Abstract
Background
Atrial fibrillation (AF) is associated with cognitive decline. Whether left atrial enlargement (LAE), a critical substrate for AF, is also associated is less well established. Therefore, we assessed the association of LAE and AF with cognitive decline in the ARIC‐NCS (Atherosclerosis Risk in Communities Neurocognitive Study).
Methods and Results
Participants (n=3391; mean age, 75±5 years; 59% women) underwent cognitive tests and 2‐dimensional echocardiograms at visit 5 (2011–2013) and follow‐up cognitive tests at visit 6 (2016–2017). LAE was defined as left atrium volume index ≥34 mL/m2. AF was ascertained using study ECGs and hospitalization discharge codes. We assessed the association of AF and LAE with (a) cognitive domain scores at visit 5 and (b) cognitive domain score changes between visit 5 and visit 6. At visit 5, compared with the reference group (without AF, normal left atrium), participants with LAE and AF had significantly lower global cognition (Z score, −0.24; 95% CI, −0.38 to −0.10), whereas participants with AF and without LAE and participants with LAE and without AF did not have lower global cognition. In longitudinal analysis, compared with the reference group, participants with AF but without LAE had significantly greater decline in global cognition (Z score, −0.13; 95% CI, −0.21 to −0.06). However, LAE, with or without AF, was not associated with greater cognitive decline.
Conclusion
Although LAE with AF was significantly associated with lower cognitive function in cross‐sectional analysis, LAE, with or without AF, was not associated with greater cognitive decline over 5 years, highlighting the importance of evaluating longitudinal cognitive function. Future studies should have longer follow‐up and evaluate left atrium function.
Keywords: atrial fibrillation, cognition, epidemiology, left atrial volume index, longitudinal cohort study
Subject Categories: Epidemiology, Cognitive Impairment, Echocardiography, Atrial Fibrillation
Clinical Perspective
What Is New?
It is unknown whether left atrial enlargement is associated with lower cognitive function.
In this study of community‐based elderly individuals, although left atrial enlargement with atrial fibrillation was significantly associated with lower cognitive function in cross‐sectional analysis, left atrial enlargement, with or without atrial fibrillation, was not associated with greater cognitive decline over 5 years.
What Are the Clinical Implications?
Our findings caution us against relying solely on cross‐sectional analysis in evaluation of cognitive function.
Future studies on this question should consider involving middle‐aged individuals, longer follow‐up duration, and evaluation of left atrial function.
Atrial fibrillation (AF), a common sustained arrhythmia that affects >2.7 million people in the United States, is associated with increased incidence of stroke, coronary heart disease, and heart failure.1, 2 More recently, compelling evidence has emerged to indicate that AF is also associated with greater risk of cognitive decline and dementia.3 Left atrial enlargement (LAE), commonly present in patients with AF, is also associated with increased incidence of stroke, coronary heart disease, and heart failure.4, 5, 6, 7 However, whether it is LAE or concomitant AF that is associated with lower cognitive function remains unclear.
A few studies have explored the association between LAE and cognitive function. van den Hurk et al found that higher left atrium (LA) volume index was associated with lower information‐processing speed.8 Karadag et al reported that LAE was associated with lower Mini‐Mental State Examination scores,9 and Alosco et al found that LAE was associated with reduced performance on the Repeatable Battery for the Assessment of Neuropsychological Status.10 All 3 studies were limited by small sample sizes, limited cognitive testing, cross‐sectional design, and not accounting for the effect of concomitant AF. In particular, cross‐sectional analysis is suboptimal because it cannot adequately control for all relevant characteristics that could differ among study participants.11
Therefore, to better characterize the relationship between LAE and cognitive function, we performed comprehensive evaluation of the association of LAE with the cognitive domains of memory, executive function, language, and global cognition among participants, with and without AF, who were enrolled in the ARIC‐NCS (Atherosclerosis Risk in Communities Neurocognitive Study). We also performed a follow‐up evaluation to evaluate longitudinal cognitive decline.
Methods
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure in accordance with ARIC study policies. Data are maintained by the ARIC study through the University of North Carolina Collaborative Studies Coordinating Center. Further information is available on the ARIC study website.
Study Population
The ARIC study is a community‐based prospective cohort study with the overall aim of understanding the development of cardiovascular diseases and their risk factors in the general population.12 In 1987 to 1989, a total of 15 792 mostly black and white men and women, aged 45 to 64 years, were recruited from 4 communities in the United States: Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis suburbs, Minnesota; and Washington County, Maryland. After a baseline examination (visit 1), participants completed 5 additional examinations in 1990 to 1992, 1993 to 1995, 1996 to 1998, 2011 to 2013, and 2016 to 2017 (visits 2–6, respectively).
ARIC‐NCS was an ancillary ARIC study designed to evaluate the role of midlife cardiovascular risk factors in determining late‐life cognitive decline and dementia. The details of this study have been previously described.13 Briefly, as part of ARIC‐NCS, 6538 participants attending the visit 5 examination in 2011 to 2013 underwent an extensive cognitive evaluation and underwent 2‐dimensional echocardiography examination as part of the main ARIC study. We excluded 1036 participants who had missing cognitive scores, inadequate echocardiographic information, or missing covariates at visit 5, yielding a final cross‐sectional sample size of 5502. In 2016 to 2017, 4003 participants took part in ARIC study visit 6. An additional 612 participants were excluded because of missing repeated cognitive scores, yielding a final longitudinal analysis sample size of 3391. Figure 1 shows the flow of study participants.
Figure 1.
