanisms, including negative regulation between RAS-ERK and PI3K-AKT (Figure 2) [16,21].
Figure 2. . RTK/MAPK feedback pathways of RAS/P13K following BRAF and MEK inhibition.
Inhibition of BRAF leads to reduced ERK-dependent feedback and increase activation of RTK and activation of alternative RAF, such as CRAF which stimulates MEK resulting in reactivation of the MAPK pathway. Inhibiting MEK concurrently with BRAF helps prevent reactivated MAPK pathway but results in increased activation of RTK and increased P13K/AKT pathway activation, which can result in treatment resistance. Alterations in EGFR that result in amplification may result in resistance to BRAF inhibition and are a possible concurrent therapeutic target. Red arrows indicate an increase of activation of pathways.
AKT: Protein kinase B; BRAF: Proto-oncogene B-Raf; CRAF: Proto-oncogene c-RAF; mTOR: Mammalian target of rapamycin; P13K: Phosphoinositide 3-kinase; RTK: Receptor tyrosine kinase; S6: p70 ribosomal protein S6 kinase.
Almost all reported cases of adult brain tumor patients with BRAF alteration have been treated with targeting therapy after progression on temozolomide and some also after bevacizumab [10]. Including the patients presented here, many cases in the literature have concurrent treatments including temozolomide, carmustine, tumor treating fields and surgically placed carmustine wafers, making assessments of efficacy difficult [22–24]. Further complicating the issue, of the 28 or more adult BRAF V600E mutant glioma case reports described in the literature, there is a mixed use of dabrafenib versus vemurafenib, with or without trametinib [10]. Dabrafenib is believed to have superior enhanced blood–brain barrier penetration compared with vemurafenib, as evidenced by increased response rates (16 vs 31%) in intracranial melanoma and, similarly, concurrent MEK with BRAF inhibition may delay resistance to therapy [10,25]. There are currently no trials to support which combination of these therapies are superior in primary brain tumors. Targeting BRAF alterations in glioma patients may yield different responses across histologies, similar to our experience and what has been observed in a Phase II basket study of a variety of malignancies [26]. A recent open-label, nonrandomized, multicohort trial evaluated 24 patients with BRAF V600E mutant brain tumors, which included PXAs, gangliogliomas and high-grade diffuse astrocytomas treated with vemurafenib. In contrast to the positive single case reports and, similar to our presented cases, an overall response rate of 25% with mixed results was seen in each group [11]. Limitations to targeting therapies in neuro-oncology patients include overcoming the blood–brain barrier [27] and intratumor heterogeneity [28,29].
Experience with BRAF targeting therapies in pediatric brain tumors is generally limited to case reports and series. This includes a complete response to vemurafenib in a 9-year-old boy with glioblastoma with a BRAF V600E mutation with no description of EGFR or loss of CDKN2A [30]. Another case series of four children with recurrent PXAs with BRAF V600E mutations were treated with vemurafenib and found progressive disease in one patient, stable in two and a partial response in another, with a median progression free survival of 5 months and overall survival of 8 months [31]. Recently, there was a preliminary analysis of a Phase I/II trial (NCT01677741) using monotherapy with dabrafenib in 32 recurrent pediatric patient brain tumor patients (of which 41% were pilocytic astrocytomas and 22% were gangliogliomas) with BRAF V600E mutations had an overall response rate of 44% [32]. In pediatric gliomas, BRAF V600E and CDKN2A mutations have a tendency to undergo malignant transformation and have poorer outcomes. Additionally, loss of CDKN2A has been identified to contribute independently to poor outcomes in BRAF V600E mutant pediatric low-grade glioma [33,34]. Based on this observation, it is possible that the loss of CDKN2A in the patients presented could also play a role in resistance to targeted therapy. However, this is contradicted by the findings in a boy with low-grade astrocytoma with a BRAF V600E mutation treated with dabrafenib for 40 weeks before progression who was found to have acquired a BRAF L514V mutation, leading to resistance after genetic analysis of tissue before and after targeted therapy [35]. These studies, similar to those in adults, are difficult to interpret given the heterogeneity of histologies and treatments and should be interpreted with caution when extrapolating to adults and vice versa.
Notably, both presented cases represent treatment with BRAF/MEK inhibitors, without other concomitant therapy, after definitive radiographic progression outside of prior radiation fields. Combinatorial multiagent regimens may be favored in the future but remain to be seen.
