Abstract
Background:
Several studies have examined the association between IPI and birth outcomes, but few have explored the association between interpregnancy interval (IPI) and postnatal outcomes.
Objective:
We examined the association between IPI and injury-related infant mortality, a leading cause of postneonatal mortality.
Methods:
We used 2011-2015 US period-linked birth-infant death vital statistics data to generate a multiyear birth cohort of non-first-born singleton births (N = 9 782 029). IPI was defined as the number of months between a live birth and the start of the pregnancy leading to the next live birth. Causes of death in the first year of life were identified using ICD-10 codes. Hazard ratios (HR) for IPI categories were estimated using Cox proportional hazards models adjusted for birth order, county poverty level, and maternal characteristics (marital status, race/ethnicity, education, age at previous birth).
Results:
After adjustment, overall infant mortality (48.1 per 10 000 births) was higher for short and long IPIs compared with IPI 18-23 months (reference): <6, aHR 1.61, 95% CI 1.54, 1.68; 6-11, aHR 1.22, 95% CI 1.17, 1.26; and 60+ months, aHR 1.12, 95% CI 1.08, 1.16. In comparison, the risk of injury-related infant mortality (4.4 per 10 000 births) decreased with longer IPIs: <6, aHR 1.77, 95% CI 1.55, 2.01; 6-11, aHR 1.41, 95% CI 1.25, 1.59; 12-17, aHR 1.25, 95% CI 1.10, 1.41; 24-59, aHR 0.78, 95% CI 0.69, 0.87; and 60+ months, aHR 0.55, 95% CI 0.48, 0.62.
Conclusion:
Unlike overall infant mortality, injury-related infant mortality decreased with IPI length. While injury-related deaths are rare, these patterns suggest that the timing between births may be a marker of risk for fatal infant injuries. The first year postpartum may be an ideal time for the delivery of evidence-based injury prevention programmes as well as family planning services.
Keywords: birth spacing, infant mortality, injury, interpregnancy interval, parity
1 |. BACKGROUND
Considerable progress has been made to reduce infant mortality in the United States over the past few decades.1 In addition to increased survival of preterm births, this decline has been partly attributed to successful public health programmes aimed at reducing infant mortality caused by unsafe sleep environments.2 At the same time, infant mortality due to sudden infant death syndrome (SIDS) and unintentional injury persist as two of the ten leading causes of infant mortality in the United States,3–5 highlighting the need for additional intervention strategies.
Examining cause-specific mortality can provide greater insight into factors that may be amenable to public health interventions. In particular, external (ie non-pathological) causes of death represent a heterogeneous grouping of potentially preventable injury-related deaths. Higher birth order and plurality have been associated with an increased risk of external causes of infant mortality, consistent with the hypothesis that divided parental time and resources combined with increased childcare responsibilities may play a role in infant injury risk.6,7 A related factor, closely spaced births, may also be associated with external causes of infant death, but has not been examined for the United States, in part because national data have not been available since the early 1990s.
Closely spaced births, often operationalised using interpregnancy interval (IPI; the time between a live birth and start of a subsequent pregnancy), have been associated with increased risks of adverse birth outcomes and infant mortality.8–10 The most common explanation for these associations is the ‘maternal depletion hypothesis’, which posits that adverse birth outcomes associated with short IPI are the result of depleted maternal nutritional stores due to insufficient recuperation time between pregnancies.11 However, recent analysis using matched designs has cast doubt on the causal effect of short IPI on adverse outcomes, arguing that previous associations have been confounded by factors such as socio-economic status (SES).12–14 A recent series of articles on birth spacing published in this journal discussed the strengths and limitations of these approaches and called for an examination of additional outcomes, beyond the perinatal period, to guide evidence-based recommendations.15,16 Limited data exist on the association between short IPI and external causes of infant death, which may operate through causal mechanisms outside of maternal nutritional depletion, such as divided parental time and resources described above. Investigating this association could further inform public health guidance on birth spacing.17
Therefore, our objective was to examine the relationship between IPI and injury-related infant mortality using recently available birth-infant death data from the United States. We use enhanced information on SES to provide better control for this potential confounding factor. In addition, we contrast our findings with those for the association between IPI and other measures of infant mortality to expand upon existing research.
