Abstract
Background
Circulating proteins are exposed to vascular endothelial layer and influence their functions. Among them, adipsin is a member of the trypsin family of peptidases and is mainly secreted from adipocytes, monocytes, and macrophages, catalyzing the rate‐limiting step of the alternative complement pathway. However, its pathophysiological role in cardiovascular disease remains to be elucidated. Here, we examined whether serum adipsin levels have a prognostic impact in patients with coronary artery disease.
Methods and Results
In 370 consecutive patients undergoing diagnostic coronary angiography, we performed a cytokine array analysis for screening serum levels of 50 cytokines/chemokines and growth factors. Among them, classification and regression analysis identified adipsin as the best biomarker for prediction of their long‐term prognosis (median 71 months; interquartile range, 55–81 months). Kaplan–Meier curve showed that higher adipsin levels (≥400 ng/mL) were significantly associated with all‐cause death (hazard ratio [HR], 4.2; 95% CI, 1.7–10.6 [P<0.001]) and rehospitalization (HR, 2.4; 95% CI, 1.7–3.5 [P<0.001]). Interestingly, higher high‐sensitivity C‐reactive protein levels (≥1 mg/L) were significantly correlated with all‐cause death (HR, 3.2; 95% CI, 1.7–5.9 [P<0.001]) and rehospitalization (HR, 1.5, 95% CI, 1.1–1.9 [P<0.01]). Importantly, the combination of adipsin (≥400 ng/mL) and high‐sensitivity C‐reactive protein (≥1 mg/L) was more significantly associated with all‐cause death (HR, 21.0; 95% CI, 2.9–154.1 [P<0.001]). Finally, the receiver operating characteristic curve demonstrated that serum adipsin levels predict the death caused by acute myocardial infarction in patients with coronary artery disease (C‐statistic, 0.847).
Conclusions
These results indicate that adipsin is a novel biomarker that predicts all‐cause death and rehospitalization in patients with coronary artery disease, demonstrating the novel aspects of the alternative complementary system in the pathogenesis of coronary artery disease.
Keywords: atherosclerosis, biomarker, coronary artery disease, prognosis
Subject Categories: Biomarkers, Coronary Artery Disease
Clinical Perspective
What Is New?
Serum adipsin levels predict all‐cause death in patients with coronary artery diseases.
Serum adipsin levels predict future incidences of acute myocardial infraction in patients with coronary artery diseases.
Elevating serum adipsin levels are associated with cancer death in patients with coronary artery diseases.
What Are the Clinical Implications?
Adipsin plays an important role in the pathogenesis of coronary artery diseases and is also useful as a novel biomarker and therapeutic target.
Serum adipsin level is a useful biomarker to predict future incidences of acute myocardial infarction and to predict new onset of cancer.
Introduction
Cardiovascular risk factors, such as hypertension, diabetes mellitus (DM), dyslipidemia, and smoking, have been used for “risk classification” of patients with coronary artery disease (CAD).1 The assessment of these factors is important for predicting future cardiovascular events.2 In particular, acute myocardial infarction (AMI) is a fatal cardiovascular event and is the most common cause of sudden cardiac death.3 AMI has become an important social issue because of the increasing number of patients with ischemic cardiomyopathy and resultant heart failure.4 It is well known that the levels of hsCRP (high sensitivity C‐reactive protein),5, 6, 7 brain natriuretic peptide (BNP),8 D‐dimer,9 and fibrinogen10 can predict the incidence of future cardiovascular events. However, these biomarkers are also increased in patients with inflammatory diseases.11 Thus, it is important to develop a novel biomarker for prediction of CAD and cardiovascular events.
Circulating cytokines/chemokines and growth factors regulate endothelial functions12 and promote the development of vascular diseases.13, 14 Indeed, vascular diseases are characterized by endothelial dysfunction, vascular smooth muscle cell (VSMC) proliferation, and inflammatory cell accumulation in the adventitial adipose tissues.15, 16, 17 Cardiovascular risk factors (eg, hypertension, DM, dyslipidemia, smoking, and aging) induce vascular oxidative stress and trigger a variety of vascular disorders including CAD.18, 19 Enhanced vascular oxidative stress promotes the secretion of cytokines/chemokines and growth factors from vascular wall cells (eg, endothelial cells, vascular smooth muscle cells, inflammatory cells, and adventitial adipocytes).15, 20 Thus, the interactions between those cells play crucial roles in the development of vascular diseases, where many cytokines/chemokines and growth factors are substantially involved.21, 22
Based on the crucial roles of those circulating factors, we attempted to screen the best biomarker for predicting prognosis and found adipsin in the serum of patients with CAD. Adipsin is a member of the trypsin family of peptidases, which was later identified to be component factor D.23, 24 Adipsin is mainly secreted from adipocytes, monocytes, and macrophages, catalyzing the rate‐limiting step of the alternative complement pathway.25 In this pathway, adipsin cleaves complement factor B and catalyzes the formation of complement 3 (C3) convertase, which leads to a hydrolysis cascade that produces various complement fragments including C3a, C3b, C5a, and C5b.25 The complement system also bridges and regulates the balance between the coagulation and fibrinolysis system.26 Indeed, the coagulation and the fibrinolysis cascade communicate through many connections including the complement system.26 For example, thrombin directly cleaves C3 and its activation fragments, and can cleave C5 into C5a.26 In contrast, it is known that thrombin activatable fibrinolysis inhibitor, which inhibits fibrinolysis, inactivates C3a and C5a.26 Importantly, we have recently demonstrated that thrombin activatable fibrinolysis inhibitor activates intracellular signaling in endothelial cells and vascular smooth muscle cells, promoting vascular remodeling.27 Additionally, the complement system augments the expression of tissue factor and plasminogen activator inhibitor‐1 by C5a and promotes the coagulation cascade.26 Thus, thrombin activatable fibrinolysis inhibitor may play a crucial role in the regulation of the complement system, which causes endothelial dysfunction, vascular smooth muscle cell proliferation, and adventitial inflammation.27 When considering the role of adipsin in the alternative complement pathway and its strong expression in the adventitial adipose tissue, we hypothesized that adipsin may potentially be involved in the pathogenesis of CAD. In the present study, we thus tested our hypothesis that serum levels of adipsin are a useful prognostic biomarker in those patients.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
The ethical review board of Tohoku University approved the study protocol, and written informed consent was obtained from all patients (No. 2008‐470). From January 2008 to June 2011, a total of 377 patients were referred for diagnostic cardiac catheterization for evaluation of chest pain and/or ECG abnormalities at our Tohoku University Hospital. The patients enrolled had evidence of myocardial ischemia on exercise ECG and/or myocardial radionuclide imaging. Patients with unstable angina or AMI were excluded from the present study. Seven of the patients were lost to follow‐up attributable to the Great East Japan earthquake disaster, March 11, 2011.
Evaluation of Coronary Artery Stenosis
Two experienced cardiologists, who were blinded to the patients’ data, including serum levels of cytokines/chemokines and growth factors, evaluated the coronary angiograms. The severity of coronary stenosis was assessed according to the American Heart Association standards. A narrowing of the lumen by >51% of the diameter was considered as a clinically significant stenosis.
Baseline Measurements
In all patients, the medical history was recorded, including details of any previous myocardial infarction, previous revascularization, angina pectoris, hypertension, previous stroke or transient ischemic attacks, DM, and smoking status. Patients with hypertension were regarded as being at risk if their blood pressure was ≥140/90 mm Hg or if they had a history of antihypertensive drug use. Patients with DM were regarded as being at risk if their fasting glucose level was ≥126 mg/dL or if they had a history of hypoglycemic drug or insulin use. Patients with dyslipidemia were regarded as being at risk if their low‐density lipoprotein cholesterol level was ≥140 mg/dL or their high‐density lipoprotein cholesterol level was ≤40 mg/dL, or if they were taking a lipid‐lowering drug. Fasting blood samples were collected for measurement of cytokines/chemokines and growth factors immediately before coronary angiogram from the antecubital vein with patients in the supine position. Serum samples were collected and were centrifuged for 10 minutes at 2500g within 30 minutes of blood collection, and aliquots were stored at −80°C. Serum levels of hsCRP were measured using the sandwich technique (Roche Diagnostics). Values of other laboratory parameters were obtained with an autoanalyzer at the Tohoku University Hospital.
Measurement of Cytokines/Chemokines and Growth Factors
Serum levels of cytokines/chemokines and growth factors were measured with a Bioplex system (Bio‐Rad) according to the manufacturer's instructions. Human cytokines/chemokines and growth factors were measured with commercially available kits (Bio‐Rad, 27‐Plex, #M50‐0KCAF0Y and 21‐Plex, #MF0‐005KMII, #171A7002M).
Immunofluorescence Staining
For immunofluorescence staining, coronary arteries obtained from patients who died of AMI were fixed with 4% phosphate‐buffered paraformaldehyde and were embedded in optimal cutting temperature. For immunostaining, we used the following primary antibodies: adipsin (200:1, Santa Cruz Biotechnology, Inc., sc‐47683) and α‐smooth muscle antibody (400:1, Sigma‐Aldrich, 113200). Tissue sections were mounted using ProLong Diamond Antifade Mountant with 4′,6‐diamidino‐2‐phenylindole (Thermo Fisher Scientific) and was visualized on an LSM780 confocal microscope (Carl Zeiss).
Follow‐Up
Information on death, rehospitalization, and revascularization was obtained annually using follow‐up questionnaires, telephone interviews, and medical records. The primary end point was the composite of all‐cause death, rehospitalization, and revascularization, while the secondary end points consisted of all‐cause death, rehospitalization, and revascularization. The primary and secondary end points were analyzed based on the time to the first occurrence. Composite cardiovascular death was defined as death caused by AMI, heat failure, stroke, sudden death, and other cardiovascular death. Rehospitalization was defined as hospital admission for any cause after enrollment in the present study. Revascularization was defined as undergoing percutaneous coronary intervention or coronary artery bypass grafting after enrollment in the study.
