Abstract
Background
Repeated episodes of limb ischemia and reperfusion (remote ischemic conditioning [RIC]) may protect the brain from ischemic reperfusion injury.
Methods and Results
We performed a phase IIb blinded dose‐escalation sham‐controlled trial in patients with hyperacute stroke, randomized 1:1 to receive RIC (four 5‐minute cycles) or sham to the nonparetic upper limb, in 3 blocks of increasing dose, starting within 6 hours of ictus. The primary outcome was trial feasibility (recruitment, attrition). Secondary outcomes included adherence, tolerability, safety (serious adverse events), plasma biomarkers at days 1 and 4 (S100‐ß protein, matrix metalloproteinase‐9, and neuron‐specific enolase), and functional outcome. Sixty participants were recruited from 2 centers (3 per month) with no loss to follow‐up: time to randomization 4 hours 5 minutes (SD 72 minutes), age 72 years (12), men 60%, blood pressure 154/80 mm Hg (25/12), National Institutes of Health Stroke Scale 8.4 (6.9), and 55% thrombolyzed. RIC was well tolerated with adherence not differing between RIC and sham, falling in both groups on day 3 (P=0.001, repeated measures ANOVA) because of discharge or transfer. S100ß increased in the sham group (mean rise 111 pg/mL [302], P=0.041, repeated measures ANCOVA) but not the RIC group. There were no differences in matrix metalloproteinase‐9, neuron‐specific enolase, number with serious adverse events (RIC 10 versus sham 10, P=0.81), deaths (2 versus 4, P=0.36), or modified Rankin Scale score (2 [interquartile range 1–4], 2 [interquartile range, 1–3]; P=0.85).
Conclusions
RIC in hyperacute stroke is feasible when given twice daily for 2 days and appears safe in a small population with hyperacute stroke. A larger phase III trial is warranted.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT02779712.
Keywords: feasibility, randomized controlled trial, remote ischemic conditioning, stroke
Subject Categories: Ischemic Stroke, Cerebrovascular Disease/Stroke
Clinical Perspective
What Is New?
This is the first randomized controlled trial in patients with hyperacute ischemic stroke to test an increasing dose of remote ischemic conditioning. Repeated dosing until day 2 was feasible in terms of adherence, and the dosing regimen for larger remote ischemic conditioning trials should consider this alongside local patient pathways.
What Are the Clinical Implications?
Beneficial clinical signals exist from several small pilot and proof‐of‐concept studies using remote ischemic conditioning after stroke and these findings warrant further testing in a well‐designed, larger phase III trial.
Introduction
Remote ischemic conditioning (RIC) uses repeated cycles of transient limb ischemia and reperfusion to induce organ protection from ischemic reperfusion injury and tolerance to subsequent ischemic events. The mechanisms underlying RIC are not fully understood but have been attributed to neurohumoral pathways linking the pre‐conditioned tissue to the brain, resulting in early and late windows of protection.1, 2
Experimentally, RIC applied before (pre‐conditioning), during (per‐conditioning), and after (post‐conditioning) an ischemic stroke decreases cerebral inflammation and edema and reduces apoptosis in the ischemic penumbra through inhibition of the mitochondrial permeability transition pore.3, 4, 5 Administration of a protein synthesis inhibitor, afferent nerve blocker or KATP channel antagonist attenuates the neuroprotective effects of RIC.4, 6 In a meta‐analysis of preclinical stroke, remote pre‐, per‐, and post‐conditioning reduced infarct volume by 35%, was effective in permanent and transient models of ischemia, and improved neurological outcome.7
Five proof‐of‐concept randomized clinical trials in stroke and RIC have been published: in populations before carotid stenting (pre‐conditioning),8 during acute ischemic stroke (per‐conditioning),9, 10 and after ischemic stroke caused by intracranial stenosis (post‐conditioning).11, 12 In RECAST‐1 (Remote Ischemic Conditioning After Stroke Trial), we demonstrated excellent intervention tolerability in patients with acute stroke.10 Although limited by a small sample size, there was also a significant decrease in National Institutes of Health Stroke Scale (NIHSS) score at day 90 and augmentation of neuroprotective proteins, plasma heat shock protein‐27 (HSP27) and phosphorylated HSP27, in the RIC group.13
In the current study, we aimed to demonstrate feasibility and safety of increasing doses of remote ischemic per‐conditioning in patients presenting to the hospital with hyperacute stroke.
Methods
Trial Design
RECAST‐2 (Remote Ischemic Conditioning After Stroke Trial 2), was a 2‐center, feasibility, dose‐escalation, outcome‐blinded, randomized, placebo‐controlled trial. The trial was conducted in accordance with the Declaration of Helsinki and the International Conference of Harmonisation of Good Clinical Practice, sponsored by the University of Nottingham (United Kingdom), received authorization from the local research ethics committee (West Midlands, April 15, 2016), and was a registered clinical trial (NCT02779712).
Patients
Adults 18 years and older were invited to participate if they had a clinical stroke with onset in the past 6 hours. Exclusion criteria were premorbid dependency (modified Rankin Scale [mRS] score >3), dementia, Glasgow Coma Scale score <8, malignancy, pregnancy, and significant comorbidities. We did not mandate that baseline neuroimaging (as part of standard care) was required before randomization, but, in practice, the brain scan and approaching the participant for the trial occurred in parallel, with the result available before trial inclusion. The trial recruited patients from University Hospitals of Derby and Burton (UHDB) NHS Foundation Trust and Nottingham University Hospitals (NUH) NHS Trust in the United Kingdom between August 2016 and April 2018. UHDB and NUH receive ≈900 and 1300 patients with strokes per year, respectively. Consent was obtained by the research practitioner from each patient or legal representative if the patient was unable to consent. Clinical and safety assessments were performed at baseline (prerandomization), day 4 (face‐to‐face), and day 90 (telephone).
