Abstract
Background
The optimal timing of surgery in relation to chemoradiation is still controversial. Retrospective analysis has demonstrated in the recent decades that the regression of adenocarcinoma can be slow and not complete until after several months. More recently, increasing pathologic Complete Response rates have been demonstrated to be correlated with longer time interval. The purpose of the trial is to demonstrate if delayed timing of surgery after neoadjuvant chemoradiotherapy actually affects pathologic Complete Response and reflects on disease-free survival and overall survival rather than standard timing.
Methods
The trial is a multicenter, prospective, randomized controlled, unblinded, parallel-group trial comparing standard and delayed surgery after neoadjuvant chemoradiotherapy for the curative treatment of rectal cancer. Three-hundred and forty patients will be randomized on an equal basis to either robotic-assisted/standard laparoscopic rectal cancer surgery after 8 weeks or robotic-assisted/standard laparoscopic rectal cancer surgery after 12 weeks.
Discussion
To date, it is well-know that pathologic Complete Response is associated with excellent prognosis and an overall survival of 90%. In the Lyon trial the rate of pCR or near pathologic Complete Response increased from 10.3 to 26% and in retrospective studies the increase rate was about 23–30%. These results may be explained on the relationship between radiation therapy and tumor regression: DNA damage occurs during irradiation, but cellular lysis occurs within the next weeks. Study results, whether confirmed that performing surgery after 12 weeks from neoadjuvant treatment is advantageous from a technical and oncological point of view, may change the current pathway of the treatment in those patient suffering from rectal cancer.
Trial registration
ClinicalTrials.gov NCT3465982.
Keywords: Radiation therapy, Minimally invasive surgery, Rectal cancer, Neoadjuvant treatment, Robotic surgery, TaTME, Timing to surgery
Background
Chemoradiotherapy is a well-known risk reducing treatment of local recurrence in the treatment of rectal cancer, followed by total mesorectal excision (TME). In low rectal tumors, surgery alone has the 30% overall survival and a local recurrence rate of about 55–65%, with a disease-free survival of 30–35% [1]. Preoperative administration of fluorouracil-based chemotherapy improved local recurrence rates to 7% [2]. The optimal timing of surgery in relation to chemoradiation is still controversial. Retrospective analysis has demonstrated in the recent decades that the regression of adenocarcinoma can be slow and not complete until after several months [3]. More recently, increasing pCR (pathological complete response) rates have been demonstrated to be correlated with longer time interval [4–6]. Conversely, several reports have shown no impact of the interval after chemoradiation on pCR and technical performance [7, 8]. In the Lyon trial the rate of pCR or near pCR increased from 10.3 to 26% [9] and in retrospective studies the increase rate was about 23–30%. These results may be explained on the relationship between radiation therapy and tumor regression: DNA (DeoxyriboNucleic Acid) damage occurs during irradiation, but cellular lysis occurs within the next weeks [10]. A recent pilot study on comparison of resonance imaging and histopathological responses at two times, has suggested that volume reduction and down-staging occur between week 9 and week 14 after neoadjuvant treatment, with a 23% pCR rate at longer time [11]. In the Stockholm III trial, a significantly lower frequency of postoperative complications was reported, even though not described in the other studies where morbidity and complications were the same. All of these studies, however, presented some biases, such as absence of randomization, the choice of surgical timing made arguably by the surgeon, tumor size and response to RCT (radiochemotherapy), different cut-off period and a limited number of recruited patients, that may have negatively or positively influenced these results [12, 13]. Delaying surgery with the aim to detect excellent responders for organ preservation, eventually, may be legitimate, even though the start of adjuvant therapy, whose advantage in pretreated rectal cancer patients is still controversial, would be delayed, and this may negatively affect survival [14, 15]. A recent meta-analysis on thirteen reports has been published, showing rates of 14 and 20% in the shorter and longer group, respectively. This meta-analysis has some biases: the pCR correlation with surgical delay could not be adjusted in a multivariate analysis with other clinico-pathological variables, the outcome (DFS and OS) of pCR, even if likely better than those without pCR as literature demonstrates, could not be directly assessed due to lack of individual patient data, the number of patients operated on in the delayed group could have been chosen using a surgical decision, different time intervals were grouped all together, no randomized trial were included in the meta-analysis, and the relevance of the reports included in was assessed by NOS scale (Newcastle–Ottawa scale), that is quite arbitrary, several reports on observation, demonstrating a higher percentage of pCR, were not included, but it is quite relevant to consider also these studies. TiMiSNAR has been developed to improve and define previous results from retrospective and review analyses.