Statistical Analysis
Categorical variables were presented as numerals and percentages. Continuous variables were presented as means±SDs. Correlations between serum adipsin levels and age, BNP, or hsCRP levels were analyzed using the Spearman correlation coefficient. Differences in serum adipsin levels in terms of sex, smoking status, and the presence of hypertension, DM, or dyslipidemia were analyzed by Fisher exact test. To determine the most appropriate cutoff points for high and low serum adipsin levels, we performed classification and regression tree (CART) analysis, which is an empirical, statistical technique based on recursive partitioning of the data space to predict the response.28 Briefly, the models were obtained by binary splitting of the data by the value of predictors, and the split variable and split point were automatically selected from possible candidate predictor values to achieve the best fit. Then, one or both “child nodes” were split into 2 regions recursively, and the process continued until some stopping rule was applied. Finally, the result of this process has been represented as a binary decision tree. We also performed the CART analysis to determine the most appropriate cutoff point for levels of BNP and hsCRP. Kaplan–Meier curves were plotted based on the cutoff points of primary end point for each biomarker. Kaplan–Meier curves were plotted for the primary end point, all‐cause death, rehospitalization, and revascularization relative to the cutoff points. The log‐rank test was applied to compare event‐free survival between groups. Cox proportional hazards model was used for univariable and multivariable analyses. To assess the nonlinear and time‐varying effects in hazard ratio (HR) of the primary end point, an HR plot was plotted. All P values were 2‐tailed, and a P<0.05 was considered to be statistically significant. All analyses were performed with R, version 3.1.3 (R Foundation for Statistical Computing, Vienna, http://www.R-project.org/).28 Additionally, an HR plot was illustrated in using the “rms” package in R.
Results
Identification of Adipsin by Multiple Screening
First, we performed cytokines array analyses to evaluate the serum levels of cytokines/chemokines and growth factors. Importantly, cytokines array showed that many cytokines were significantly elevated in patients with CAD compared with those without it. Then, we performed the CART analysis to evaluate the best biomarker for the prediction of the primary end point, ie, the composite of all‐cause death, rehospitalization, and revascularization. Importantly, among the 50 cytokines/chemokines and growth factors examined, serum levels of adipsin were identified as the best biomarker for poor prognosis in patients with CAD (Figure S1). Baseline characteristics of the 370 patients are shown in Table 1. The mean age was 64±13 years, 66% of them were men, and 49 (13%) died after a mean of 3.2 years (range, 1.5–4.8 years). Among them, 62% had hypertension, 34% had DM, 50% had dyslipidemia, and 30% had a history of smoking. The cutoff point of serum adipsin level was 400 ng/mL as determined by the CART analysis. Baseline characteristics of the groups with higher (≥400 ng/mL) and lower (<400 ng/mL) levels of serum adipsin are shown in Table 1. Additionally, serum levels of cytokines/chemokines and growth factors in the groups with higher (≥400 ng/mL) and lower (<400 ng/mL) levels of serum adipsin are shown in Figure S2. Interestingly, serum adipsin levels were not associated with classic risk factors for atherosclerosis, including smoking, DM, dyslipidemia, and aging except hypertension (Figure S3).
Table 1.
Baseline Patient Characteristics
| All Patients (N=370) | Adipsin ≥400 ng/mL (n=260) | Adipsin <400 ng/mL (n=110) | P Value | |
|---|---|---|---|---|
| Age, y | 63.9±13.1 | 66.5±11.9 | 61.2±13.8 | <0.001 |
| Men, % | 66.4 | 67.5 | 65.2 | 0.582 |
| Family history of IHD, % | 5.1 | 4.9 | 5.3 | 0.846 |
| Medical history, % | ||||
| Hypertension | 62.0 | 66.0 | 58.0 | 0.060 |
| DM | 34.2 | 39.6 | 28.8 | 0.010 |
| Dyslipidemia | 50.1 | 55.5 | 44.7 | 0.015 |
| Smoking, % | 30.2 | 34.3 | 25.9 | 0.037 |
| Angiographic findings, % | ||||
| No coronary stenosis | 58.1 | 54.4 | 67.0 | |
| 1‐Vessel disease | 21.5 | 23.0 | 17.9 | |
| 2‐Vessel disease | 11.7 | 13.2 | 8.0 | |
| 3‐Vessel disease | 8.7 | 9.4 | 7.1 | |
| eGFR, mL/min per 1.73/m2 | 56.6±28.8 | 48.3±26.8 | 65.0±28.4 | <0.001 |
| LVEF, % | 38.9±34.0 | 36.1±33.8 | 41.7±34.1 | 0.058 |
| Total cholesterol, mg/dL | 156.4±67.3 | 147.2±69.1 | 165.6±64.5 | 0.002 |
| LDL, mg/dL | 88.0±46.6 | 85.9±45.1 | 90.1±48.1 | 0.302 |
| HDL, mg/dL | 43.1±20.9 | 42.0±20.3 | 44.3±21.2 | 0.211 |
| Triglycerides, mg/dL | 120.5±110.5 | 113.8±109.8 | 127.3±111.0 | 0.158 |
| Body weight, kg | 55.2±23.2 | 53.5±23.3 | 56.9±22.9 | 0.098 |
| BMI | 19.0±10.1 | 18.3±10.2 | 19.6±9.9 | 0.152 |
| Systolic BP, mm Hg | 106.5±50.3 | 107.2±50.1 | 105.8±50.6 | 0.743 |
| Diastolic BP, mm Hg | 61.6±29.6 | 61.1±28.9 | 62.1±30.3 | 0.681 |
| HbA1c | 5.9±1.4 | 6.0±1.3 | 5.8±1.4 | 0.005 |
| hsCRP, mg/L | 2.0±3.2 | 2.2±3.2 | 1.8±3.2 | 0.221 |
| Medication, % | ||||
| Aspirin | 26.3 | 34.3 | 18.2 | <0.001 |
| β‐Blockers | 32.3 | 34.7 | 29.9 | 0.432 |
| Statins | 45.6 | 46.8 | 44.3 | 0.129 |
| ACEIs | 17.0 | 21.5 | 12.5 | 0.001 |
| ARBs | 17.2 | 21.1 | 13.3 | 0.021 |
| CCBs | 69.2 | 57.4 | 81.1 | <0.001 |
Values are expressed as average±SD unless otherwise indicated. ACEIs indicates angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BMI, body mass index; BP, blood pressure; CCBs, calcium channel blockers; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; hsCRP, high‐sensitivity C‐reactive protein; IHD, ischemic heart disease; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction.
Long‐term Outcomes
We also examined the causes of death during the follow‐up period in this population. During the median follow‐up period of 71 months (interquartile range, 55‐81 months), 226 patients (61%) reached the primary end point, mainly all‐cause death (Figure 1A). Regarding the serum adipsin levels, 45 of 260 patients (17%) died in the higher group, while 6 of 110 (5%) died in the lower group (Figure 1B). In the present study, among the 199 patients who were admitted to hospitals, 61 underwent coronary artery bypass grafting or percutaneous coronary intervention. In particular, in the higher and lower adipsin level groups, 158 (61%) and 41 (37%) were admitted to hospitals (Figure 1C), and 49 (19%) and 12 (11%) underwent coronary revascularization (Figure 1D), respectively. Notably, 4 deaths (8.9%) attributable to AMI occurred in the higher adipsin level group, whereas there was no AMI in the lower adipsin level group (Figure S4A). Thus, in this study population, serum adipsin levels had a significantly higher specificity for predicting death caused by AMI. Regarding “other cardiovascular death” (aneurysm rupture, peripheral ischemia, aortic dissection, and cerebrovascular events excluding AMI), 7 patients (3%) with higher adipsin levels and 3 (3%) with lower adipsin levels died (Table 2). In addition, 12 (5%) and 3 (3%) cancer deaths were noted in the higher and lower groups, respectively (Table 2). Importantly, serum adipsin levels were significantly elevated in patients with cancer death compared with those without it (Figure S5). Regarding “other causes of death (eg, accident, pneumonia, respiratory failure, and renal failure),” 10 patients (4%) with higher adipsin levels and no patients (0%) with lower adipsin levels died (Table 2). Detailed causes of death were not available in the remaining 9 (4%) (Table 2).
Figure 1.
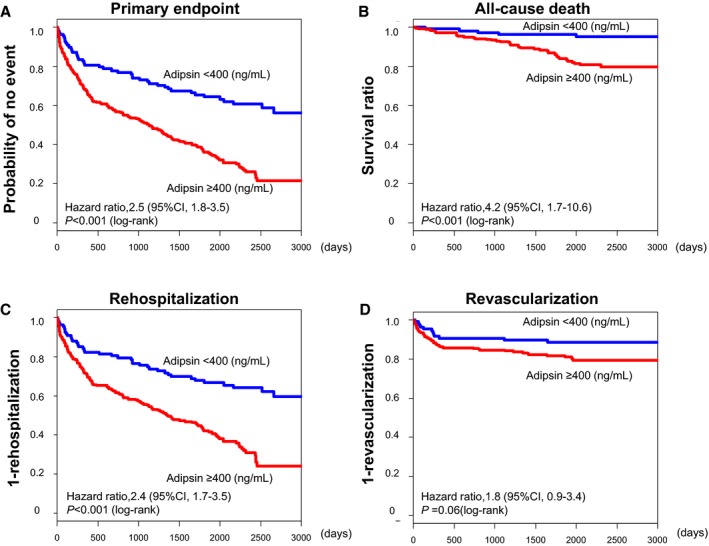
Kaplan–Meier curves in patients with coronary artery disease. Higher adipsin levels were significantly associated with (A) primary end point, (B) all‐cause death, and (C) rehospitalization, but not with (D) revascularization. The primary end point was a composite of all‐cause death, rehospitalization, and revascularization. Revascularization was defined as percutaneous coronary intervention or coronary artery bypass grafting during the follow‐up period.