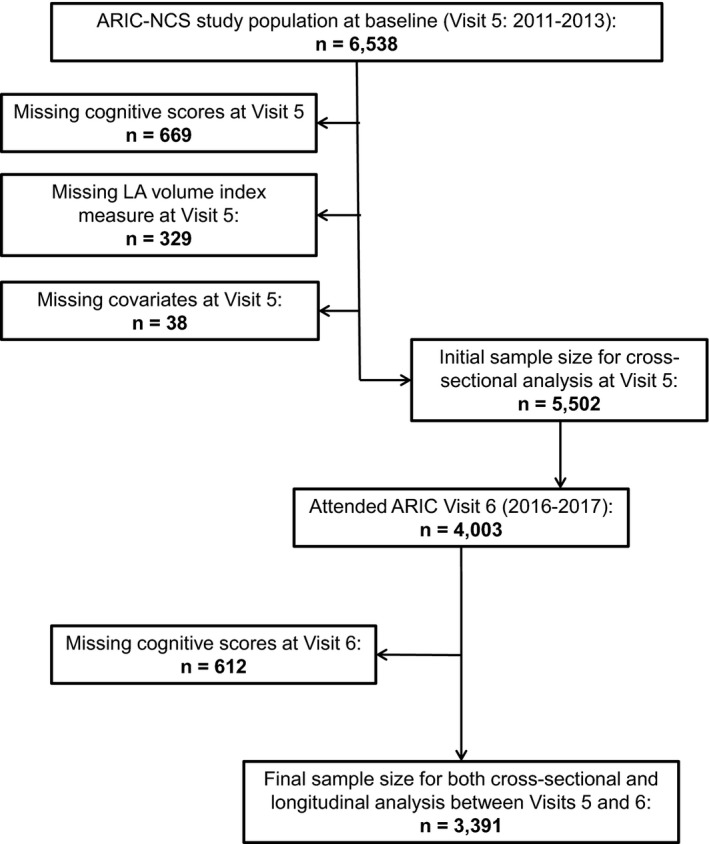
Flow diagram of study participants. ARIC‐NCS indicates Atherosclerosis Risk in Communities Neurocognitive Study; LA, left atrium.
The ARIC study and ARIC‐NCS have been approved by institutional review boards at all participating institutions. Participants provided written informed consent before being examined.
Study Variables
Measures of cognitive function
A neuropsychological test battery was administered to participants by trained examiners in a standardized order during one session in a quiet room. Examiner performance was monitored by audiotape recording. Recordings were reviewed locally and shared across centers to ensure consistency with testing procedures and standardization across study sites. The specific cognitive tests used in our study and the method in which they were standardized and combined were described in detail previously.14, 15 Briefly, for the memory cognitive domain, tests were Delayed Word Recall, immediate and delayed Logical Memory, and Incidental Learning (from Wechsler Memory Scale–III). For the executive function domain, tests were Digit Symbol Substitution and Digit Span Backwards (from Wechsler Adult Intelligence Scale–Revised) and Trail Making Test parts A and B. For the language cognitive domain, tests were Word Finding (or Controlled Oral World Association Test), Animal Naming, and Boston Naming Test. We then used factor analysis to derive scores for memory, executive function, language, and global cognition (a scaled combination of the 3 different cognitive domains). This structured approach for describing common covariation among a set of observed indicators leverages data from multiple cognitive tests to provide more robust measures of domain‐specific function than those provided by individual tests.16 The interpretation of our factor scores is similar to Z scores because they were scaled to have a mean of 0 and a variance of 1. Finally, to define mild cognitive impairment or dementia among participants, a panel of neurologists and neuropsychologists reviewed the aforementioned cognitive tests and followed diagnostic criteria proposed by the National Institute of Aging–Alzheimer's Association workgroups.13
Echocardiography and LAE
LA size was assessed with 2‐dimensional echocardiography during visit 5. The design and quality control details have been described previously.17 Size measurement was performed at the end of systole using biplane disk summation and indexed to body surface area to derive an LA volume index per American Society of Echocardiography guidelines.18 LAE was defined as left atrial volume index ≥34 mL/m2 and used as a categorical variable for analysis.
Prevalent AF
Prevalent AF at visit 5 was ascertained using 12‐lead ECG performed during study visits 1 to 5 and previous hospitalization for AF based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes 427.31 or 427.32.19 Presence or absence of AF was used as a binary variable for analysis.
Covariates
The following covariates were collected and included in the adjustment model as they were believed to represent strong confounders in the association between LAE and cognitive scores: age, sex, race (black or white), study center, educational level (high school graduate versus not), apolipoprotein E genotype (0, 1, or 2 ε4 alleles), smoking (never, former, or current), body mass index, systolic and diastolic blood pressure, use of antihypertensive medication, use of anticoagulants, diabetes mellitus, stroke, coronary heart disease or myocardial infarction, and heart failure. Hypertension was defined as systolic blood pressure of ≥140 mm Hg, diastolic blood pressure of ≥90 mm Hg, or use of blood pressure–lowering medications. Diabetes mellitus was defined as a fasting glucose level of ≥126 mg/dL or a nonfasting glucose level of ≥200 mg/dL, a self‐reported physician diagnosis of diabetes mellitus, or use of diabetes mellitus medications. Stroke was self‐reported at visit 1 and then defined by subsequent reported incidences by annual telephone interviews, visit examination, ARIC study community hospital records, physician questionnaires, and informant interviews. Coronary heart disease was defined by self‐reported physician diagnoses at visit 1, evidence of myocardial infarction on ECG, and adjudicated cases between visit 1 and visit 5. Heart failure was defined according to Gothenburg criteria, an ICD‐9 code of 428.x from hospitalization records, a previously identified heart failure diagnosis, or reported use of heart failure medications within the previous 2 weeks before the examination.20
Statistical Analysis
We categorized participants into 4 groups: group 1 (reference group), participants without AF and without LAE; group 2, participants with AF but without LAE; group 3, participants with LAE but without AF; and group 4, participants with LAE and with AF. For cross‐sectional analysis, we used general linear models to assess the association of LAE and AF cognitive domain factor scores at visit 5. Longitudinal change in cognitive domain factor scores was computed as an absolute difference between cognitive domain factor scores at visit 5 and visit 6. We used general linear models to assess the association of LAE and AF with longitudinal change in cognitive domain factor scores. For both cross‐sectional and longitudinal analyses, model 1 was adjusted for age, sex, race study center, and educational level. Model 2 was additionally adjusted for apolipoprotein E genotype, smoking status, body mass index, systolic and diastolic blood pressure, use of antihypertensive medication, diabetes mellitus, stroke, coronary heart disease or myocardial infarction, and heart failure. Model 3 was additionally adjusted for use of vitamin K antagonists or direct oral anticoagulants.