2 |. METHODS
2.1 |. Data source
We used the 2011-2015 period-linked birth-infant death vital statistics files.18 The period-linked numerator files consist of all deaths within 1 year of birth occurring in a given calendar year, retrospectively linked to their corresponding birth certificate data from the same or prior year. The period-linked denominator files consist of all births occurring in a given calendar year. These files are used by the National Center for Health Statistics to tabulate infant mortality rates by various maternal and infant characteristics on the birth certificate. We linked deaths in the numerator files to their corresponding birth records in the denominator files using unique identifiers to create a single multiyear birth cohort file, which we used for multiple regression analyses. Maternal identifiers were not available to link multiple or repeat births to the same woman. Ethical approval was not required as the deidentified data are publically available through a data use agreement with the National Center for Health Statistics.
The analytic sample included non-first-born singleton births to residents in states using the 2003 revised US birth certificate during 2011-2015. Given the small number of infant deaths due to external causes per year (~1400),4 we pooled across years 2011-2015 to ensure stable estimates. We restricted analyses to resident births among states using the 2003 revised US birth certificate because information to determine IPI had not been available since 1993.19 This information was reinstated with the 2003 revision; however, states adopted the 2003 revised US birth certificate at different times. Newly revised items on the birth certificate were first released in the 2011 period-linked national data files.20 By 2011, 36 states had adopted the revision and 48 states had adopted by 2015 (representing 88% of all US non-first-born, singleton resident births during 2011-2015 period). Detailed information on birth certificate revisions and its implications for IPI analyses are described elsewhere.19,21
2.2 |. Measures
The linked birth-infant death data provide information on maternal and infant characteristics at birth from birth certificates and information on age and cause of death from death certificates. We also merged additional data from the 2011-2015 American Community Survey on the percentage of families living below the federal poverty threshold in the mother’s county of residence.22
2.2.1 |. Birth certificate measures
The exposure variable was IPI, defined as the number of months between a live birth and the start of the pregnancy leading to the next live birth. This information is obtained from three data items collected on the birth certificate—’Date of current birth’, ‘Date of last live birth’, and ‘obstetric estimate of gestational age’—which have high agreement with medical records and other nationally representative estimates of IPI.21,23,24 The data file includes a recoded variable, live birth interval (in months), which is calculated by subtracting the date of last live birth from the date of current birth. We calculated IPI by subtracting the obstetric estimate of gestational age (in weeks converted to months) from the recoded live birth interval. We categorised IPI as <6, 6-11, 12-17, 18-23, 24-59, and 60+ months, consistent with other studies.9,11,25 Plural births could not be included in the calculation of IPI because information on ‘date of last live birth’ for multiples does not necessarily capture information on the preceding pregnancy. IPI was calculated using information on the birth certificate and not by linking births within mothers, which is currently not possible with national birth certificate data.
Descriptive information from the birth certificate on maternal sociodemographic and health characteristics included: maternal age at the current birth, race/ethnicity, level of education, marital status, principal source of payment at delivery, use of the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) during pregnancy, pre-pregnancy body mass index (BMI), and pre-pregnancy smoking. Maternal age at prior birth was derived by subtracting IPI from her current age and grouped into 5-year age categories. Infant characteristics included birth order, gestational age (using the obstetric estimate), birthweight, and sex. Maternal race/ ethnicity was classified consistent with 1997 Office of Management and Budget standards.26 Infant sex refers to the sex (male/female) assigned on the birth certificate at the time of the birth.
2.2.2 |. Death certificate measures
Information on the age at death (days) and cause of death was obtained from the death certificate. We categorised infant deaths by age into either neonatal (<28 days) or postneonatal (28-365 days) infant death and subcategories of early neonatal (<7), late neonatal (7-27), early postneonatal (28-90), early middle postneonatal (91-182), late middle postneonatal (183-273), and late postneonatal (274-365).
