Abstract
Background
Differences in hospital proximity and nongeographic factors affect disparities in hospital quality for heart disease, but their relative contributions are unknown. The current study quantifies the influences of these factors on the white‐black gap in high‐ and low‐quality hospital use for acute myocardial infarction (AMI) and coronary artery bypass grafting (CABG) surgery.
Methods and Results
We used Medicare claims to identify fee‐for‐service Medicare beneficiaries aged 65 and older hospitalized during 2009–2011 with AMI (n=384 443) and CABG (n=71 411). Hospital quality was measured using publicly available AMI mortality rates. In national and regional analyses, we used conditional multinomial logit models to estimate the white‐black gap in high‐ and low‐quality hospital use and decompose the gap into geographic and nongeographic contributions. Overall, more whites used high‐quality hospitals for both conditions (34.8% versus 32.4% for AMI; 39.0% versus 29.9% for CABG; P<0.001), but after accounting for distance to hospitals, the white‐black gap was significant only for CABG (9.1%; P<0.001). The nongeographic component was significant for both conditions (3.4% for AMI and 7.7% for CABG; P<0.001) and accounted for nearly the entire gap for CABG. In contrast, hospital geographic proximity was not significant. In regional analyses, white beneficiaries had higher rates of high‐quality hospital use in the Northeast (CABG) and South (AMI and CABG), whereas black had higher rates of high‐quality hospital use in the Midwest (AMI).
Conclusions
White‐black differences in high‐quality hospital use were significant for CABG and related to nongeographic factors. Interventions should consider health system and contextual reasons for these disparities.
Keywords: coronary artery disease, disparities, hospital, quality of care
Subject Categories: Health Services, Quality and Outcomes
Clinical Perspective
What Is New?
Studies have documented a white‐black gap in high‐quality hospital use for coronary heart disease, but the contributions of hospital geographic proximity and nongeographic factors to the gap have not been quantified.
Nationally, the gap was significant for open heart surgery but not for myocardial infarction, and largely due to nongeographic factors.
Significant regional differences were observed, most notably black patients with myocardial infarction had higher rates of high‐quality hospital use in the Midwest.
What Are the Clinical Implications?
This suggests that disparities in coronary heart disease treatment quality, in particular elective high‐risk procedures, are driven by factors above and beyond geography.
Further studies are needed to understand the relationship between nongeographic factors such as physician referral networks and disparities in high‐quality hospital use.
Introduction
Despite marked improvements in treatments for patients with coronary heart disease (CHD),1 large white‐black gaps in CHD quality of care remain.2, 3, 4 The causes of white‐black disparities in CHD treatment may be explained in part by differences in the hospitals where black and white patients seek care.5, 6, 7, 8, 9, 10, 11
Research has shown that black patients with acute myocardial infarction (AMI) or undergoing coronary artery bypass grafting (CABG) surgery are more likely to receive care at low‐quality hospitals.5, 8, 9, 10 and less likely to receive care at high‐quality hospitals6, 7, 11 compared with their white peers. Some studies portray a more complex picture, showing that the magnitude of the disparity varies according to acuity of the condition. In particular, gaps may be smaller for AMI, an emergent condition, than for CABG, which is often elective.7 Yet other studies have shown significant regional variations in hospital quality for heart disease,12 with potential consequences for disparities.13 The pathways leading to racial gaps in high‐ and low‐quality hospital use are incompletely understood.
Given the importance of travel distance in hospital choice,14, 15, 16 white‐black gaps in the use of hospitals with different quality could be attributable to racial differences in geographic access to high‐ and low‐quality hospitals. However, evidence shows that blacks are less likely to use high‐quality hospitals even when they live closer to these hospitals than whites.6, 11 For instance, black patients with CHD are more likely to bypass top‐ranked hospitals for elective cardiac procedures and seek care at lower‐quality hospitals located farther from their homes,7 suggesting that nongeographic factors significantly influence hospital use.
Nongeographic sources of disparities in the quality of hospitals that blacks and whites use are likely complex and can be categorized into patient factors (eg, differences in patients’ preferences and attitudes toward particular types of hospitals, or socioeconomic status), provider factors (eg, provider bias or differences in the hospitals to which physicians treating whites and blacks have access), and healthcare system factors (eg, healthcare organizational culture). For the purpose of the current study, we refer to the array of factors influencing hospital choice independent of geographic access as “nongeographic factors.”
To date, no study has assessed the contributions of geographic access and nongeographic factors to racial gaps in high‐ and low‐quality hospital use. However, understanding the relative contributions of these factors to the overall gap is likely to be important for more targeted interventions aimed at narrowing these gaps. The current study addresses this shortcoming in the literature by using innovative decomposition methods to quantify the contributions of racial differences in geographic access and nongeographic factors to the white‐black gaps in the use of high‐ and low‐quality hospitals for CHD. We hypothesized that both geographic and nongeographic factors contribute to the white‐black gaps in high‐ and low‐quality hospital use and that nongeographic factors will have a larger contribution for CABG than for AMI because of the elective nature of CABG surgery. We conducted national analyses, as well as separate analyses for each of the US Census regions.