To account for attrition between visit 5 and visit 6, we conducted a sensitivity analysis using inverse probability of attrition weights (model 4). The weights for each individual were calculated at visit 5 and visit 6 using the inverse of the estimated probabilities of being alive at the time of follow‐up and attending the visit given that the participant was alive. The weights were stabilized by the baseline variables of age, sex, race, study center, and apolipoprotein E genotype. In addition, to account for differences in cognitive function at baseline, we adjusted for cognitive domain scores at visit 5 (model 5).
We conducted a secondary analysis to evaluate the association between LAE and change in cognitive function, with and without adjustment for AF. Model 1 was adjusted for age, sex, race study center, and educational level. Model 2 was additionally adjusted for apolipoprotein E genotype, smoking status, body mass index, systolic and diastolic blood pressure, use of antihypertensive medication, diabetes mellitus, stroke, coronary heart disease or myocardial infarction, heart failure, and use of an anticoagulant. Model 3 was additionally adjusted for AF. A sensitivity analysis of model 3 using inverse probability of attrition weights was also conducted (model 4).
All analyses were performed using SAS, Version 9.4 (SAS Inc, Cary, NC), or STATA 14.0 (StataCorp LP, College Station, TX). We report means with SDs for continuous variables and counts with percentages for categorical variables. All P values reported were 2 sided, and the statistical significance threshold was set as P<0.05.
Results
Study Population
Table 1 lists the clinical characteristics of our analysis cohort at visit 5. The mean age of participants was 74.4 years (SD, 5.1 years); 59% were women, and 21% were black. The prevalence of AF was 6%, and the prevalence of LAE was 13%. Mean (SD) cognitive domain scores at baseline are also provided in Table 1. Compared with the other groups, there were more men in group 4 (LAE and AF). In addition, group 4 participants had the highest LA volume index and the highest prevalence of stroke, coronary heart disease, and heart failure. Notably, mean unadjusted cognitive scores in every cognitive domain at visit 5 were lower in group 4 than in all other groups. Unadjusted cognitive domain scores were lower in all groups at the follow‐up visit 6 than during visit 5; this is represented visually in Figure 2.
Table 1.
Participant Characteristics by LAE and AF Status, Visit 5 (2011–2013), ARIC‐NCS
| Characteristics | Group 1 | Group 2 | Group 3 | Group 4 | P Value |
|---|---|---|---|---|---|
| (Normal LA/No AF) | (Normal LA/AF) | (LAE/No AF) | (LAE/AF) | ||
| No. | 2842 | 107 | 337 | 105 | … |
| Age, mean (SD), y | 74 (5) | 77 (5) | 76 (5) | 77 (4) | <0.0001 |
| Female sex, n (%) | 1737 (61) | 58 (54) | 171 (51) | 42 (40) | <0.0001 |
| Black race, n (%) | 627 (22) | 8 (7) | 74 (22) | 8 (8) | <0.0001 |
| Less than high school education, n (%) | 291 (10) | 9 (8) | 43 (13) | 12 (11) | 0.64 |
| Current smoker, n (%) | 154 (5) | 6 (6) | 13 (4) | 1 (1) | 0.03 |
| Body mass index, mean (SD), kg/m2 | 29 (6) | 30 (6) | 29 (5) | 30 (6) | 0.06 |
