Abstract
Background
Poor adherence to cardioprotective drugs remains a concern among patients for secondary prevention. A better understanding of adherence fluctuations before and after critical health events may inform approaches for addressing or preventing poor adherence. Therefore, we assessed trajectories of adherence to lipid‐lowering drugs before and after acute coronary syndrome (ACS) or stroke and identified post‐ACS/stroke trajectories’ predictors.
Methods and Results
We conducted a cohort study of patients hospitalized for ACS or stroke in Alberta, Canada, using administrative health data between 2009 and 2015. Patients using lipid‐lowering drugs in the 2 years pre‐hospitalization and had post‐discharge follow‐up ≥365 days were included. We used group‐based trajectory modeling to assess adherence trajectories and multinomial logistic regression to assess trajectories’ predictors. In total, 10 623 patients were included. The average age was 69 years, and 65% were men. Five trajectories were identified in both periods: nearly perfect, gradual increase, gradual decline, rapid decline, and poor adherence throughout. Of patients who were poor adherers, rapidly or gradually declining pre‐hospitalization, 2395/3588 (66.8%) switched to gradual increase or perfect adherence post discharge. Conversely, of patients gradually increasing or nearly perfect before, only 4822/7035 (68.5%) were nearly perfect adherers after. Main predictors of poor post‐ACS/stroke trajectories included older age, female sex, lack of immediate post discharge follow‐up, and prior trajectories.
Conclusions
This study suggests that adherence post‐ACS/stroke is highly variable and emphasizes the importance for clinicians to recognize that post‐discharge adherence will likely change negatively for prior good adherers. Adherence‐enhancing interventions should occur both early and late following discharge.
Keywords: acute coronary syndrome, adherence trajectories, lipid‐lowering drugs, pre‐post hospitalization, stroke
Subject Categories: Acute Coronary Syndromes, Intracranial Hemorrhage, Lipids and Cholesterol, Secondary Prevention, Epidemiology
Clinical Perspective
What Is New?
To our knowledge, this is the first population‐based cohort study that assessed trajectories of adherence to lipid‐lowering drugs before and after hospitalization for acute coronary syndrome or stroke; 5 trajectories were identified in both periods: nearly perfect, gradual increase, gradual decline, rapid decline, and poor adherence throughout.
A significant proportion (ie, 67%) of patients who had poor adherence trajectories before their hospitalization, switched to good adherence trajectories after discharge.
Conversely, of patients who had good adherence trajectories before hospitalization, 27% turned to poor adherence trajectories, immediately or later after the event.
What Are the Clinical Implications?
Clinicians need to be cognizant of the changes and initiate adherence‐enhancing interventions before hospital discharge and during follow‐up visits.
Although patients who were poor adherers before the event deserve full consideration to help them improve their adherence and avoid or minimize the risk of recurrent events; attention should be paid to all patients as even those who were good adherers before the event often negatively change their adherence patterns.
Introduction
Acute coronary syndrome (ACS) and stroke are critical events that can cause death or major functional limitations.1 The risk of ACS or stroke is particularly high for patients with cardiovascular risk factors such as hypertension, dyslipidemia, and smoking.2, 3, 4 Therefore, to prevent such events, strategies targeting high‐risk group patients include lifestyle modification and the prescription of drugs for life‐long use.
Among the strategies, lipid‐lowering drug (LLD) therapy reduces the risk of myocardial infarction, stroke, and cardiovascular mortality by about 25% to 30%.5, 6, 7 However, evidence clearly shows that the long‐term use of these drugs is challenging for patients. Indeed, 40% to 75% of patients discontinue their LLD therapy within 1 year after initiation.8 Moreover, patients who experienced a non‐fatal ACS or stroke may increase their adherence to LLD9, 10 because of the severity of the event, their fear of recurrent events, and increased support by healthcare professionals and family members. However, evidence also suggest that some patients may become poor adherers.9, 10 For example, in a study of 113 296 patients hospitalized for acute myocardial infarction, 19.7% had increased adherence to LLD therapy while 16.3% had decreased adherence in the post‐discharge year.9
One of the difficulties in estimating adherence is how to account for fluctuations in adherence over time. The majority of previous studies evaluating LLD adherence post‐ACS summarized adherence as a single estimate for all of the follow‐up period (ie, as an average proportion of days covered [PDC] by drugs over the entire follow‐up). However, it is more likely that patients may have periods of good or poor adherence over time. For example, some patients may be good adherers in the early periods following the event and then subsequently decrease their adherence over time or vice versa. Others may sustain good or poor adherence throughout the entire time. Previous studies have not accounted for this and have not evaluated the underlying longitudinal patterns (trajectories) of use after an ACS or stroke. Furthermore, how prior adherence trajectories translate into post‐ACS or stroke adherence trajectories is also unknown. Such information is important to healthcare professionals to anticipate their patients’ behavior to their LLD use after life‐threatening events to help them maintain or improve their adherence, thus avoiding or minimizing the risk of recurrent events.