Underlying cause of death was coded using the International Classification of Disease, tenth revision (ICD-10), and used to determine external causes of infant deaths (ie injury-related) from birth to 365 days: accidents (unintentional injuries; V01-X59); assault (homicide; *U01, X85-Y09); complications of medical and surgical care (Y40-Y84); and other external causes (Y10-Y36). Accidental suffocation and strangulation in bed (W75) is the most common external cause of death code among infants6,7 and one of three codes used to classify sudden unexpected infant death (SUID) (W75, R95, and R99). Due to diagnostic shifts in classification of sleep-related deaths over time from R95 to W75,3,27,28 we examined the following alternative groupings of injury-related ICD-10 codes: external causes excluding W75, external causes plus two additional causes of death used to classify SUID (SIDS [R95] and other ill-defined and unspecified causes of mortality [R99]), and SUID alone (W75, R95, and R99). Unintentional injury and homicide were examined separately in sensitivity analyses.
2.3 |. Statistical analysis
Tabulations of births and deaths were based on period-linked data to be consistent with national reporting of infant mortality rates by maternal and infant characteristics, which rely on period-linked data. For deaths, we tabulated infant deaths overall, by age at the time of death, and cause of death categories across IPI categories; rates were calculated using births as the denominator. We used the statistical weights provided in the period-linked data20 to account for unlinked infant deaths (<1%), to ensure that counts of deaths were consistent with national reports.
Using the multiyear birth cohort file, we fit Cox proportional hazard models to estimate hazard rate ratios (HR) by age at time of death and cause of death categories. We selected a reference group of 18-23 months to be consistent with other studies showing this interval has the lowest risk of adverse birth outcomes.12,29,30 Time at risk was based on age in days. For surviving infants, births occurring during 2011-2014 were censored after 365 days of life. For births occurring in 2015, data on deaths occurring in 2016 were not available at the time of analysis; thus, time at risk for these infants was censored at the age of the infant (in days) as of 31 December 2015. Infants who died from causes that were not the outcome under evaluation were censored at their age of death. For neonatal deaths, infants who died after 27 days were censored at 28 days. Graphs of Schoenfeld residuals showed uniform distributions, indicating the Cox model proportionality assumption was not violated. Additionally, cumulative and instantaneous hazard of external cause of death curves by IPI categories were generated using the Kaplan-Meier survival function estimator. In situations such as ours, where the risk of outcome was rare, this estimator provides a reasonable approximation of risk even in the presence of competing risks.6,7,31
Confounders were selected based on the literature and whether the variable was associated (and preceded) the interpregnancy interval and outcome.6,7,16,25,32 Our final models were adjusted for: maternal age at prior birth, race/ethnicity, and birth order; and, at the time of the current birth, maternal marital status, education, and county poverty level. Pre-pregnancy BMI, pre-pregnancy smoking, source of payment at delivery, and WIC use during pregnancy were not included in final models, since they were measured at the time of the current birth and may have represented a factor along the causal pathway. However, we considered this latter group of variables as proxy indicators of socio-economic and health status and additionally adjusted for these characteristics in sensitivity analysis to assess consistency of findings. Consistent with previous studies,33 we further adjusted for preterm birth in sensitivity analyses to assess potential attenuation of associations due to mediation by gestational age. Additionally, we ran adjusted models with an interaction term between IPI categories and birth year (2011-2015) and IPI categories and indicators of SES (eg maternal education, marital status) to assess variation in risk of external cause of death over time and SES indicators, respectively. The significance of the interaction terms was assessed using a likelihood ratio test.
Analyses were performed in 2018-2019 using Stata/MP and Stata/SE, version 14.