| Systolic blood pressure, mean (SD), mm Hg | 129 (17) | 125 (16) | 132 (19) | 126 (20) | <0.0001 |
| Diastolic blood pressure, mean (SD), mm Hg | 67 (10) | 66 (10) | 64 (11) | 63 (13) | <0.0001 |
| Antihypertensive use, n (%) | 2015 (71) | 91 (85) | 273 (81) | 95 (90) | <0.0001 |
| Anticoagulant use, n (%) | 63 (2) | 41 (38) | 13 (4) | 76 (72) | <0.0001 |
| Diabetes mellitus, n (%) | 810 (29) | 37 (35) | 91 (27) | 37 (35) | 0.21 |
| Prevalent stroke, n (%) | 64 (2) | 6 (6) | 19 (6) | 11 (10) | <0.0001 |
| CHD/MI, n (%) | 328 (12) | 27 (25) | 60 (18) | 32 (30) | <0.0001 |
| Heart failure, n (%) | 61 (2) | 18 (17) | 13 (4) | 31 (30) | <0.0001 |
| Left atrial volume index, mean (SD), mL/m2 | 23 (5) | 26 (5) | 39 (5) | 44 (8) | <0.0001 |
| Dementia, n (%) | 25 (1) | 2 (2) | 6 (2) | 1 (1) | 0.35 |
| Mild cognitive impairment, n (%) | 474 (17) | 18 (17) | 49 (15) | 25 (24) | 0.18 |
| Cognitive domain score, mean (SD) | |||||
| Memory | |||||
| Visit 5 | 0.24 (0.7) | 0.06 (0.7) | 0.08 (0.8) | 0.02 (0.7) | <0.0001 |
| Visit 6 | 0.02 (0.8) | −0.13 (0.8) | −0.15 (0.8) | −0.26 (0.7) | <0.0001 |
| Executive function | |||||
| Visit 5 | 0.26 (0.8) | 0.23 (0.7) | 0.07 (0.9) | −0.07 (0.6) | <0.0001 |
| Visit 6 | −0.06 (0.9) | −0.23 (0.7) | −0.23 (0.9) | −0.42 (0.7) | <0.0001 |
| Language | |||||
| Visit 5 | 0.22 (0.8) | 0.28 (0.8) | 0.12 (0.8) | 0.05 (0.7) | 0.02 |
| Visit 6 | 0.03 (0.8) | −0.06 (0.8) | −0.10 (0.8) | −0.21 (0.8) | <0.0001 |
| Global cognition | |||||
| Visit 5 | 0.31 (0.8) | 0.29 (0.7) | 0.12 (0.8) | 0.03 (0.6) | <0.0001 |
| Visit 6 | 0.01 (0.9) | −0.14 (0.7) | −0.17 (0.9) | −0.33 (0.7) | 0.001 |
P values calculated using χ2 or ANOVA tests. AF indicates atrial fibrillation; ARIC‐NCS, Atherosclerosis Risk in Communities Neurocognitive Study; CHD, coronary heart disease; LA, left atrium; LAE, left atrial enlargement; MI, myocardial infarction.
Figure 2.
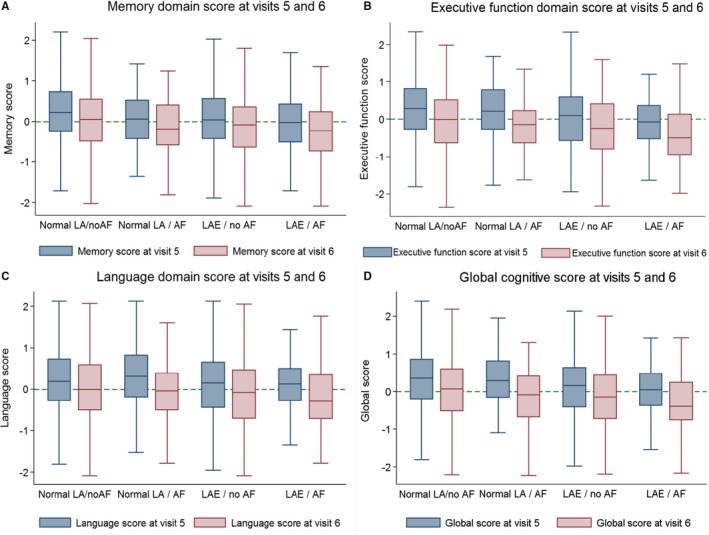
Unadjusted cognitive domain scores at ARIC‐NCS (Atherosclerosis Risk in Communities Neurocognitive Study) visit 5 and visit 6. A, Memory domain scores. B, Executive function scores. C, Language domain scores. D, Global cognition scores. The line through each box plot indicates the median value, whereas the 75th and 25th percentiles are depicted by the edges of each box. A dotted reference line is at 0. The whiskers extend to lower quartile—1.5 interquartile range (IQR) and upper quartile—1.5 IQR. There were few outside values; therefore, they were not plotted. AF indicates atrial fibrillation; LA, left atrium; LAE, left atrial enlargement.
Association of LAE and AF With Cognitive Function
Table 2 shows the cross‐sectional association of LAE and AF with cognitive domain scores at visit 5. Compared with participants without LAE or AF (group 1), those with LAE and prevalent AF (group 4) had significantly lower cognitive domain scores in executive function, language, and global cognition, even after adjusting for cardiovascular risk factors and anticoagulant use. Compared with group 1, LAE without AF (group 3) was not associated with lower cognitive domain scores after multivariable adjustment. Finally, prevalent AF without LAE (group 2) also was not associated with lower cognitive domain scores when compared with group 1.
Table 2.
