Abstract
The muscle thin filament protein troponin (Tn) regulates contraction of vertebrate striated muscle by conferring Ca2+ sensitivity to the interaction of actin and myosin. Troponin C (TnC), the Ca2+ binding subunit of Tn contains two homologous domains and four divalent cation binding sites. Two structural sites in the C-terminal domain of TnC bind either Ca2+ or Mg2+, and two regulatory sites in the N-terminal domain are specific for Ca2+. Interactions between TnC and the inhibitory Tn subunit troponin I (TnI) are of central importance to the Ca2+ regulation of muscle contraction and have been intensively studied. Much remains to be learned, however, due mainly to the lack of a three-dimensional structure for TnI. In particular, the role of amino acid residues near the C-terminus of TnI is not well understood. In this report, we prepared a mutant TnC which contains a single Trp-26 residue in the N-terminal, regulatory domain. We used fluorescence lifetime and quenching measurements to monitor Ca2+- and Mg2+-dependent changes in the environment of Trp-26 in isolated TnC, as well as in binary complexes of TnC with a Trp-free mutant of TnI or a truncated form of this mutant, TnI(1–159), which lacked the C-terminal 22 amino acid residues of TnI. We found that full-length TnI and TnI(1–159) affected Trp-26 similarly when all four binding sites of TnC were occupied by Ca2+. When the regulatory Ca2+-binding sites in the N-terminal domain of TnC were vacant and the structural sites in the C-terminal domain of were occupied by Mg2+, we found significant differences between full-length TnI and TnI(1–159) in their effect on Trp-26. Our results provide the first indication that the C-terminus of TnI may play an important role in the regulation of vertebrate striated muscle through Ca2+-dependent interactions with the regulatory domain of TnC.
Keywords: muscle, regulation, troponin, calcium, fluorescence
Vertebrate striated muscle contraction is regulated by Ca2+ and requires the proteins troponin (Tn)2 and tropomyosin (Tm), located on the actin-containing thin filaments. Tn is composed of three subunits: TnC, which binds Ca2+, TnI, which inhibits actomyosin ATPase activity, and TnT, which binds Tm (for recent reviews, see Refs. 1–5). The purpose of this paper is to help delineate functionally important interactions between TnC and TnI.
The crystal structure of vertebrate fast skeletal muscle TnC shows a dumbbell-shaped molecule with two globular domains, each of which contains a pair of Ca2+-binding sites (6–8). The sites in the N-terminal domain are regulatory and Ca2+-specific, while the sites in the C-terminal domain bind both Ca2+ and Mg2+ and play a structural role. Despite many studies over the past quarter century, much remains to be learned about the three-dimensional structure of TnI and the nature of its extensive and complex interactions with TnC (see Ref. 9 for recent review). TnI binds to TnC in an antiparallel orientation, with the N-terminus of TnC binding the C-terminus of TnI and vice versa (10, 11). The N-terminal region (residues 1–47) and a centrally located inhibitory segment (residues 96–116) of TnI have long been established as sites of interaction with TnC (12). The inhibitory segment, like whole TnI, binds to actin and inhibits actomyosin ATPase activity in the absence of Ca2+. This inhibition can be reversed by TnC in the presence of Ca2+ (12). Binding of Ca2+ to the regulatory sites of TnC induces localized conformational changes which result in the exposure of a hydrophobic pocket in the N-terminal domain. This pocket interacts with a segment of TnI, residues 115–131, which overlaps the C-terminal part of the TnI inhibitory region (13, 14). Pearlstone et al. (15) prepared a spectral probe mutant, F29W, of chicken fast skeletal muscle TnC. In this mutant, Phe-29, which is near one of the regulatory Ca2+ binding sites in the N-terminal domain, was replaced by a single Trp residue. The quantum yield of the steady-state fluorescence of F29W increased about threefold in response to Ca2+ binding to the N-terminal, regulatory sites of TnC. Binding of Mg2+ to the structural sites in the C-terminal domain of TnC did not affect the fluorescence properties of F29W.
Little is known about interactions between TnC and the most C-terminal region of TnI. A deletion mutant, TnI1–156, of chicken fast skeletal muscle TnI, equivalent to our TnI(1–159), mimicked the ability of full-length TnI to inhibit actomyosin ATPase in the absence of TnC and to release the inhibition when TnC and Ca2+ were added. Inhibition by full-length TnI was maintained when TnC and Mg2+ (but not Ca2+) were added, but inhibition by TnI1–156 was 80% reversed under these conditions (11). Thus, the C-terminal 26 residues which are missing from TnI1–156 appear to play a role in conferring Ca2+ sensitivity to the regulation of muscle contraction. The structural basis for this role is not yet well understood, but a recent, detailed investigation (16) of the functional properties of a series of C-terminal deletion mutants of TnI led to the conclusion that the entire C-terminal region of TnI is needed for full regulatory activity. Ramos (16) suggested that deletion of the C-terminal 26 residues of TnI may reduce the affinity of TnI for the thin filament, thereby allowing TnC to reverse the inhibitory activity of TnI1–156, even in the absence of Ca2+.