Methods
Data and Study Sample
Because of the sensitive nature of the data collected for this study, access to the data set requires entering a data use agreement with the Centers for Medicare & Medicaid Services (CMS). Requests from qualified researchers may be sent to the CMS via its Research Data Assistance Center (https://www.resdac.org/).
We used MedPAR files, which contain data on hospital discharges for Medicare fee‐for‐service beneficiaries, to identify black and white beneficiaries aged 65 or older admitted to the hospital between July 2009 and December 2011 with 2 conditions: AMI (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM] code 410 except 410.x2, subsequent episode of care) and CABG (ICD‐9‐CM codes 36.10–36.19). We excluded beneficiaries with health maintenance organizations or discontinuous part A/B enrollment because of incomplete data; beneficiaries transferred from another hospital, as transfers are influenced by different factors from those determining the initial admission; and beneficiaries who had a previous AMI or underwent coronary revascularization during a 6‐month lead‐in period for which we had data, as these patients may have different reasons for selecting particular hospitals compared with first‐episode patients. We restricted the CABG cohort to cases not occurring in the setting of a primary AMI diagnosis (87%) because these cases were more likely elective. We also limited the sample to beneficiaries living in core‐based statistical areas (CBSAs; metropolitan and micropolitan areas anchored by an urban center), excluding rural residents whose hospital choices may differ systematically from those of CBSA residents.
We also excluded beneficiaries who were treated in a CBSA other than their CBSA of residence, as they may have been traveling at the time of their admission.
Finally, we retained only CBSAs with sizeable black and white populations and at least 10 black AMI and CABG cases to ensure the robustness of our comparisons. The final AMI cohort included 35 561 black and 307 813 white beneficiaries admitted to 2681 hospitals in 253 CBSAs. The final CABG cohort included 3055 black and 40 933 white beneficiaries admitted to 1168 hospitals in 110 CBSAs.
Variables of Interest
Key study variables included patient race and 2 hospital attributes: quality and distance from the hospital to each patient's home. We defined race as black or white, based on Medicare data. We assessed hospital quality using the CMS 30‐day risk‐standardized AMI mortality rates publicly available on Hospital Compare. CMS did not report mortality measures specific to CABG during the study period. Thus, we used the AMI measure for both conditions, since it may in fact reflect a hospital's overall quality of care for CHD. We identified low‐quality hospitals as hospitals ranking in the top quintile and high‐quality hospitals as hospitals ranking in the bottom quintile of the mortality distribution for all hospitals in the AMI cohort. Hospitals with mortality rates in the middle 3 quintiles were classified as medium‐quality hospitals. For consistency, we applied the same rankings for each hospital in both cohorts. We measured home‐to‐hospital distance as the straight‐line distance, using patients’ home zip code centroids and hospitals’ street addresses. In addition, we performed sensitivity analyses using different cutoffs for high and low quality at the 15th and 25th percentiles of the mortality distribution.
Other patient‐level variables used to describe our study sample included age, sex, and a comorbidity index17 obtained from the hospital claims data.
Regression Models
Since patients living in urban areas have numerous hospitals to choose from, our analyses employed the conditional logit regression model.18 In this model, the probability that a patient uses a particular hospital is a function of the attributes of all the hospitals to which the patient could have reasonably been admitted. Thus, the model explicitly accounts for the attributes of the hospital chosen by the patient as well as those of hospitals not chosen. This is accomplished by fitting the model to a file that includes, for each patient, information on each hospital that the patient could have used; an indicator variable identifies the hospital actually used. Further, patient race does not appear as a main effect in this model; rather, it is interacted with the hospital attributes to assess whether the effects of distance and hospital quality on the likelihood of using a hospital differ between blacks and whites.
To estimate the models, we first developed the set of hospitals to which each patient could have been admitted (ie, “hospital choice sets”), defined as all hospitals available within 100 miles of patients’ home. Although some of the more distant hospitals in these choice sets may have never been used by patients, in the conditional logit model, hospitals never used do not affect estimates.18
We modeled the probability that a patient uses a particular hospital as a function of quality (high, medium, and low) and the home‐to‐hospital distance for all hospitals in the choice set. Because the relationship between distance and hospital use is nonlinear, we specified distance using a binary indicator variable for the closest hospital in each choice set and a set of indicator variables for incremental distance categories (ie, 0–2, 2–4, 4–6, 6–8, 8–10, 10–15, 15–30, 30–60, and >60 miles from the closest hospital). By interacting race with hospital attributes, we effectively estimated distinct coefficients for blacks and whites.
Decompositions
We used the regression estimates to decompose the overall gap between blacks and whites in high‐ and low‐quality hospital use into 2 components: (1) racial differences in the geographic availability of high‐ and low‐quality hospitals and (2) racial differences in nongeographic factors influencing which hospitals patients use. We conceptualized the first component as the difference in the probabilities that black patients would use a high‐quality (or a low‐quality) hospital in the hypothetical scenario where they face the sets of hospitals available to white patients versus when they face their own choice sets. We conceptualized the second component as the difference in the probabilities that white patients and black patients would use a high‐quality (or a low‐quality) hospital if they both faced white patients’ choice sets.