Association of LAE and AF Status With Baseline Cognitive Domain Scores, Visit 5 (2011–2013), ARIC‐NCS
| Cognitive Domain | Model | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|---|
| (Normal LA/No AF) (n=2842) | (Normal LA/AF) (n=107) | (LAE/No AF) (n=337) | (LAE/AF) (n=105) | ||
| Memory | 1 | Reference | −0.12 (−0.25 to 0.01) | −0.04 (−0.11 to 0.04) | −0.08 (−0.21 to 0.05) |
| 2 | Reference | −0.13 (−0.26 to 0.01) | −0.03 (−0.10 to 0.05) | −0.07 (−0.20 to 0.07) | |
| 3 | Reference | −0.13 (−0.27 to 0.01) | −0.03 (−0.10 to 0.05) | −0.07 (−0.23 to 0.08) | |
| 4 | Reference | −0.14 (−0.26 to −0.01) | −0.06 (−0.13 to 0.01) | −0.06 (−0.21 to 0.08) | |
| Executive function | 1 | Reference | −0.05 (−0.17 to 0.07) | −0.08 (−0.15 to −0.01)a | −0.28 (−0.40 to −0.15)a |
| 2 | Reference | −0.01 (−0.14 to 0.11) | −0.05 (−0.12 to 0.02) | −0.20 (−0.33 to −0.07)a | |
| 3 | Reference | −0.04 (−0.17 to 0.09) | −0.04 (−0.12 to 0.02) | −0.25 (−0.40 to −0.10)a | |
| 4 | Reference | −0.04 (−0.16 to 0.08) | −0.07 (−0.14 to −0.01) | −0.24 (−0.38 to −0.10)a | |
| Language | 1 | Reference | 0.03 (−0.10 to 0.16) | −0.02 (−0.09 to 0.06) | −0.16 (−0.29 to −0.03)a |
| 2 | Reference | 0.04 (−0.09 to 0.17) | −0.01 (−0.08 to 0.07) | −0.14 (−0.27 to −0.01)a | |
| 3 | Reference | −0.01 (−0.14 to 0.13) | −0.01 (−0.08 to 0.07) | −0.23 (−0.38 to −0.07) | |
| 4 | Reference | −0.01 (−0.14 to 0.12) | −0.03 (−0.10 to 0.04) | −0.22 (−0.36 to −0.07) | |
| Global cognition | 1 | Reference | −0.04 (−0.15 to 0.08) | −0.07 (−0.14 to −0.01)a | −0.23 (−0.35 to −0.12)a |
| 2 | Reference | −0.02 (−0.13 to 0.10) | −0.04 (−0.11 to 0.02) | −0.18 (−0.30 to −0.06)a | |
| 3 | Reference | −0.04 (−0.17 to 0.08)a | −0.04 (−0.11 to 0.02) | −0.24 (−0.38 to −0.10)a | |
| 4 | Reference | −0.05 (−0.16 to 0.06) | −0.07 (−0.13 to −0.01) | −0.23 (−0.36 to −0.11)a |
Model 1, adjusted for age, sex, race/study center, and educational level. Model 2, model 1+apolipoprotein E genotype, smoking, body mass index, systolic and diastolic blood pressure, use of antihypertensive medication, diabetes mellitus, stroke, coronary heart disease or myocardial infarction, and heart failure. Model 3, model 2+anticoagulant use. Model 4, model 3+adjustment for mild cognitive impairment and dementia. Data represented are changes in factor scores. AF indicates atrial fibrillation; ARIC‐NCS, Atherosclerosis Risk in Communities Neurocognitive Study; LA, left atrium; LAE, left atrial enlargement.
Indicates a significant association (95% CI below zero).
Table S1 shows the cross‐sectional association between LAE and cognitive domain scores at visit 5, with and without adjusting for AF. Participants with LAE had lower executive and global cognitive function than participants with normal LA size, even after adjustment for confounders and AF.
In addition, we evaluated the association of LAE and AF at visit 5 with cognitive domain scores at visit 6 (Table S2). Although group 4 (LAE/AF) was associated with lower executive function and global cognition domain scores at visit 6, after adjustment for cognitive domain scores at visit 5, the associations were no longer significant. In addition, participants in group 2 (normal LA/AF) had lower executive function and global cognition domain scores at visit 6, even after adjustment for cognitive domain scores at visit 5. Table S3 shows the association between LAE and AF at visit 5 with cognitive domain scores at visit 6, with and without adjusting for AF. Consistent with our findings in Table S2, compared with normal LA, participants with LAE at visit 5 did not have lower cognitive domain scores at visit 6.
Association of LAE and AF With Cognitive Function Decline
Table 3 shows the association of LAE and AF with longitudinal change in cognitive domain scores between visit 5 and visit 6 (2011–2017). Compared with group 1, AF without LAE (group 2) had greater decline in cognitive domain scores in executive function, language, and global cognition, even after multivariable adjustment. However, participants with LAE, with or without AF (group 3 and 4), did not have significantly greater decline in cognitive domain scores compared with group 1.
Table 3.
Association of LAE and AF Status With Longitudinal Change in Cognitive Domain Scores Between Visit 5 (2011–2013) and Visit 6 (2016–2017), ARIC‐NCS
| Cognitive Domain | Model | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|---|