As outlined above, a better understanding of the different longitudinal patterns of LLD use before and after a non‐fatal ACS or stroke and their modifying factors is needed to inform approaches for addressing non‐adherence and preventing recurrent events. We thus planned the present study to assess longitudinal changes in adherence to LLD following a non‐fatal ACS or stroke and to assess some factors that may contribute to explain these changes in Alberta, Canada.
Methods
Data, Research Methods, and Material Sharing
This study used administrative data provided by Alberta Health, the Ministry of Health of the Province of Alberta, Canada. The authors do not have authorization to share the data with others. Requests for data from Alberta Health related to the study can be sent to Health.InfoRequest@gov.ab.ca. The authors did not have any special access privileges to these data that others would not have.
The research methods and all other research materials are included in the present report or referenced.
Study Design
We conducted a retrospective cohort study of patients hospitalized for a non‐fatal ACS or stroke between 2009 and 2015. Trajectory patterns of LLD adherence were summarized and assessed in the 1‐year periods before admission and following discharge.
Sources of Data
Combined data from the comprehensive provincial healthcare administrative databases and vital statistics file were used in all analyses. These databases maintain current demographic information, and billable medical services claims including inpatient and outpatient visits and medical procedures, for all patients within Alberta universal publicly funded healthcare system. More specifically, we combined databases from Alberta Services (ie, Population and Vital Statistic Data), Alberta Health (Discharge Abstract Database, Ambulatory Care Classification Database, and Alberta Physician Claims database), and Alberta Blue Cross/PIN (Medications database). The population and vital statistic data contain information on patient sex, age, marital status, immigration and emigration data, date of death, and cause of death according to the World Health Organization algorithm using International Classification of Diseases, Ninth and Tenth Revision (ICD‐9; ICD‐10) codes. The Discharge Abstract Database contains all hospital services, length of stay, diagnosis (with ICD codes), and procedure intervention while in hospital. Data and coding accuracy are routinely validated, both provincially and centrally. The Ambulatory Care Classification Database includes emergency department visits data (start, end date, and procedure interventions). This database allows clear distinction of emergency department visits from other physician visits. The Alberta Physician Claims Database contains the date of service, ICD code associated with the claim, procedure and billing information, and the specialty of the billing physician. The medications database contains information on drug class, ATC codes, name, generic and brand name, strength and dosage, date of dispensation, quantities dispensed, and the number of days covered by the dispensed drugs. The prescriber, the dispensed pharmacy and costs were also recorded. This database captures 95% of all dispensed drugs, irrespective of age. Accuracy and validity are routinely checked through computerized processing.
All databases were linked at patient level based on personal health number.
Study Population
We selected all patients who were hospitalized for a non‐fatal ACS or stroke (ICD‐10 codes: I20, I21, I24, I60, I61, I62, I63, I64) between 2009 and 2015 and registered in Alberta's universal health insurance plan.
All patients had to be LLD users (ie, any LLD) in the 2‐year before hospitalization (ie, patients who had at least one LLD dispensation between −730 to −366 days as well as −365 to −1 days before hospitalization) (Figure 1). We excluded patients who died in the year following their discharge from hospital and those who lost insurance coverage (eg, moved out of province), as well as those with insufficient follow‐up (ie, <365 days before and after hospitalization). These criteria were necessary to ensure sufficient data were available to robustly estimate adherence patterns in the year before to and after the non‐fatal ACS/stroke event. Each included patient would have 12 months of LLD dispensation information, irrespective of adherence level, which is required to estimate an adherence trajectory over time.11
Figure 1.
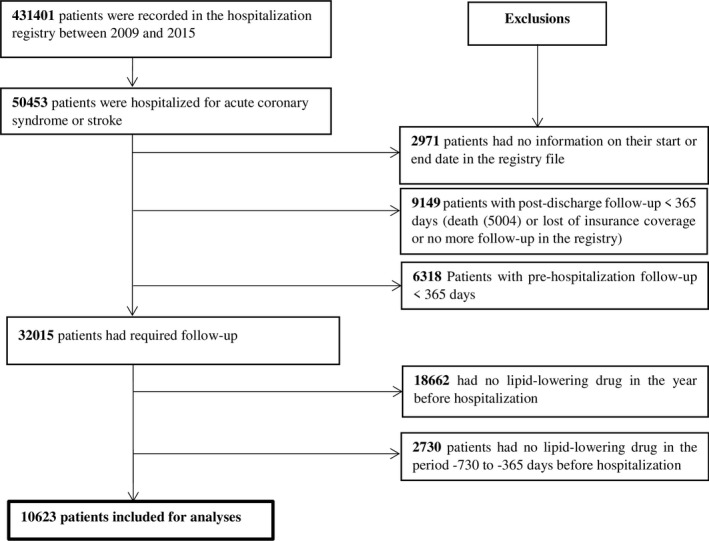
Selection of the study population.