In the present study, we have prepared a mutant, TnCF26W, of rabbit fast skeletal muscle TnC, which is equivalent to the spectral probe mutant of Pearlstone et al. (15) and contains a single Trp at position 26. We used TnCF26W in fluorescence lifetime and quenching studies of both isolated TnC and TnC in binary complexes with a Trp-free mutant of full-length rabbit fast skeletal muscle TnI or a truncated form of this mutant, TnI(1–159), which lacked the C-terminal 22 amino acid residues of the full-length TnI. Our results suggest for the first time that the functional importance of the C-terminal segment (residues 160–181) of TnI may be attributed, at least in part, to interactions with the regulatory, N-terminal domain of TnC.
MATERIALS AND METHODS
Protein preparation.
In these studies we used recombinant forms of TnC and TnI whose sequences were based on those of the rabbit fast skeletal muscle proteins. Our full-length TnI, dCW-TnI, was a Cys-less, Trp-less mutant in which the Cys-48 and Cys-64 of the wild-type protein had been replaced by Ala, Cys-133 had been replaced by Ser and Trp-160 had been replaced by Phe, prepared as described previously (17). We prepared the deletion mutant TnI(1–159) by substituting a stop codon for the Trp-160 codon in the cDNA for dCW-TnI. We prepared wild-type TnC as described previously (18). We prepared the single-Trp TnC mutant TnCF26W by substituting a Trp codon for the Phe-26 codon in the cDNA for wild-type TnC. We screened clones containing the designed mutations by DNA sequencing. For construction of TnI expression plasmids, we excised NcoI–EcoRI fragments, which contained TnI-coding sequences from double-stranded M13mp18/TnI (19) and subcloned them into NcoI–EcoRI sites of pTrc99. We expressed and purified TnCF26W as described by Kobayashi et al. (18). We expressed and purified TnI mutants as described previously (19, 20). Protein concentrations were measured by their absorbance at 280 nm. Extinction coefficients for TnCF26W and dCW-TnI were calculated using the method of Gill and von Hippel (21). Complexes of TnCF26W and either dCW-TnI or TnI(1–159) were formed by first mixing equimolar amounts of TnC and TnI in a buffer of 6 M urea, 20 mM Tris, pH 8.0, 1 M NaCl, 1 mM DTT, and 5 mM CaCl2. The complexes were dialyzed against a series of buffers containing 0.1 mM DTT and decreasing concentrations of urea and NaCl and purified by ion-exchange HPLC using a Pharmacia Mono-Q column.
Fluorescence measurements.
Quantum yields and quenching of Trp fluorescence were measured on an Instruments, S.A. model Spex Fluoromax 2 fluorimeter at 25°C, with polarizers set to magic angle conditions, and an excitation wavelength of 295 nm. Quantum yields of Trp-26 in TnCF26W were calculated using the method of Parker and Rees (22), with Trp in water (23) as the standard. Measurements were made in a buffer solution containing 20 mM Tris, pH 8, 0.3 M KCl, and 2 mM EGTA, the same buffer plus 20 mM MgCl2, and the same buffer plus 2.5 mM CaCl2.
Iodide quenching was measured by titrating protein solutions with 6 M KI and observing the decrease in fluorescence intensity at the emission maximum. Samples were prepared in 20 mM Tris (pH 8.0), 2 mM EGTA, 0.1 M KCl, and 1 mM DTT. Acrylamide quenching was measured in a buffer solution containing 20 mM Tris, pH 8, 0.3 M KCl, and 2 mM EGTA, plus either 1 mM DTT (for TnCF26W and TnCF26W-TnI(1–159)) or 0.1 mM DTT (for TnCF26W–dCW-TnI). Acrylamide quenching data was corrected for the absorbance of acrylamide at the excitation wavelength (24) and when it was nonlinear it was fit to the equation:
| [1] |
where Fo and F are the fluorescence intensity in the absence and presence, respectively, of quencher, Ks.v. is the Stern–Volmer quenching constant, [Q] is the concentration of quencher, and V is a static quenching term. NATA, a small indole-containing molecule, was used as a standard to determine the amount of quenching expected in a fully exposed Trp side chain.