| (Normal LA/No AF) (n=2842) | (Normal LA/AF) (n=107) | (LAE/No AF) (n=337) | (LAE/AF) (n=105) | ||
| Memory | 1 | Reference | 0.05 (−0.07 to 0.16) | −0.01 (−0.07 to 0.06) | −0.03 (−0.14 to 0.08) |
| 2 | Reference | 0.06 (−0.06 to 0.17) | −0.01 (−0.08 to 0.05) | −0.01 (−0.13 to 0.10) | |
| 3 | Reference | 0.05 (−0.06 to 0.17) | −0.01 (−0.08 to 0.05) | −0.02 (−0.16 to 0.11) | |
| 4 | Reference | 0.06 (−0.05 to 0.17) | −0.01 (−0.08 to 0.05) | −0.02 (−0.14 to 0.11) | |
| 5 | Reference | 0.02 (−0.08 to 0.12) | −0.02 (−0.08 to 0.04) | −0.03 (−0.15 to 0.08) | |
| 6 | Reference | 0.02 (−0.09 to 0.12) | −0.03 (−0.09 to 0.03) | −0.04 (−0.15 to 0.08) | |
| Executive function | 1 | Reference | −0.13 (−0.23 to −0.04)a | 0.02 (−0.04 to 0.07) | 0.01 (−0.09 to 0.10) |
| 2 | Reference | −0.13 (−0.22 to −0.03)a | 0.01 (−0.04 to 0.07) | −0.01 (−0.10 to 0.10) | |
| 3 | Reference | −0.13 (−0.23 to −0.03)a | 0.01 (−0.04 to 0.07) | −0.01 (−0.12 to 0.12) | |
| 4 | Reference | −0.15 (−0.24 to −0.05)a | 0.01 (−0.04 to 0.07) | 0.01 (−0.10 to 0.11) | |
| 5 | Reference | −0.16 (−0.25 to −0.07)a | 0.01 (−0.05 to 0.06) | −0.05 (−0.16 to 0.05) | |
| 6 | Reference | −0.16 (−0.25 to −0.07)a | 0.01 (−0.06 to 0.05) | −0.06 (−0.16 to 0.04) | |
| Language | 1 | Reference | −0.13 (−0.23 to −0.03)a | −0.02 (−0.08 to 0.04) | −0.04 (−0.14 to 0.06) |
| 2 | Reference | −0.13 (−0.23 to −0.03)a | −0.02 (−0.08 to 0.04) | −0.03 (−0.13 to 0.08) | |
| 3 | Reference | −0.12 (−0.22 to −0.01)a | −0.02 (−0.08 to 0.04) | −0.01 (−0.13 to 0.11) | |
| 4 | Reference | −0.11 (−0.21 to −0.01)a | −0.02 (−0.08 to 0.04) | −0.01 (−0.12 to 0.10) | |
| 5 | Reference | −0.12 (−0.21 to −0.03)a | −0.02 (−0.08 to 0.03) | −0.06 (−0.16 to 0.05) | |
| 6 | Reference | −0.12 (−0.21 to −0.03)a | −0.03 (−0.08 to 0.03) | −0.06 (−0.17 to 0.04) | |
| Global cognition | 1 | Reference | −0.11 (−0.19 to −0.02)a | 0.02 (−0.03 to 0.07) | −0.02 (−0.11 to 0.06) |
| 2 | Reference | −0.10 (−0.18 to −0.02)a | 0.02 (−0.03 to 0.07) | −0.02 (−0.11 to 0.06) | |
| 3 | Reference | −0.11 (−0.20 to −0.02)a | 0.02 (−0.03 to 0.07) | −0.04 (−0.14 to 0.06) | |
| 4 | Reference | −0.13 (−0.21 to −0.05)a | 0.02 (−0.03 to 0.07) | −0.04 (−0.13 to 0.05) | |
| 5 | Reference | −0.13 (−0.21 to −0.06)a | 0.01 (−0.03 to 0.06) | −0.07 (−0.16 to 0.02) | |
| 6 | Reference | −0.14 (−0.22 to −0.06)a | 0.01 (−0.04 to 0.05) | −0.07 (−0.16 to 0.01) |
Model 1, adjusted for age, sex, race/study center, and educational level. Model 2, model 1+apolipoprotein E genotype, smoking, body mass index, systolic and diastolic blood pressure, use of antihypertensive medication, diabetes mellitus, stroke, coronary heart disease or myocardial infarction, and heart failure. Model 3, model 2+anticoagulant use. Model 4, model 3+takes into account inverse probability of attrition weights. Model 5, model 4+adjustment for baseline scores at visit 5. Model 6, model 5+adjustment for mild cognitive impairment and dementia. Data represented are changes in factor scores. AF indicates atrial fibrillation; ARIC‐NCS, Atherosclerosis Risk in Communities Neurocognitive Study; LA, left atrium; LAE, left atrial enlargement.
Indicates a significant association (95% CI below zero).
Of participants who attended visit 5, only 62% returned for visit 6 and had a complete cognitive evaluation. To account for potential bias caused by attrition, we conducted a sensitivity analysis using inverse probability of attrition weights; our results were essentially unchanged (model 4). Furthermore, to account for the difference in baseline cognitive domain scores among groups at visit 5, we additionally adjusted for cognitive domain scores at visit 5; our results remained unchanged (model 5).
Discussion
In a large community‐based cohort study that comprised elderly individuals, based on cross‐sectional analysis, participants with LAE and AF had lower cognitive performance in executive function and language domain than those with normal LA size and without AF. However, after a longitudinal follow‐up of 5 years, these participants (with LAE and AF) did not have greater cognitive decline, compared with those with normal LA size and without AF. In contrast, in the same 5‐year follow‐up, participants with AF and normal LA size had greater longitudinal decline in executive, language, and global cognitive function compared with those with normal LA size and without AF.