Intervention
RIC was performed by trained trial staff immediately after randomization and included 4 cycles of intermittent limb ischemia: alternating 5 minutes of inflation (20 mm Hg above systolic blood pressure [BP]) followed by 5 minutes of deflation of a standard upper arm BP cuff in the nonparetic arm. The control group received a sham procedure mimicking the intervention protocol, but cuff inflation only reached 30 mm Hg. The intervention was performed manually using a standard BP cuff. Preclinically, repeated RIC cycles are more effective than a single set of cycles14; hence, we increased the dose in 3 phases: the first 20 participants received 1 “dose” of RIC/sham, ie 4 cycles of cuff inflation and deflation; participants 21 to 40 received a second dose (4 cycles of RIC/sham) 1 hour after the first dose; and the final 20 participants (41–60) were also administered twice‐daily dosing starting the following morning up to and including day 4 (total 8 doses). Delivery time of each cycle (seconds) and reasons for discontinuation were recorded.
Primary Outcome
The primary outcome was trial feasibility describing recruitment rate, time to recruitment, number recruited per center, and attrition to follow‐up.
Secondary Outcomes
Tolerability
Tolerability of increasing doses of RIC: duration cuff tolerated, number of cycles, adherence to RIC, and reasons for poor adherence.
Clinical measures and safety
Clinical secondary outcomes included safety: vascular events (recurrent stroke, myocardial infarction, limb ischemia, and venous thromboembolism), death, neurological deterioration (increase in NIHSS ≥4 points), neurovascular limb damage, and tissue injury. Comparison of serious adverse events (SAEs) by treatment with thrombolysis provided further assessment of safety in this subgroup. Function was assessed at day 90 by telephone interview blinded to treatment allocation: dependency (mRS),15 disability (Barthel Index),16 Zung depression scale,17 quality of life (EuroQol‐5D), and cognition (Modified Telephone Interview for Cognitive Status [TICS‐M]).18
Laboratory measures
Immediately before treatment and on day 4 (±1), blood samples were collected for: (1) surrogate markers of brain injury, which might be attenuated if RIC improves ischemic reperfusion injury (plasma S100ß protein, matrix metalloproteinase [MMP‐9], neuron‐specific enolase [NSE], by multiplex technology, Merck Millipore Ltd); S‐100ß, NSE, and MMP‐9 also act as surrogate markers of infarct volume and prognosis in acute ischemic stroke19, 20; and (2) HSP27 (DouSet ELISA, R&D Systems), which is a biomarker implicated in the mechanisms of ischemic conditioning10 and is neuroprotective in experimental stroke.13 Assays and analysis of data were performed blinded to treatment allocation.
Sample Size
No formal power calculation was performed for this feasibility study. Considering resources, competing trials and time, recruiting 60 participants from 2 centers was deemed an appropriate number, at an anticipated rate of recruitment of 1.5 recruits per center per month, to inform a larger trial on application of RIC in the hyperacute setting in terms of trial feasibility and increasing RIC dose.
Randomization and Blinding
Participants were recruited using web‐based randomization with computerized minimization distributing the patients on a 1:1 ratio into RIC or sham groups. Minimization variables were age (≥70 years), sex (male), NIHSS (≥10), and systolic BP (≥160 mm Hg). The research practitioner delivering the intervention could not be blinded. Adjudicated SAEs and outcomes, day 90 interview, laboratory measures, and statistical analyses were performed blinded to treatment allocation.
Statistical Analysis
Baseline characteristics and functional outcomes of RIC and control groups were compared using chi‐square or Fisher exact tests for binary data; continuous data were compared using t test or Mann–Whitney U tests; and recurrent clinical events were compared using hazard ratios and univariate Cox regression analyses (SPSS version 24, IBM). Additionally, day 90 mRS was compared using ordinal logistic regression. Repeated measures ANOVA with no covariate adjustment compared adherence to treatment between groups. Repeated measures ANCOVA, adjusting for baseline NIHSS, compared plasma biomarkers taken on day 1 and day 4, with further adjustment using Sidak multiple comparisons test (SPSS version 24 and Prism 7 for Mac OS X version 7.0c). Associations between S100ß and functional outcome were tested using Pearson's correlation coefficient. Subgroup analyses were not performed at a dose level since numbers were considered too small. Data in the figures are mean±SD unless otherwise stated. Statistical significance was taken at P<0.05.
Data Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Patients
The trial commenced in August 2016, completed follow‐up in August 2018, and enrolled 60 participants (31 and 29 in the RIC and sham groups, respectively; 20 per dose block) (Figure 1). The mean age of all participants was 72 years (SD 12), 60% were men, mean BP was 154/80 mm Hg (SD 25/12 mm Hg), and median NIHSS was 6 (interquartile range, 3–11). There were no baseline statistical differences between groups for age, sex, and baseline stroke severity (Table 1). The RIC group was randomized later (254 versus 195 minutes, P=0.003), contained more participants with diabetes mellitus (33% versus 7%, P=0.02), and had a lower mean systolic BP (146 versus 162 mm Hg, P=0.01). The final diagnosis was ischemic stroke in 55 cases (92%), transient ischemic attack in 4 cases (7%), and functional disorder in 1 case (2%).
Figure 1.