Table 2.
Causes of Death
| All Patients (N=370) | Adipsin ≥400 ng/mL (n=260) | Adipsin <400 ng/mL (n=110) | P Value | |
|---|---|---|---|---|
| AMI | 1.1 (4) | 1.5 (4) | 0 (0) | 0.323 |
| Other cardiovascular cause | 2.7 (10) | 2.7 (7) | 2.7 (3) | 1.000 |
| Cancer | 4.1 (15) | 4.6 (12) | 2.7 (3) | 0.567 |
| Pneumonia | 0.8 (3) | 1.1 (3) | 0 (0) | 0.558 |
| Other causes | 2.7 (10) | 3.8 (10) | 0 (0) | 0.037 |
| Unknown | 2.4 (9) | 3.5 (9) | 0 (0) | 0.063 |
Values are expressed as percentage (absolute number). Other cardiovascular cause, cardiovascular death excluding acute myocardial infarction (AMI); other causes, known cause of death excluding AMI, other cardiovascular cause, cancer, and pneumonia.
Prognostic Impacts of Serum Adipsin Levels
We next examined whether plasma levels of BNP and serum levels of hsCRP and adiponectin could predict all‐cause death, rehospitalization, and revascularization. The cutoff points of BNP, hsCRP, and adiponectin were determined as 100 pg/mL, 1 mg/L, and 9373 ng/mL, respectively, by the CART analysis. Higher plasma BNP levels were associated with the primary end point (Figure S6A), all‐cause death (Figure S6B), and rehospitalization (Figure S6C), but not with revascularization (Figure S6D). This was also the case with serum hsCRP levels (Figure S7) and serum adiponectin levels (Figure S8). Next, we examined the ability of combinations of serum adipsin levels with plasma BNP or serum hsCRP levels to predict prognosis compared with each level alone. Serum adipsin levels were significantly correlated with plasma BNP and adiponectin levels (Figure S9A and S9B), but not those of hsCRP levels (Figure S9C). We further compared the performance of the combinations of adipsin and these biomarkers in predicting prognosis compared with each level alone. The combination of higher serum adipsin levels and higher plasma BNP levels was more significantly associated with the primary end point (Figure 2A), all‐cause death (Figure 2B), and rehospitalization (Figure 2C), but not with revascularization (Figure 2D), than lower serum adipsin levels or lower plasma BNP levels alone. Additionally, the combination of higher serum levels of adipsin and hsCRP levels was more significantly associated with the primary end point (Figure 3A), all‐cause death (Figure 3B), and rehospitalization (Figure 3C), but not with revascularization (Figure 3D), than lower serum levels of adipsin or hsCRP alone. Moreover, we fitted univariable Cox proportional hazard model utilizing 50 proteins as the independent variables. The significance was assessed using Bonferroni correction. As a result, the proportional hazard assumption was satisfied in all candidate covariates except for TNF‐related apoptosis‐inducing ligand (TRAIL). The additive Cox proportional hazard model with spline representation was fit for TRAIL, although it was not significant with P=0.876. Adipsin and cyclophilin A were still significant with adjusted P<0.001 by Bonferroni correction. Finally, we used Cox proportional hazards model for multivariable analysis. In multivariable analyses including age, sex, body mass index, glycated hemoglobin, presence of hypertension, dyslipidemia, DM, smoking status, aspirin, statins, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, estimated glomerular filtration rate, BNP levels, and hsCRP levels, serum adipsin levels remained associated with the primary end point and rehospitalization, but not with all‐cause death or revascularization (Table 3). Next, we analyzed the nonlinear and time‐varying effects in HRs of the primary end point by an HR plot (Figure S10). HR plot revealed that serum adipsin levels were an accurate biomarker for predicting the primary end point in patients with CAD. There was a slightly worsening trend in prognosis, but the prognostic effect of adipsin level according to time was not significant and independent of the length of follow‐up period. Additionally, to assess adipsin's performance of prediction, we conducted simulation study as follows. First, we randomly separated the data set into the training set with three fourths of the total observations, and the validation set with one fourth of the observations. Next, we fit the CART model using the training set to identify the protein for prediction of the primary end point and its threshold. Finally, we defined an indicator to classify the risk strata using the detected protein and its threshold, and we fit the Cox proportional hazard model using the indicator in the training and the validation sets. We repeated this simulation for 1000 runs and calculated the median (interquartile range) of the cutoff of adipsin and the HRs in the training and the validation sets of the iterations. Of the 1000 runs of the simulation, adipsin was selected 945 times as the predictor of the primary end point with the cutoff of 395.2326 (395.2326–396.3145) and the HR of 2.52 (2.35–2.72) in the training set and 2.32 (1.87–2.88) in the validation set.
Figure 2.
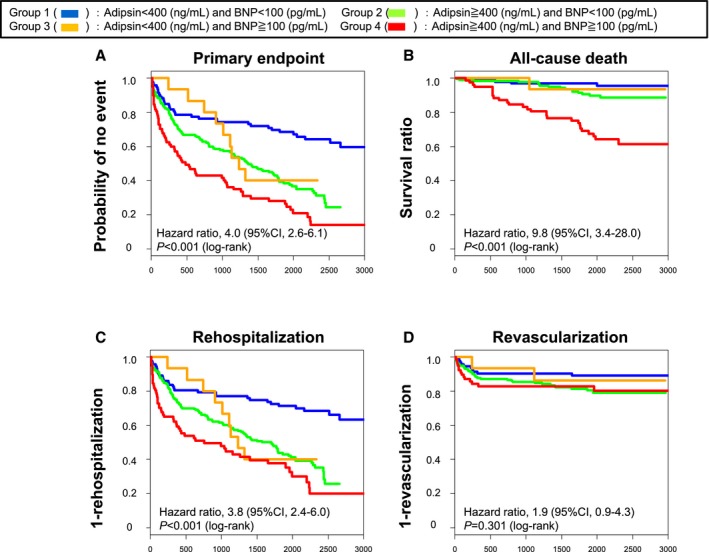
Kaplan–Meier curves based on the levels of adipsin and brain natriuretic peptide (BNP). The combination of adipsin levels (≥400 ng/mL) and BNP levels (≥100 pg/mL) was more significantly associated with (A) primary end point, (B) all‐cause death, and (C) rehospitalization than the combination of adipsin levels (<400 ng/mL) and BNP levels (<100 pg/mL).
Figure 3.
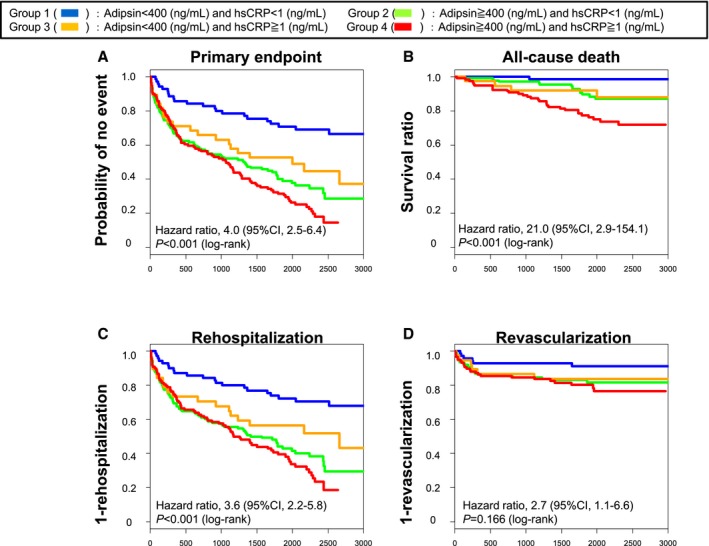
Kaplan–Meier curves based on the levels of adipsin and hsCRP (high sensitivity C‐reactive protein). The combination of adipsin levels (≥400 ng/mL) and hsCRP levels (≥1 mg/L) was more significantly associated with (A) primary end point, (B) all‐cause death, (C) rehospitalization, and (D) revascularization than the combination of adipsin levels (<400 ng/mL) and hsCRP levels (<1 mg/L).
Table 3.