Outcome
The outcome of this study was change in adherence trajectories to LLD following the hospitalization for non‐fatal ACS or stroke. To assess adherence trajectories, we first calculated monthly PDCs by any LLD for each patient in each period (ie, 12 monthly PDCs before and after the event). The PDCs were calculated using prescription fills date and number of days’ supply. Practically, the total number of days covered by any LLD in each 30‐day period was divided by 30″. Thus, the PDCs could range from 0 (0 days covered/30 days) to 1 (30 covered/30 days). Each monthly PDC was dichotomized as ≥80% to define good adherence in a given month.11, 12 Next, we used group‐based trajectory modeling to group patients in different adherence trajectories (see Analysis section for more details).11
Predictors of Adherence Trajectories
The following variables were assessed as potential predictors of changes in adherence: sex, age area of residence (urban versus rural), and comorbidities at the time of hospitalization. Comorbidities included hypertension, diabetes mellitus, history of ischemic heart disease, heart failure, chronic kidney disease, mental health issue, asthma, and chronic obstructive pulmonary disease (see Table S1 for codes used for the definitions). We also assessed the total number of days spent in hospital for the index ACS or stroke, critical care medicine during hospitalization for the index event (yes versus no), cardiovascular surgery or neurosurgery during hospitalization, and the 30‐day post‐discharge follow‐up (ie, follow‐up with a specialist, a general practitioner, or other health professional). Finally, we assessed the total number of drugs used in the year before hospitalization, and alcohol dependence syndrome (yes versus no).
Statistical Analysis
We used group‐based trajectory modeling to assess the adherence trajectories of patients before and after hospitalization. Group‐based trajectory modeling is a method used to identify latent groups (clusters) of individuals following a similar progression of an outcome over time.13, 14 For a given outcome, it assumes that the population is composed of a mixture of finite numbers of underlying trajectories.13, 14 Specifically, based on individuals’ adherence patterns over time, the probability of belonging to each potential adherence group is modeled. Because PDCs were not normally distributed, we dichotomized each monthly PDC as ≥0.8 and we then used a logit function to estimate the models’ parameters. In practice, several regression models are estimated simultaneously through maximization of a likelihood that combines the information from all models. Within each group, adherence is modeled as a smooth function of time using up to a fourth order polynomial.11, 14 As suggested by Nagin,13 we first considered quadratic polynomial order to describe the shape of the latent trajectories and to retain the number of trajectories. Quadratic form has the capacity to capture alternative trajectories of change.13 To this end, a one‐group model was first assessed as suggested, then we consecutively increase the number of groups (trajectories). At each step, the probability that an individual belongs to a given group is calculated and then each individual is classified in the group where his/her membership probability is the highest. For each step, we used the Bayesian Information Criterion13 to select the best model (model with lowest Bayesian Information Criterion preferred). However, in the first step of selection of the number of trajectories, the Bayesian Information Criterion continued to increase as the number of groups increases and was not useful in identifying the number of trajectories. Therefore, we used recommendations based on the principle of parsimony, the minimum group size of 5%, and the objective of the research13 to select clinically meaningful trajectories. After obtaining the number of trajectories, we varied the order of the polynomials describing the shape by considering cubic and quartic orders to further describe the shape of the trajectories.
A multivariable logistic regression analysis was conducted to identify factors predicting each adherence trajectory during the 1‐year post‐discharge period. To this end, all covariates mentioned above and the patient's adherence trajectory in the year before hospitalization were considered in the model. We then used backward selection to retain the predictors (P value for variables to stay in the model was 0.10).
The analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). Trajectories are modeled using Proc Traj, a SAS procedure for group‐based trajectories modeling (https://www.andrew.cmu.edu/user/bjones/download.htm).
This research was approved by the Health Ethics Research Board of the University of Alberta (PRO 00060499). All data provided by Alberta Health were fully anonymized. Patient consent was not required to have access to the data.
Results
In total, 50 453 patients were hospitalized for non‐fatal ACS or stroke in the study period and 32 015 met our initial inclusion criteria (survived with pre‐hospitalization and post‐discharge follow‐up ≥365 days). Among the 32 015 patients, 10 623 patients had at least one LLD dispensation between −730 to −366 days as well as −365 to −1 days before hospitalization. The mean age was 69 years and the majority of patients were men. Hypertension, history of ischemic heart disease, diabetes mellitus, and mental health issues were the most frequent health conditions (Table 1).