Methods/design
The trial is a multicenter, prospective, randomized controlled, unblinded, parallel-group trial comparing standard and delayed surgery after neoadjuvant chemoradiotherapy for the curative treatment of rectal cancer. Three-hundred and forty patients will be randomized on an equal basis to either robotic-assisted/standard laparoscopic rectal cancer surgery after 8 weeks or robotic-assisted/standard laparoscopic rectal cancer surgery after 12 weeks (Fig. 1). Eight weeks are the current standard interval to surgery after neodjuvant treatment, while 12 weeks represent the “minimum” longer time interval to determine further tumor modifications and the “a priori” choice to avoid hypothetic surgical detrimental effect (postoperative complications related to radiation therapy). The recruiting interval will be of 5 years and the follow-up period will end 5 years after the last patient is randomized.
Fig. 1.
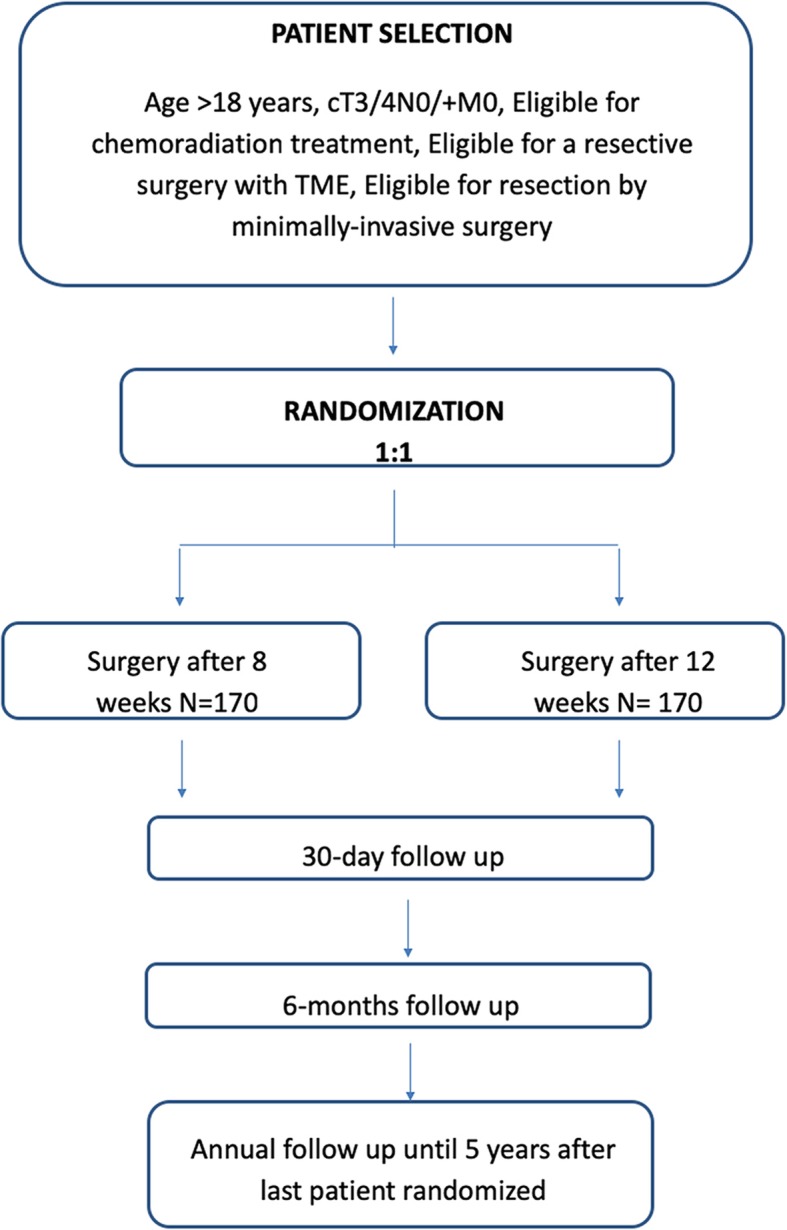
Flow chart of TiMiSNAR Trial
The trial has been held in Alessandria at SS. Antonio e Biagio e Cesare Arrigo Community Hospital, Italy and in others National Academic and not-Academic Centers, list of which is available at https://www.timisnar.it.