To operationalize these concepts, we employed the estimated model coefficients and the hospital choice sets for black and white patients to calculate the predicted probabilities that a black patient would use each hospital in each black patient's choice set, that a black patient would use each hospital in each white patient's choice set, and that a white patient would use each hospital in each white patient's choice set. Predicted probabilities were then summed across hospitals within the same quality level and averaged by race. (Decomposition details are provided in Data S1.)
We developed standard errors for the predicted probabilities and for differences between predicted probabilities using the clustered bootstrap,19 where the clusters were the CBSAs. To test the statistical significance of racial differences in probabilities of hospital use, we employed t‐statistics constructed using the bootstrap standard errors.
We conducted analyses for the United States as a whole and for each Census region separately.
The study was approved by the UCLA institutional review board, and a waiver of consent was obtained.
Results
Descriptive Data
For both conditions, blacks were younger, more often female, and had higher comorbidity compared with whites (Table). For AMI, similar proportions of white and black beneficiaries had a high‐quality hospital as the closest or second‐closest hospital. However, a slightly higher proportion of white beneficiaries was admitted to a high‐quality hospital (Table). On the other hand, a higher proportion of white beneficiaries had a low‐quality hospital as the closest or second‐closest hospital, but similar proportions of white and black beneficiaries were admitted to these hospitals (Table). For CABG, higher proportions of white beneficiaries had a high‐quality hospital as the closest or second‐closest hospital and were admitted to high‐quality hospitals (Table). Proportions of white and black beneficiaries having a low‐quality hospital as the closest or second‐closest hospital, and admitted to a low‐quality hospital, were similar (Table). Study hospital characteristics are summarized in the online supplement (Table S1). Of note, hospitals in the CABG cohort represent a subset of the hospitals in the AMI cohort, which are larger, more likely teaching tertiary care centers with cardiac surgery capabilities.
Table 1.
Characteristics of Black and White Medicare Beneficiaries Admitted for AMI or CABG at US Hospitals During 2009–2011
| Patient Characteristic | AMI | CABG | ||
|---|---|---|---|---|
| White (N=307 813) | Black (N=35 561) | White (N=40 933) | Black (N=3055) | |
| Regiona | ||||
| Northeast | 25.6% | 17.8% | 20.2% | 14.2% |
| South | 39.7% | 48.5% | 46.5% | 56.5% |
| Midwest | 22.7% | 26.2% | 24.6% | 24.0% |
| West | 12.0% | 7.4% | 38.7% | 5.3% |
| Age group (y), %a | ||||
| 65–69 | 11.9 | 23.0 | 19.4 | 32.6 |
| 70–74 | 14.8 | 17.7 | 27.9 | 29.5 |
| 75–79 | 17.1 | 17.7 | 26.3 | 22.3 |
| 80–84 | 20.3 | 16.5 | 18.9 | 12.1 |
| 85+ | 35.9 | 25.2 | 6.7 | 3.2 |
| Female, %a | 52.0 | 60.3 | 30.7 | 46.8 |
| Comorbidity index,b mean (SD)a | 6.9 (8.1) | 8.3 (8.4) | 5.1 (7.1) | 5.9 (7.6) |
| High‐quality hospital is closest or second‐closest hospital, %c | 48.2 | 48.3 | 49.9 | 45.0 |
| Admitted to a high‐quality hospital, %a | 34.8 | 32.4 | 39.0 | 29.9 |
| Low‐quality hospital is closest or second‐closest hospital, %d | 27.0 | 24.1 | 23.1 | 24.6 |
| Admitted to a low‐quality hospital, % | 11.2 | 11.0 | 9.7 | 10.2 |
AMI indicates acute myocardial infarction; CABG indicates coronary artery bypass grafting surgery.
P<0.001 for all white‐black comparisons.
The comorbidity index represents a weighted summary of 30 prevalent comorbidities identified from secondary diagnoses present at hospital discharge. The index is calculated using a previously published methodology.17
P<0.001 for the CABG cohort only.
P<0.001 for the AMI cohort only.
Regression Results
The conditional logit model estimates revealed that, as expected, both blacks and whites were more likely to use the closest hospital and that increasing distance was negatively associated with using a hospital. The effects of distance on the probability of hospital use were similar across races and study conditions (Table S2). With respect to quality, both white and black beneficiaries were more likely to use high‐ and medium‐quality hospitals compared with low‐quality hospitals (Table S2).
Decompositions
Figures 1 and 2 present results from the decompositions of the national white‐black gap in the use of high‐ and low‐quality hospitals for AMI and CABG, where the gap is the difference between the white and black probabilities of use.
Figure 1.