Table 1.
Characteristics of Study Population
| Characteristics | Total Sample (N=10 623) |
|---|---|
| Mean age, y (SD) | 69.09 (11.52) |
| Age in categories | |
| <55 y | 1063 (10.0%) |
| 55 to <65 y | 2373 (22.3%) |
| 65 to <75 y | 3115 (29.3%) |
| 75 to <85 y | 2977 (28.0%) |
| ≥85 y | 1095 (10.3%) |
| Female sex | 3671 (34.6%) |
| Area of residence in the year before hospitalization (urban vs rural) | 8426 (79.3%) |
| Event responsible for hospitalization | |
| Angina pectoris | 2562 (24.1%) |
| Myocardial infarction | 5312 (50.0%) |
| Other acute coronary syndrome | 345 (3.3%) |
| Stroke | 2404 (22.6%) |
| Morbidities at index hospitalization | |
| Hypertension | 8746 (82.3%) |
| History of ischemic heart disease | 6646 (62.6%) |
| Heart failure | 2193 (20.6%) |
| Diabetes mellitus | 5490 (51.7%) |
| Chronic obstructive pulmonary disease | 1877 (17.7%) |
| Asthma | 1391 (13.1%) |
| Mental health issues | 5311 (50.0%) |
| Chronic kidney disease | 1126 (10.6%) |
| First event length of stay, median (IQR) | 4 (3–8) |
| 30‐d post‐discharge follow‐up | |
| No | 507 (4.8%) |
| Cardiologist or neurologist | 3365 (31.7%) |
| General practitioner | 6319 (59.5%) |
| Other professional | 432 (4.1%) |
| Critical care medicine during index hospitalization (yes vs no) | 217 (2.0%) |
| Cardiovascular surgery or neurosurgery during index hospitalization (yes vs no) | 475 (4.5%) |
| Alcohol dependence syndrome | 164 (1.5%) |
| Median number of different drugs in the year before hospitalization (IQR) | 11 (7–15) |
IQR indicates interquartile range.
We identified 5 adherence trajectories in both periods. Figure 2 illustrates the adherence trajectories in the 1‐year period before hospitalization: 10.4% were poor adherent throughout the 1‐year period, 6.4% had a rapid decline in adherence, 16.8% had a gradual decline in adherence, 17.0% had a gradual increase to reach good adherence, and 49.5% of patients were nearly perfect adherers. In the post‐discharge period, 4.6% were poor adherers throughout the 1‐year period, 5.4% rapidly declined, 13.9% gradually declined, 16.2% gradually increased to good adherence, and 59.7% of patients were nearly perfect adherers (Figure 3).
Figure 2.
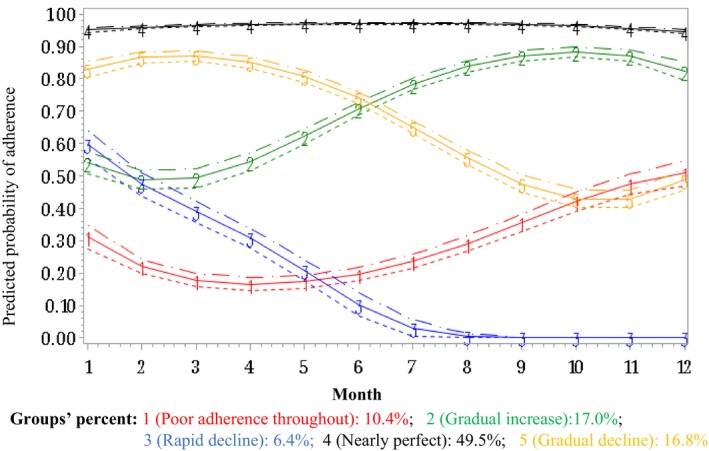
Group‐based trajectories of adherence to lipid‐lowering drugs in the year before a hospitalization for acute coronary syndrome or stroke. For ease of comparison of trajectories between periods, we considered trajectory 1 as “poor adherence throughout” as, although the shape of this trajectory suggests a slight increase, the patients of this group started the period with poor adherence and did not reach good adherence at the end of the period.
Figure 3.