The Primary Endpoint is pCR; secondary endpoints are: DFS (disease-free survival), OS (overall survival), postoperative complications (Clavien-Dindo classification), reintervention, late complications (Clavien-Dindo classification), radiation toxicity, chemotherapy toxicity, QoL (quality of life), Functional status.
Inclusion Criteria are: age > 18 years, cT3/4 N0/+M0 confirmed on CT-scan (Computed Tomography Scan), MRI (Magnetic Resonance Imaging - stratification for T3a-b-c-d), tumor starting from the distal or medium rectum (even those crossing the peritoneal reflection at distal margin, within 15 cm from the anal margin), histologically-proven adenocarcinoma of the rectum, eligible for a resective surgery with TME (low anterior resection, intersphyncteric resection, abdominoperineal resection), eligible for resection by minimally-invasive surgery (standard or robotic-assisted laparoscopic procedure, all robotic systems will be accepted), eligible for chemoradiation treatment, able to give written informed consent, capable of completing required questionnaires at time of consent (provided questionnaires are available in a language spoke fluently by the participant).
Main exclusion Criteria are: metastatic disease, squamous carcinoma of the anal canal, unable to complete neoadjuvant treatment.
Patients will be randomized on a 1:1 basis to receive minimally-invasive rectal cancer surgery 8 or 12 weeks after neoadjuvant treatment and will be allocated a unique trial number.
Participants will be randomized using Sealed Envelope Ltd. 2017 Online Simple randomization service. Allocation concealment will be ensured, as the service will not release the randomization code until the patient has been recruited into the trial, which takes place after all baseline measurements have been completed.
An informed consent to participate has been prepared and will be obtained by all the participants.
All patients who give consent for participation and who fulfil the inclusion criteria will be randomized. Randomization will be requested by the staff member responsible for recruitment and clinical interviews from all participating centers. Due to the nature of the intervention neither participants nor staff can be blinded to allocation, but are strongly inculcated not to disclose the allocation status of the participant at the follow up assessments.
All the involved centers have to respect the following criteria: site able to perform robotic-assisted and standard laparoscopic rectal cancer surgery and TaTME (transanal total mesorectal excision); site able to provide standard neoadjuvant treatment, both chemo and radiation therapy; predicted capability to recruit a minimum of 15 patients per year to the trial.
Neoadjuvant treatment consists in long course radiation therapy with IMRT (Intensity Modulated RadioTherapy – 50-54 Gy in 25–28 fractions; an optional boost is suggested) associated to concomitant chemotherapy treatment (Capecitabine 825 mg /m2/ twice daily during radiation therapy).
Several studies have compared IMRT of rectal cancer to 3D Conformal Radiotherapy. Although results from comparative randomized clinical trial are not available yet, IMRT is usually associated with less dose to organ at risk, such as urinary bladder, small bowel and anal sphincters (in selected cases). This is translated into better clinical outcomes, in terms of gastrointestinal toxicity, genitourinary toxicity and skin side effects [16–20].
Restaging and treatment-efficacy assessment after Neoadjuvant therapy
The MERCURY study group has developed an MRI-based tumor regression grading (ymrTRG) system by applying the principles of histopathological tumor regression grade (ypTRG) [21].
Recently, a pilot study from UK has defined two groups of patients divided into favourable vs unfavourable responders based on the following three factors:
ymrT
ymrTRG
Change in volume
ymrT is based on the interpretation of local extent of persistent tumor signal intensity relative to the layers of bowel wall on T2-weighted images. Tumor response is evaluated as either replacement of tumor signal by low signal intensity fibrosis (dark stroma) or the development of high signal intensity mucin pools, that are not considered to be tumor.
ymrTRG is based on principles similar to the pathological ypTRG system described by Dworak and subsequently modified by Mandard.
Change in volume, better defined as percentage volume reduction is calculated multiplying tumor length, width and height, using the following formula:
Time interval to surgery in this trial are 8 weeks and 12 weeks after treatment, that are the standard and the expected “minimum” longer time interval to determine further tumor modifications. Post-treatment staging for evaluation of postneoadjuvant treatment response, eventually, will depend on MRI evaluation at week 7 for patients in both the two arms; a MRI evaluation will be repeated at week 11 for patients randomized in the delayed arm.
A Thoraco-abdominal CT-Scan with and without contrast enhancement will be performed at week 6 after neoadjuvant surgery, for restaging of potential disseminated disease.