Conclusion
In this case series, minimal clinical benefit was seen after targeting BRAF V600E in anaplastic PXA and glioblastoma with concurrent dabrafenib and trametinib. These cases suggest that targeting BRAF alterations through BRAF/MEK inhibition in the treatment of high-grade diffuse gliomas in adult patients will not be as successful as it is in other BRAF mutated cancers. Based on studies of different malignancies, EGFR-amplified gliomas with BRAF alterations may be resistant to BRAF inhibition. To avoid publication biases, there is a need to report poor responders in brain tumor patients treated with molecularly targeted therapies in BRAF and other alterations. Additional studies are needed to understand when these targeting agents could play a role in combination with other treatments to overcome redundant oncogenic molecular pathways.
Executive summary.
Targeting BRAF mutations has shown to be beneficial for a variety of cancers; however, there is limited high-level evidence of the efficacy in primary brain tumors.
Concurrent inhibition of BRAF and MEK with dabrafenib and trametinib did not produce a durable treatment effect in pretreated adults with recurrent glioblastoma or anaplastic pleomorphic xanthoastrocytoma with mutations in BRAFV600E.
Similar to other cancers, a gain of function mutations in EGFR could lead to resistance to BRAF-targeting therapies in primary brain tumors and may be a concurrent therapeutic target with BRAF-inhibiting treatments.
Heterogenicity of BRAF-targeting therapy in primary brain tumors in the literature makes an evaluation of the efficacy of these therapies difficult and requires additional investigation.
Acknowledgments
The authors thank the patients and their families.
Footnotes
Financial & competing interests disclosure
This research is supported by Award Number P30CA042014 from the National Cancer Institute. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Gagan J, Van Allen EM. Next-generation sequencing to guide cancer therapy. Genome Med. 7(1), 80 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ascierto PA, Kirkwood JM, Grob J-J. et al. The role of BRAF V600 mutation in melanoma. J. Transl. Med. 10(1), 85 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subbiah V, Kreitman RJ, Wainberg ZA. et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J. Clin. Oncol. 36(1), 7–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Odogwu L, Mathieu L, Blumenthal G. et al. FDA approval summary: dabrafenib and trametinib for the treatment of metastatic non‐small-cell lung cancers harboring BRAF V600E mutations. Oncologist 23(6), 740–745 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Long GV, Hauschild A, Santinami M. et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N. Engl. J. Med. 377(19), 1813–1823 (2017). [DOI] [PubMed] [Google Scholar]; •• Provides high-level evidence that concurrent inhibition of BRAF and MEK with dabrafenib and trametinib can provide a durable treatment response in BRAF V600E mutant melanoma which may be applicable to primary brain tumors.
- 6.Chen R, Smith-Cohn M, Cohen AL, Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics 14(2), 284–297 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Provides overview and summary of primary brain tumors with BRAF alterations.
- 7.Penman CL, Faulkner C, Lowis SP, Kurian KM. Current understanding of BRAF alterations in diagnosis, prognosis, and therapeutic targeting in pediatric low-grade gliomas. Front. Oncol. 5, 54 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones DTW, Kocialkowski S, Liu L. et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 68(21), 8673–8677 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forshew T, Tatevossian RG, Lawson AR. et al. Activation of the ERK/MAPK pathway: a signature genetic defect in posterior fossa pilocytic astrocytomas. J. Pathol. 218(2), 172–181 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Maraka S, Janku F. BRAF alterations in primary brain tumors. Discov. Med. 26(141), 51–60 (2018). [PubMed] [Google Scholar]; •• Comprehensive review of the pathology of primary brain tumors with BRAF alterations and summary of known cases with BRAF targeting therapy.
- 11.Kaley T, Touat M, Subbiah V. et al. BRAF inhibition in BRAFV600E.mutant gliomas: results from the VE-BASKET study. J. Clin. Oncol. 36(35), 3477–3484 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Phase II open-label, nonrandomized, basket study that shows different responses across histologies to 24 patients BRAF V600E mutant primary brain tumors treated with vemurafenib.
- 12.Prahallad A, Sun C, Huang S. et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 483(7388), 100–103 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Richman SD, Seymour MT, Chambers P. et al. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J. Clin. Oncol. 27(35), 5931–5937 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Roth AD, Tejpar S, Delorenzi M. et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J. Clin. Oncol. 28(3), 466–474 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Yao T-W, Zhang J, Prados M, Weiss WA, James CD, Nicolaides T. Acquired resistance to BRAF inhibition in BRAFV600E mutant gliomas. Oncotarget 8(1), 583–595 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Shows preclinical data that EGFR upregulation is a resistance mechanism to BRAF inhibition in glioma cell lines and that concurrent inhibition of EGFR can overcome resistance.