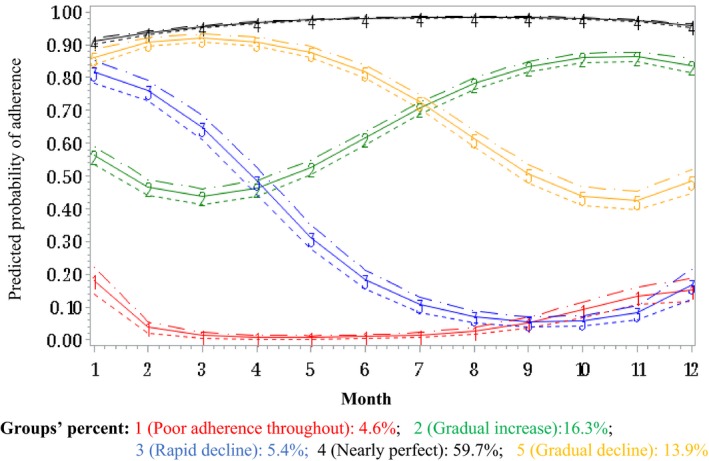
Group‐based trajectories of adherence to lipid‐lowering drugs in the year after hospitalization for acute coronary syndrome or stroke.
Interestingly, only 4505 (42.41%) patients had similar adherence trajectory after their ACS or stroke hospitalization (Table 2). In general, most patients maintained good adherence or had improvements in their adherence trajectory after hospitalization. Of the 3588 patients who were poor adherers throughout, rapidly or gradually declining before their hospitalization, 2395 (66.8%) switched to gradually increased or perfect adherence after discharge. Conversely, of patients who were nearly perfect adherers or gradually increased their adherence to reach good adherence before hospitalization, only 4822/7035 (68.5%) were nearly perfect adherers in the year post discharge.
Table 2.
Adherence Trajectory Groups Before and After Hospitalization for an Acute Coronary Syndrome or Stroke
| Pre‐ACS/Stroke Adherence Group | Post‐ACS/Stroke Adherence Group | |||||
|---|---|---|---|---|---|---|
| Poor Adherence Throughout | Rapid Decline | Gradual Decline | Gradual Increase | Nearly Perfect | Total | |
| Poor adherence throughout | 57 (5.59) | 89 (8.73) | 196 (19.23) | 202 (19.82) | 475 (46.61) | 1019 |
| Rapid decline | 128 (16.84) | 81 (10.66) | 135 (17.76) | 131 (17.24) | 285 (37.50) | 760 |
| Gradual decline | 130 (7.19) | 104 (5.75) | 273 (15.09) | 405 (22.39) | 897 (49.59) | 1809 |
| Gradual increase | 60 (3.50) | 68 (3.97) | 262 (15.30) | 297 (17.35) | 1025 (59.87) | 1712 |
| Nearly perfect | 126 (2.37) | 180 (3.38) | 574 (10.78) | 646 (12.14) | 3797 (71.33) | 5323 |
| Total | 501 | 522 | 1440 | 1681 | 6479 | 10 623 |
Values in parenthesis are proportions within the pre‐ACS/stroke adherence group. ACS indicates acute coronary syndrome
In regression analysis, we identified different factors that were associated with post‐discharge adherence trajectories (Table 3). The length of hospital stay during index hospitalization, the number of drugs, and adherence trajectory in the year before hospitalization were predictive of all non‐perfect adherence trajectories in the year after. The trajectory of “poor adherence throughout” also had unique predictors, including sex, living in a rural area, heart failure, mental health issue, and angina pectoris versus other ACS. Patients in this group were also more likely to be aged >85 years.
Table 3.
Factors Associated With Trajectories of Adherence to Lipid‐Lowering Drugs After a Hospitalization for an Acute Coronary Syndrome or Stroke in Multinomial Logistic Regression
| Predictors | Poor Adherence Throughout (1) vs Nearly Perfect | Rapid Decline (3) vs Nearly Perfect | Gradual Decline (5) vs Nearly Perfect | Gradual Increase (2) vs Nearly Perfect |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
|
Pre‐event adherence trajectory group (ref: nearly perfect adherence [4]) | ||||
| Rapid decline (3) | 21.00 (15.54–28.38)* | 7.54 (5.59–10.18)* | 3.35 (2.66–4.21)* | 3.25 (2.59–4.09)* |
| Gradual decline (5) | 5.18 (3.95–6.80)* | 2.68 (2.08–3.46)* | 2.06 (1.75–2.42)* | 2.83 (2.45–3.28)* |
| Poor adherence throughout (1) | 5.61 (3.96–7.95)* | 4.97 (3.75–6.58)* | 2.90 (2.39–3.52)* | 2.89 (2.39–3.49)* |
| Gradual increase (2) | 1.89 (1.36–2.64)* | 1.48 (1.11–1.98)* | 1.72 (1.46–2.03)* | 1.77 (1.52–2.07)* |
| Age (ref: <55 y) | ||||