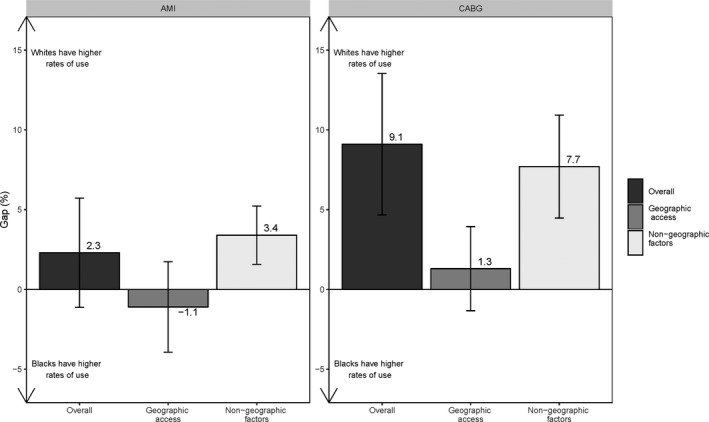
The national white‐black gap in high‐quality hospital use for acute myocardial infarction (AMI) and coronary artery bypass grafting (CABG) surgery.
Figure 2.
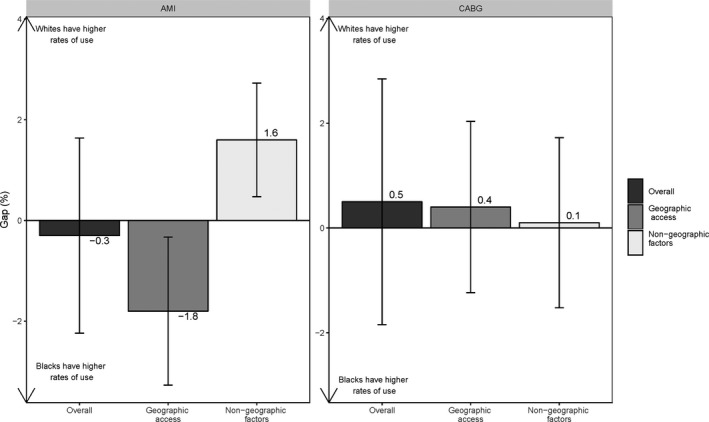
The national white‐black gap in low‐quality hospital use for acute myocardial infarction (AMI) and coronary artery bypass grafting (CABG) surgery.
Nationally, the white‐black gap in high‐quality hospital use was positive for both study conditions (ie, white beneficiaries had higher use), but it was statistically significant only for CABG (2.3%, P=0.18 for AMI; 9.1%, P<0.001 for CABG). However, the component attributable to racial differences in nongeographic factors was positive and significant for both conditions (3.4%, P<0.001 for AMI; 7.7%, P<0.001 for CABG), meaning that, given similar geographic proximity of high‐quality hospitals, white beneficiaries would be, on average, more likely than black beneficiaries to use those hospitals. Further, nearly all of the CABG gap was due to the nongeographic component. On the other hand, the component attributable to racial differences in the geographic availability of high‐quality hospitals was small and not significant for either condition (−1.1%, P=0.44 for AMI; and 1.3%, P=0.31 for CABG).
The overall gap in low‐quality hospital use was small and not statistically significant for either condition (0.3%, P=0.79 for AMI; −0.5%, P=0.40 for CABG) (Figure 2). In the case of AMI, both gap components were significant, although they worked in opposite directions, showing that, although white beneficiaries had more low‐quality hospitals closer to their homes, they were no more likely to use low‐quality hospitals than black beneficiaries were, because of nongeographic factors (eg, better referrals).
While not shown, given the lower probability of high‐quality hospital use and similar probability of low‐quality hospital use, black beneficiaries had a higher probability of medium‐quality hospital use than did white beneficiaries (Table S2).
Additional decompositions performed by US Census region are presented in Figures 3 and 4 and Figures S1 and S2. For AMI, white beneficiaries had a significantly higher probability of high‐quality hospital use in the South primarily because of differences in nongeographic factors (Figure 3). In the Midwest, however, black beneficiaries had a significantly higher probability of high‐quality hospital use for AMI because of better geographic proximity (Figure 3). For CABG, white beneficiaries had a significantly higher probability of high‐quality hospital use in the Northeast and South regions (Figure 4). The component attributable to geographic proximity was uniformly not significant, while the component attributable to differences in nongeographic factors was significant in 3 of 4 regions, favoring whites (Figure 4).
Figure 3.
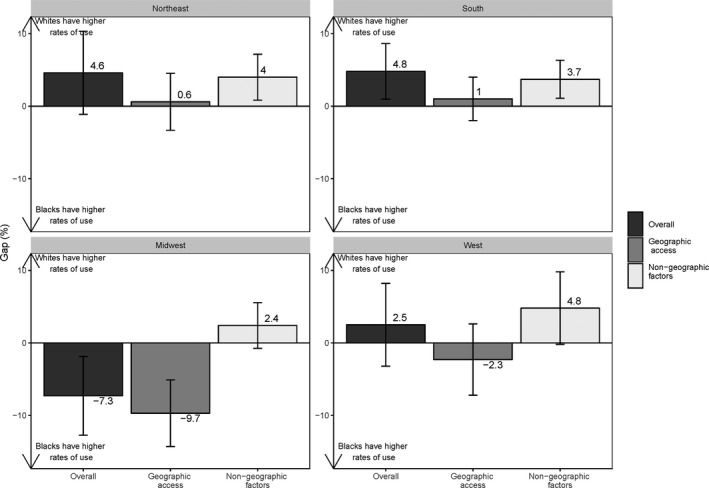
Regional white‐black gaps in high‐quality hospital use for acute myocardial infarction (AMI).