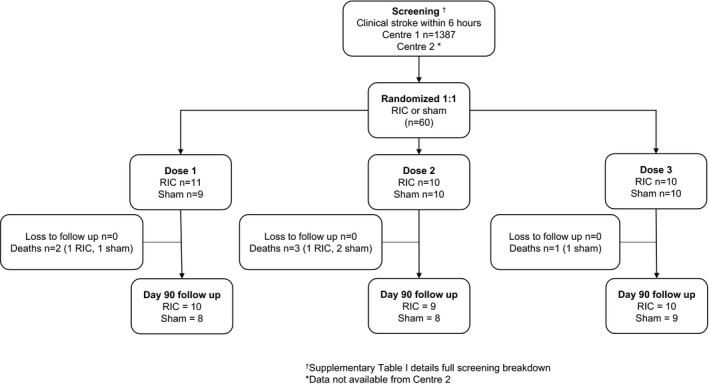
Trial flow. RIC indicates remote ischemic conditioning.
Table 1.
Baseline Characteristics
| Characteristic | RIC | Sham |
|---|---|---|
| No. | 31 | 29 |
| Age, y (SD)a | 70.9 (13.4) | 73.7 (10.2) |
| Mena | 21 (70) | 15 (50.0) |
| BP, mm Hg | ||
| Systolica | 146 (24) | 162 (23) |
| Diastolic | 78 (12) | 83 (11) |
| Heart rate | 77 (13) | 80 (18) |
| Admission ECG in AF | 11 (36.7) | 12 (40.0) |
| NIHSSa | 6 [3–9] | 7 [3–12] |
| GCS | 15 [14–15] | 15 [14–15] |
| Premorbid mRS | 0 [0–2] | 0 [0–1] |
| Stroke to randomization, min | 254 [254–343] | 199 [149–261] |
| Admission to randomization, min | 195 [174–277] | 93 [66–168] |
| Thrombolyzed | 16 (51.6) | 17 (58.6) |
| Mechanical thrombectomy | 0 (0) | 0 (0) |
| Final diagnosisb | ||
| Ischemic stroke | 28 (90.3) | 27 (93.1) |
| TIA | 2 (6.5) | 2 (6.9) |
| Hemorrhagic stroke | 0 (0) | 0 (0) |
| Clinical syndrome | ||
| Total anterior circulation | 6 (20.0) | 5 (16.7) |
| Partial anterior circulation | 9 (30.0) | 14 (46.7) |
| Lacunar | 9 (30.0) | 8 (26.7) |
| Posterior circulation | 4 (13.3) | 0 (0.0) |
| Medical history | ||
| Hypertension | 14 (46.7) | 11 (36.7) |
| Diabetes mellitus | 10 (33.3) | 2 (6.7) |
| Known AF | 12 (40.0) | 10 (33.3) |
| Hyperlipidemia | 14 (46.7) | 9 (30.0) |
| Stroke | 9 (30.0) | 5 (16.7) |
| TIA | 6 (20.0) | 2 (6.7) |
| Ischemic heart disease | 5 (16.7) | 4 (13.3) |
| Peripheral vascular disease | 1 (3.3) | 1 (3.3) |
Data are expressed as mean values (SD), median [interquartile range], or number (percentage). AF indicates atrial fibrillation; BP, blood pressure; GCS, Glasgow Coma Scale; NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale; TIA, transient ischemic attack.
Minimization variables.
One participant was diagnosed with functional disorder in the remote ischemic conditioning (RIC) group.
Trial Feasibility
The recruitment rate averaged 1.5 participants per center per month (n=20 center 1, n=40 center 2). The main reasons for exclusion included presentation >6 hours from onset of symptoms (42.5%), presenting outside of working hours (13.4%), and nonstroke diagnoses (12.5%) (Table S1). The median time to randomization was 255 minutes (interquartile range, 186–298), with 33 (55%) receiving thrombolysis. There were no losses to follow‐up. The sham appeared feasible since when asked at day 90 which intervention patients received, 56 (93%) did not know, 2 (4%) were incorrect, and 2 were (4%) correct.
Compliance
RIC was well tolerated with no statistical differences between RIC and sham regarding duration of cuff inflation (Figure 2). Adherence was high in the first 2 days but there was a significant fall on day 3 (dose 5) in the RIC and sham groups to 40% and 43%, respectively (P=0.001, repeated measures ANOVA), with no between‐group differences (P=0.64) secondary to either early discharge or the participant moving to another rehabilitation setting. In the first 48 hours, procedure intolerance in the RIC group (cuff pressure intolerance, headache, agitation) leading to incomplete treatment of 4 cycles occurred in 5 of 62 (8%) offered doses (Table S2).
Figure 2.
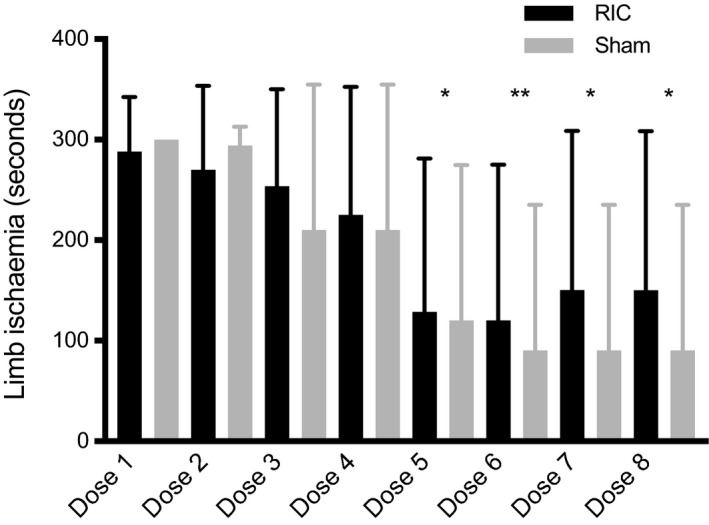
Adherence to remote ischemic conditioning (RIC) or sham by dose number and mean total duration of limb ischemia (seconds±SD). Maximum length of cuff inflation is 300 seconds per dose (4× 5 minutes per cycle). Compared with dose 1, there is a significant fall in adherence over time from day 3 (*P=0.001, **P<0.001 repeated measures ANOVA), with no between‐group differences (P=0.64). “n” sham/RIC=dose 1: 29/31; dose 2: 19/21; and doses 3 to 8: 10/10.