| ≥55 to <65 y | 0.86 (0.58–1.26) | 1.07 (0.75–1.54) | 0.81 (0.66–1.00)* | 0.87 (0.70–1.07) |
| ≥65 to 75 y | 0.73 (0.50–1.08) | 1.02 (0.71–1.46) | 0.84 (0.68–1.03) | 1.02 (0.83–1.25) |
| ≥75 to <85 y | 1.19 (0.82–1.72) | 1.32 (0.93–1.89) | 0.90 (0.73–1.11) | 1.16 (0.94–1.43) |
| ≥85 y | 2.40 (1.61–3.58)* | 2.23 (1.50–3.31)* | 0.90 (0.69–1.17) | 1.05 (0.81–1.36) |
| Sex (women vs men) | 1.53 (1.25–1.88)* | 1.16 (0.96–1.41) | 0.98 (0.86–1.11) | 1.05 (0.93–1.18) |
| Asthma (yes vs no) | 1.06 (0.80–1.40) | 0.76 (0.57–1.01) | 0.77 (0.64–0.93)* | 0.88 (0.75–1.05) |
| Heart failure | 1.44 (1.13–1.84)* | 1.00 (0.78–1.27) | 1.03 (0.88–1.20) | 1.09 (0.94–1.26) |
| History of ischemic heart disease | 0.89 (0.72–1.10) | 1.09 (0.89–1.33) | 1.12 (0.98–1.27) | 1.13 (1.00–1.27)* |
| Mental health issue | 1.26 (1.03–1.55)* | 1.15 (0.96–1.39) | 1.02 (0.90–1.15) | 0.95 (0.85–1.07) |
| Chronic kidney disease | 1.30 (0.96–1.75) | 1.41 (1.06–1.87)* | 1.21 (1.00–1.47)* | 1.03 (0.85–1.24) |
| Index event (ref: other ACS) | ||||
| Angina pectoris | 0.45 (0.27–0.76)* | 1.58 (0.81–3.08) | 0.73 (0.53–1.01) | 0.82 (0.60–1.11) |
| Myocardial infarction | 0.37 (0.23–0.60)* | 1.11 (0.58–2.15) | 0.70 (0.51–0.95)* | 0.61 (0.45–0.83)* |
| Stroke | 0.95 (0.58–1.56) | 1.91 (0.98–3.73) | 0.85 (0.61–1.18) | 1.01 (0.74–1.39) |
| Number of drugs in the year before | 1.01 (1.00–1.03)* | 1.01 (0.99–1.03) | 1.01 (1.00–1.02)* | 1.01 (1.00–1.03)* |
| Area of residence (urban vs rural) | 0.66 (0.52–0.83)* | 0.99 (0.79–1.25) | 1.01 (0.88–1.17) | 0.94 (0.82–1.08) |
| Index event hospital days | 1.03 (1.02–1.04)* | 1.02 (1.01–1.03)* | 1.01 (1.00–1.01)* | 1.01 (1.01–1.02)* |
| 30‐d follow‐up (ref: No.) | ||||
| Cardiologist or neurologist | 0.42 (0.27–0.66)* | 0.61 (0.41–0.93)* | 0.97 (0.73–1.30) | 0.82 (0.63–1.06) |
| General practitioner | 0.66 (0.44–0.99)* | 0.76 (0.51–1.12) | 0.98 (0.74–1.30) | 0.78 (0.61–1.01) |
| Other | 0.53 (0.29–0.97)* | 0.75 (0.43–1.33) | 1.07 (0.72–1.59) | 1.07 (0.76–1.51) |
| Cardiovascular surgery or neurosurgery during hospitalization (ref: No.) | 0.96 (0.59–1.54) | 1.08 (0.70–1.66) | 0.81 (0.59–1.11) | 1.31 (1.02–1.67)* |
How to read the results (example of the variables pre‐event adherence trajectories and sex): For example, patients whose adherence “rapidly declined” before the hospitalization for ACS or stoke are more likely to be “poor adherer throughout” in the year post‐ACS or stroke (OR: 21) rather than being “nearly perfect adherer” when compared with patients who were “nearly perfect adherer” before hospitalization. Women are more likely to be “poor adherer throughout” in the year post‐ACS or stroke (OR: 1.53) rather than “nearly perfect adherer” when compared with men. ACS indicates acute coronary syndrome; OR, odds ratio.
Statistical significant association.
Discussion
We identified 5 adherence trajectories in both pre‐ and post hospitalization for ACS or stroke going from nearly perfect trajectories to poor adherence in all the follow‐up. The results suggest that a hospitalization for ACS or stroke has significant positive or negative impact on medication adherence. Indeed, only 42% had similar adherence trajectory after their event. Some patients who had positive trajectories (nearly perfect or gradually increasing) in the year before the event did not maintain good adherence after. Conversely, other patients who had negative adherence trajectories in the year before the event switched to good adherence trajectories after.