- 16.Corcoran RB, André T, Atreya CE. et al. Combined BRAF, EGFR, and MEK inhibition in patients with BRAFV600E.mutant colorectal cancer. Cancer Discov. 8(4), 428–443 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corcoran RB, Ebi H, Turke AB. et al. EGFR-mediated reactivation of MAPK signaling contributes to insensitivity of BRAF-mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2(3), 227–235 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoeflich KP, O’Brien C, Boyd Z. et al. In vivo antitumor activity of MEK and phosphatidylinositol 3-kinase inhibitors in basal-like breast cancer models. Clin. Cancer Res. 15(14), 4649–4664 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Yu CF, Liu Z-X, Cantley LG. ERK negatively regulates the epidermal growth factor-mediated interaction of Gab1 and the phosphatidylinositol 3-kinase. J. Biol. Chem. 277(22), 19382–19388 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Liao RS, Ma S, Miao L, Li R, Yin Y, Raj GV. Androgen receptor-mediated non-genomic regulation of prostate cancer cell proliferation. Transl. Androl. Urol. 2(3), 187–196 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem. Sci. 36(6), 320–328 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreck KC, Guajardo A, Lin DDM, Eberhart CG, Grossman SA. Concurrent BRAF/MEK inhibitors in BRAF V600-mutant high-grade primary brain tumors. J. Natl Compr. Canc. Netw. 16(4), 343–347 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Beba Abadal K, Walsh MA, Yachnis AT, Tran DD, Ghiaseddin AP. Eleven month progression-free survival on vemurafenib monotherapy in a patient with recurrent and metastatic BRAF V600E-mutated glioblastoma WHO grade 4. JCO Precis. Oncol. 1, 1–5 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Meletath SK, Pavlick D, Brennan T. et al. Personalized treatment for a patient with a BRAF V600E mutation using dabrafenib and a tumor treatment fields device in a high-grade glioma arising from ganglioglioma. J. Natl Compr. Canc. Netw. 14(11), 1345–1350 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Arozarena I, Wellbrock C. Overcoming resistance to BRAF inhibitors. Ann. Transl. Med. 5(19), 387–387 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hyman DM, Puzanov I, Subbiah V. et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N. Engl. J. Med. 373(8), 726–736 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Tellingen O, Yetkin-Arik B, de Gooijer MC, Wesseling P, Wurdinger T, de Vries HE. Overcoming the blood–brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updat. 19, 1–12 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Brastianos PK, Nayyar N, Rosebrock D. et al. Resolving the phylogenetic origin of glioblastoma via multifocal genomic analysis of pre-treatment and treatment-resistant autopsy specimens. NPJ Precis. Oncol. 1(1), 33 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahlokozera T, Vellimana AK, Li T. et al. Biological and therapeutic implications of multisector sequencing in newly diagnosed glioblastoma. Neuro Oncol. 20(4), 472–483 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson GW, Orr BA, Gajjar A. Complete clinical regression of a BRAF V600E-mutant pediatric glioblastoma multiforme after BRAF inhibitor therapy. BMC Cancer 14(1), 258 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chamberlain MC. Salvage therapy with BRAF inhibitors for recurrent pleomorphic xanthoastrocytoma: a retrospective case series. J. Neurooncol. 114(2), 237–240 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Kieran MW, Bouffet E, Broniscer A. et al. Efficacy and safety results from a Phase I/IIa study of dabrafenib in pediatric patients with BRAF V600-mutant relapsed refractory low-grade glioma. J. Clin. Oncol. 36(Suppl. 15), 10506–10506 (2018). [Google Scholar]; •• Preliminary analysis of a Phase I/II trial (NCT01677741) that uses monotherapy with dabrafenib in 32 recurrent pediatric brain tumor patients with BRAF V600E mutations had an overall response rate of 44%.
- 33.Lassaletta A, Zapotocky M, Mistry M. et al. Therapeutic and prognostic implications of BRAF V600E in pediatric low-grade gliomas. J. Clin. Oncol. 35(25), 2934–2941 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mistry M, Zhukova N, Merico D. et al. BRAF mutation and CDKN2A deletion define a clinically distinct subgroup of childhood secondary high-grade glioma. J. Clin. Oncol. 33(9), 1015–1022 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Yao Z, Jonsson P. et al. A secondary mutation in BRAF confers resistance to RAF inhibition in a BRAFV600E -mutant brain tumor. Cancer Discov. 8(9), 1130–1141 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]