Lifetime measurements were performed using the frequency domain fluorometry technique as previously described (25–27). Excitation at 295 nm was provided by the frequency-doubled output of a rhodamine 6G dye laser synchronously pumped by a mode-locked argon ion laser. The dye laser was cavity dumped at 3.77 MHz, and measurements at higher frequencies were performed using the harmonic content of the picosecond pulses (28–30). Emission was observed at 350 nm using a 350 nm interference filter. All measurements were performed under magic angle conditions.
RESULTS
Fluorescence Properties of Isolated TnCF26W
The effects of Ca2+-binding site occupancy on the steady-state fluorescence of Trp-26, the only Trp residue in TnCF26W, were essentially the same as those previously obtained with the equivalent TnC mutant from chicken fast skeletal muscle (15). Binding of Mg2+ to sites in the C-terminal domain of TnCF26W caused no change in quantum yield compared to the apo form, while binding of Ca2+ to the N-terminal domain resulted in a greater than threefold increase in quantum yield. The wavelength of the maximum fluorescence emission, λmax, was essentially invariant (see Table I).
TABLE I.
Spectral Properties of Trp-26 in TnCF26W
| TnCF26W | TnCF26W-dCW-TnI | TnCF26W-TnI(1–159) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| EGTA | Mg2+ | Ca2+ | EGTA | Mg2+ | Ca2+ | EGTA | Mg2+ | Ca2+ | |
| λmax (nm) | 343 | 342 | 345 | 344 | 344 | 330 | 344 | 344 | 330 |
| Q | 0.12 | 0.12 | 0.39 | 0.11 | 0.11 | 0.28 | 0.091 | 0.095 | 0.2l |
| τmean (ns) | 3.25 | 3.74 | 7.08 | 3.24 | 3.52 | 5.03 | 3.30 | 3.51 | 5.0l |
| τ1 (ns) | 3.34 | 5.14 | 7.26 | 3.47 | 6.10 | 5.22 | 3.42 | 4.14 | 5.16 |
| τ2 (ns) | 0.40 | 2.75 | 1.48 | 0.49 | 3.04 | 0.66 | 0.40 | 2.57 | 0.68 |
| τ3 (ns) | 0.31 | 0.34 | 0.2l | ||||||
| A1 | 0.80 | 0.55 | 0.86 | 0.63 | 0.07 | 0.75 | 0.73 | 0.40 | 0.80 |
| A2 | 0.20 | 0.21 | 0.14 | 0.37 | 0.54 | 0.25 | 0.27 | 0.36 | 0.20 |
| A3 | 0.24 | 0.38 | 0.24 | ||||||
Note. Ai, fractional amplitude of component lifetime τi; Q, quantum yield; λmax, wavelength at which maximum fluorescence emission was observed; τmean, mean lifetime.
The mean fluorescence lifetime of Trp-26 increased by 15% when Mg2+ bound to the apo form of TnCF26W, and doubled when Ca2+ bound. The heterogeneity of the fluorescence decay increased in the Mg2+-bound, but not the Ca2+-bound, form, requiring a three-exponential rather than a two-exponential fit for the data. The long lifetime and high quantum yield of Trp-26 in the Ca2+-bound form of TnCF26W indicate a significant absence of both static and solvent quenching.
A heterogeneous decay such as is seen here can be an indication of multiple species of Trp. Since our protein has a single Trp it may be an indication that the protein exists in more than one conformation simultaneously. However, this is only one of many possible causes of lifetime heterogeneity, which include protein dynamics, spectral relaxation, and other molecular interactions (24).
Fluorescence Properties of the TnC-TnI Complexes
Formation of a complex of TnCF26W and dCW-TnI had little effect on the steady-state fluorescence of Trp-26 in the absence of Ca2+. The quantum yields and emission maxima of Trp-26 in both EGTA and Mg2+ were only slightly changed by complex formation. In the presence of Ca2+, however, formation of a complex with dCW-TnI resulted in a 14-nm blue shift in the emission maximum and a 30% reduction in the quantum yield of Trp-26.
Formation of a complex of TnCF26W and TnI(1–159) in the absence of Ca2+ resulted in only slightly lower quantum yields of Trp-26 than those obtained from the complex with full-length dCW-TnI, and no difference in the emission maxima. In the presence of Ca2+, however, the quantum yield of the TnCF26W–TnI(1–159) complex was 25% lower than the already diminished (compared to isolated TnCF26W) quantum yield of the TnCF26W–dCW-TnI complex. Both the TnCF26W–TnI(1–159) and TnCF26W–dCW-TnI complexes had the same blue-shifted λmax of 330 nm in the presence of Ca2+, indicating a substantial decrease in the exposure of Trp-26.