The association between AF and cognitive decline has been extensively reported,3, 21, 22, 23 and a few small studies have also evaluated the association between LA size and cognitive function. van den Hurk et al found an association between higher LA volume index and lower information‐processing and executive functioning, but did not account for AF nor assess the association with longitudinal change in cognitive function.8 Karadag et al reported a cross‐sectional association between LAE and lower Mini‐Mental State Examination scores in patients with sinus rhythm, but did not evaluate specific cognitive domains nor assess association with longitudinal change in cognitive function.9 Yet, another cross‐sectional study found that greater LA diameter was associated with lower language and memory scores, but it only involved 50 participants and it did not assess longitudinal change in cognitive performance.10
Our study advances the field on several fronts. We comprehensively evaluated cognitive function by performing a battery of tests in specific cognitive domains: memory, executive, and language. Furthermore, by categorizing our study participants into 4 groups, we were able to evaluate separately the effects of LAE and AF in stratified analysis and not only rely on statistical adjustment for AF. By doing so, we identified a cross‐sectional association between LAE and AF (when both conditions are present) and lower cognitive function. However, in longitudinal analysis, LAE, with or without AF, was not associated with greater cognitive decline over 5 years. Our additional longitudinal cognitive analysis highlights the limitations of relying only on cross‐sectional analysis: even with multivariable adjustment, cross‐sectional analysis cannot adequately control for all relevant characteristics that could differ among study participants at a single time point. Finally, although we did not observe a decline in cognitive performance in participants with LAE, we confirmed the established relationship of AF to greater cognitive decline in the executive and language domains, as reported by Nishtala et al in the Framingham and Offspring cohort.24
There are several potential limitations that may have bearing on our findings. First, AF was identified from hospitalization discharges and study ECGs; thus, we could have missed asymptomatic AF or AF managed exclusively in an outpatient setting. However, we and others have previously shown that the validity of AF ascertainment using hospitalizations is acceptable and that incidence rates of AF in the ARIC study are consistent with other population‐based studies.2, 19 Second, data on AF duration are not available in the ARIC study; hence, we were not able to account for the possible effect of AF duration.25 Third, we defined LAE as LA volume index ≥34 mL/m2 during a single echocardiographic measurement at visit 5 and used it as a categorical variable for analysis; it is possible this may have contributed to a type 2 error. Fourth, it is possible that we were underpowered to detect an association between LAE and greater cognitive decline (the number of participants with both LAE and AF was 105). To overcome this limitation, we conducted a secondary analysis to assess the association of LAE with longitudinal change in cognitive function, adjusting for AF (the number of participants with LAE was higher; n=442). Once again, we did not find a significant relationship between LAE and greater longitudinal cognitive decline in the secondary analysis (Table S4). Fifth, compared with the groups 1 to 3, the cognitive domain scores in participants with LAE and AF were the lowest for both at visit 5. Hence, the lower absolute cognitive scores in participants with LAE and AF may have precluded detection of further cognitive decline. To address this concern, we adjusted for baseline cognitive scores, and our results remain unchanged. Sixth, the length of follow‐up for our current study was 5 years; this follow‐up time may not be long enough to detect changes in decreased baseline cognitive function. Sixth, our study was composed of elderly individuals; it may well be that LAE is associated with greater cognitive decline in middle‐aged but not elderly individuals. Finally, we acknowledge that although ARIC study participants at inception of the cohort reflected the general population 30 years ago, participants surviving to visit 5 and 6 may be somewhat healthier than a similar‐age community. Finally, we only evaluated LA size and did not assess LA function. Kamel et al have previously demonstrated that LA dysfunction, as measured by increased P‐wave terminal force in lead V1, was associated with increased risk of stroke.26 It is, therefore, possible that LA dysfunction may play a greater role in cognitive decline rather than LA size.
On the basis of a large community‐based cohort study, we did not find LAE to be significantly associated with greater cognitive decline. Additional studies of middle‐aged to late age individuals with longer follow‐up are needed to confirm our findings. Future studies should also evaluate LA function in relation to change in cognitive performance.
Sources of Funding
Dr Alonso is supported by American Heart Association grant 16EIA26410001. Dr Chen is supported by National Institutes of Health (NIH) grants R01HL126637 and R01HL141288. Dr Shah is supported by NIH grants K08HL116792, R01HL135008, and R01HL143224. The ARIC (Atherosclerosis Risk in Communities) study is performed as a collaborative study, supported by National Heart, Lung, and Blood Institute (NHLBI) contracts (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, and HHSN268201700004I). Neurocognitive data are collected by 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, and 2U01HL096917 from the NIH (NHLBI, National Institute of Neurological Disorders and Stroke, National Institute on Aging, and National Institute on Deafness and Other Communication Disorders), and with previous brain magnetic resonance imaging examinations funded by R01‐HL70825 from the NHLBI. Funding for laboratory testing and biospecimen collection at ARIC visit 6 was supported by grant R01DK089174 from the National Institutes of Diabetes and Digestive and Kidney Diseases of the NIH. The views expressed in this article are those of the authors and do not necessarily represent the views of the NHLBI, the NIH, or the US Department of Health and Human Services.
Disclosures
Dr Shah receives research support through Brigham and Women's Hospital from Novartis. He serves as a consultant for Bellerophon Therapeutics and Philips Ultrasound. The remaining authors have no disclosures to report.
Supporting information
Table S1. Association of Left Atrial Enlargement Status With Baseline Cognitive Domain Scores, Visit 5 (2011–2013), ARIC‐NCS
Table S2. Association of Left Atrial Enlargement and Atrial Fibrillation Status at Visit 5 (2011–2013) With Cognitive Domain Scores at Visit 6 (2016–2017), ARIC‐NCS
Table S3. Association of Left Atrial Enlargement Status at Visit 5 (2011–2013) With Cognitive Domain Scores at Visit 6 (2016–2017), ARIC‐NCS
Table S4. Association of Left Atrial Enlargement Status With Longitudinal Change in Cognitive Domain Scores, Visit 5 and 6, 2011–2017, ARIC‐NCS
Acknowledgments
The authors thank the staff and participants of the ARIC (Atherosclerosis Risk in Communities) study for their important contributions.
(J Am Heart Assoc. 2019;8:e013197 DOI: 10.1161/JAHA.119.013197.)