Figure 4.
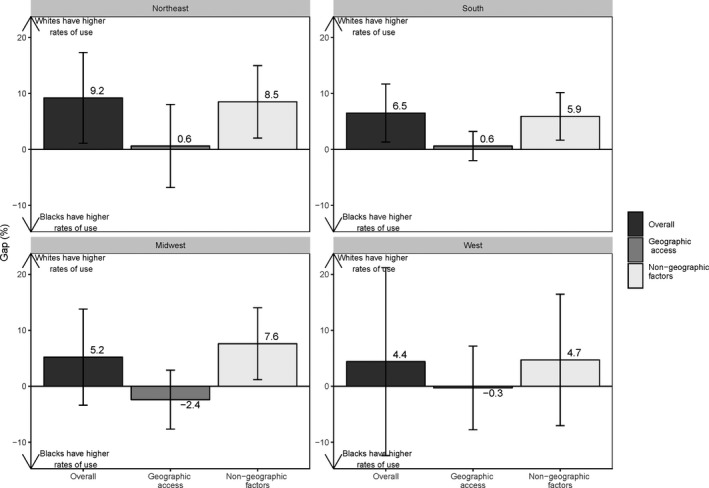
Regional white‐black gaps in high‐quality hospital use for coronary artery bypass grafting (CABG) surgery.
For low‐quality hospitals (Figures S1 and S2), the overall gap and the components attributable to differences in geographic and nongeographic factors were largely not significant, except for the Midwest, where black beneficiaries had a lower probability of low‐quality hospital use because of lower geographic proximity of low‐quality hospitals.
Sensitivity analyses for the high‐ and low‐quality hospital definitions yielded gaps and components of similar magnitude and direction.
Discussion
Among fee‐for‐service Medicare beneficiaries with CHD, we found that whites lived overall closer to high‐quality hospitals and were more likely to be admitted to these hospitals than were blacks, although differences were significant only for CABG.
Moreover, we found that white‐black differences in high‐quality hospital use for CABG were primarily related to differences in nongeographic factors, meaning that given similar geographic proximity, white beneficiaries were more likely to use high‐quality hospitals. On the other hand, black beneficiaries had higher probabilities of medium‐quality hospital use, and white‐black differences in low‐quality hospital use were not significant for both conditions. Distinct patterns emerged in regional analyses. White beneficiaries had a higher probability of high‐quality hospital use in the Northeast and South (although the results did not reach statistical significance for the overall AMI gap in the Northeast), mainly associated with differences in nongeographic factors, while black beneficiaries with AMI had a higher probability of high‐quality hospital use in the Midwest, mainly related to better geographic availability of high‐quality hospitals. Regional gaps in low‐quality hospital use were not significant except, again, in the Midwest, where black beneficiaries had a lower probability of low‐quality hospital use.
The current findings build on earlier work describing white‐black disparities in hospital quality for CHD treatment, yet differ from prior work in important ways. First, in contrast to prior analyses,6, 20, 21 this study found no significant racial gaps in high‐quality hospital use for AMI. Second, we found no significant disparities in low‐quality hospital use for either condition. The divergent results may have several explanations, including differences in sample size (prior studies used fewer metro areas and patients or incorporated nonmetro areas) or differences in hospital quality definitions (eg, mortality, process of care, or reputation‐based measures).
Our results also suggest that national‐level analyses of disparities in high‐ and low‐quality hospital use may mask important geographic variations. For example, the finding that the overall gap in high‐quality use for AMI is not significant misses the fact that patterns of use differ strikingly in the Midwest, where blacks have higher rates of use than whites related to better geographic access, and the South, where whites have higher rates of use in association with differences in nongeographic factors influencing hospital use. However, in the case of CABG, whites had higher rates of high‐quality hospital use across all regions (albeit significant only in the Northeast and South), mainly related to nongeographic factors. Taken together, the findings from regional analyses seem to suggest that a more geographically targeted, condition‐specific approach is needed for interventions to reduce CHD disparities.
The most striking finding of this analysis is the relatively large and significant white‐black gap in high‐quality hospital use for CABG, which stands in sharp contrast to the nonsignificant gap for AMI. Moreover, decomposition results show that the gap is almost entirely attributable to white‐black differences in nongeographic factors. Unlike AMI, which requires urgent treatment at the closest hospital, elective CABG surgery is a planned procedure. Thus, healthcare referral systems, social contextual factors, and patient characteristics potentially influencing hospital choice are likely to play a larger role in what types of hospitals patients use. These factors may influence high‐quality hospital use for blacks and whites differently.