All MRI exams are collected and sent to the Promoting Center for final revision by a well-trained Pelvic MRI expert radiologist. Every participating center must fill in a structured MRI form according to the fac-simile provided by the ESGAR (European Society of Gastrointestinal and Abdominal Radiology) [22].
Surgery
Minimally-invasive mesorectal resection is required: both robotic or standard laparoscopic approach or TaTME will be accepted, in accordance with each surgeon’s usual practice. The specifics of each operation will be at the discretion of the operating surgeon (e.g. port-site placement, mobilization of the splenic flexure, inferior mesenteric artery/vein division, high versus low vascular division etc.), as well as the decision to convert to an open operation. Conversion to open operation is defined as the use of a laparotomy wound for any part of the mesorectal dissection. All participating centers are allowed and suggested to use Indocyanine Green test (ICG), wherever available, but it is not mandatory. Several studies have shown that ICG test could reduce anastomotic leakage and thus postoperative complications, that are important in light of the secondary endpoints. A recent systematic review and meta-analysis by Blanco-Colino et al. has shown that ICG fluorescence imaging seems to reduce AL rates following colorectal surgery for cancer [23].
Post-operative care and follow up
Post-operative care and follow up will be as per institutional protocol, but patients must be reviewed at 30 days, and 6 months post-operatively at a minimum. Any further visits will be according to local standard clinical practice. All patients will be followed up as per protocol until 5 years after the last patient has been randomized.
Statistical evaluation
Sample size
The primary endpoint is the pCR rate. Based on the published results from prospective studies on delayed time interval or observation only and on retrospective study for standard time interval, we assume that the mean rate of pCR in the standard treatment is about 15%, while the mean pCR rate in the observation treatment or longer time interval is 30%. To determine this difference, 270 patients are required, using a two-group continuity corrected χ2 test of equal proportions, assuming an α error of 4.9% and a power of 80% (MedCalc Version 17.9.7); an interim analysis on efficacy will be performed when half of events will be observed. The conservative Haybittle-Peto [24] boundary will be used as a stopping guidance in order to perform the final analysis at the significance level of 4.9%, two sides. Considering results from the pilot study reported on section 1, the percentage of unfavourable patients is 20% (favourable MRI tumor regression grade is defined as grades 1, 2 and 3; unfavourable MRI regression as grades 4 and 5). In addition, a meta-analysis on results from five randomized European clinical trials for locally advanced rectal cancer, has confirmed this rate of “poor” responders subgroup, identified by having no pCR and no DFS within 2 years [25]. In computing the sample size, we assume that the percentage of missing data will be 5%. A total of 340 patients, 170 for each arm, is intended to be enrolled, eventually. Patients will be randomized on a 1:1 basis to receive minimally-invasive rectal cancer surgery 8 or 12 weeks after neoadjuvant treatment and will be allocated a unique trial number. A computer-generated software with block randomization criteria will be used to ensure treatment groups are well-balanced for timing of surgery. All enrolled patients’ data will be registered in a prospective electronic database (ACCESS, MICROSOFT OFFICE Professional Plus 2010, regular licensed).
All data will be entered by means of case report forms. Original study forms will be entered and kept on file at the Coordinator site (SS. Antonio e Biagio e Cesare Arrigo Hospital). When a form is selected, the participating site staff will pull that form, copy it, and sent the copy to the DCC (Data Coordinating Center) for re-entry. Participant files are to be stored in numerical order and stored in a secure and accessible place and manner. Participant files will be maintained in storage for a period of 5 years after completion of the study.
The DCC will send monthly email reports with information on missing data, missing forms, and missing visits. Personnel at the Core Coordinating Center and the Participating Sites should review these reports for accuracy and report any discrepancies to the DCC.
Statistical analysis
All efficacy outcomes will be assessed in the intention-to-treat population, which includes all enrolled patients who did not violate the eligibility criteria. pCR, OS and DFS will be assessed from the time of treatment allocation to local progression, death or disease progression. Patients who will not die and will not experience local of distant disease progression at the date of study cutoff will be censored at the last available information on status.
Time-to-event data will be analyzed by the Kaplan-Meier method and compared with the log-rank test. Cox proportional hazards model will be used to adjust the treatment effect for baseline prognostic factors.
Serious adverse events reporting (SAE)
Any SAE considered to be reasonably related to the investigational treatment or study participation, have to be promptly notified.
This must be done by email within 24 h of the initial observation of the event. The principal investigator will decide if these events are related to the trial treatment (i.e. unrelated, likely related, and not assessable) and the decision will be recorded on the Serious Adverse Event form, if necessary with the reasoning of the principal investigator.