Cox Hazard Model for Univariate and Multivariate Analysis
| HR | 95% CI | P Value | Adjusted HR | 95% CI | P Value | |
|---|---|---|---|---|---|---|
| Primary end point | ||||||
| Adipsin ≥400 ng/mL | 2.5 | 1.8 to 3.5 | <0.001 | 2.0 | 1.2 to 3.2 | 0.007 |
| Men, % | 0.9 | 0.7 to 1.2 | 0.673 | … | … | … |
| Age, y | 1.0 | 1.0 to 1.0 | <0.001 | 1.0 | 1.0 to 1.0 | 0.163 |
| BNP ≥100 pg/mL | 2.1 | 1.6 to 2.6 | <0.001 | 1.8 | 1.2 to 2.8 | 0.006 |
| hsCRP ≥1 mg/L | 1.6 | 1.2 to 2.1 | <0.001 | 1.1 | 0.7 to 1.6 | 0.734 |
| BMI | 1.0 | 0.9 to 1.0 | 0.007 | 1.0 | 0.9 to 1.0 | 0.138 |
| Hypertension, % | 1.3 | 0.9 to 1.7 | 0.114 | … | … | … |
| DM, % | 1.5 | 1.1 to 2.0 | 0.003 | 1.2 | 0.7 to 1.9 | 0.520 |
| Smoke, % | 1.0 | 0.7 to 1.3 | 0.931 | … | … | … |
| Dyslipidemia, % | 1.0 | 0.8 to 1.3 | 0.973 | … | … | … |
| HbA1c, % | 1.2 | 1.1 to 1.3 | 0.005 | 1.3 | 1.1 to 1.5 | 0.009 |
| ACEIs, % | 1.0 | 0.7 to 1.4 | 0.922 | … | … | … |
| ARBs, % | 1.5 | 1.1 to 2.1 | 0.014 | 1.4 | 0.9 to 2.1 | 0.120 |
| CCBs, % | 0.9 | 0.7 to 1.1 | 0.305 | … | … | … |
| Statins, % | 1.2 | 0.9 to 1.5 | 0.267 | … | … | … |
| Aspirin, % | 1.7 | 1.3 to 2.1 | <0.001 | 1.4 | 1.0 to 1.8 | 0.045 |
| eGFR, mL/min per 1.73/m2 | 1.0 | 1.0 to 1.0 | 0.001 | 1.0 | 1.0 to 1.0 | 0.397 |
| All‐cause death | ||||||
| Adipsin ≥400 ng/mL | 4.2 | 1.7 to 10.6 | <0.001 | 2.8 | 0.9 to 9.0 | 0.082 |
| Men, % | 2.0 | 1.0 to 4.0 | 0.052 | … | … | … |
| Age, y | 1.0 | 1.0 to 1.1 | 0.001 | 1.0 | 1.0 to 1.1 | 0.354 |
| BNP ≥100 pg/mL | 3.6 | 2.0 to 6.7 | <0.001 | 3.8 | 1.6 to 9.2 | 0.003 |
| hsCRP ≥1 mg/L | 3.2 | 1.7 to 5.9 | <0.001 | 1.8 | 0.8 to 4.1 | 0.131 |
| BMI | 1.0 | 0.9 to 1.0 | 0.177 | … | … | … |
| Hypertension, % | 1.4 | 0.7 to 2.6 | 0.318 | … | … | … |
| DM, % | 1.5 | 0.9 to 2.7 | 0.139 | … | … | … |
| Smoke, % | 1.5 | 0.9 to 2.7 | 0.154 | … | … | … |
| Dyslipidemia, % | 1.3 | 0.7 to 2.3 | 0.412 | … | … | … |
| HbA1c, % | 0.9 | 0.7 to 1.2 | 0.555 | … | … | … |
| ACEIs, % | 1.8 | 1.1 to 2.9 | 0.032 | 1.5 | 0.7 to 3.2 | 0.273 |
| ARBs, % | 0.6 | 0.3 to 1.2 | 0.169 | … | … | … |
| CCBs, % | 0.6 | 0.4 to 1.0 | 0.053 | … | … | … |
| Statins, % | 1.1 | 0.7 to 2.0 | 0.618 | … | … | … |
| Aspirin, % | 1.0 | 0.6 to 1.7 | 0.927 | … | … | … |
| eGFR, mL/min per 1.73/m2 | 1.0 | 1.0 to 1.0 | 0.016 | 1.0 | 1.0 to 1.0 | 0.993 |
| Rehospitalization | ||||||
| Adipsin ≥400 ng/mL | 2.4 | 1.7 to 3.5 | <0.001 | 2.1 | 1.2 to 3.7 | 0.006 |
| Men, % | 0.9 | 0.8 to 1.2 | 0.508 | … | … | … |
| Age, y | 1.0 | 1.0 to 1.1 | <0.001 | 1.0 | 1.0 to 1.0 | 0.219 |
| BNP ≥100 pg/mL | 2.0 | 1.5 to 2.6 | <0.001 | 2.0 | 1.3 to 3.1 | 0.003 |
| hsCRP ≥1 mg/L | 1.5 | 1.1 to 1.9 | 0.007 | 0.9 | 0.6 to 1.4 | 0.697 |
| BMI | 1.0 | 0.9 to 1.0 | 0.010 | 1.0 | 0.9 to 1.0 | 0.246 |
| Hypertension, % | 1.1 | 0.8 to 1.6 | 0.382 | … | … | … |
| DM, % | 1.5 | 1.1 to 2.0 | 0.006 | 1.0 | 0.6 to 1.7 | 0.930 |
| Smoke, % | 0.9 | 0.7 to 1.2 | 0.616 | … | … | … |
| Dyslipidemia, % | 0.9 | 0.7 to 1.2 | 0.632 | … | … | … |
| HbA1c, % | 1.2 | 1.0 to 1.4 | 0.001 | 1.3 | 1.1 to 1.6 | 0.001 |
| ACEIs, % | 0.9 | 0.6 to 1.3 | 0.561 | … | … | … |
| ARBs, % | 1.8 | 1.3 to 2.5 | 0.001 | 1.7 | 1.2 to 2.6 | 0.008 |
| CCBs, % | 0.9 | 0.8 to 1.5 | 0.418 | … | … | … |
| Statins, % | 1.1 | 0.9 to 1.5 | 0.418 | … | … | … |
| Aspirin, % | 1.7 | 1.3 to 2.2 | <0.001 | 1.5 | 1.1 to 2.0 | 0.016 |
| eGFR, mL/min per 1.73/m2 | 1.0 | 1.0 to 1.0 | 0.003 | 1.0 | 1.0 to 1.0 | 0.550 |
| Revascularization | ||||||
| Adipsin ≥400 ng/mL | 1.8 | 1.0 to 3.4 | 0.062 | … | … | … |
| Men, % | 1.2 | 0.7 to 2.1 | 0.451 | … | … | … |
| Age, y | 1.0 | 1.0 to 1.1 | 0.011 | 1.0 | 1.0 to 1.0 | 0.818 |
| BNP ≥100 pg/mL | 1.3 | 0.8 to 2.2 | 0.285 | … | … | … |
| hsCRP ≥1 mg/L | 1.4 | 0.8 to 2.3 | 0.186 | … | … | … |
| BMI | 1.0 | 1.0 to 1.1 | 0.757 | … | … | … |
| Hypertension, % | 2.4 | 1.3 to 4.7 | 0.008 | 1.4 | 0.6 to 3.3 | 0.459 |
| DM, % | 3.5 | 2.0 to 5.9 | <0.001 | 2.0 | 0.9 to 4.4 | 0.069 |
| Smoke, % | 1.4 | 0.8 to 2.4 | 0.186 | … | … | … |
| Dyslipidemia, % | 2.0 | 1.1 to 3.5 | 0.018 | 0.8 | 0.3 to 2.4 | 0.659 |
| HbA1c, % | 1.4 | 1.2 to 1.6 | <0.001 | 1.2 | 1.0 to 1.6 | 0.093 |
| ACEIs, % | 1.5 | 0.9 to 2.4 | 0.136 | 1.2 | 0.7 to 2.1 | 0.482 |
| ARBs, % | 1.4 | 0.8 to 2.4 | 0.272 | … | … | … |
| CCBs, % | 0.6 | 0.4 to 1.0 | 0.040 | 0.9 | 0.5 to 1.5 | 0.616 |
| Statins, % | 2.4 | 1.5 to 4.0 | <0.001 | 2.6 | 1.0 to 6.7 | 0.040 |
| Aspirin, % | 2.4 | 1.7 to 3.5 | <0.001 | 1.6 | 1.0 to 2.6 | 0.047 |
| eGFR, mL/min per 1.73/m2 | 1.0 | 1.0 to 1.0 | 0.001 | 1.0 | 1.0 to 1.0 | 0.064 |
ACEIs indicates angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BMI, body mass index; BNP, brain natriuretic peptide; CCBs, calcium channel blockers; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HR, hazard ratio; hsCRP, high‐sensitivity C‐reactive protein.
Serum Adipsin Levels Predict Deaths Caused by AMI
During the follow‐up period, 5 patients had an incidence of AMI, and 4 of them died. Importantly, all of them were in the group with higher serum adipsin levels (Table 2). Here, we performed receiver operating characteristic analysis, which showed that serum adipsin levels were useful for predicting death caused by AMI (C‐statistic, 0.84 [sensitivity, 64.8%, specificity, 100%]) (Figure S11A). Furthermore, serum adipsin levels predicted the future incidence of AMI in patients with CAD (C‐statistic, 0.809 [sensitivity, 64.9%, specificity, 100%]) (Figure S11B). Moreover, receiver operating characteristic curve showed that plasma BNP levels effectively predicted death caused by AMI (C‐statistic, 0.806; 95% CI, 0.706–0.907 [sensitivity, 68.8%, specificity, 100%]) (Figure S12A), and future incidence of AMI in patients with CAD (C‐statistic, 0.793; 95% CI, 0.708–0.877 [sensitivity, 68.9%, specificity, 100%]) (Figure S12B). However, serum hsCRP levels may not be useful for predicting death caused by AMI (C‐statistic, 0.506; 95% CI, 0.188–0.905 [sensitivity, 52.4%, specificity, 75.0%]) (Figure S12C), or for predicting future incidence of AMI in patients with CAD (C‐statistic, 0.589; 95% CI, 0.298–0.879 [sensitivity, 52.5%, specificity, 80.0%]) (Figure S12D). Thus, in this study population, serum adipsin levels had a significantly higher specificity for predicting the death caused by AMI (Figure S11A).
Enhanced Adipsin Expression in Atherosclerotic Unstable Plaque
Adipsin is secreted from adipocytes, monocytes, and macrophages.25 Moreover, serum adipsin levels were elevated in patients with CAD and predicted death caused by AMI. Thus, we hypothesized that adipsin is also expressed in coronary atherosclerotic plaque in patients with CAD. To test this hypothesis, we performed immunostaining for adipsin in coronary arteries obtained from patients who died of AMI. Importantly, expression of adipsin was especially higher in the atherosclerotic plaque and adventitial adipocyte tissue in a patient (Figure 4), suggesting that adipsin is potentially involved in the unstabilization of atherosclerotic plaque in patients with CAD.
Figure 4.