In the presence of EGTA, formation of the TnC–TnI complex using either dCW-TnI or TnI(1–159) had little effect on either the individual or the mean lifetimes of Trp-26. In the presence of Ca2+, the mean lifetimes of both complexes were about 30% lower than those of isolated TnCF26W. A difference between the two complexes could be seen in the presence of Mg2+, when the structural sites in the C-terminal domain of TnC were occupied by Mg2+ and the regulatory Ca2+-binding sites in the N-terminal domain of TnC were vacant. Formation of the TnCF26W–dCW-TnI complex in the Mg2+-bound form greatly reduced the contributions of the longest of three lifetimes, thereby lowering the mean lifetime and leaving a less heterogeneous system. When TnI(1–159) was used to form the TnC–TnI complex, the mean lifetime in the Mg2+-bound form was also reduced, but the lifetime heterogeneity was more comparable to that seen in isolated TnCF26W.
Fluorescence Quenching by Acrylamide
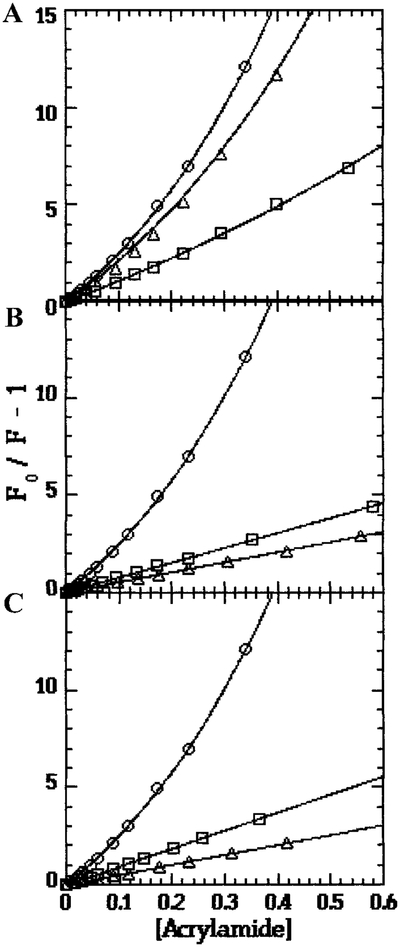
Acrylamide quenching showed no significant difference in the exposure of Trp-26 between the Mg2+ and Ca2+ bound forms of isolated TnCF26W. Both samples showed some static quenching, as revealed by the upward curvature of the plots (Table II and Fig. 1A).
TABLE II.
Quenching of Trp-26 Fluorescence
| TnCF26W | TnCF26W-dCW-TnI | TnCF26W-TnI(1–159) | NATA | ||||
|---|---|---|---|---|---|---|---|
| Mg2+ | Ca2+ | Mg2+ | Ca2+ | Mg2+ | Ca2+ | ||
| τo_(ns) | 3.74 | 7.08 | 3.52 | 5.2 | 3.51 | 5.01 | 3.02 |
| Ks.v. (M−1)a | 9.88 | 17.98 | 7.63 | 5.18 | 9.28 | 5.09 | 20 |
| V (M−1)a | 0.45 | 1.14 | 1.53 | ||||
| kq (M ns)−1a | 2.64 | 2.54 | 2.17 | 1.00 | 2.64 | 1.02 | 6.62 |
| kq/Ka | 0.40 | 0.38 | 0.33 | 0.15 | 0.40 | 0.15 | 1.00 |
| Ks.v. (M−1)b | 0.97 | 2.17 | 1.57 | 1.56 | 1.97 | 0.39 | 14.3 |
| kq (M ns)−1b | 0.26 | 0.31 | 0.45 | 0.30 | 0.56 | 0.08 | 4.74 |
| kq/Kb | 0.055 | 0.065 | 0.094 | 0.063 | 0.118 | 0.016 | 1.00 |
Note. kq is the bimolecular quenching constant calculated from the Stern–Volmer constant Ks.v. by the equation Ks.v. = kq*τo; τo is the lifetime of the sample in the absence of quencher; Ks is the bimolecular quenching constant for the standard NATA.
Acrylamide quenching.
Iodide quenching.
FIG. 1.
Acrylamide Quenching of Trp-26 in TnCF26W. (○) NATA standard, (○) Sample in Ca2+, (□) Sample in Mg2+. Samples: (A) isolated TnCF26W; (B) TnCF26W-dCW-TnI complex; (C) TnCF26W-TnI(1–159) complex.