The investigator is obligated to assess the relationship between investigational treatment and the occurrence of each AE/SAE. A “reasonable possibility” is meant to convey that there are facts/evidence or arguments to suggest a causal relationship, rather than a relationship cannot be ruled out. The investigator will use clinical judgement to determine the relationship. Alternative causes, such as natural history of the underlying diseases, concomitant therapy, other risk factors, and the temporal relationship of the event to the investigational product will be considered and investigated.
End of the study
The end of the study is defined as 5 years after the date that the last patient has been randomized to the trial.
Research ethics approval
The protocol, site-specific informed consent forms, participant education and recruitment materials, and other requested documents — and any subsequent modifications — also has been reviewed and approved by SS. Antonio e Biagio e Cesare Arrigo Hospital Ethical Committee on 31 May 2018.
Discussion
To date, it is well-know that pCR is associated with excellent prognosis and an overall survival of 90% [1]. In the Lyon trial the rate of pCR or near pCR increased from 10.3 to 26% [2] and in retrospective studies the increase rate was about 23–30%. These results may be explained on the relationship between radiation therapy and tumor regression: DNA damage occurs during irradiation, but cellular lysis occurs within the next weeks [3]. In the Stockholm III trial, a significantly lower frequency of postoperative complications was reported, even though not described in the other studies where morbidity and complications were the same.
There are several audiences for this trial: Oncologists, Surgeons, Radiation oncologists, Patients and the public, Academia, General Practitioners.
Another crucial point of the trial is the use of a structured MRI report, as recommended by the European Society of Gastrointestinal and Abdominal Radiology (ESGAR) [22], for primary staging and for restaging after neoadjuvant treatment. One of the goals of the trial is to determine whether MRI can specifically depict cancer local diffusion and predict downstaging and be used as a good prognostic instrument. High quality MRI, indeed, allows further subclassification of cT3, which is recommended by European Society for Medical Oncology (ESMO) guidelines and it is useful in stratifying and selecting patients with indication to neoadjuvant treatment before surgery.
In summary, the optimal interval between adjuvant chemoradiation and surgery may give the opportunity to optimize patients, initiate an individualized and “targeted” treatment, and favor organ preservation.
TiMiSNAR (NCT3465982 – https://www.timisnar.it) results, whether confirmed that performing surgery after 12 weeks from neoadjuvant treatment is advantageous from a technical and oncological point of view, may change the current pathway of the treatment in those patient suffering from rectal cancer.
Acknowledgements
Not Applicable
Administrative Information
Organisational structure and responsibilities
Principal Investigator
Design and conduct of TiMiSNAR
Preparation of protocol and revisions
Preparation of investigators brochure (IB) and CRFs [Case Report Forms]
Organising steering committee meetings
Managing CTO [Clinical Trials Office]
Publication of study reports
Members of TMC [Trial Management Committee]
Steering committee (SC)
Agreement of final protocol
All lead investigators will be steering committee members
Recruitment of patients and liasing with principal investigator
Reviewing progress of study and if necessary agreeing changes to the protocol and/or investigators brochure to facilitate the smooth running of the study
Trial Management Committee (TMC)
(Principle investigator, Administrator (D.ssa Marinella Bertolotti))
Study planning
Organisation of steering committee meetings
Provide annual SUSAR [Serious unexpected suspected adverse events] reporting
Responsible for trial master file
Advice for lead investigators
Data verification
Randomization
Data Manager
Maintenance of trial IT system and data entry
Data verification
Lead Investigators
In each participating center a lead investigator (senior surgeon) will be identified, to be responsible for identification, recruitment, data collection and completion of CRFs, along with follow up of study patients and adherence to study protocol and investigators brochure. Lead investigators will be steering committee members
Primary sponsor
Azienda Ospedaliera SS. Antonio e Biagio e Cesare Arrigo di Alessandria, Italy.