2.4 |. Missing data
In our analytic sample, information on IPI was missing for 7.5% of births. All other covariates had missing values of less than 5%. We conducted sensitivity analyses using multiple imputation. Our imputation models used chained equations with predictive mean matching, generating 50 imputations, and included all covariates from the final models described above, plus the survival time and log of the survival. We collapsed continuous variables into categories (eg grouped age at death in days to weeks) and applied frequency weights for computational efficiency. We compared findings from the main complete case analysis (with continuous variables), frequency-weighted complete case analysis (with collapsed variables), and frequency-weighted multiple imputation analysis (with collapsed variables).
3 |. RESULTS
During 2011-2015, IPI information was available for 9 782 029 non-first-born singleton births to residents who gave birth in states using the 2003 revised birth certificate. Of these births, the percentage that followed an IPI < 6, 6-11, 12-17, 18-23, 24-59, and 60+ months were 4.8%, 10.8%, 13.5%, 11.7%, 37.5%, and 21.7%, respectively. In general, compared with the other IPI categories, women having the shortest (<12 months) and longest (60+ months) IPI had similar covariate profiles (Table 1), which included lower SES and higher pre-pregnancy BMI. Births among the shortest and longest IPI groups were also more likely to be preterm and low birthweight. Being a fourth-or-higher-born infant was more common among the shorter interval groups; no differences in infant sex were found.
TABLE 1.
Per cent distribution of selected maternal and infant characteristics by interpregnancy interval among singleton, second‐born, or higher births: 2003 revised US birth certificate data linked to infant death data, 2011‐2015
| Interpregnancy Interval (months) % of total |
|||||||
|---|---|---|---|---|---|---|---|
| Total | <6 (4.8%) | 6‐11 (10.8%) | 12‐17 (13.5%) | 18‐23 (11.7%) | 24‐59 (37.5%) | 60+ (21.7%) | |
| Maternal and infant characteristics | N = 9 782 029 | N = 471 165 | N = 1 056 915 | N = 1 319 210 | N = 1 148 398 | N = 3 667 239 | N = 2 119 102 |
| Maternal characteristics at birth | |||||||
| County of residence at birth (% below poverty threshold)a | |||||||
| Less than 10% | 34.9 | 28.0 | 34.0 | 37.7 | 38.3 | 35.7 | 31.9 |
| 10%‐19% | 59.7 | 64.8 | 60.3 | 57.6 | 57.1 | 59.0 | 62.1 |
| 20%‐29% | 5.2 | 6.8 | 5.4 | 4.5 | 4.4 | 5.1 | 5.8 |
| 30% or greater | 0.3 | 0.4 | 0.2 | 0.2 | 0.2 | 0.3 | 0.3 |
| County of residence at birth (% poverty level, mean) | 12.0 | 12.8 | 12.1 | 11.7 | 11.6 | 11.9 | 12.3 |
| Maternal age at prior birth (years) | |||||||
| Under 20 | 13.0 | 15.7 | 9.2 | 9.4 | 8.3 | 12.8 | 19.4 |
| 20‐24 | 31.9 | 37.0 | 29.9 | 26.0 | 25.6 | 30.7 | 40.8 |
| 25‐29 | 30.7 | 26.3 | 30.2 | 32.5 | 32.8 | 31.5 | 28.0 |
| 30‐34 | 18.9 | 15.2 | 21.8 | 24.1 | 25.2 | 19.8 | 10.3 |
| 35‐39 | 5.0 | 5.2 | 7.8 | 7.3 | 7.4 | 4.8 | 1.4 |
| 40‐54 | 0.5 | 0.6 | 1.0 | 0.8 | 0.8 | 0.4 | 0.1 |
| Maternal age at prior birth (years, mean) | 26.1 | 25.7 | 26.9 | 27.4 | 27.3 | 26.2 | 24.1 |