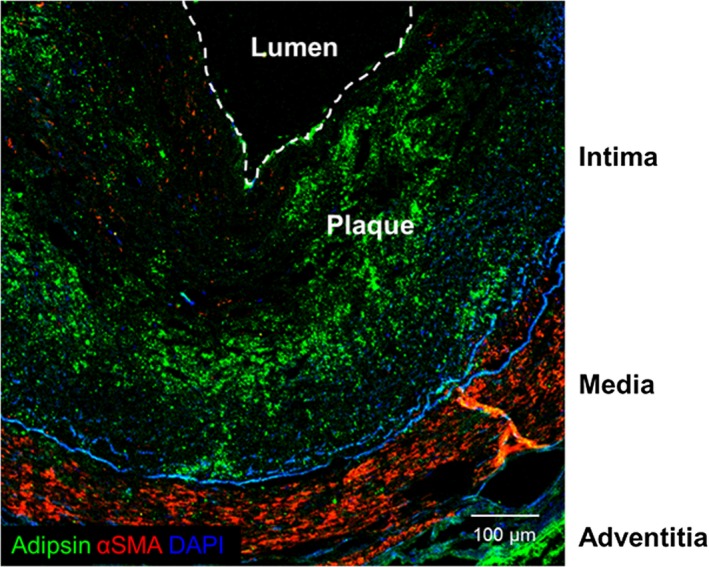
Adipsin expression in the atherosclerotic plaque in patients with acute myocardial infarction. Representative double‐immunostaining for adipsin (Alexa‐fluor 488, green), α‐smooth muscle actin (αSMA) (Cy3; red), and 4′,6‐diamidino‐2‐phenylindole (DAPI) (blue) of the human coronary artery. Scale bar, 100 μm.
Discussion
The major findings of the present study are that: (1) the rates of all‐cause death and rehospitalization were significantly higher in patients with higher serum adipsin levels than in those with lower serum adipsin levels; (2) serum adipsin levels, when combined with BNP or hsCRP levels, had better predicting capabilities; and (3) serum adipsin levels predicted the incidences and deaths caused by AMI in patients with CAD. These results indicate that serum adipsin levels have prognostic impacts for all‐cause death and rehospitalization in patients with CAD and that combination of the adipsin levels and other established biomarkers (hsCRP and BNP) further enhances the prognostic impacts in those patients.
Adipsin in the Pathogenesis of Atherosclerosis
Adipokines are secreted from adipose tissue29 and may promote the development of CAD.30 In a clinical study, it was reported that plasma adipsin levels predict the occurrence of ischemic stroke.31 However, no study has ever addressed the role of adipsin in the pathogenesis of atherosclerosis in animals or humans. In the present study, by using a cytokines array, we demonstrated that serum levels of adipsin predict the long‐term prognosis of patients with CAD. Indeed, higher adipsin levels were significantly associated with all‐cause death and rehospitalization compared with lower adipsin levels. Thus, adipsin could be a potentially novel biomarker that reflects the development of atherosclerosis, although further analyses using genetically modified animal models are needed. It is widely known that circulating levels of BNP,32 homocysteine,33 oxidized low‐density lipoprotein cholesterol,34 and CRP35 can predict future revascularization. However, circulating levels of these biomarkers are also elevated in patients with other diseases. Thus, serum adipsin levels could be a more specific biomarker that reflects the presence of atherosclerosis. Interestingly, adipsin is secreted from adipocytes and also from monocytes and macrophages, catalyzing the rate‐limiting step of the alternative complement pathway.25 Additionally, adipsin is closely associated with the metabolic state and insulin resistance in mice.23 A recent clinical study demonstrated that plasma adipsin levels are correlated with insulin resistance in patients with obesity.36 In contrast, adipsin is mechanistically important for pancreatic β‐cell function in patients with type 2 DM.29 Thus, the interpretation of these reports is complex but indicates the potential role of adipsin in the pathogenesis of atherosclerosis.
Recently, Meloche et al37 reported that bromodomain protein 4 is a trigger not only for pulmonary arterial hypertension but also for calcification and remodeling processes in patients with CAD. Bromodomain protein 4 is an important regulator of the proliferation/apoptosis balance by modulating gene expression38 and plays crucial roles in post‐DNA damage events.39 In the present study, the cancer deaths were significantly increased in the group with higher serum adipsin levels compared with the group with lower serum adipsin levels. This may suggest that adipsin is involved in the pathogenesis of atherosclerosis and cancer.
In addition to adipsin,25 adipose tissues also secrete various inflammatory cytokines (eg, tumor necrosis factor‐α) and chemokines (eg, monocyte chemoattractant protein), which leads to insulin resistance, dyslipidemia, and adipose tissue dysfunction,40, 41 and unstabilizes coronary atherosclerotic plaques.42 Increased levels of serum adipsin are closely related to the increased metabolic disturbances.43 Moreover, adipsin promotes lipid accumulation and adipocyte differentiation.44 Consistently, we found a strong expression of adipsin in the coronary atherosclerotic plaque of patients with AMI. Moreover, in the present study, 4 deaths caused by AMI were in the group with higher adipsin levels, whereas there were no deaths in the group with lower adipsin levels. Indeed, receiver operating characteristic curves clearly showed that serum adipsin levels predicted the incidence of and deaths caused by AMI in patients with CAD. Thus, adipsin may be involved in unstabilization of coronary atherosclerotic plaque and could be a novel biomarker to predict the future incidence of AMI.
Comparison Between Adipsin and Other Biomarkers
In the present study, we compared serum levels of adipsin with other biomarkers (BNP and hsCRP). It is well known that higher plasma BNP levels predict adverse prognosis,45 including the risk of cardiovascular death.8 Additionally, plasma levels of processed forms of BNP (N‐terminal pro‐BNP) can predict the incidence of revascularization.32 Moreover, higher serum levels of hsCRP (>1 mg/L) predict future cardiovascular events.6 Similarly, in the present study, higher serum hsCRP levels were associated with all‐cause death and rehospitalization. However, serum hsCRP levels are elevated not only in patients with CAD but also in those with infectious diseases,6 which may limit the usefulness of hsCRP as a specific biomarker for cardiovascular events. In the present study, there was no correlation between serum levels of adipsin and those of hsCRP. Thus, we consider that adipsin is secreted from adipose tissue while CRP is produced mainly by the liver and indirectly reflects the levels of circulating inflammatory cytokines.19 We also examined whether combination of adipsin and other biomarkers could better predict prognosis than each biomarker alone. Indeed, the combination of adipsin and BNP levels significantly better predicted the incidence of all‐cause death and rehospitalization than each biomarker alone. This was also the case with hsCRP. These results suggest that the combination of adipsin levels and other biomarkers enhances the prognostic impacts for patients with CAD. BNP and hsCRP levels are frequently used as useful biomarkers in the clinical practice. Thus, additional use of adipsin could further improve clinical practice with biomarkers.
Adipsin Levels Predict Cancer Death
Cardiovascular diseases are the major cause of death in patients with CAD.46 However, the causes of death in patients with CAD are often noncardiac, especially after the establishment of medical therapies and coronary interventions.47 Recently, it has been noted that patients with cardiovascular diseases are at increased risk of cancer death.48, 49 In the present study, 15 patients died as a result of cancer during the follow‐up. Interestingly, serum adipsin levels were significantly elevated in patients who died of cancer. Indeed, the risk factors for atherosclerosis and cancer are common, such as advanced age, DM, dyslipidemia, and smoking, all of which induce systemic inflammation and enhanced oxidative stress. In the present study, cancer death was also a major cause of death, demonstrating that patients with CAD are at increased risk of cancer death. Some medications for cardiovascular diseases may have influenced the incidence of cancer.50 Indeed, patients with higher adipsin levels had a higher frequency of use of aspirin, statins, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, and calcium channel blockers than those with lower adipsin levels. Here, it has been reported that angiotensin receptor blockers could be associated with a modestly increased risk of cancer,51 whereas a recent study has denied this notion.52 Additionally, calcium channel blockers and statins do not increase the new occurrence of cancer,53, 54 and aspirin reduces cancer deaths.55 Thus, medications in patients with higher adipsin levels may not have had a strong impact on the occurrence of cancer death.
Atherosclerotic diseases are closely associated with vascular inflammation.6, 7, 56 Recently, it has been reported that anti‐inflammatory therapy targeting the interleukin (IL) 1β innate immunity pathway with canakinumab significantly decreased the occurrences of cardiovascular events and the new onset of cancers (CANTOS [Canakinumab Anti‐Inflammatory Thrombosis Outcomes Study]).57 This study has directly demonstrated that inflammation promotes atherosclerosis and cancers. In the present study, serum adipsin levels were significantly elevated in patients with cancer death compared with those without it. Indeed, adipsin‐mediated effects on alternative complement pathway may have augmented coagulation and inflammation in patients with CAD. Here, we consider the potential role of higher levels of circulating cytokines/chemokines and growth factors to promote atherosclerosis and cancer. In the present study, serum levels of IL‐1α, IL‐15, IL‐17, leukemia inhibitory factor, macrophage colony‐stimulating factor, tumor necrosis factor‐β, granulocyte macrophage colony‐stimulating factor, and monocyte chemotactic protein‐1 were significantly elevated in patients with lower serum adipsin levels compared with those with higher adipsin levels. In contrast, serum levels of IL‐16, cutaneous T‐cell–attracting chemokine, adiponectin, and cyclophilin A were significantly elevated in patients with higher serum adipsin levels compared with those with lower adipsin levels. Indeed, it has been reported that inflammatory cytokines are associated with cancer development.58 Thus, elevated levels of cytokines/chemokines in patients with higher adipsin may have contributed to the development of atherosclerosis and cancer in the present study.
Alternative Complimentary System and Atherosclerosis
Adipsin is mainly secreted from adipocytes, monocytes, and macrophages, catalyzing the rate‐limiting step of the alternative complement pathway.25 In this pathway, adipsin cleaves complement factor B and catalyzes the formation of C3 convertase, which leads to a hydrolysis cascade that produces various complement fragments including C3a, C3b, C5a, and C5b.25 The complimentary system stimulates macrophages to phagocytose bacterium and induces inflammation.25 In the present study, serum adipsin levels predicted future incidence of AMI in patients with CAD. Consistently, it has been reported that higher serum levels of adipsin were associated with an increased 10‐year risk of ischemic stroke among middle‐aged men.31 Thus, the present study shows the new aspects of the alternative complementary system that promotes atherosclerosis.