In the Ca2+-bound forms, both the TnCF26W–dCW-TnI and TnCF26W–TnI(1–159) complexes had kq values that were 60% lower than the kq of isolated TnCF26W (Table II), showing a substantial reduction in the exposure of Trp-26 to acrylamide. A difference between the two complexes could be seen in their Mg2+-bound forms. Under these conditions the kq values of isolated TnCF26W and the TnCF26W–TnI(1–159) complex were identical, but the kq of the TnCF26W–dCW-TnI complex was 20% lower. These results suggest that the portions of TnI, which shield Trp-26 in the Ca2+-bound form are located within residues 1–159, but residues 160–181 at the C-terminus of TnI are needed for blocking access to Trp-26 in the Mg2+-bound form of the TnC–TnI complex. The plots of Fo/F for both complexes are linear in both the Ca2+- and Mg2+-bound forms, indicating an absence of static quenching (Figs. 1b and 1c).
Fluorescence Quenching by Iodide
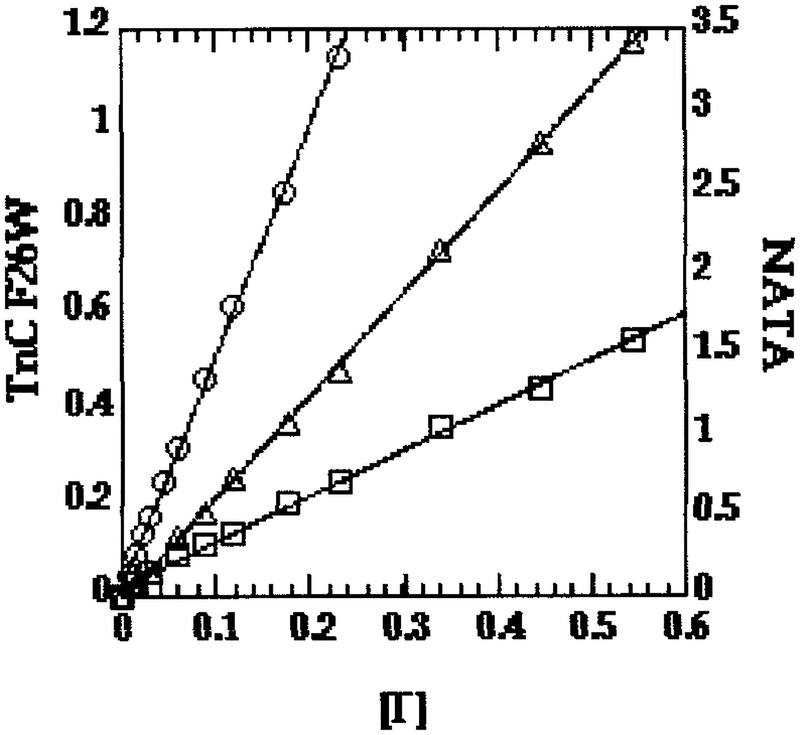
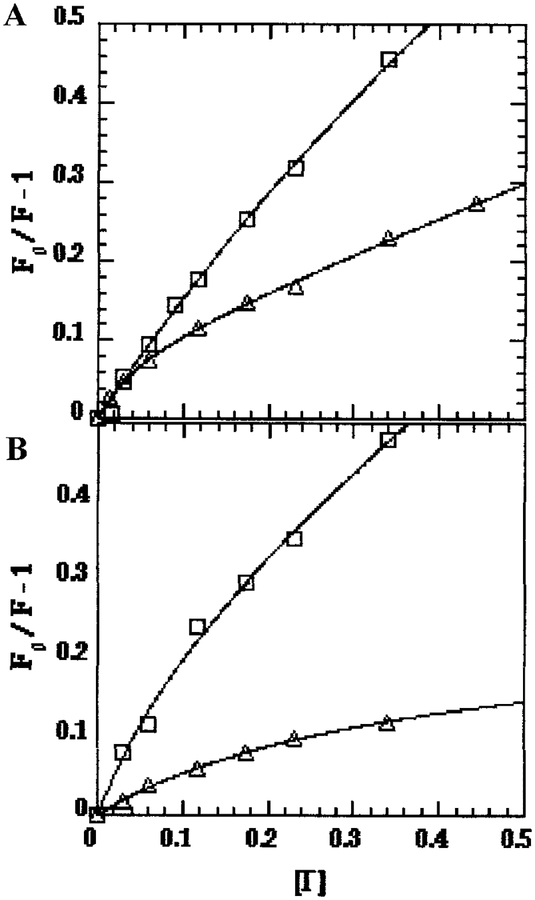
Because of its charge and large size, iodide is considered to be a probe of surface exposure, whereas smaller, uncharged quenchers such as acrylamide can penetrate some distance into aqueous cavities below the surface and are considered to be probes of exposure to solvent. As shown by the kq values for iodide quenching (Table II), there was very little surface exposure of Trp-26 in any of our samples, typically about 5% that of the NATA standard in solution. For isolated TnCF26W, Trp-26 was about 20% less exposed to iodide in Mg2+ than in Ca2+. Linear plots of Fo/F for isolated TnCF26W were observed in both Mg2+ and Ca2+ (Fig. 2), indicating no charge effects. For both the TnCF26W–dCW-TnI (Fig. 3A) and TnCF26W–TnI(1–159) (Fig. 3B) complexes, downward curvatures of the Fo/F plots in both Mg2+ and Ca2+ indicated electrostatic interactions with iodide in the vicinity of Trp-26, as demonstrated in several examples in a review by Eftink and Ghiron (31). Both TnC–TnI complexes showed a stronger downward curvature in Ca2+ than in Mg2+, indicating a stronger electrostatic interaction with iodide in the presence of Ca2+. A plausible explanation for the downward curvature is that one or more positive charges from Lys and Arg residues in the basic protein TnI raise the local concentration of the negatively charged iodide quencher near Trp-26 of TnC. Since TnI and the N-terminal domain of TnC interact more closely in Ca2+ than in Mg2+, it is not surprising to find that the downward curvature of the Fo/F plots is stronger in Ca2+ than in Mg2+.