Abbreviations
- CT
Computed Tomography
- DFS
Disease-free survival
- DNA
DeoxyriboNucleic Acid
- ESGAR
European Society of Gastrointestinal and Abdominal Radiology
- ICG
Indocyanine Green test
- IMRT
Intensity Modulated Radiotherapy
- MRI
Magnetic Resonance Imaging
- NOS
The Newcastle-Ottawa Scale
- OS
Overall Survival
- pCR
pathologic Complete Response
- QoL
Quality of Life
- RCT
radiochemotherapy
- TaTME
transanal total mesorectal excision
- TME
Total Mesorectal Excision
- ymrTRG
MRI-based tumor regression grading
- ypTRG
pathologic tumor regression grading
- ESMO
European Society for Medical Oncology
Authors’ contributions
IM, FP1, EB, SG1, GR, EC, LB1, LB2, GP, FP2, CND, RP, EM1, TC, EM2, UE, RD, RR, FP3, AC, BM, SG2, PB1, PB2, RB, CC, VT, ET, VF, MR, FP4, GN, PF have made substantial contributions to the conception or design of the work. VT is the statistician involved in the analysis and interpretation of data. All authors read and approved the final manuscript.
Funding
No funds have been utilized for this trial.
Availability of data and materials
NOT APPLICABLE (the current manuscript doesn’t contain any data related to patients; it’s only a draft).
Ethics approval and consent to participate
The present study has obtained Ethical Approval by SS. Antonio e Biagio e Cesare Arrigo Hospital Ethical Committee on 31 May 2018 and informed consent to participate in the study has been elaborated and will be obtained from participants.
Consent for publication
NOT APPLICABLE.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Igor Monsellato, Email: igor.monsellato@ospedale.al.it.
Filippo Alongi, Email: filippo.alongi@sacrocuore.it.
Elisa Bertocchi, Email: elisa.bertocchi@sacrocuore.it.
Stefania Gori, Email: stefania.gori@sacrocuore.it.
Giacomo Ruffo, Email: giacomo.ruffo@sacrocuore.it.
Elisa Cassinotti, Email: cassinotti.elisa@gmail.com.
Ludovica Baldarti, Email: ludovica.baldarti@gmail.com.
Luigi Boni, Email: luigi.boni@policlinico.mi.it.
Graziano Pernazza, Email: gpernazza@hsangiovanni.roma.it.
Fabio Pulighe, Email: fabiopulighe@gmail.com.
Carlo De Nisco, Email: carlodenisco@gmail.com.
Roberto Perinotti, Email: roberto.perinotti@aslbi.piemonte.it.
Emilio Morpurgo, Email: emilio.morpurgo@aulss6.veneto.it.
Tania Contardo, Email: tania.contardo@aulss6.veneto.it.
Enzo Mammano, Email: enzo.mammano@aulss6.veneto.it.
Ugo Elmore, Email: elmore.ugo@hsr.it.
Roberto Delpini, Email: delpini.roberto@hsr.it.
Riccardo Rosati, Email: rosati.riccardo@unisr.it.
Federico Perna, Email: fedefez@hotmail.com.
Andrea Coratti, Email: corattian@gmail.com.
Benedetta Menegatti, Email: menegatti.b@virgilio.it.
Sergio Gentilli, Email: sergio.gentilli@med.unipmn.it.
Paolo Baroffio, Email: paolo.baroffio@maggioreosp.novara.it.
Piero Buccianti, Email: p.buccianti@ao-pisa.toscana.it.
Riccardo Balestri, Email: r.balestri@ao-pisa.toscana.it.
Cristina Ceccarelli, Email: c.ceccarelli19@gmail.com.
Valter Torri, Email: valter.torri@marionegri.it.
Elena Traverso, Email: elena.traverso@ospedale.al.it.
Vittorio Fusco, Email: vfusco@ospedale.al.it.
Maura Rossi, Email: mrossi@ospedale.al.it.
Fabio Priora, Email: fpriora@ospedale.al.it.
G. Numico, Email: gianmauro.numico@ospedale.al.it
Paola Franzone, Email: pfranzone@ospedale.al.it.