| Maternal race/ethnicity | |||||||
| Non‐Hispanic white | 52.2 | 45.2 | 54.8 | 60.8 | 61.0 | 52.9 | 41.2 |
| Non‐Hispanic black | 14.5 | 21.6 | 15.9 | 12.0 | 11.5 | 13.5 | 17.0 |
| Hispanic | 25.9 | 26.6 | 22.4 | 20.0 | 20.2 | 25.8 | 34.4 |
| Otherb | 7.4 | 6.6 | 7.0 | 7.2 | 7.4 | 7.7 | 7.5 |
| Marital status | |||||||
| Not married | 36.6 | 53.8 | 37.9 | 29.2 | 28.3 | 34.6 | 44.6 |
| Married | 63.5 | 46.2 | 62.1 | 70.8 | 71.8 | 65.5 | 55.4 |
| Maternal educational attainment | |||||||
| No high school diploma or GED | 18.2 | 27.0 | 19.7 | 15.8 | 14.8 | 17.0 | 20.8 |
| High school diploma or GED | 25.9 | 34.0 | 27.2 | 22.9 | 22.4 | 25.5 | 28.2 |
| Some college | 21.2 | 21.1 | 20.4 | 18.9 | 19.0 | 21.3 | 24.2 |
| Bachelor’s degree or higher | 34.7 | 17.9 | 32.7 | 42.5 | 43.8 | 36.2 | 26.8 |
| Source of payment for delivery | |||||||
| Private | 44.0 | 25.7 | 40.4 | 49.7 | 51.7 | 46.0 | 38.7 |
| Medicaid | 46.5 | 64.3 | 49.4 | 40.3 | 38.9 | 45.0 | 51.8 |
| Self‐pay | 4.7 | 4.8 | 5.2 | 5.2 | 4.7 | 4.4 | 4.8 |
| Other | 4.8 | 5.2 | 5.1 | 4.8 | 4.7 | 4.7 | 4.8 |
| WIC use during pregnancy | |||||||
| Yes | 46.2 | 62.8 | 48.1 | 39.3 | 38.0 | 44.4 | 53.5 |
| No | 53.8 | 37.2 | 51.9 | 60.7 | 62.0 | 55.7 | 46.5 |
| Pre‐pregnancy body mass index | |||||||
| Underweight | 3.2 | 3.1 | 3.6 | 3.6 | 3.8 | 3.7 | 2.3 |
| Normal | 43.1 | 37.6 | 43.8 | 43.8 | 48.2 | 48.1 | 37.0 |
| Overweight | 26.8 | 27.6 | 27.6 | 26.3 | 24.9 | 25.1 | 29.1 |
| Obese | 26.9 | 31.7 | 31.7 | 26.2 | 23.1 | 23.1 | 31.6 |
| Pre‐pregnancy smoking statusc | |||||||
| No | 88.7 | 84.3 | 88.6 | 91.1 | 91.1 | 89.0 | 86.3 |
| Yes | 11.3 | 15.7 | 11.4 | 8.9 | 8.9 | 11.0 | 13.7 |
| Infant characteristics at birth | |||||||
| Birth order | |||||||
| Second‐born | 52.9 | 44.4 | 50.2 | 54.5 | 55.9 | 54.6 | 50.4 |
| Third‐born | 27.6 | 27.7 | 25.9 | 24.6 | 25.0 | 27.6 | 31.5 |
| Fourth‐born or higher | 19.6 | 27.9 | 23.9 | 20.9 | 19.1 | 17.8 | 18.1 |
| Gestational week at birth | |||||||
| <37 (preterm birth) | 7.6 | 11.1 | 7.8 | 6.4 | 6.2 | 7.0 | 9.3 |
| 37‐38 (early term) | 26.3 | 31.9 | 26.9 | 24.9 | 24.6 | 25.8 | 27.4 |
| 39‐40 (term) | 61.2 | 53.4 | 60.3 | 63.0 | 63.8 | 62.5 | 58.8 |
| 41 (late term) | 4.6 | 3.2 | 4.6 | 5.2 | 5.1 | 4.5 | 4.3 |
| 42 or over (post‐term birth) | 0.3 | 0.4 | 0.5 | 0.4 | 0.4 | 0.3 | 0.3 |
| Birthweight (grams)d | |||||||
| <1500 | 0.9 | 1.3 | 0.8 | 0.6 | 0.6 | 0.8 | 1.3 |
| 1500–2499 | 4.6 | 6.9 | 4.6 | 3.7 | 3.6 | 4.2 | 5.9 |
| 2500 or over | 94.5 | 91.8 | 94.7 | 95.7 | 95.8 | 95.0 | 92.8 |
| Sex | |||||||
| Female | 48.9 | 48.9 | 48.9 | 48.9 | 48.8 | 48.8 | 48.9 |
| Male | 51.2 | 51.1 | 51.1 | 51.1 | 51.2 | 51.2 | 51.1 |
Note: Unweighted counts of average live births per year were used. GED stands for General Education Diploma. Missing values were <4% of the following characteristics: maternal educational attainment (1.1%), source of payment for delivery (1.0%), Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) use during pregnancy (2.0%), pre‐pregnancy body mass index (3.6%), pre-pregnancy smoking status (3.8%), and imputed birthweight (<0.1%).