FIG. 2.
Iodide quenching of Trp-26 in isolated TnCF26W. (○) NATA standard, (○) Sample in Ca2+, (□) Sample in Mg2+.
FIG. 3.
Iodide quenching of Trp-26 in TnCF26W when in complex with TnI. (○) Sample in Ca2+, (□) Sample in Mg2+. Samples: (A) TnCF26W-dCW-TnI complex; (B) TnCF26W-TnI(1–159) complex.
Downward curvature of Stern–Volmer plots may also result from the existence of multiple protein conformations, which might also cause the observed heterogeneity in the lifetimes (31). In isolated TnCF26W, however, the curves are linear despite lifetime heterogeneity, and no downward curvature is observed in any sample when neutral acrylamide is used as the quencher. Therefore the particular combination of a negatively charged iodide quencher and the presence of TnI must be the cause of the observed curvature, making electrostatic interaction between iodide and positive charges in the vicinity of Trp-26 the most likely cause.
In cases where downward curvature of Fo/F plots were seen, the Stern–Volmer constant Ks.v. was taken to be the initial slope (31). In the Mg2+-bound forms, the initial slopes suggest that there was a nearly 50% increase in iodide quenching in both complexes compared to isolated TnCF26W. This was much more likely due to charge effects than to any increased exposure of Trp-26. The Mg2+-bound forms of our samples were also interesting in that the TnCF26W–TnI(1–159) complex yielded the highest kq value. This is reminiscent of what was seen with acrylamide quenching, where TnI(1–159) was less effective than full-length dCW-TnI at shielding Trp-26 from solvent effects in Mg2+. The situation is different in the Ca2+-bound forms, where the kq value for the TnCF26W–TnI(1–159) complex is about one sixth those of the TnCF26W–dCW-TnI complex and the isolated TnCF26W. It is also noteworthy that, in the Ca2+-bound forms, the kq values for isolated TnCF26W and for the TnCF26W–dCW-TnI complex were nearly identical. This may be explained by competing effects which cancel each other out during the iodide quenching. A lower exposure of Trp-26 in the complex, as indicated by the acrylamide data (see above and Table I), would reduce kq, while introduction of an electrostatic attraction would increase kq. It should be noted that we were limited in the range of iodide concentration over which data could be collected, because the exposure of Trp-26, observed as a steady growth in the amount of quenching, increased over time at ionic strengths greater than approximately 0.5.
DISCUSSION
Crystal structures of TnC with the regulatory sites filled (8) and empty (32, 33) showed that, in the Mg2+ bound form, the native Phe-26 side chain is only partially accessible to solvent on the outside surface of TnC. When Ca2+ binds to the regulatory sites in the N-terminal domain, Phe-26 is shifted so that a portion of the side chain is exposed in the middle of a newly formed hydrophobic pocket. Changes in the fluorescence properties of Trp-26 in TnCF26W, therefore, are good indicators for Ca2+-dependent changes in the interaction of TnC and TnI. Consistent with the earlier results of Pearlstone et al. (15), we found that steady-state measurements of fluorescence emission maxima and quantum yields failed to show any changes in the environment of Trp-26 when Mg2+ bound to the C-terminal domain of the apo form of isolated TnCF26W. Judging by changes in the length and heterogeneity of the lifetimes, however, we found that binding of Mg2+ to the C-terminal sites did have some effect on the environment of Trp-26. Binding of Ca2+ to the N-terminal, regulatory sites of TnCF26W had a large effect on Trp-26 fluorescence, tripling the quantum yield and doubling the mean lifetime. Ca2+ binding did not change the exposure of Trp-26 to solvent, as measured by acrylamide quenching, although exposure to iodide quenching actually increased. The iodide effect may be explained by a charge-induced rearrangement of negatively charged side chains of nearby Asp and Glu residues in the vicinity of Trp-26.