The behavior of increasing or decreasing adherence to LLD has been previously observed in patients hospitalized for acute myocardial infarction.9, 10, 15 Indeed, Kronish et al studied adherence to statin therapy after hospitalization for myocardial infarction and found that 33% of patients with pre‐hospitalization PDC ≥0.80 became non‐adherent in the post‐discharge year, while 38% of patients with PDC <0.80 became adherent after.10
Why adherence changes so remarkably is difficult to ascertain. Despite obvious factors that should promote higher adherence (fear of recurrent events, support of healthcare professionals and family members), these results showed that other factors influenced adherence including pill burden post discharge, age, sex, and type of initial event. Experiencing ACS or stroke, despite being a good adherer to LLD, may have affected the trust and perceptions of some patients in these medications and therefore may explain why some turned to poor adherence trajectories in the year following their hospitalization. Indeed, the lack of (immediate) therapeutic benefit is a factor of poor adherence to cardiovascular medications.16 Clinicians need to be cognizant of these changes as a non‐negligible proportion of patients who were good adherers to LLDs and possibly other cardioprotective drugs before an ACS or stroke will become poor adherers, immediately or later after the event. Therefore, considering that poor adherence to LLD is associated with a high risk of cardiovascular disease and mortality,8, 17 clinicians should initiate adherence enhancing interventions for those patients before hospital discharge and during follow‐up visits. Such interventions should address the patients’ post‐ACS or stroke perceptions on the effectiveness and usefulness of the drugs, preventing them from stopping or decreasing the use of LLDs and other cardiovascular medications.
On the other hand, some patients who had negative adherence trajectories before the hospitalization positively changed their trajectories in the year after the hospitalization. Indeed, 29% of patients who had positive trajectories (gradually increasing or nearly perfect adherence) in the post‐discharge year were in negative trajectories groups in the year before hospitalization. For those patients, hospitalization may have acted as a “wake‐up call” in regard to their health and the need to use their LLD. A study by Librero et al that assessed trajectories of adherence to statin therapy and other drugs following hospitalization for coronary heart disease identified 3 trajectories for statin use: nearly perfect adherers (74.9%), rapid declining (7.6%) and gradually declining groups (17.5%).12 However, the study did not account for trajectories before hospitalization. Differences in the characteristics of the study populations, the length of follow‐up (9 versus 12 months in our study) and the inclusion of patients who died during the 9‐month follow‐up may contribute to explain differences in the number of trajectories between the 2 studies.
Our results highlight groups of patients and timelines for possible interventions to improve or maintain adherence after an ACS or stroke. Indeed, interventions before discharge from hospital and during early post‐discharge follow‐up may help to improve adherence for patients who are “poor adherers in all the follow‐up” or “rapidly decreasing”. For patients who are “lately decreasing”, follow‐up and interventions (eg re‐education) 6 months after their discharge from hospital may help reinforce their drug adherence. These interventions should target patients regardless of their pre‐hospitalization adherence trajectories as our results showed that even patients with nearly perfect adherence in the year before hospitalization can decline to poor adherence over time.
Strengths of our study include the large sample size, our ability to account for adherence before hospitalization in our analysis and to appreciate the pattern of change, and the use of group‐based modeling to better capture the longitudinal patterns of medication adherence. However, our study also has some limitations. The use of drug refills data to assess adherence is among these limitations. Indeed, adherence based on drugs refill data assumes that drugs filled or refilled by patients are properly used. This assumption can lead to overestimating adherence for patients who partially use their refilled drugs. A second limitation is that some factors that may affect medication adherence (eg, fatigue or muscle aches which are common side effects of LLD drugs, patients’ education level, perceived effectiveness of drugs, etc) were not possible to take into account in our analysis in determining factors predicting the trajectories. Moreover, given the retrospective observational nature of the data, limitations which are inherent to these study designs may not have been fully addressed with our methods. However, no randomized controlled trials or similar designs can address the research question of interest and thus observational studies, as we have conducted, are required.
Conclusions
This study suggests that adherence following a non‐fatal ACS/stroke is highly variable. It is important for clinicians to recognize that adherence before a major event does not translate into similar adherence following the event. Additional follow‐up and interventions targeted to improving post‐discharge adherence is recommended. Although patients who were poor adherers before the event deserve full consideration to help them improve their adherence and to avoid or minimize the risk of recurrent events, attention should be paid to all patients as even those who were good adherers before the event often negatively change their adherence patterns. These activities should occur both early and later following discharge to maximize adherence and ultimately reduce further downstream sequalae in patients experiencing non‐fatal ACS or stroke.
Sources of Funding
This work was supported by a grant from the Alberta Institute of Health Economics.