Safety and Clinical Outcomes
There was no difference in the number of participants with an SAE (Table 2 and Table S3) and no episodes of limb ischemia or injury. The mortality rate was 10% with 4 deaths in the sham group (n=1 extension/recurrent ischemic stroke, n=1 hemorrhagic transformation of infarction, n=1 early neurological deterioration, n=1 gradual decline) and 2 in the RIC group (n=1 hemorrhagic transformation of infarction, n=1 malignancy). Extension and recurrent ischemic stroke were more frequent in the sham group (6 versus 2 events) (unadjusted hazard ratio, 0.28; 95% CI, 0.06–1.37 [P=0.12]). A total of 83% of recurrent cerebrovascular events occurred in the first 48 hours. RIC appeared to be safely administered in the thrombolyzed cohort, with no differences in SAEs between groups (Table S4).
Table 2.
Summary of Secondary Clinical Outcomes and SAEs
| SAE | RIC (n=31) | Sham (n=29) | HR (95% CI) | P Value |
|---|---|---|---|---|
| No with SAE | ||||
| Any SAE | 10 (32.3) | 10 (34.5) | 0.81 (0.33–1.96) | 0.81 |
| Fatal | 2 (6.5) | 4 (13.8) | 0.46 (0.8–2.5) | 0.36 |
| All stroke and NDa | ||||
| Extension/recurrent ischemic stroke | 2 (6.5) | 6 (20.7) | 0.28 (0.06–1.37) | 0.12 |
| Symptomatic HTI | 2 (6.5) | 1 (3.4) | 1.85 (0.17–20.38) | 0.62 |
| Early ND | 1 (3.2) | 0 (0.0) | ··· | ··· |
| Seizure | 0 (0.0) | 1 (3.4) | ··· | ··· |
| TIA | 1 (3.2) | 0 (0.0) | ··· | ··· |
| MI | 0 (0.0) | 0 (0.0) | ··· | ··· |
| VTE | ||||
| PE | 0 (0.0) | 1 (3.4) | ··· | ··· |
| DVT | 0 (0.0) | 0 (0.0) | ··· | ··· |
Analyses performed using unadjusted Cox regression. DVT indicates deep vein thrombosis; HR, hazard ratio; MI, myocardial infarction; PE, pulmonary embolism; SAE, serious adverse event; TIA, transient ischemic attack; VTE, venous thromboembolism.
One participant in the sham group had neurological deterioration (ND) and hemorrhagic transformation of infarction (HTI), and 1 participant in the remote ischemic conditioning (RIC) group had HTI and recurrent stroke (only the first event is counted in regression analyses).
Laboratory Measures
Plasma S100ß increased significantly in the sham group from day 1 to day 4, mean difference, 111 pg/mL (95% CI, 5.6–216 [P=0.041], repeated measures ANOVA, adjusted for baseline NIHSS) (Figure 3). No differences in plasma S100ß were present in the RIC group between days 1 and 4 (mean difference, 27.5 pg/mL; 95% CI, −14.5 to 69.5 [P=0.187]) nor were there significant differences between groups at day 4 (adjusted P=0.35). Day 4 plasma S100ß correlated significantly with baseline NIHSS (Pearson's correlation coefficient, r=0.561; P<0.001) and day 90 mRS (r=0.41, P=0.006). MMP‐9 concentration was nonsignificantly higher in the sham group compared with the RIC group (change from baseline, mean difference, 15.3 ng/mL [95% CI, −2.6 to 33.2], P=0.09). There were no differences between groups with respect to NSE. Heat shock protein 27 assays proved unreliable and data are not presented.
Figure 3.
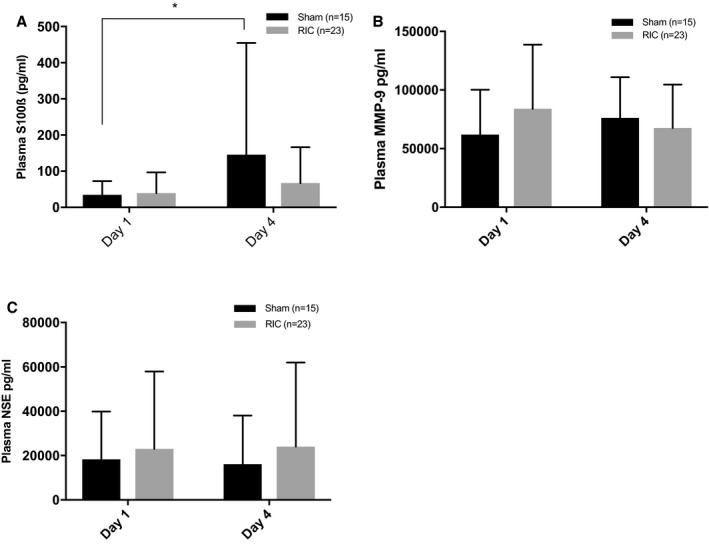
Plasma S100ß (A), matrix metalloproteinase‐9 (MMP‐9, B), and neuron‐specific enolase (NSE, C) on days 1 and 4 by treatment group. S100ß levels increase by day 4 in the sham group from 34.5 pg/mL (SD 37.8) to 145.6 pg/mL (309.1), mean difference 111 pg/mL (95% CI, 5.6–216; P=0.041*). There were no significant between‐group differences at day 4. Analysis by repeated measures ANCOVA, Sidak correction for multiple comparisons, and adjusted for baseline stroke severity. RIC indicates remote ischemic conditioning.