In the absence of Ca2+ and Mg2+, complex formation between TnCF26W and full-length dCW-TnI had only slight effects on the fluorescence properties of Trp-26. When the C-terminal sites of TnCF26W were occupied by Mg2+, the effects were still quite small, observable only as shorter and less heterogeneous lifetimes than in isolated TnCF26W. Acrylamide quenching showed that complex formation with dCW-TnI partially shielded Trp-26 from solvent in the Mg2+ bound form, while increased exposure to iodide indicated that the basic dCW-TnI increased the net positive charge in the vicinity of Trp-26.
When the regulatory sites of TnCF26W were occupied by Ca2+, complex formation with dCW-TnI had a pronounced effect on the fluorescence properties of Trp-26. There was a large blue shift in the emission maximum, indicating a change to a more hydrophobic environment. This is consistent with acrylamide quenching data, which showed that the exposure of Trp-26 to solvent was substantially reduced. We also observed a significantly lower quantum yield and a shorter mean lifetime upon complex formation with dCW-TnI. The simultaneous occurrence of all these changes is strong evidence for closer interactions between dCW-TnI and the N-terminal domain of TnCF26W.
The effects of Ca2+ and Mg2+ binding on the environment of Trp-26 in the TnC–TnI complex were different when residues 160–181 at the C-terminus of TnI were removed. Lifetime measurements in the apo and Ca2+-bound forms showed no significant differences between the complexes formed using full-length dCW-TnI or the truncated TnI(1–159). In the Mg2+-bound form, however, the Trp-26 fluorescence lifetime was more heterogeneous in the complex formed with TnI(1–159). In this respect the TnCF26W–TnI(1–159) complex more closely resembled isolated TnCF26W than did the TnCF26W–dCW-TnI complex.
Acrylamide quenching and iodide quenching measurements yielded the same percentage difference in kq values when comparing the Mg2+-bound forms of the TnCF26W–dCW-TnI and TnCF26W–TnI(1–159) complexes. This could mean that, in the Mg2+-bound form, both the exposure to solvent and sensitivity to surface charge effects of Trp-26 were increased by C-terminal truncation of TnI. In the Ca2+-bound form, acrylamide quenching showed that the solvent exposure of Trp-26 was not changed by C-terminal truncation of TnI, but sensitivity to surface charge effects as measured by iodide quenching was much reduced. Our results are consistent with previous studies which showed that TnI segment 115–131 binds to the N-terminal hydrophobic pocket of TnC which is exposed when Ca2+ binds to the regulatory sites (13, 14). Our new observations of the effects of TnI C-terminal truncation on the fluorescence properties of TnC Trp-26 indicate that a short segment at the C-terminus of TnI interacts with the N-terminal, regulatory domain of TnC in their binary complex, providing the first clue as to what role the C-terminus of TnI may play in the regulation of vertebrate striated muscle.
ACKNOWLEDGMENTS
This work was supported by NIH Grants R01-AR-41161 and T32-AR-07592 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and by NIH Grant RR-08119 to the Center for Fluorescence Spectroscopy.
Footnotes
Abbreviations used: IAANS, 2-[4′-(iodoacetamido)anilino]-napthalene-6-sulfonic acid; IAEDANS, N-(iodoacetyl)-N′-(1-sulfo-5-naphthyl)ethylenediamine; Tn, Troponin; TnC, Troponin C; TnCF26W, mutant of troponin C in which Phe-26 is replaced by Trp; TnI, Troponin I; dCW-TnI, a Cys-less, Trp-less TnI mutant (17); TnI(1–159), deletion mutant of dCW-TnI containing residues 1–159; TnT, Troponin T; Tm, tropomyosin; NATA, N-Acetyl-l-tryptophanamide.