Disclosures
None.
Supporting information
Table S1. International Classification of Diseases, Ninth and Tenth Revisions (ICD‐9 and ‐10 Codes Used to Assess Comorbidities
Acknowledgments
This study is based in part on data provided by Alberta Health. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of the Government of Alberta nor the funder (Institute of Health Economics). Neither the Government nor Alberta Health nor the Institute of Health Economics express any opinion in relationship to this study.
Author Contributions: Prof Eurich, Dr Zongo, Prof Simpson, and Prof Johnson designed the study and Eurich and Johnson acquired the data. Prof Eurich and Dr Zongo analyzed the data and Prof Eurich, Dr Zongo, and Prof Simpson drafted the manuscript. All authors revised it critically for important intellectual content and approved the final version to be published. All authors are accountable for the work and integrity of the work. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. Eurich and Zongo had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
(J Am Heart Assoc. 2019;8:e013857 DOI: 10.1161/JAHA.119.013857.)
References
- 1. Levine DA, Davydow DS, Hough CL, Langa KM, Rogers MA, Iwashyna TJ. Functional disability and cognitive impairment after hospitalization for myocardial infarction and stroke. Circ Cardiovasc Qual Outcomes. 2014;7:863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hajar R. Risk factors for coronary artery disease: historical perspectives. Heart Views. 2017;18:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet. 2014;383:999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O'Donnell CJ, Elosua R. Cardiovascular risk factors. Insights from Framingham Heart Study. Rev Esp Cardiol. 2008;61:299–310. [PubMed] [Google Scholar]
- 5. Thavendiranathan P, Bagai A, Brookhart MA, Choudhry NK. Primary prevention of cardiovascular diseases with statin therapy: a meta‐analysis of randomized controlled trials. Arch Intern Med. 2006;166:2307–2313. [DOI] [PubMed] [Google Scholar]
- 6. Cheung BM, Lauder IJ, Lau CP, Kumana CR. Meta‐analysis of large randomized controlled trials to evaluate the impact of statins on cardiovascular outcomes. Br J Clin Pharmacol. 2004;57:640–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cholesterol Treatment Trialists C , Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Banach M, Stulc T, Dent R, Toth PP. Statin non‐adherence and residual cardiovascular risk: there is need for substantial improvement. Int J Cardiol. 2016;225:184–196. [DOI] [PubMed] [Google Scholar]
- 9. Hickson RP, Robinson JG, Annis IE, Killeya‐Jones LA, Korhonen MJ, Cole AL, Fang G. Changes in statin adherence following an acute myocardial infarction among older adults: patient predictors and the association with follow‐up with primary care providers and/or cardiologists. J Am Heart Assoc. 2017;6:e007106 DOI: 10.1161/JAHA.117.007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kronish IM, Ross JS, Zhao H, Muntner P. Impact of hospitalization for acute myocardial infarction on adherence to statins among older adults. Circ Cardiovasc Qual Outcomes. 2016;9:364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Franklin J, Shrank W, Pakes J, Sanfélix Gimeno G, Matlin O, Brennan T, Choudhry N. Group‐based trajectory models: a new approach to classifying and predicting long‐term medication adherence. Med Care. 2013;51:789–796. [DOI] [PubMed] [Google Scholar]
- 12. Librero J, Sanfelix‐Gimeno G, Peiro S. Medication adherence patterns after hospitalization for coronary heart disease. A population‐based study using electronic records and group‐based trajectory models. PLoS One. 2016;11:e0161381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nagin DS. Group‐Based Modeling of Development. Cambridge, MA: Harvard University Press; 2005:214. [Google Scholar]
- 14. Jones BL, Nagin DS. Advances in group‐based trajectory modeling and an SAS procedure for estimating them. Sociol Methods Res. 2007;35:542–571. [Google Scholar]
- 15. de Vries FM, Denig P, Vegter S, Bos HJ, Postma MJ, Hak E. Does a cardiovascular event change adherence to statin treatment in patients with type 2 diabetes? A matched cohort design. Curr Med Res Opin. 2015;31:595–602. [DOI] [PubMed] [Google Scholar]
- 16. Ferdinand KC, Senatore FF, Clayton‐Jeter H, Cryer DR, Lewin JC, Nasser SA, Fiuzat M, Califf RM. Improving medication adherence in cardiometabolic disease: practical and regulatory implications. J Am Coll Cardiol. 2017;69:437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rodriguez F, Maron DJ, Knowles JW, Virani SS, Lin S, Heidenreich PA. Association of statin adherence with mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2019;4:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. International Classification of Diseases, Ninth and Tenth Revisions (ICD‐9 and ‐10 Codes Used to Assess Comorbidities