These days, it has been reported that lower circulating adiponectin levels increase insulin resistance, further develop arteriosclerosis, and contribute to the development of cardiovascular disease.59, 60, 61, 62, 63 As shown in Figure S2, serum adiponectin levels are positively correlated with serum adipsin levels in the present study. This result is in contrast to the previous reports.62, 63 Considering the previous reports, it is easy to understand whether adipsin and adiponectin are inversely correlated, but it is conceivable that the present findings suggest a new aspect of the relationship between adipsin and adiponectin. Adipsin is also known as complement factor D, playing central roles in the complement system alternative pathway.29 The definitive difference between adipsin and adiponectin is thought to be a difference in the involvement in the immune system. However, at this time, there are no data that are sufficient for proof, which is a subject to be studied in future studies.
Future Perspectives
In the present study, we were able to demonstrate that serum adipsin levels predict the need for future revascularization. Additionally, receiver operating characteristic curves clearly showed that serum adipsin levels predict the incidence of and deaths caused by AMI in patients with CAD. Indeed, 5 patients developed AMI during the follow‐up, and 4 of them died. Importantly, all of the patients with AMI during the follow‐up period had higher serum adipsin levels, suggesting that serum levels of adipsin may be useful in identifying patients at high risk for AMI. Thus, further analyses with an increased number of patients may provide further evidence of adipsin as a biomarker for future occurrence of AMI.
Study Limitations
Several limitations should be mentioned for the present study. First, the detailed mechanisms of adipsin‐mediated development of atherosclerosis remain to be elucidated. Second, the number of patients in the present study was relatively small. However, despite the small sample size, serum adipsin levels had a significant prognostic impact to predict AMI. Additionally, this limitation did not affect the validity of the main result of the present study. Future prospective analysis in a large cohort will further elucidate the importance of serum levels of adipsin. Next, the association between adipsin and other complement factors is important. However, in the present study, we did not measure other complement factors. This point remains to be addressed in future studies.
Conclusions
In the present study, we were able to demonstrate, for the first time, that higher serum adipsin levels in patients with CAD predict all‐cause death and rehospitalization. Combining adipsin levels with other established biomarkers (BNP or hsCRP levels) would further enhance the prognostic impacts of serum adipsin levels in patients with CAD. Importantly, the present study demonstrates that higher adipsin levels in patients with CAD can predict future death caused by AMI or cancer. Thus, serum adipsin levels could be a novel and useful biomarker in the super‐aging society.
Sources of Funding
This work was supported in part by grants‐in‐aid for Scientific Research (15H02535, 15H04816, and 15K15046), all of which are from the Ministry of Education, Culture, Sports, Science and Technology, Tokyo, Japan; grants‐in‐aid for Scientific Research from the Ministry of Health, Labour, and Welfare, Tokyo, Japan (10102895); and grants‐in‐aid for Scientific Research from the Japan Agency for Medical Research and Development, Tokyo, Japan (15ak0101035 h0001, 16ek0109176 h0001, and 17ek0109227 h0001).
Disclosures
None.
Supporting information
Figure S1. Classification and regression tree (CART). CART analyses were performed to determine the best biomarker for predicting prognosis in patients with coronary artery disease.Figure S2. Boxplots of serum levels of cytokines/chemokines and growth factors. Serum levels of interleukin (IL) 15, IL‐16, IL‐17, cutaneous T‐cell–attracting chemokine (CTACK), leukemia inhibitory factor (LIF), granulocyte macrophage colony‐stimulating factor (GM‐CSF), adiponectin, and cyclophilin A (CyPA) were elevated in patients with higher serum levels of adipsin.
Figure S3. Serum levels of adipsin and cardiovascular risk factors. Distribution of serum adipsin levels based on advanced age (>66 years), hypertension, smoking, diabetes mellitus, dyslipidemia, and sex. Results are expressed by boxplots analyses.
Figure S4. Kaplan–Meier curve based on serum adipsin levels. Serum adipsin levels were not associated with acute myocardial infarction death (A) or with composite cardiovascular death (B). Cardiovascular death was considered if the death was caused by acute myocardial infarction. Composite cardiovascular death was defined if the death was caused by acute myocardial infraction, heart failure, stroke, or other cardiovascular causes.
Figure S5. Boxplot for serum adipsin levels. Serum adipsin levels were elevated in patients with future occurrences of cancer death.
Figure S6. Kaplan–Meier curve based on plasma brain natriuretic peptide (BNP) levels. Higher BNP levels were significantly associated with (A) primary end point, (B) all‐cause death, and (C) rehospitalization, but not with (D) revascularization. The primary end point was a composite of all‐cause death, rehospitalization, and revascularization. Revascularization was defined as percutaneous coronary intervention or coronary artery bypass grafting.
Figure S7. Kaplan–Meier curve based on serum high‐sensitivity C‐reactive protein (hsCRP) levels. Higher hsCRP levels were significantly associated with (A) primary end point, (B) all‐cause death, (C) rehospitalization, but not with (D) revascularization. The primary end point was a composite of all‐cause death, rehospitalization, and revascularization. Revascularization was defined as percutaneous coronary intervention or coronary artery bypass grafting.
Figure S8. Kaplan–Meier curve based on serum adiponectin levels. Higher adiponectin levels were significantly associated with (A) primary end point, (B) all‐cause death, (C) rehospitalization, but not with (D) revascularization. The primary end point was a composite of all‐cause death, rehospitalization, and revascularization. Revascularization was defined as percutaneous coronary intervention or coronary artery bypass grafting.
Figure S9. Scatter plots showing the relationship between serum adipsin levels and other variables. Scatter plots showed that serum levels of adipsin were correlated with (A) plasma levels of brain natriuretic peptide and (C) those of adiponectin, but not those of high‐sensitivity C‐reactive protein (hsCRP) (B) in Spearman's correlation analysis.
Figure S10. Hazard ratio (HR) plot and histogram for serum adipsin levels. HR plot revealed that serum adipsin levels were an accurate biomarker for predicting primary end point in patients with coronary artery disease, independent of the length of follow‐up period. Solid, broken, dotted, and red lines indicate HR plot, 95% CIs, smoothed control line, and log(HR)0, respectively. Blue bars show histogram.
Figure S11. Receiver operating characteristic curve for serum adipsin levels.
Figure S12. Receiver operating characteristic (ROC) curve for plasma brain natriuretic peptide (BNP) and serum high‐sensitivity C‐reactive protein (hsCRP) levels. The ROC curve revealed that plasma BNP levels were an accurate biomarker of predicting (A) death caused by AMI and (B) AMI incidence in patients with coronary artery disease (CAD), whereas serum hsCRP levels were not useful for predicting (C) death caused by AMI or (D) AMI incidence in patients with CAD.
Acknowledgments
We are grateful to the members in the Department of Cardiovascular Medicine at Tohoku University for their valuable technical assistance, especially Yumi Watanabe, Ai Nishihara, and Hiromi Yamashita.
(J Am Heart Assoc. 2019;8:e013716 DOI: 10.1161/JAHA.119.013716.)