Generated from restricted‐use data files merged by county Federal Information Processing Standard Publication 6‐4 (FIPS) county codes with families below the poverty threshold in the mother’s county of residence at the time of birth.
Includes Asian or Pacific Islander, American Indian or Alaska Native, Other, and Hispanic origin unknown or not stated.
In the three months prior to pregnancy.
Missing birthweight values are imputed from the previous record with the same period of gestation, race, sex, and plurality.
Among our analytic sample, there were 47 011 infant deaths, which corresponded to an infant mortality rate of 48.1 per 10 000 births (Table 2). Rates of neonatal and postneonatal deaths were 28.4 and 19.6 per 10 000 births, respectively. The infant death rate was highest for IPI < 6 months (87.0) and decreased to 56.9, 44.9, and 41.2 per 10 000 births for IPI of 6-11, 12-17, and 18-23 months, respectively, before increasing slightly to 41.7 for IPI of 24-29 months and more appreciably to 51.6 for IPI of 60+ months (Figure S1). Neonatal mortality rates, which accounted for approximately 60% of all infant deaths, demonstrated a similar J-shaped pattern with the highest rate per 10 000 births found among the shortest IPI (45.9) and longest IPI (35.3). Postneonatal mortality rates decreased with increasing IPI length—from 41.1 (IPI < 6 months) to 16.3 per 10 000 births (IPI of 60+ months).
TABLE 2.
Rate of infant death by all‐cause, neonatal, postneonatal, and external cause categories among singleton, second‐born, or higher births: 2003 revised US birth certificate data linked to infant death data, 2011‐2015
| Interpregnancy Interval (IPI) in months | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total deaths | <6 | 6–11 | 12–17 | 18–23 | 24–59 | 60+ | ||||||||
| Number of births (2011–2015) | 9 782 029 | 47 11 65 | 10 56 915 | 1 319 210 | 1 148 398 | 3 667 239 | 2 119 102 | |||||||
| Cause of death | n | Ratea | n | Ratea | n | Ratea | n | Ratea | n | Ratea | n | Ratea | n | Ratea |
| All causes | 47 011 | 48.1 | 4098 | 87.0 | 6012 | 56.9 | 5927 | 44.9 | 4737 | 41.2 | 15 299 | 41.7 | 10 938 | 51.6 |
| Age at death (days) | ||||||||||||||
| Total neonatal (0–27) | 27 802 | 28.4 | 2161 | 45.9 | 3087 | 29.2 | 3214 | 24.4 | 2667 | 23.2 | 9192 | 25.1 | 7482 | 35.3 |
| Early neonatal (under 7) | 21 643 | 22.1 | 1678 | 35.6 | 2393 | 22.6 | 2460 | 18.6 | 2082 | 18.1 | 7131 | 19.4 | 5900 | 27.8 |