To begin with, blacks and whites may have access to physicians practicing in distinct referral networks. This explanation is supported by prior research showing that the care of black patients is concentrated among a small number of physicians who report lower access to hospitals and advanced technologies.22, 23 Further, a recent analysis of physician networks for cardiac surgery showed that networks serving communities with high and low black populations have different characteristics.24 In particular, physicians in networks serving predominantly blacks are more isolated, with potentially negative consequences for referral access. Altogether, this body of evidence suggests that physician networks may be a driver of the nongeographic white‐black gap component and ultimately lead to disparities in high‐quality hospital use.
In addition, black and white patients may prefer to use hospitals with different attributes. For example, studies have shown that black beneficiaries are more likely to use teaching hospitals compared with their white peers.25, 26 Furthermore, blacks may have higher levels of distrust in the healthcare system because of experience with prior discrimination.27 As a result, blacks may be less willing to seek care at particular institutions that are perceived as practicing unjust care toward people of color, and may prefer to use hospitals they assume will be more sensitive to their needs and more devoted to their welfare.28, 29 Therefore, unobserved racial differences in hospital preferences might contribute to the nongeographic component of the gap.
Finally, differences in medical decision making may also contribute to the observed gap. Provider bias and stereotyping30, 31 and differences in patient‐provider communication32 could lead to treatment misconceptions and reinforce distrust in healthcare institutions, ultimately contributing to differences in the referral process. However, whether these factors influence the racial gap in high‐quality hospital use remains unclear. While prior analyses suggest that black patients are more often treated by lower‐quality cardiac surgeons,33, 34, 35 the underlying reasons for these differences (eg, differences in admitting privileges, physician assessments of hospital quality, bias, or perceptions of patient preferences) are not known.
Limitations
Several study limitations merit discussion. First, we have not directly examined black and white patients’ choices for hospital care. Rather, we are describing patterns of high‐ and low‐quality hospital use.
In addition, while this type of decomposition is commonly used in econometrics and has been previously employed to explain health disparities,36, 37 it does rely on assumptions. Specifically, the method assumes that, under the hypothetical scenario where black patients are faced with white patients’ hospital choices, the contribution of unexplained (nongeographic) factors influencing the gap (eg, black patient preferences and access to referral systems) remains constant (ie, as observed when patients face their actual choices).
Second, analyses were based on hospital claims data, which lack information on patient preferences and provider referrals. Although we could not explore the specific provider and patient factors potentially contributing to the nongeographic component of the gap, our findings strongly suggest that nongeographic factors are primarily responsible for the white‐black gaps in high‐quality hospital use and can help focus future interventions on these types of factors.
Third, data for this study (2009–2011) may not reflect the current state of disparities in CHD care. However, several other studies published as recently as 2016 continue to show black‐white disparities in coronary care.38 Further, CMS did not report CABG‐specific hospital‐level outcome measures at the time of this study, leading us to use the AMI hospital‐level mortality measure to rank hospital quality for both cohorts. It is possible that using the AMI measure for both conditions led to potential misclassification in the setting of discordant performance for AMI and CABG. The assumption that the quality of care for different CHD conditions is correlated at the hospital level should be further tested.
Fourth, in regional analyses, some components did not reach statistical significance, suggesting that analyses may have been underpowered because of the smaller samples, particularly in the West. Fifth, the current analysis is limited to Medicare beneficiaries aged 65 and older. Since age and insurance are both known to influence hospital choice,39, 40 findings may not be generalizable to the younger populations or to other vulnerable categories (eg, the uninsured).
Finally, the use of straight‐line distances has its limitations, as travel conditions may be different for urban areas with varied population density and infrastructure. To lessen this issue, our study included only urban (metropolitan) areas. Further, other studies have used straight‐line distance to approximate travel distance to hospitals. More granular analyses may be needed to capture local variations in travel conditions.
Conclusions
Overall, we found racial gaps in the use of high‐quality hospitals for CHD, in particular for elective CABG. With few exceptions (ie, the Midwest), these gaps were primarily related to factors above and beyond geographic access. To address disparities, a better understanding of what underlies racial differences in nongeographic factors influencing hospital use is needed. Furthermore, given the regional variations shown by our analyses, more granular market‐ or metro area–level analyses may be better suited to identify the contributions of geographic and nongeographic (eg, system or patient) factors to the racial gap in high‐quality hospital use for CHD.
Sources of Funding
This research was supported by R01HL118410 from the National Heart, Lung, and Blood Institute.
Disclosures
Drs Popescu, Huckfeldt, and Escarce receive funding from the AHRQ. Dr Pane has no disclosures to report.
Supporting information
Data S1. Supplemental methods.
Table S1. Characteristics of Hospitals Treating Black and White Medicare Beneficiaries Admitted With AMI or Undergoing CABG During 2009–2011
Table S2. Model Coefficients, Standard Errors, and Statistical Significance
Figure S1. Regional white‐black gaps in low‐quality hospital use for AMI.
Figure S2. Regional white‐black gaps in low‐quality hospital use for CABG.
(J Am Heart Assoc. 2019;8:e011964 DOI: 10.1161/JAHA.119.011964.)