References
- 1.Morino M, Giraudo G. Laparoscopic total mesorectal excision-the Turin experience. Recent Results Cancer Res. 2005;165:167–179. doi: 10.1007/3-540-27449-9_18. [DOI] [PubMed] [Google Scholar]
- 2.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H. Raab R; German rectal Cancer study group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 3.Glimelius B. Optimal time intervals between pre-operative radiotherapy or chemotherapy and surgery in rectal cancer? Front Oncol. 2014;4:50. doi: 10.3389/fonc.2014.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrelli F, Sgroi G, Sarti E, Barni S. Increasing the interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer. A meta-analysis of published studies. Ann Surg. 2016;263:458–464. doi: 10.1097/SLA.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 5.Erlandsson J, Holm T, Pettersson D, Berglund Å, Cedermark B, Radu C, Johansson H, Machado M, Hjern F, Hallböök O, Syk I, Glimelius B, Martling A. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017;18(3):336–346. doi: 10.1016/S1470-2045(17)30086-4. [DOI] [PubMed] [Google Scholar]
- 6.Kaytan-Saglam E, Balik E, Saglam S, Akgün Z, Ibis K, Keskin M, Dagoglu N, Kapran Y, Gulluoglu M. Delayed versus immediate surgery following short-course neoadjuvant radiotherapy in resectable (T3N0/N+) rectal cancer. J Cancer Res Clin Oncol. 2017;143(8):1597–1606. doi: 10.1007/s00432-017-2406-6. [DOI] [PubMed] [Google Scholar]
- 7.Lefevre JH, Mineur L, Kotti S, Rullier E, Rouanet P, de Chaisemartin C, Meunier B, Mehrdad J, Cotte E, Desrame J, Karoui M, Benoist S, Kirzin S, Berger A, Panis Y, Piessen G, Saudemont A, Prudhomme M, Peschaud F, Dubois A, Loriau J, Tuech JJ, Meurette G, Lupinacci R, Goasgen N, Parc Y, Simon T, Tiret E. Effect of interval (7 or 11 weeks) between neoadjuvant radiochemotherapy and surgery on complete pathologic response in rectal cancer: A multicenter, Randomised, Controlled Trial (GRECCAR-6) J Clin Oncol. 2016;34(31):3773–3780. doi: 10.1200/JCO.2016.67.6049. [DOI] [PubMed] [Google Scholar]
- 8.Foster JD, Ewings P, Falk S, Cooper EJ, Roach H, West NP, Williams-Yesson BA, Hanna GB. Francis NK; STARRCAT investigators. Surgical timing after chemoradiotherapy for rectal cancer, analysis of technique (STARRCAT): results of a feasibility multi-Centre randomized controlled trial. Tech Coloproctol. 2016;20(10):683–693. doi: 10.1007/s10151-016-1514-7. [DOI] [PubMed] [Google Scholar]
- 9.Francois Yves, Nemoz Chantal J., Baulieux Jacques, Vignal Jacques, Grandjean Jean-Paul, Partensky Christian, Souquet Jean Christophe, Adeleine Patrice, Gerard Jean-Pierre. Influence of the Interval Between Preoperative Radiation Therapy and Surgery on Downstaging and on the Rate of Sphincter-Sparing Surgery for Rectal Cancer: The Lyon R90-01 Randomized Trial. Journal of Clinical Oncology. 1999;17(8):2396–2396. doi: 10.1200/JCO.1999.17.8.2396. [DOI] [PubMed] [Google Scholar]
- 10.Bujko K. Timing of surgery following preoperative therapy in rectal cancer: there is no need for a prospective randomized trial. Dis Colon Rectum. 2012;55(3):e31. doi: 10.1097/DCR.0b013e31823f86cb. [DOI] [PubMed] [Google Scholar]
- 11.West MA, Dimitrov BD, Moyses HE, Kemp GJ, Loughney L, White D, Grocott MP, Jack S, Brown G. Timing of surgery following neoadjuvant chemoradiotherapy in locally advanced rectal cancer - A comparison of magnetic resonance imaging at two time points and histopathological responses. Eur J Surg Oncol. 2016;42(9):1350–1358. doi: 10.1016/j.ejso.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Tran CL, Udani S, Holt A, Arnell T, Kumar R, Stamos MJ. Evaluation of safety of increased time interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer. Am J Surg. 2006;192(6):873–877. doi: 10.1016/j.amjsurg.2006.08.061. [DOI] [PubMed] [Google Scholar]
- 13.Lefevre JH, Parc Y, Tiret E. French Research Group of Rectal Cancer Surgery (GRECCAR). Increasing the interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer. Ann Surg. 2015;262(6):e116. doi: 10.1097/SLA.0000000000000771. [DOI] [PubMed] [Google Scholar]