Functional Outcomes
There were no significant differences between groups with respect to functional outcomes, although the trial was not large enough to detect these (Table 3 and Figure 4). Telephone data collection at day 90 was feasible for measures of dependency (mRS), disability (Barthel index, BI), mood (Zung), cognition (TICS‐M), and quality of life (EuroQoL), similar to other large trials.21
Table 3.
Functional Outcome by Group at Day 4 and Day 90
| Functional Measure | RIC | Sham | P Value |
|---|---|---|---|
| Day 4a | n=30 | n=24 | |
| NIHSS | 6.4 (9.4) | 9.5 (12.8) | 0.30 |
| Change NIHSS | −3.0 (3.8) | −1.4 (6.3) | 0.35 |
| Day 90 | n=31 | n=29 | |
| mRS (/6) | |||
| Median | 2 [1–4] | 2 [1–3] | 0.85 |
| mRS 3 to 6 | 12 (40) | 14 (46.6) | 0.46 |
| Barthel Index (/100) | 100 [65–100] | 100 [57.5–100] | 0.89 |
| Zung depression scoreb | 46.25 [33.75–53.75] | 42.5 [37.5–52.5] | 0.94 |
| EuroQoL HUIb | 0.514 (0.377) | 0.482 (0.393) | 0.77 |
| EuroQoL VASb | 70.8 (23.0) | 69.8 (19.2) | 0.87 |
| TICS‐Mb | 23 [20–25] | 23.5 [21–27] | 0.89 |
Data are expressed as mean (SD), median [interquartile range], or number (percentage). Imputed value for death: Barthel index −5; National Institutes of Health Stroke Scale (NIHSS) 42. mRS indicates modified Rankin Scale.
Day 4 NIHSS—sham n=24, remote ischemic conditioning (RIC) n=30 (data missing attributable to early discharge or refused). Analyzed by independent t test, Mann–Whitney U test, or chi‐square test as appropriate.
Number for EuroQoL Health Utilities Index (HUI): 24 (sham)/28 (RIC), EuroQoL visual analogue scale (VAS) 22/24, Zung 17/16, and Modified Telephone Interview for Cognitive Status (TICS‐M) 14/14. Number reduced by: (1) carers answering on behalf of participants who could not respond (n=17), (2) refused to answer questions on mood and cognition, and (3) death (n=6).
Figure 4.
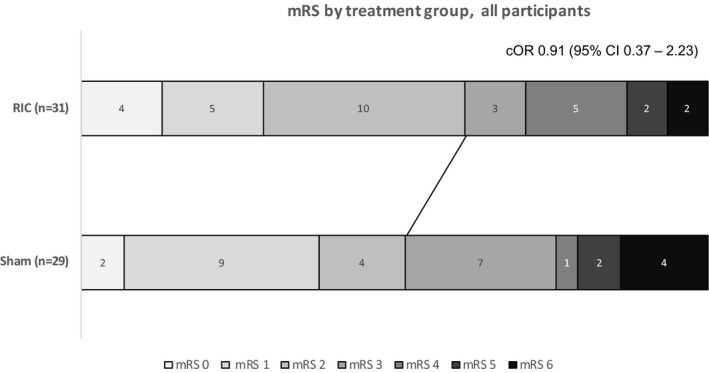
Day 90 modified Rankin Scale (mRS) score by treatment group. Unadjusted common odds ratios (cORs) and 95% CIs comparing groups are analyzed by ordinal logistic regression. There was no significant interaction when treatment*thrombolysis was introduced into the model. The line demarcates dichotomy at functional independence, an mRS of ≤2. RIC indicates remote ischemic conditioning.
Discussion
RECAST‐2 has demonstrated the feasibility of conducting a randomized controlled trial of remote ischemic per‐conditioning in hyperacute stroke across 2 centers in terms of recruitment, intervention delivery, attrition, compliance of increasing dose to day 2, and use of an effective sham.
RECAST‐2 is the first stroke and RIC trial to evaluate alternative dosing strategies. Overall, the optimal dosing and method of application of RIC in stroke remains unclear. There is noticeable heterogeneity in completed and ongoing clinical trials ranging from daily administration using both arms in post‐conditioning secondary prevention studies (cuff pressure to 200 mm Hg),11, 12 to single lower limb application using cuff pressures 120 mm Hg above the systolic BP in acute ischemic stroke.22 Strategies appear to be based on the population studied rather than from information provided by preclinical data. Importantly, an experimental dose‐finding study in postconditioned stroke rats determined that 3 cycles of 5/5 minutes limb ischemia and reperfusion was more effective than 15/15 seconds and 8/8 minutes.4 Previous trials have delivered RIC daily for up to 300 days,11, 12 initiated in the subacute phase after stroke. It is therefore feasible to deliver RIC for a prolonged period using an automated machine. We chose the maximum dose to stop at day 4 since this covers the hyperacute phase and prolonged effects of the treatment are anticipated.23 We also expected it would not be possible to administer RIC using a manual BP cuff for longer than this, which proved to be the case. In RECAST‐2, repeated dosing until day 2 was feasible in terms of adherence, and the dosing regimen for larger RIC trials should consider this alongside local patient pathways. The main reason for treatment discontinuation was not cuff pressure intolerance but transfer of the participant to a different setting or discharge home.