References
- 1. Nguyen HN, Fujiyoshi A, Abbott RD, Miura K. Epidemiology of cardiovascular risk factors in Asian countries. Circ J. 2013;77:2851–2859. [DOI] [PubMed] [Google Scholar]
- 2. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 3. Hao K, Takahashi J, Ito K, Miyata S, Nihei T, Nishimiya K, Tsuburaya R, Matsumoto Y, Sakata Y, Yasuda S, Shimokawa H. Clinical characteristics of patients with acute myocardial infarction who did not undergo primary percutaneous coronary intervention‐report from the MIYAGI‐AMI registry study. Circ J. 2015;79:2009–2016. [DOI] [PubMed] [Google Scholar]
- 4. Hao K, Yasuda S, Takii T, Ito Y, Takahashi J, Ito K, Nakayama M, Shiba N, Fukumoto Y, Shimokawa H. Urbanization, life style changes and the incidence/in‐hospital mortality of acute myocardial infarction in Japan: report from the MIYAGI‐AMI Registry Study. Circ J. 2012;76:1136–1144. [DOI] [PubMed] [Google Scholar]
- 5. Memon L, Spasojevic‐Kalimanovska V, Bogavac‐Stanojevic N, Kalimanovska‐Ostric D, Jelic‐Ivanovic Z, Spasic S, Topic A. Association of C‐reactive protein with the presence and extent of angiographically verified coronary artery disease. Tohoku J Exp Med. 2006;209:197–206. [DOI] [PubMed] [Google Scholar]
- 6. Ridker PM. High‐sensitivity C‐reactive protein, inflammation, and cardiovascular risk: from concept to clinical practice to clinical benefit. Am Heart J. 2004;148:S19–S26. [DOI] [PubMed] [Google Scholar]
- 7. Ridker PM, Cook N. Clinical usefulness of very high and very low levels of C‐reactive protein across the full range of Framingham Risk Scores. Circulation. 2004;109:1955–1959. [DOI] [PubMed] [Google Scholar]
- 8. Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. [DOI] [PubMed] [Google Scholar]
- 9. Cushman M, Lemaitre RN, Kuller LH, Psaty BM, Macy EM, Sharrett AR, Tracy RP. Fibrinolytic activation markers predict myocardial infarction in the elderly. The cardiovascular health study. Arterioscler Thromb Vasc Biol. 1999;19:493–498. [DOI] [PubMed] [Google Scholar]
- 10. Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, Wilson AC, Folsom AR, Wu K, Benderly M, Goldbourt U, Willeit J, Kiechl S, Yarnell JW, Sweetnam PM, Elwood PC, Cushman M, Psaty BM, Tracy RP, Tybjaerg‐Hansen A, Haverkate F, de Maat MP, Fowkes FG, Lee AJ, Smith FB, Salomaa V, Harald K, Rasi R, Vahtera E, Jousilahti P, Pekkanen J, D'Agostino R, Kannel WB, Wilson PW, Tofler G, Arocha‐Pinango CL, Rodriguez‐Larralde A, Nagy E, Mijares M, Espinosa R, Rodriquez‐Roa E, Ryder E, Diez‐Ewald MP, Campos G, Fernandez V, Torres E, Marchioli R, Valagussa F, Rosengren A, Wilhelmsen L, Lappas G, Eriksson H, Cremer P, Nagel D, Curb JD, Rodriguez B, Yano K, Salonen JT, Nyyssonen K, Tuomainen TP, Hedblad B, Lind P, Loewel H, Koenig W, Meade TW, Cooper JA, De Stavola B, Knottenbelt C, Miller GJ, Cooper JA, Bauer KA, Rosenberg RD, Sato S, Kitamura A, Naito Y, Palosuo T, Ducimetiere P, Amouyel P, Arveiler D, Evans AE, Ferrieres J, Juhan‐Vague I, Bingham A, Schulte H, Assmann G, Cantin B, Lamarche B, Despres JP, Dagenais GR, Tunstall‐Pedoe H, Woodward M, Ben‐Shlomo Y, Davey Smith G, Palmieri V, Yeh JL, Rudnicka A, Ridker P, Rodeghiero F, Tosetto A, Shepherd J, Ford I, Robertson M, Brunner E, Shipley M, Feskens EJ, Kromhout D, Dickinson A, Ireland B, Juzwishin K, Kaptoge S, Lewington S, Memon A, Sarwar N, Walker M, Wheeler J, White I, Wood A. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta‐analysis. JAMA. 2005;294:1799–1809. [DOI] [PubMed] [Google Scholar]
- 11. Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton‐Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, Benjamin EJ, D'Agostino RB, Vasan RS. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. [DOI] [PubMed] [Google Scholar]
- 12. Satoh K. AMPKα2 regulates hypoxia‐inducible factor‐1α stability and neutrophil survival to promote vascular repair after ischemia. Circ Res. 2017;120:8–10. [DOI] [PubMed] [Google Scholar]
- 13. Satoh K, Satoh T, Kikuchi N, Omura J, Kurosawa R, Suzuki K, Sugimura K, Aoki T, Nochioka K, Tatebe S, Miyamichi‐Yamamoto S, Miura M, Shimizu T, Ikeda S, Yaoita N, Fukumoto Y, Minami T, Miyata S, Nakamura K, Ito H, Kadomatsu K, Shimokawa H. Basigin mediates pulmonary hypertension by promoting inflammation and vascular smooth muscle cell proliferation. Circ Res. 2014;115:738–750. [DOI] [PubMed] [Google Scholar]
- 14. Shimokawa H. 2014 Williams Harvey Lecture: importance of coronary vasomotion abnormalities‐from bench to bedside. Eur Heart J. 2014;35:3180–3193. [DOI] [PubMed] [Google Scholar]
- 15. Satoh K, Matoba T, Suzuki J, O'Dell MR, Nigro P, Cui Z, Mohan A, Pan S, Li L, Jin ZG, Yan C, Abe J, Berk BC. Cyclophilin A mediates vascular remodeling by promoting inflammation and vascular smooth muscle cell proliferation. Circulation. 2008;117:3088–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Satoh K, Kikuchi N, Kurosawa R, Shimokawa H. PDE1C negatively regulates growth factor receptor degradation and promotes VSMC proliferation. Circ Res. 2015;116:1098–1100. [DOI] [PubMed] [Google Scholar]
- 17. Shimokawa H, Sunamura S, Satoh K. RhoA/Rho‐kinase in the cardiovascular system. Circ Res. 2016;118:352–366. [DOI] [PubMed] [Google Scholar]
- 18. Satoh K, Nigro P, Matoba T, O'Dell MR, Cui Z, Shi X, Mohan A, Yan C, Abe J, Illig KA, Berk BC. Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II‐induced aortic aneurysms. Nat Med. 2009;15:649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Satoh K, Shimokawa H. High‐sensitivity C‐reactive protein: still need for next‐generation biomarkers for remote future cardiovascular events. Eur Heart J. 2014;35:1776–1778. [DOI] [PubMed] [Google Scholar]
- 20. Nigro P, Satoh K, O'Dell MR, Soe NN, Cui Z, Mohan A, Abe J, Alexis JD, Sparks JD, Berk BC. Cyclophilin A is an inflammatory mediator that promotes atherosclerosis in apolipoprotein E‐deficient mice. J Exp Med. 2011;208:53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suzuki K, Satoh K, Ikeda S, Sunamura S, Otsuki T, Satoh T, Kikuchi N, Omura J, Kurosawa R, Nogi M, Numano K, Sugimura K, Aoki T, Tatebe S, Miyata S, Mukherjee R, Spinale FG, Kadomatsu K, Shimokawa H. Basigin promotes cardiac fibrosis and failure in response to chronic pressure overload in mice. Arterioscler Thromb Vasc Biol. 2016;36:636–646. [DOI] [PubMed] [Google Scholar]
- 22. Kudo S, Satoh K, Nogi M, Suzuki K, Sunamura S, Omura J, Kikuchi N, Kurosawa R, Satoh T, Minami T, Ikeda S, Miyata S, Shimokawa H. SmgGDS as a crucial mediator of the inhibitory effects of statins on cardiac hypertrophy and fibrosis: novel mechanism of the pleiotropic effects of statins. Hypertension. 2016;67:878–889. [DOI] [PubMed] [Google Scholar]
- 23. Flier JS, Cook KS, Usher P, Spiegelman BM. Severely impaired adipsin expression in genetic and acquired obesity. Science. 1987;237:405–408. [DOI] [PubMed] [Google Scholar]
- 24. Rosen BS, Cook KS, Yaglom J, Groves DL, Volanakis JE, Damm D, White T, Spiegelman BM. Adipsin and complement factor D activity: an immune‐related defect in obesity. Science. 1989;244:1483–1487. [DOI] [PubMed] [Google Scholar]
- 25. White RT, Damm D, Hancock N, Rosen BS, Lowell BB, Usher P, Flier JS, Spiegelman BM. Human adipsin is identical to complement factor D and is expressed at high levels in adipose tissue. J Biol Chem. 1992;267:9210–9213. [PubMed] [Google Scholar]
- 26. Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8:776–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Satoh T, Satoh K, Yaoita N, Kikuchi N, Omura J, Kurosawa R, Numano K, Al‐Mamun E, Siddique MA, Sunamura S, Nogi M, Suzuki K, Miyata S, Morser J, Shimokawa H. Activated TAFI promotes the development of chronic thromboembolic pulmonary hypertension: a possible novel therapeutic target. Circ Res. 2017;120:1246–1262. [DOI] [PubMed] [Google Scholar]
- 28. R Core Team . R: A language and environment for statistical computing. Vienna, Austria: R foundation for statistical computing; 2012. Available at: http://www.R‐project.org/. Accessed February 24, 2017. [Google Scholar]
- 29. Lo JC, Ljubicic S, Leibiger B, Kern M, Leibiger IB, Moede T, Kelly ME, Chatterjee Bhowmick D, Murano I, Cohen P, Banks AS, Khandekar MJ, Dietrich A, Flier JS, Cinti S, Bluher M, Danial NN, Berggren PO, Spiegelman BM. Adipsin is an adipokine that improves β cell function in diabetes. Cell. 2014;158:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luc G, Empana JP, Morange P, Juhan‐Vague I, Arveiler D, Ferrieres J, Amouyel P, Evans A, Kee F, Bingham A, Machez E, Ducimetiere P. Adipocytokines and the risk of coronary heart disease in healthy middle aged men: the PRIME Study. Int J Obes (Lond). 2010;34:118–126. [DOI] [PubMed] [Google Scholar]
- 31. Prugger C, Luc G, Haas B, Arveiler D, Machez E, Ferrieres J, Ruidavets JB, Bingham A, Montaye M, Amouyel P, Yarnell J, Kee F, Ducimetiere P, Empana JP. Adipocytokines and the risk of ischemic stroke: the PRIME Study. Ann Neurol. 2012;71:478–486. [DOI] [PubMed] [Google Scholar]
- 32. Fujimoto H, Suzuki T, Aizawa K, Sawaki D, Ishida J, Ando J, Fujita H, Komuro I, Nagai R. Processed B‐type natriuretic peptide is a biomarker of postinterventional restenosis in ischemic heart disease. Clin Chem. 2013;59:1330–1337. [DOI] [PubMed] [Google Scholar]
- 33. Breuckmann F, Naber C, Beckert J, Schmermund A, Haude M, Baumgart D, Erbel R. Post‐interventional homocysteine levels: failure as a predictive biomarker of in‐stent restenosis. Int J Cardiol. 2006;108:20–25. [DOI] [PubMed] [Google Scholar]
- 34. Suzuki T, Kohno H, Hasegawa A, Toshima S, Amaki T, Kurabayashi M, Nagai R, Suzuki T, Amaki T, Nagai R, Hasegawa A, Toshima S, Kurabayashi MH, Shimada K, Nakamura H, Teramoto T, Yamaguchi H, Nishiyama S, Takahashi H, Michishita I, Sugano Z, Konoshi K. Diagnostic implications of circulating oxidized low density lipoprotein levels as a biochemical risk marker of coronary artery disease. Clin Biochem. 2002;35:347–353. [DOI] [PubMed] [Google Scholar]