REFERENCES
- 1.Leavis PC, and Gergely J (1984) CRC Crit. Rev. Biochem 16, 235–305. [DOI] [PubMed] [Google Scholar]
- 2.Zot A, and Potter JD (1987) Annu. Rev. Biophys. Biophys. Chem 16, 535–559. [DOI] [PubMed] [Google Scholar]
- 3.Farah C, and Reinach FC (1995) FASEB J. 9, 755–767. [DOI] [PubMed] [Google Scholar]
- 4.Tobacman LS (1996) Annu. Rev. Physiol 58, 447–481. [DOI] [PubMed] [Google Scholar]
- 5.Squire JM, and Morris EP (1998) FASEB J. 12, 761–771. [DOI] [PubMed] [Google Scholar]
- 6.Herzberg O, and James MN (1985) Nature 313, 653–659. [DOI] [PubMed] [Google Scholar]
- 7.Sundaralingam M, Bergstrom R, Strasburg G, Rao ST, Roychowdhury P, Greaser M, and Wang BC (1985) Science 227, 945–948. [DOI] [PubMed] [Google Scholar]
- 8.Houdusse A, Love ML, Dominguez R, Grabarek Z, and Cohen C (1997) Structure 5, 1695–1711. [DOI] [PubMed] [Google Scholar]
- 9.Perry SV (1999) Mol. Cell Biochem. 190, 9–32. [PubMed] [Google Scholar]
- 10.Kobayashi T, Tao T, Gergely J, and Collins JH (1994)J. Biol. Chem 269, 5725–5729. [PubMed] [Google Scholar]
- 11.Farah CS, Miyamoto CA, Ramos CH, da Silva AC, Quaggio RB, Fujimori K, Smillie LB, and Reinach FC (1994) J. Biol. Chem 269, 5230–5240. [PubMed] [Google Scholar]
- 12.Syska H, Wilkinson JM, Grand RJA, and Perry SV (1976) Biochem. J 153, 375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKay RT, Tripet BP, Hodges RS, and Sykes BD (1997)J. Biol. Chem 272, 28494–28500. [DOI] [PubMed] [Google Scholar]
- 14.Tripet B, Van Eyk J, and Hodges RS (1997) J. Mol. Biol 271, 728–750. [DOI] [PubMed] [Google Scholar]
- 15.Pearlstone JR, Borgford T, Chandra M, Oikawa K, Kay CM, Herzberg O, Moult J, Herklotz A, Reinach FC, and Smillie LB (1992) Biochemistry 31, 6545–6553. [DOI] [PubMed] [Google Scholar]
- 16.Ramos CHI (1999) J. Biol. Chem 274, 18189–18195. [DOI] [PubMed] [Google Scholar]
- 17.Zhao X, Kobayashi T, Malak H, Gryczynski I, Lakowicz J, Wade R, and Collins JH (1995) J. Biol. Chem 270, 15507–15514. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi T, Zhao X, Wade R, and Collins JH (1999) Biochim. Biophys. Acta 1430, 214–221. [DOI] [PubMed] [Google Scholar]
- 19.Kluwe L, Maeda K, and Maéda Y (1993) FEBS Lett. 232, 83–88. [DOI] [PubMed] [Google Scholar]
- 20.Fujita S, Maeda K, and Maéda Y (1992) J. Biochem 112, 306–308. [DOI] [PubMed] [Google Scholar]
- 21.Gill SC, and von Hippel PH (1989) Anal. Biochem 182, 319–326. [DOI] [PubMed] [Google Scholar]
- 22.Parker CA, and Rees WT (1960) Analyst (London) 85, 587–600. [Google Scholar]
- 23.Chen RF (1967) Anal. Let 1, 35–42. [Google Scholar]
- 24.Lakowicz JR (1983) in Principles of Fluorescence Spectroscopy (Lakowicz JR, Ed.), pp. 258–295, Plenum Press, New York. [Google Scholar]
- 25.Lakowicz JR, and Maliwal BP (1985) Biophys. Chem 21, 61–78. [DOI] [PubMed] [Google Scholar]
- 26.Lakowicz JR, Laczko G, and Gryczynski I (1986) Rev. Sci. Instrum 57, 2499–2506. [Google Scholar]
- 27.Laczko G, Lakowicz JR, Gryczynski I, Gryczynski Z, and Malak H (1990) Rev. Sci. Instrum 61, 2331–2337. [Google Scholar]
- 28.Berndt K, Duerr H, and Palme D (1982) Opt. Commun 42, 419–422. [Google Scholar]
- 29.Gratton E, and Lopez-Delgado R (1980) Nuovo Cimento B56, 110–124. [Google Scholar]
- 30.Gratton E, James DM, Rosato N, and Weber G (1984) Rev. Sci. Instrum 55, 486–494. [Google Scholar]
- 31.Eftink MR, and Ghiron CA (1981) Anal. Biochem 114, 199–227. [DOI] [PubMed] [Google Scholar]
- 32.Herzberg O, Moult J, and James MNG (1986) J. Biol. Chem 261, 2638–2644. [PubMed] [Google Scholar]
- 33.Strynadka NC, and James MN (1989) Annu. Rev. Biochem 58, 951–998. [DOI] [PubMed] [Google Scholar]