References
- 1. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 2. Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. [DOI] [PubMed] [Google Scholar]
- 3. Kalantarian S, Stern TA, Mansour M, Ruskin JN. Cognitive impairment associated with atrial fibrillation: a meta‐analysis. Ann Intern Med. 2013;158:338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sanfilippo AJ, Abascal VM, Sheehan M, Oertel LB, Harrigan P, Hughes RA, Weyman AE. Atrial enlargement as a consequence of atrial fibrillation: a prospective echocardiographic study. Circulation. 1990;82:792–797. [DOI] [PubMed] [Google Scholar]
- 5. Benjamin EJ, D'Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham heart study. Circulation. 1995;92:835–841. [DOI] [PubMed] [Google Scholar]
- 6. Kizer JR, Bella JN, Palmieri V, Liu JE, Best LG, Lee ET, Roman MJ, Devereux RB. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle‐aged and elderly adults: the strong heart study (SHS). Am Heart J. 2006;151:412–418. [DOI] [PubMed] [Google Scholar]
- 7. Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol. 2014;63:493–505. [DOI] [PubMed] [Google Scholar]
- 8. van den Hurk K, Reijmer YD, van den Berg E, Alssema M, Nijpels G, Kostense PJ, Stehouwer CD, Paulus WJ, Kamp O, Dekker JM, Biessels GJ. Heart failure and cognitive function in the general population: the Hoorn study. Eur J Heart Fail. 2011;13:1362–1369. [DOI] [PubMed] [Google Scholar]
- 9. Karadag B, Ozyigit T, Ozben B, Kayaoglu S, Altuntas Y. Relationship between left atrial volume index and cognitive decline in elderly patients with sinus rhythm. J Clin Neurosci. 2013;20:1074–1078. [DOI] [PubMed] [Google Scholar]
- 10. Alosco ML, Gunstad J, Jerskey BA, Clark US, Hassenstab JJ, Xu X, Poppas A, Cohen RA, Sweet LH. Left atrial size is independently associated with cognitive function. Int J Neurosci. 2013;123:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Salthouse TA. Neuroanatomical substrates of age‐related cognitive decline. Psychol Bull. 2011;137:753–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. The ARIC investigators. The atherosclerosis risk in communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 13. Knopman DS, Gottesman RF, Sharrett AR, Wruck LM, Windham BG, Coker L, Schneider AL, Hengrui S, Alonso A, Coresh J, Albert MS, Mosley TH Jr. Mild cognitive impairment and dementia prevalence: the atherosclerosis risk in communities neurocognitive study (ARIC‐NCS). Alzheimers Dement (Amst). 2016;2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schneider AL, Sharrett AR, Gottesman RF, Coresh J, Coker L, Wruck L, Selnes OA, Deal J, Knopman D, Mosley TH. Normative data for 8 neuropsychological tests in older blacks and whites from the atherosclerosis risk in communities (ARIC) study. Alzheimer Dis Assoc Disord. 2015;29:32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rawlings AM, Bandeen‐Roche K, Gross AL, Gottesman RF, Coker LH, Penman AD, Sharrett AR, Mosley TH. Factor structure of the ARIC‐NCS neuropsychological battery: an evaluation of invariance across vascular factors and demographic characteristics. Psychol Assess. 2016;28:1674–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gross AL, Power MC, Albert MS, Deal JA, Gottesman RF, Griswold M, Wruck LM, Mosley TH Jr, Coresh J, Sharrett AR, Bandeen‐Roche K. Application of latent variable methods to the study of cognitive decline when tests change over time. Epidemiology. 2015;26:878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shah AM, Cheng S, Skali H, Wu J, Mangion JR, Kitzman D, Matsushita K, Konety S, Butler KR, Fox ER, Cook N, Ni H, Coresh J, Mosley TH, Heiss G, Folsom AR, Solomon SD. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community‐dwelling elderly persons: the atherosclerosis risk in communities study. Circ Cardiovasc Imaging. 2014;7:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 19. Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African‐Americans: the atherosclerosis risk in communities (ARIC) study. Am Heart J. 2009;158:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kwok CS, Loke YK, Hale R, Potter JF, Myint PK. Atrial fibrillation and incidence of dementia: a systematic review and meta‐analysis. Neurology. 2011;76:914–922. [DOI] [PubMed] [Google Scholar]
- 22. Chen LY, Lopez FL, Gottesman RF, Huxley RR, Agarwal SK, Loehr L, Mosley T, Alonso A. Atrial fibrillation and cognitive decline: the role of subclinical cerebral infarcts: the atherosclerosis risk in communities study. Stroke. 2014;45:2568–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen LY, Norby FL, Gottesman RF, Mosley TH, Soliman EZ, Agarwal SK, Loehr LR, Folsom AR, Coresh J, Alonso A. Association of atrial fibrillation with cognitive decline and dementia over 20 years: the ARIC‐NCS (atherosclerosis risk in communities neurocognitive study). J Am Heart Assoc. 2018;7:e007301 DOI: 10.1161/JAHA.117.007301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nishtala A, Piers RJ, Himali JJ, Beiser AS, Davis‐Plourde KL, Saczynski JS, McManus DD, Benjamin EJ, Au R. Atrial fibrillation and cognitive decline in the Framingham heart study. Heart Rhythm. 2018;15:166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diener HC, Hart RG, Koudstaal PJ, Lane DA, Lip GYH. Atrial fibrillation and cognitive function: JACC review topic of the week. J Am Coll Cardiol. 2019;73:612–619. [DOI] [PubMed] [Google Scholar]
- 26. Kamel H, Soliman EZ, Heckbert SR, Kronmal RA, Longstreth WT Jr, Nazarian S, Okin PM. P‐wave morphology and the risk of incident ischemic stroke in the multi‐ethnic study of atherosclerosis. Stroke. 2014;45:2786–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Association of Left Atrial Enlargement Status With Baseline Cognitive Domain Scores, Visit 5 (2011–2013), ARIC‐NCS
Table S2. Association of Left Atrial Enlargement and Atrial Fibrillation Status at Visit 5 (2011–2013) With Cognitive Domain Scores at Visit 6 (2016–2017), ARIC‐NCS
Table S3. Association of Left Atrial Enlargement Status at Visit 5 (2011–2013) With Cognitive Domain Scores at Visit 6 (2016–2017), ARIC‐NCS
Table S4. Association of Left Atrial Enlargement Status With Longitudinal Change in Cognitive Domain Scores, Visit 5 and 6, 2011–2017, ARIC‐NCS