The absence of any SAEs relating to limb ischemia or injury, especially in the thrombolyzed cohort, is reassuring. The safety of RIC in hyperacute stroke, however, requires further evaluation since this is a small population. RIC has potential antiplatelet effects,24 which may be beneficial in ischemic stroke, but could exacerbate hemorrhagic transformation of infarction or lead to deterioration if administered in intracerebral hemorrhage before confirmation of the diagnosis. One pre‐hospital RIC trial, however, reported no clinical deterioration in 37 participants with primary intracerebral haemorrhage.9
The majority of recurrent cerebrovascular events occurred within the first 48 hours, reflecting early ischemic reperfusion injury, which can manifest clinically as recurrent ischemia, hemorrhagic transformation of infarction, cerebral edema, and expansion of the original infarct. The trial was not powered to detect reductions in these events, but we observed tendency in favor of RIC towards reduced risk of recurrent fatal and nonfatal stroke. In addition, there are biochemical signals of efficacy evidenced by increased plasma biomarkers of brain injury (S100ß) in the placebo group not seen in the RIC group. S100ß is a recognized surrogate marker of infarct volume and functional outcome,19 and in this study correlated significantly with baseline stroke severity and day 90 mRS.
It is recognized that RIC leads to an immediate period of ischemic tolerance lasting 1 to 2 hours, followed by a second window of protection 12 to 24 hours later, lasting 48 to 72 hours.25 Preclinically, alteplase combined with RIC has an additive effect26 and a single dose of RIC can have long‐lasting protective effects for up to 6 days.23 Further, the time window of RIC application in experimental models extends up to 6 hours post‐ictus27 and combining per‐ and post‐conditioning may tackle both early and late phases of ischemic reperfusion injury. Per‐conditioning was more effective at reducing infarct volume than a pre‐conditioning stimulus in one study28 but this difference is not borne out in meta‐analysis of experimental data.7 Since strokes are difficult to predict, per‐conditioning is a viable strategy in acute ischemia, whereas pre‐conditioning may be more suited towards high‐risk populations, eg, before carotid intervention8 or after a transient ischemic attack.
A recent Cochrane Review exploring RIC for preventing and treating ischemic stroke has highlighted the paucity of published randomized clinical trials in this area.29 Interestingly, recurrence in ischemic stroke (by end of trial) was significantly reduced.8, 11, 12, 29 In updating the meta‐analysis with results from RECAST‐1,10 RECAST‐2, and Che et al,30 and organizing groups into pre‐, per‐, and post‐conditioning trials, RIC significantly reduces the composite outcome of recurrent vascular events (odds ratio, 0.27; 95% CI, 0.13–0.59) (Figure 5.8, 10, 11, 12, 30 This is consistent with secondary analyses in the cardiac literature (RIC and acute myocardial infarction) where recurrent cardiovascular and cerebrovascular events were reduced by half.31 It is not intuitive that brief periods of RIC can lead to protection from vascular events at much later time points (and repeated doses may be required) but the finding deserves further exploration in clinical trials.
Figure 5.
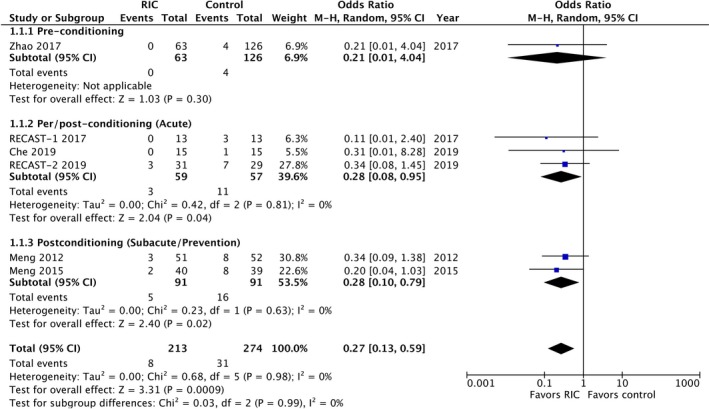
Recurrent vascular events (nonfatal and fatal stroke, nonfatal and fatal myocardial infarction) in randomized controlled trials assessing remote ischemic conditioning (RIC) in stroke. RECAST indicates Remote Ischemic Conditioning After Stroke Trial; Ref, reference number.
Study Limitations
RECAST‐2 was a high‐quality randomized trial strengthened by assessment of multiple doses, concealment of allocation, and blinded outcome assessments. Limitations include the inability to blind the investigator who performed the intervention, potentially introducing bias into RIC/sham compliance. Second, small sample size, which is sufficient to answer questions of feasibility, introduces risk that other findings may be due to chance, especially since there was an imbalance in systolic BP and diabetes mellitus between groups at baseline. A larger randomized trial is needed to further evaluate efficacy and safety. Third, no participants undergoing mechanical thrombectomy were included (as a result of the need to deliver RIC manually and logistics of transfer to another site). We are unable to comment on the safety of RIC in mechanical thrombectomy but RIC seems feasible and safe in an observational study of mechanical thrombectomy.32 Finally, because of a limited budget, we did not perform any mechanistic neuroimaging studies that determine recanalization or reperfusion rates; however, whilst use of RIC in acute ischemic stroke seems most likely to benefit patients with ischemic reperfusion injury, there is suggestion that in patients with persisting occlusion, RIC may still reduce infarct risk.9
Conclusions
RIC in hyperacute ischemic stroke is feasible, appears safe, and can be administered in repeated doses reliably for 2 days. It is an attractive prospect since it bears low cost and would be simple to administer. A larger phase III trial is warranted.