- 14.Breugom AJ, Swets M, Bosset JF, Collette L, Sainato A, Cionini L, Glynne-Jones R, Counsell N, Bastiaannet E, van den Broek CB, Liefers GJ, Putter H, van de Velde CJ. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysisof individual patient data. Lancet Oncol. 2015;16(2):200–207. doi: 10.1016/S1470-2045(14)71199-4. [DOI] [PubMed] [Google Scholar]
- 15.Bujko K, Glimelius B, Valentini V, Michalski W, Spalek M. Postoperative chemotherapy in patients with rectal cancer receiving preoperative radio(chemo)therapy: a meta-analysis of randomized trials comparing surgery +/− a fluoropyrimidine and surgry + a fluoropyrimidine +/− oxaliplatin. Eur J Surg Oncol. 2015;41(6):713–723. doi: 10.1016/j.ejso.2015.03.233. [DOI] [PubMed] [Google Scholar]
- 16.Dapper H, Rodríguez I, Münch S, Peeken JC, Borm K, Combs SE, Habermehl D. Impact of VMAT-IMRT compared to 3D conformal radiotherapy on anal sphincter dose distribution in neoadjuvant chemoradiation of rectal cancer. Radiat Oncol. 2018;13(1):237. doi: 10.1186/s13014-018-1187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwak Y-K, Lee S-W, Kay CS, Park HH. Intensity-modulated radiotherapy reduces gastrointestinal toxicity in pelvic radiation therapy with moderate dose. PLoS One. 2017;12:e0183339. doi: 10.1371/journal.pone.0183339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arbea L, Ramos LI, Martínez-Monge R, Moreno M, Aristu J. Intensity- modulated radiation therapy (IMRT) vs. 3D conformal radiotherapy (3DCRT) in locally advanced rectal cancer (LARC): dosimetric comparison and clinical implications. Radiat Oncol. 2010;5:17. doi: 10.1186/1748-717X-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamashita H, Ishihara S, Nozawa H, et al. Comparison of volumetric- modulated arc therapy using simultaneous integrated boosts (SIB-VMAT) of 45 Gy/55 Gy in 25 fractions with conventional radiotherapy in preoperative chemoradiation for rectal cancers: a propensity score case-matched analysis. Radiat Oncol. 2017;12:156. doi: 10.1186/s13014-017-0894-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simson DK, Mitra S, Ahlawat P, et al. Prospective study of neoadjuvant chemoradiotherapy using intensity-modulated radiotherapy and 5 fluorouracil for locally advanced rectal cancer - toxicities and response assessment. Cancer Manag Res. 2018;10:519–526. doi: 10.2147/CMAR.S142076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel UB, Taylor F, Blomqvist L, George C, Evans H, Tekkis P, Quirke P, Sebag-Montefiore D, Moran B, Heald R, Guthrie A, Bees N, Swift I, Pennert K, Brown G. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol. 2011;29(28):3753–3760. doi: 10.1200/JCO.2011.34.9068. [DOI] [PubMed] [Google Scholar]
- 22.Beets-Tan Regina G. H., Lambregts Doenja M. J., Maas Monique, Bipat Shandra, Barbaro Brunella, Curvo-Semedo Luís, Fenlon Helen M., Gollub Marc J., Gourtsoyianni Sofia, Halligan Steve, Hoeffel Christine, Kim Seung Ho, Laghi Andrea, Maier Andrea, Rafaelsen Søren R., Stoker Jaap, Taylor Stuart A., Torkzad Michael R., Blomqvist Lennart. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. European Radiology. 2017;28(4):1465–1475. doi: 10.1007/s00330-017-5026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanco-Colino R., Espin-Basany E. Intraoperative use of ICG fluorescence imaging to reduce the risk of anastomotic leakage in colorectal surgery: a systematic review and meta-analysis. Techniques in Coloproctology. 2017;22(1):15–23. doi: 10.1007/s10151-017-1731-8. [DOI] [PubMed] [Google Scholar]
- 24.Haybittle J. L. Repeated assessment of results in clinical trials of cancer treatment. The British Journal of Radiology. 1971;44(526):793–797. doi: 10.1259/0007-1285-44-526-793. [DOI] [PubMed] [Google Scholar]
- 25.Valentini Vincenzo, van Stiphout Ruud G.P.M., Lammering Guido, Gambacorta Maria A., Barba Maria C., Bebenek Marek, Bonnetain Franck, Bosset Jean F., Bujko Krzysztof, Cionini Luca, Gerard Jean P., Rödel Claus, Sainato Aldo, Sauer Rolf, Minsky Bruce D., Collette Laurence, Lambin Philippe. Selection of appropriate end-points (pCR vs 2yDFS) for tailoring treatments with prediction models in locally advanced rectal cancer. Radiotherapy and Oncology. 2015;114(3):302–309. doi: 10.1016/j.radonc.2015.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
NOT APPLICABLE (the current manuscript doesn’t contain any data related to patients; it’s only a draft).