| Late neonatal (7–27) | 6159 | 6.3 | 483 | 10.3 | 694 | 6.6 | 754 | 5.7 | 585 | 5.1 | 2061 | 5.6 | 1582 | 7.5 |
| Total postneonatal (28–365) | 19 210 | 19.6 | 1937 | 41.1 | 2925 | 27.7 | 2714 | 20.6 | 2071 | 18.0 | 6107 | 16.7 | 3456 | 16.3 |
| Early postneonatal (28–90) | 8775 | 9.0 | 840 | 17.8 | 1259 | 11.9 | 1205 | 9.1 | 965 | 8.4 | 2831 | 7.7 | 1674 | 7.9 |
| Early middle postneonatal (91–182) | 6422 | 6.6 | 694 | 14.7 | 1011 | 9.6 | 925 | 7.0 | 676 | 5.9 | 2028 | 5.5 | 1088 | 5.1 |
| Late middle postneonatal (183–273) | 2643 | 2.7 | 276 | 5.9 | 433 | 4.1 | 372 | 2.8 | 286 | 2.5 | 820 | 2.2 | 455 | 2.1 |
| Late postneonatal (274–365) | 1370 | 1.4 | 127 | 2.7 | 222 | 2.1 | 212 | 1.6 | 143 | 1.2 | 427 | 1.2 | 239 | 1.1 |
| External causes and SUID (ICD-10 codes) | ||||||||||||||
| All external causesb | 4344 | 4.4 | 531 | 11.3 | 752 | 7.1 | 697 | 5.3 | 477 | 4.2 | 1305 | 3.6 | 581 | 2.7 |
| Accidents/unintentional injuries | 3435 | 3.5 | 399 | 8.5 | 591 | 5.6 | 548 | 4.2 | 383 | 3.3 | 1049 | 2.9 | 466 | 2.2 |
| Assault/homicide | 634 | 0.6 | 96 | 2.0 | 113 | 1.1 | 100 | 0.8 | 69 | 0.6 | 172 | 0.5 | 83 | 0.4 |
| Complications of medical/ surgical carec | 29 | 0.0 | -- | -- | -- | -- | -- | -- | -- | -- | 10 | 0.0 | -- | -- |
| Other external causes (undetermined intent) | 246 | 0.3 | 35 | 0.7 | 43 | 0.4 | 45 | 0.3 | 23 | 0.2 | 74 | 0.2 | 25 | 0.1 |
| External causes excluding W75d | 1966 | 2.0 | 264 | 5.6 | 347 | 3.3 | 316 | 2.4 | 210 | 1.8 | 577 | 1.6 | 252 | 1.2 |
| External causes and SUIDe | 11 573 | 11.8 | 1399 | 29.7 | 1957 | 18.5 | 1744 | 13.2 | 1255 | 10.9 | 3616 | 9.9 | 1602 | 7.6 |
| SUID onlyf | 9607 | 9.8 | 1134 | 24.1 | 1610 | 15.2 | 1429 | 10.8 | 1045 | 9.1 | 3039 | 8.3 | 1350 | 6.4 |
Note: Weighted counts of infant deaths are presented. SUID, sudden unexpected infant death. ‘‐‐’ suppressed due to fewer than 10 infant deaths.
Per 10 000 live births.
ICD‐10 codes: U01, V01‐Y84. Accidents/unintentional injuries (V01‐X59); assault/homicide (U01, X85‐Y09); complications of medical/surgical care (Y40‐Y84); other external causes (Y10‐Y36) of undetermined intent.
The underlying cause of death will underestimate the number of deaths in which this complication is a factor, unless the cause of or reason for the medical/surgical care is not reported.
ICD‐10 codes: U01, V01‐W74, W76‐Y84.
ICD‐10 codes: U01, VO1‐Y84, R95, R99.
ICD‐10 codes: R95, R99, W75.