References
- 1. Anderson JL, Morrow DA. Acute myocardial infarction. N Engl J Med. 2017;376:2053–2064. [DOI] [PubMed] [Google Scholar]
- 2. Brown TM, Deng LQ, Becker DJ, Bittner V, Levitan EB, Rosenson RS, Safford MM, Muntner P. Trends in mortality and recurrent coronary heart disease events after an acute myocardial infarction among Medicare beneficiaries, 2001–2009. Am Heart J. 2015;170:249–255. [DOI] [PubMed] [Google Scholar]
- 3. Bolorunduro OB, Kiladejo AV, Animashaun IB, Akinboboye OO. Disparities in revascularization after ST elevation myocardial infarction (STEMI) before and after the 2002 IOM report. J Natl Med Assoc. 2016;108:119–123. [DOI] [PubMed] [Google Scholar]
- 4. Chaudhry SI, Khan RF, Chen J, Dharmarajan K, Dodson JA, Masoudi FA, Wang Y, Krumholz HM. National trends in recurrent AMI hospitalizations 1 year after acute myocardial infarction in Medicare beneficiaries: 1999–2010. J Am Heart Assoc. 2014;3:e001197 DOI: 10.1161/JAHA.114.001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Skinner J, Chandra A, Staiger D, Lee J, McClellan M. Mortality after acute myocardial infarction in hospitals that disproportionately treat black patients. Circulation. 2005;112:2634–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Popescu I, Cram P, Vaughan‐Sarrazin MS. Differences in admitting hospital characteristics for black and white Medicare beneficiaries with acute myocardial infarction. Circulation. 2011;123:2710–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Popescu I, Nallamothu BK, Vaughan‐Sarrazin MS, Cram P. Racial differences in admissions to high‐quality hospitals for coronary heart disease. Arch Intern Med. 2010;170:1209–1215. [DOI] [PubMed] [Google Scholar]
- 8. Rangrass G, Ghaferi AA, Dimick JB. Explaining racial disparities in outcomes after cardiac surgery the role of hospital quality. JAMA Surg. 2014;149:223–227. [DOI] [PubMed] [Google Scholar]
- 9. Jha AK, Orav EJ, Li Z, Epstein AM. Concentration and quality of hospitals that care for elderly black patients. Arch Intern Med. 2007;167:1177–1182. [DOI] [PubMed] [Google Scholar]
- 10. Khera R, Vaughan‐Sarrazin M, Rosenthal GE, Girotra S. Racial disparities in outcomes after cardiac surgery: the role of hospital quality. Curr Cardiol Rep. 2015;17:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dimick J, Ruhter J, Sarrazin MV, Birkmeyer JD. Black patients more likely than whites to undergo surgery at low‐quality hospitals in segregated regions. Health Aff (Millwood). 2013;32:1046–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suter LG, Li SX, Grady JN, Lin ZQ, Wang YF, Bhat KR, Turkmani D, Spivack SB, Lindenauer PK, Merrill AR, Drye EE, Krumholz HM, Bernheim SM. National patterns of risk‐standardized mortality and readmission after hospitalization for acute myocardial infarction, heart failure, and pneumonia: update on publicly reported outcomes measures based on the 2013 release. J Gen Intern Med. 2014;29:1333–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jha AK, Orav EJ, Epstein AM. Low‐quality, high‐cost hospitals, mainly in south, care for sharply higher shares of elderly black, Hispanic, and Medicaid patients. Health Aff (Millwood). 2011;30:1904–1911. [DOI] [PubMed] [Google Scholar]
- 14. Porell FW, Adams EK. Hospital choice models: a review and assessment of their utility for policy impact analysis. Med Care Res Rev. 1995;52:158–195. [DOI] [PubMed] [Google Scholar]
- 15. Luft HS, Garnick DW, Mark DH, Peltzman DJ, Phibbs CS, Lichtenberg E, McPhee SJ. Does quality influence choice of hospital? JAMA. 1990;263:2899–2906. [PubMed] [Google Scholar]
- 16. Cohen MA, Lee HL. The determinants of spatial distribution of hospital utilization in a region. Med Care. 1985;23:27–38. [DOI] [PubMed] [Google Scholar]
- 17. Thompson NR, Fan YR, Dalton JE, Jehi L, Rosenbaum BP, Vadera S, Griffith SD. A new Elixhauser‐based comorbidity summary measure to predict in‐hospital mortality. Med Care. 2015;53:374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McFadden D. Conditional logit analysis of qualitative choice behavior In: Zaremba P, ed. Frontiers in Econometrics. New York: Academic Press; 1973:105–142. [Google Scholar]
- 19. Cameron AC, Miller DL. A practitioner's guide to cluster‐robust inference. J Hum Resour. 2015;50:317–372. [Google Scholar]
- 20. Hasnain‐Wynia R, Kang R, Landrum MB, Vogeli C, Baker DW, Weissman JS. Racial and ethnic disparities within and between hospitals for inpatient quality of care: an examination of patient‐level Hospital Quality Alliance measures. J Health Care Poor Underserved. 2010;21:629–648. [DOI] [PubMed] [Google Scholar]