Sources of Funding
The trial was funded by UHDB.
Disclosures
Bath is Stroke Association Professor of Stroke Medicine and is an NIHR Senior Investigator. The remaining authors have no disclosures to report.
Supporting information
Table S1. Reasons for Exclusion From Center 1
Table S2. Reasons for Noncompliance
Table S3. Serious Adverse Events
Table S4. Serious Adverse Events and Clinical Outcomes by Thrombolysis
Acknowledgments
We thank National Institute of Health Research (NIHR) Clinical Research Network staff in participant recruitment, Nottingham Stroke Trials Unit for completing follow‐up, and Dr Iskandar Idris for reviewing unblinded safety data.
England designed the trial, analyzed the data, and wrote the article. Hedstrom and Jackson screened for participants, acquired the data, and helped revise the article. O'Sullivan processed and analyzed blood biomarkers and revised the article. Woodhouse provided statistical support. Sprigg and Bath contributed to trial design and management, and revised the article.
(J Am Heart Assoc. 2019;8:e013572 DOI: 10.1161/JAHA.119.013572.)
References
- 1. Hess DC, Blauenfeldt RA, Andersen G, Hougaard KD, Hoda MN, Ding Y, Ji X. Remote ischaemic conditioning‐a new paradigm of self‐protection in the brain. Nat Rev Neurol. 2015;11:698–710. [DOI] [PubMed] [Google Scholar]
- 2. Hausenloy DJ, Barrabes JA, Botker HE, Davidson SM, Di Lisa F, Downey J, Engstrom T, Ferdinandy P, Carbrera‐Fuentes HA, Heusch G, Ibanez B, Iliodromitis EK, Inserte J, Jennings R, Kalia N, Kharbanda R, Lecour S, Marber M, Miura T, Ovize M, Perez‐Pinzon MA, Piper HM, Przyklenk K, Schmidt MR, Redington A, Ruiz‐Meana M, Vilahur G, Vinten‐Johansen J, Yellon DM, Garcia‐Dorado D. Ischaemic conditioning and targeting reperfusion injury: a 30 year voyage of discovery. Basic Res Cardiol. 2016;111:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ren C, Gao X, Niu G, Yan Z, Chen X, Zhao H. Delayed postconditioning protects against focal ischemic brain injury in rats. PLoS One. 2008;3:e3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sun J, Li T, Luan Q, Deng J, Li Y, Li Z, Dong H, Xiong L. Protective effect of delayed remote limb ischemic postconditioning: role of mitochondrial K(ATP) channels in a rat model of focal cerebral ischemic reperfusion injury. J Cereb Blood Flow Metab. 2012;32:851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun J, Luan Q, Dong H, Song W, Xie K, Hou L, Xiong L. Inhibition of mitochondrial permeability transition pore opening contributes to the neuroprotective effects of ischemic postconditioning in rats. Brain Res. 2012;1436:101–110. [DOI] [PubMed] [Google Scholar]
- 6. Ren C, Yan Z, Wei D, Gao X, Chen X, Zhao H. Limb remote ischemic postconditioning protects against focal ischemia in rats. Brain Res. 2009;1288:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weir P, Maguire R, O'Sullivan SE, England TJ. Remote ischaemic conditioning in experimental stroke: a systematic review and meta‐analysis (UK stroke forum). Int J Stroke. 2018;13:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao W, Meng R, Ma C, Hou B, Jiao L, Zhu F, Wu W, Shi J, Duan Y, Zhang R, Zhang J, Sun Y, Zhang H, Ling F, Wang Y, Feng W, Ding Y, Ovbiagele B, Ji X. Safety and efficacy of remote ischemic preconditioning in patients with severe carotid artery stenosis before carotid artery stenting: a proof‐of‐concept, randomized controlled trial. Circulation. 2017;135:1325–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hougaard KD, Hjort N, Zeidler D, Sorensen L, Norgaard A, Hansen TM, von Weitzel‐Mudersbach P, Simonsen CZ, Damgaard D, Gottrup H, Svendsen K, Rasmussen PV, Ribe LR, Mikkelsen IK, Nagenthiraja K, Cho TH, Redington AN, Botker HE, Ostergaard L, Mouridsen K, Andersen G. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: a randomized trial. Stroke. 2014;45:159–167. [DOI] [PubMed] [Google Scholar]
- 10. England TJ, Hedstrom A, O'Sullivan S, Donnelly R, Barrett DA, Sarmad S, Sprigg N, Bath PM. RECAST (remote ischemic conditioning after stroke trial): a pilot randomized placebo controlled phase II trial in acute ischemic stroke. Stroke. 2017;48:1412–1415. [DOI] [PubMed] [Google Scholar]
- 11. Meng R, Asmaro K, Meng L, Liu Y, Ma C, Xi C, Li G, Ren C, Luo Y, Ling F, Jia J, Hua Y, Wang X, Ding Y, Lo EH, Ji X. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology. 2012;79:1853–1861. [DOI] [PubMed] [Google Scholar]
- 12. Meng R, Ding Y, Asmaro K, Brogan D, Meng L, Sui M, Shi J, Duan Y, Sun Z, Yu Y, Jia J, Ji X. Ischemic conditioning is safe and effective for octo‐ and nonagenarians in stroke prevention and treatment. Neurotherapeutics. 2015;12:667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shimada Y, Tanaka R, Shimura H, Yamashiro K, Urabe T, Hattori N. Phosphorylation enhances recombinant HSP27 neuroprotection against focal cerebral ischemia in mice. Neuroscience. 2014;278:113–121. [DOI] [PubMed] [Google Scholar]