- 35. Park DW, Lee CW, Yun SC, Kim YH, Hong MK, Kim JJ, Park SW, Park SJ. Prognostic impact of preprocedural C reactive protein levels on 6‐month angiographic and 1‐year clinical outcomes after drug‐eluting stent implantation. Heart. 2007;93:1087–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vasilenko MA, Kirienkova EV, Skuratovskaia DA, Zatolokin PA, Mironyuk NI, Litvinova LS. The role of production of adipsin and leptin in the development of insulin resistance in patients with abdominal obesity. Dokl Biochem Biophys. 2017;475:271–276. [DOI] [PubMed] [Google Scholar]
- 37. Meloche J, Lampron MC, Nadeau V, Maltais M, Potus F, Lambert C, Tremblay E, Vitry G, Breuils‐Bonnet S, Boucherat O, Charbonneau E, Provencher S, Paulin R, Bonnet S. Implication of inflammation and epigenetic readers in coronary artery remodeling in patients with pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2017;37:1513–1523. [DOI] [PubMed] [Google Scholar]
- 38. Meloche J, Potus F, Vaillancourt M, Bourgeois A, Johnson I, Deschamps L, Chabot S, Ruffenach G, Henry S, Breuils‐Bonnet S, Tremblay E, Nadeau V, Lambert C, Paradis R, Provencher S, Bonnet S. Bromodomain‐containing protein 4: the epigenetic origin of pulmonary arterial hypertension. Circ Res. 2015;117:525–535. [DOI] [PubMed] [Google Scholar]
- 39. Belkina AC, Denis GV. BET domain co‐regulators in obesity, inflammation and cancer. Nat Rev Cancer. 2012;12:465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor‐α: direct role in obesity‐linked insulin resistance. Science. 1993;259:87–91. [DOI] [PubMed] [Google Scholar]
- 41. Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA. 2003;100:7265–7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nasu K, Tsuchikane E, Katoh O, Fujita H, Surmely JF, Ehara M, Kinoshita Y, Tanaka N, Matsubara T, Asakura Y, Asakura K, Terashima M, Suzuki T. Plaque characterisation by virtual histology intravascular ultrasound analysis in patients with type 2 diabetes. Heart. 2008;94:429–433. [DOI] [PubMed] [Google Scholar]
- 43. Gursoy Calan O, Calan M, Yesil Senses P, Unal Kocabas G, Ozden E, Sari KR, Kocar M, Imamoglu C, Senses YM, Bozkaya G, Bilgir O. Increased adipsin is associated with carotid intima media thickness and metabolic disturbances in polycystic ovary syndrome. Clin Endocrinol (Oxf). 2016;85:910–917. [DOI] [PubMed] [Google Scholar]
- 44. Song NJ, Kim S, Jang BH, Chang SH, Yun UJ, Park KM, Waki H, Li DY, Tontonoz P, Park KW. Small molecule‐induced complement factor D (adipsin) promotes lipid accumulation and adipocyte differentiation. PLoS One. 2016;11:e0162228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Horwich TB, Hamilton MA, Fonarow GC. B‐type natriuretic peptide levels in obese patients with advanced heart failure. J Am Coll Cardiol. 2006;47:85–90. [DOI] [PubMed] [Google Scholar]
- 46. Roger VL, Go AS, Lloyd‐Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dunlay SM, Redfield MM, Weston SA, Therneau TM, Hall Long K, Shah ND, Roger VL. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol. 2009;54:1695–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hasin T, Gerber Y, McNallan SM, Weston SA, Kushwaha SS, Nelson TJ, Cerhan JR, Roger VL. Patients with heart failure have an increased risk of incident cancer. J Am Coll Cardiol. 2013;62:881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hasin T, Gerber Y, Weston SA, Jiang R, Killian JM, Manemann SM, Cerhan JR, Roger VL. Heart failure after myocardial infarction is associated with increased risk of cancer. J Am Coll Cardiol. 2016;68:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ocampo NV, Tafreshi J, Hauschild CL, Pai RG. Cardiovascular medications and risk of cancer. Am J Cardiol. 2011;108:1045–1051. [DOI] [PubMed] [Google Scholar]
- 51. Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC. Angiotensin‐receptor blockade and risk of cancer: meta‐analysis of randomised controlled trials. Lancet Oncol. 2010;11:627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bhaskaran K, Douglas I, Evans S, van Staa T, Smeeth L. Angiotensin receptor blockers and risk of cancer: cohort study among people receiving antihypertensive drugs in UK General Practice Research Database. BMJ. 2012;344:e2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rosenberg L, Rao RS, Palmer JR, Strom BL, Stolley PD, Zauber AG, Warshauer ME, Shapiro S. Calcium channel blockers and the risk of cancer. JAMA. 1998;279:1000–1004. [DOI] [PubMed] [Google Scholar]
- 54. Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta‐analysis. JAMA. 2006;295:74–80. [DOI] [PubMed] [Google Scholar]
- 55. Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long‐term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. [DOI] [PubMed] [Google Scholar]
- 56. Ridker PM. A test in context: high‐sensitivity C‐reactive protein. J Am Coll Cardiol. 2016;67:712–723. [DOI] [PubMed] [Google Scholar]
- 57. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida‐Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 58. Grinberg‐Bleyer Y, Ghosh S. A novel link between inflammation and cancer. Cancer Cell. 2016;30:829–830. [DOI] [PubMed] [Google Scholar]
- 59. Sattar N, Wannamethee G, Sarwar N, Tchernova J, Cherry L, Wallace AM, Danesh J, Whincup PH. Adiponectin and coronary heart disease: a prospective study and meta‐analysis. Circulation. 2006;114:623–629. [DOI] [PubMed] [Google Scholar]
- 60. Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama‐Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat‐derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. [DOI] [PubMed] [Google Scholar]
- 61. Chougule D, Nadkar M, Venkataraman K, Rajadhyaksha A, Hase N, Jamale T, Kini S, Khadilkar P, Anand V, Madkaikar M, Pradhan V. Adipokine interactions promote the pathogenesis of systemic lupus erythematosus. Cytokine. 2018;111:20–27. [DOI] [PubMed] [Google Scholar]
- 62. Martinez HR, Escamilla‐Ocanas CE, Camara‐Lemarroy CR, Gonzalez‐Garza MT, Tenorio‐Pedraza JM, Hernandez‐Torre M. CSF concentrations of adipsin and adiponectin in patients with amyotrophic lateral sclerosis. Acta Neurol Belg. 2017;117:879–883. [DOI] [PubMed] [Google Scholar]
- 63. Natarajan R, Hagman S, Hamalainen M, Leppanen T, Dastidar P, Moilanen E, Elovaara I. Adipsin is associated with multiple sclerosis: a follow‐up study of adipokines. Mult Scler Int. 2015;2015:371734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Classification and regression tree (CART). CART analyses were performed to determine the best biomarker for predicting prognosis in patients with coronary artery disease.Figure S2. Boxplots of serum levels of cytokines/chemokines and growth factors. Serum levels of interleukin (IL) 15, IL‐16, IL‐17, cutaneous T‐cell–attracting chemokine (CTACK), leukemia inhibitory factor (LIF), granulocyte macrophage colony‐stimulating factor (GM‐CSF), adiponectin, and cyclophilin A (CyPA) were elevated in patients with higher serum levels of adipsin.
Figure S3. Serum levels of adipsin and cardiovascular risk factors. Distribution of serum adipsin levels based on advanced age (>66 years), hypertension, smoking, diabetes mellitus, dyslipidemia, and sex. Results are expressed by boxplots analyses.
Figure S4. Kaplan–Meier curve based on serum adipsin levels. Serum adipsin levels were not associated with acute myocardial infarction death (A) or with composite cardiovascular death (B). Cardiovascular death was considered if the death was caused by acute myocardial infarction. Composite cardiovascular death was defined if the death was caused by acute myocardial infraction, heart failure, stroke, or other cardiovascular causes.
Figure S5. Boxplot for serum adipsin levels. Serum adipsin levels were elevated in patients with future occurrences of cancer death.
Figure S6. Kaplan–Meier curve based on plasma brain natriuretic peptide (BNP) levels. Higher BNP levels were significantly associated with (A) primary end point, (B) all‐cause death, and (C) rehospitalization, but not with (D) revascularization. The primary end point was a composite of all‐cause death, rehospitalization, and revascularization. Revascularization was defined as percutaneous coronary intervention or coronary artery bypass grafting.
Figure S7. Kaplan–Meier curve based on serum high‐sensitivity C‐reactive protein (hsCRP) levels. Higher hsCRP levels were significantly associated with (A) primary end point, (B) all‐cause death, (C) rehospitalization, but not with (D) revascularization. The primary end point was a composite of all‐cause death, rehospitalization, and revascularization. Revascularization was defined as percutaneous coronary intervention or coronary artery bypass grafting.
Figure S8. Kaplan–Meier curve based on serum adiponectin levels. Higher adiponectin levels were significantly associated with (A) primary end point, (B) all‐cause death, (C) rehospitalization, but not with (D) revascularization. The primary end point was a composite of all‐cause death, rehospitalization, and revascularization. Revascularization was defined as percutaneous coronary intervention or coronary artery bypass grafting.
Figure S9. Scatter plots showing the relationship between serum adipsin levels and other variables. Scatter plots showed that serum levels of adipsin were correlated with (A) plasma levels of brain natriuretic peptide and (C) those of adiponectin, but not those of high‐sensitivity C‐reactive protein (hsCRP) (B) in Spearman's correlation analysis.
Figure S10. Hazard ratio (HR) plot and histogram for serum adipsin levels. HR plot revealed that serum adipsin levels were an accurate biomarker for predicting primary end point in patients with coronary artery disease, independent of the length of follow‐up period. Solid, broken, dotted, and red lines indicate HR plot, 95% CIs, smoothed control line, and log(HR)0, respectively. Blue bars show histogram.
Figure S11. Receiver operating characteristic curve for serum adipsin levels.
Figure S12. Receiver operating characteristic (ROC) curve for plasma brain natriuretic peptide (BNP) and serum high‐sensitivity C‐reactive protein (hsCRP) levels. The ROC curve revealed that plasma BNP levels were an accurate biomarker of predicting (A) death caused by AMI and (B) AMI incidence in patients with coronary artery disease (CAD), whereas serum hsCRP levels were not useful for predicting (C) death caused by AMI or (D) AMI incidence in patients with CAD.