- 21. Barnato AE, Lucas FL, Staiger D, Wennberg DE, Chandra A. Hospital‐level racial disparities in acute myocardial infarction treatment and outcomes. Med Care. 2005;43:308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bach PB, Pham HH, Schrag D, Tate RC, Hargraves JL. Primary care physicians who treat blacks and whites. N Engl J Med. 2004;351:575–584. [DOI] [PubMed] [Google Scholar]
- 23. Komaromy M, Grumbach K, Drake M, Vranizan K, Lurie N, Keane D, Bindman AB. The role of black and Hispanic physicians in providing health care for underserved populations. N Engl J Med. 1996;334:1305–1310. [DOI] [PubMed] [Google Scholar]
- 24. Hollingsworth JM, Funk RJ, Garrison SA, Owen‐Smith J, Kaufman SR, Landon BE, Birkmeyer JD. Differences between physician social networks for cardiac surgery serving communities with high versus low proportions of black residents. Med Care. 2015;53:160–167. [DOI] [PubMed] [Google Scholar]
- 25. Kahn KL, Pearson ML, Harrison ER, Desmond KA, Rogers WH, Rubenstein LV, Brook RH, Keeler EB. Health care for black and poor hospitalized Medicare patients. JAMA. 1994;271:1169–1174. [PubMed] [Google Scholar]
- 26. Iwashyna TJ, Curlin FA, Christakis NA. Racial, ethnic, and affluence differences in elderly patients’ use of teaching hospitals. J Gen Intern Med. 2002;17:696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Armstrong K, Putt M, Halbert CH, Grande D, Schwartz JS, Liao KJ, Marcus N, Demeter MB, Shea JA. Prior experiences of racial discrimination and racial differences in health care system distrust. Med Care. 2013;51:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Armstrong K, McMurphy S, Dean LT, Micco E, Putt M, Halbert CH, Schwartz JS, Sankar P, Pyeritz RE, Bernhardt B, Shea JA. Differences in the patterns of health care system distrust between blacks and whites. J Gen Intern Med. 2008;23:827–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grande D, Shea JA, Armstrong K. Perceived community commitment of hospitals: an exploratory analysis of its potential influence on hospital choice and health care system distrust. Inquiry. 2013;50:312–321. [DOI] [PubMed] [Google Scholar]
- 30. Sabin JA, Rivara FP, Greenwald AG. Physician implicit attitudes and stereotypes about race and quality of medical care. Med Care. 2008;46:678–685. [DOI] [PubMed] [Google Scholar]
- 31. Fincher C, Williams JE, MacLean V, Allison JJ, Kiefe CI, Canto J. Racial disparities in coronary heart disease: a sociological view of the medical literature on physician bias. Ethn Dis. 2004;14:360–371. [PubMed] [Google Scholar]
- 32. White‐Means SI, Osmani AR. Racial and ethnic disparities in patient‐provider communication with breast cancer patients: evidence from 2011 MEPS and experiences with cancer supplement. Inquiry. 2017;54:46958017727104 DOI: 10.1177/0046958017727104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Epstein AJ, Gray BH, Schlesinger M. Racial and ethnic differences in the use of high‐volume hospitals and surgeons. Arch Surg. 2010;145:179–186. [DOI] [PubMed] [Google Scholar]
- 34. Mukamel DB, Murthy AS, Weimer DL. Racial differences in access to high‐quality cardiac surgeons. Am J Public Health. 2000;90:1774–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mukamel DB, Weimer DL, Mushlin AI. Referrals to high‐quality cardiac surgeons: patients’ race and characteristics of their physicians. Health Serv Res. 2006;41:1276–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sen B. Using the Oaxaca‐Blinder decomposition as an empirical tool to analyze racial disparities in obesity. Obesity (Silver Spring). 2014;22:1750–1755. [DOI] [PubMed] [Google Scholar]
- 37. Holmes GM, Freburger JK, Ku LJE. Decomposing racial and ethnic disparities in the use of postacute rehabilitation care. Health Serv Res. 2012;47:1158–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Burton BN, Munir NA, Labastide AS, Sanchez RA, Gabriel RA. An update on racial disparities with 30‐day outcomes after coronary artery bypass graft under the Affordable Care Act. J Cardiothorac Vasc Anesth. 2019;33:1890–1898. [DOI] [PubMed] [Google Scholar]
- 39. Tai WT, Porell FW, Adams EK. Hospital choice of rural Medicare beneficiaries: patient, hospital attributes, and the patient‐physician relationship. Health Serv Res. 2004;39:1903–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Popescu I, Heslin KC, Coffey RM, Washington RE, Barrett ML, Karnell LH, Escarce JJ. Differences in use of high‐quality and low‐quality hospitals among working‐age individuals by insurance type. Med Care. 2017;55:148–154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental methods.
Table S1. Characteristics of Hospitals Treating Black and White Medicare Beneficiaries Admitted With AMI or Undergoing CABG During 2009–2011
Table S2. Model Coefficients, Standard Errors, and Statistical Significance
Figure S1. Regional white‐black gaps in low‐quality hospital use for AMI.
Figure S2. Regional white‐black gaps in low‐quality hospital use for CABG.