- 14. Ren C, Wang P, Wang B, Li N, Li W, Zhang C, Jin K, Ji X. Limb remote ischemic per‐conditioning in combination with post‐conditioning reduces brain damage and promotes neuroglobin expression in the rat brain after ischemic stroke. Restor Neurol Neurosci. 2015;33:369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rankin J. Cerebral vascular accidents in patients over the age of 60. 2. Prognosis. Scott Med J. 1957;2:200–215. [DOI] [PubMed] [Google Scholar]
- 16. Wilkinson PR, Wolfe CD, Warburton FG, Rudd AG, Howard RS, Ross‐Russell RW, Beech R. Longer term quality of life and outcome in stroke patients: is the Barthel index alone an adequate measure of outcome? Qual Health Care. 1997;6:125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zung WWK. A self‐rating depression scale. Arch Gen Psychiatry. 1965;12:63–70. [DOI] [PubMed] [Google Scholar]
- 18. Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- 19. Missler U, Wiesmann M, Friedrich C, Kaps M. S‐100 protein and neuron‐specific enolase concentrations in blood as indicators of infarction volume and prognosis in acute ischemic stroke. Stroke. 1997;28:1956–1960. [DOI] [PubMed] [Google Scholar]
- 20. Ramos‐Fernandez M, Bellolio MF, Stead LG. Matrix metalloproteinase‐9 as a marker for acute ischemic stroke: a systematic review. J Stroke Cerebrovasc Dis. 2011;20:47–54. [DOI] [PubMed] [Google Scholar]
- 21. RIGHT‐2Investigators . Prehospital transdermal glyceryl trinitrate in patients with ultra‐acute presumed stroke (RIGHT‐ 2): an ambulance‐based, randomised, sham‐controlled, blinded, phase 3 trial. Lancet. 2019;393:1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pico F, Rosso C, Meseguer E, Chadenat ML, Cattenoy A, Aegerter P, Deltour S, Yeung J, Hosseini H, Lambert Y, Smadja D, Samson Y, Amarenco P. A multicenter, randomized trial on neuroprotection with remote ischemic per‐conditioning during acute ischemic stroke: the remote ischemic conditioning in acute brain infarction study protocol. Int J Stroke. 2016;11:938–943. [DOI] [PubMed] [Google Scholar]
- 23. Hildebrandt HA, Kreienkamp V, Gent S, Kahlert P, Heusch G, Kleinbongard P. Kinetics and signal activation properties of circulating factor(s) from healthy volunteers undergoing remote ischemic pre‐conditioning. JACC Basic Transl Sci. 2016;1:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qian YX, Dai KS, Zhao LL, Yang XJ. Effects of remote ischemic post‐conditioning on platelet activation of AMI patients. Exp Ther Med. 2018;16:1273–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hausenloy DJ, Yellon DM. The second window of preconditioning (SWOP) where are we now? Cardiovasc Drugs Ther. 2010;24:235–254. [DOI] [PubMed] [Google Scholar]
- 26. Hoda MN, Siddiqui S, Herberg S, Periyasamy‐Thandavan S, Bhatia K, Hafez SS, Johnson MH, Hill WD, Ergul A, Fagan SC, Hess DC. Remote ischemic perconditioning is effective alone and in combination with intravenous tissue‐type plasminogen activator in murine model of embolic stroke. Stroke. 2012;43:2794–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma J, Ma Y, Dong B, Bandet MV, Shuaib A, Winship IR. Prevention of the collapse of pial collaterals by remote ischemic perconditioning during acute ischemic stroke. J Cereb Blood Flow Metab. 2017;37:3001–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hahn CD, Manlhiot C, Schmidt MR, Nielsen TT, Redington AN. Remote ischemic per‐conditioning: a novel therapy for acute stroke? Stroke. 2011;42:2960–2962. [DOI] [PubMed] [Google Scholar]
- 29. Zhao W, Zhang J, Sadowsky MG, Meng R, Ding Y, Ji X. Remote ischaemic conditioning for preventing and treating ischaemic stroke. Cochrane Database Syst Rev. 2018;7:Cd012503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Che R, Zhao W, Ma Q, Jiang F, Wu L, Yu Z, Zhang Q, Dong K, Song H, Huang X, Ji X. rt‐PA with remote ischemic postconditioning for acute ischemic stroke. Ann Clin Transl Neurol. 2019;6:364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sloth AD, Schmidt MR, Munk K, Kharbanda RK, Redington AN, Schmidt M, Pedersen L, Sorensen HT, Botker HE. Improved long‐term clinical outcomes in patients with ST‐elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J. 2014;35:168–175. [DOI] [PubMed] [Google Scholar]
- 32. Zhao W, Che R, Li S, Ren C, Li C, Wu C, Lu H, Chen J, Duan J, Meng R, Ji X. Remote ischemic conditioning for acute stroke patients treated with thrombectomy. Ann Clin Transl Neurol. 2018;5:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Reasons for Exclusion From Center 1
Table S2. Reasons for Noncompliance
Table S3. Serious Adverse Events
Table S4. Serious Adverse Events and Clinical Outcomes by Thrombolysis