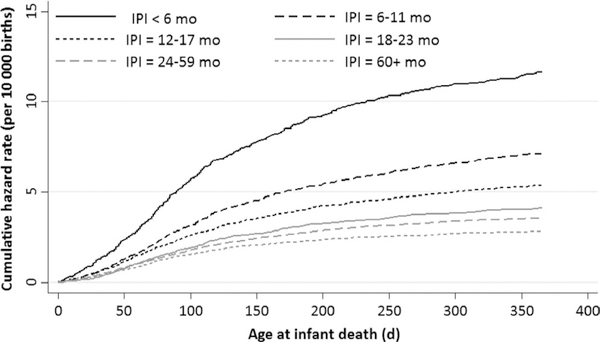
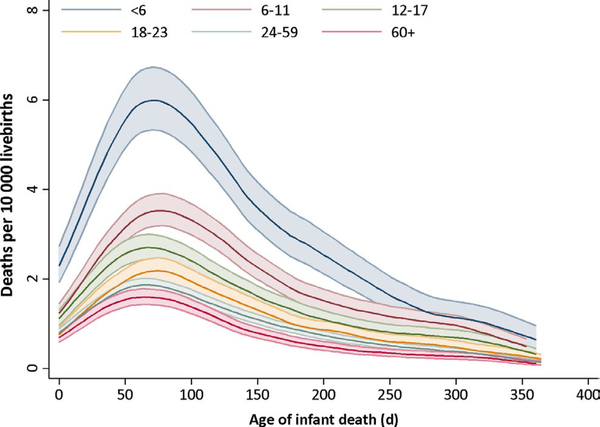
The rates of infant deaths due to external causes and SUID were 4.4 and 9.8 per 10 000 births, respectively (Table 2). In contrast to the overall and neonatal death rates, external cause and alternative groupings demonstrated declines with increasing IPI length, similar to postneonatal mortality (Figures S1 and S2). The cumulative and instantaneous hazard functions for infant mortality due to external causes by IPI category, respectively, show the steepest increases in risk were during the first 30-150 days of life (Figures 1 and 2).
FIGURE 1.
Cumulative hazard function of external cause of infant mortality by interpregnancy interval categories
FIGURE 2.
Instantaneous hazard function of external cause of infant mortality by interpregnancy interval categories. Footnote to Figure 2. The darker line indicates the point estimate for each IPI category. The shaded area in the same colour around each line corresponds to the 95% confidence interval estimates
After adjustment, the HRs for all-cause and neonatal infant mortality were highest for IPI < 6, 6-11, and 60+ months (all-cause aHR 1.61, 1.22, and 1.12; neonatal aHR 1.60, 1.13, and 1.40, respectively), whereas the aHRs for postneonatal mortality decreased from 1.61 (<6 months) to 0.77 (60+ months) relative to IPI of 18-23 months (Table 3). Similarly, infant mortality due to all external causes and alternative groupings of these cause of death codes showed diminishing risk with increasing length of IPI. In particular, the risk of infant mortality due to external causes was 77% higher among women with IPI < 6 months and reduced by 45% among women with IPI of 60+ months compared to 18-23 months. Finally, when we examined specific external causes in sensitivity analyses, the aHRs for infant deaths due to unintentional injuries decreased across length of IPI from 1.73 (95% CI 1.43, 2.09) for IPI < 6 months to 0.54 (95% CI: 0.45, 0.66) for 60+ months relative to IPI of 18-23 months; the corresponding aHRs for homicide decreased from 1.83 (95% CI 1.22, 2.76) to 0.51 (95% CI: 0.33, 0.77) (data not shown in tables).
TABLE 3.
Hazard ratios of interpregnancy interval and infant death among singleton, second‐born, or higher births by ICD‐10 grouping: 2003 revised US birth certificate data linked to infant death data, 2011‐2015
| Interpregnancy Interval (months) | ||||||
|---|---|---|---|---|---|---|
| < 6 | 6–11 | 12–17 | 18–23 | 24–59 | 60+ | |
| Cause of death | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| All causes | ||||||
| Unadjusted | 2.12 (2.03, 2.21) | 1.38 (1.32, 1.43) | 1.09 (1.05, 1.13) | 1.00 (Reference) | 1.02 (0.98, 1.05) | 1.27 (1.23, 1.32) |
| Adjusteda | 1.61 (1.54, 1.68) | 1.22 (1.17, 1.26) | 1.06 (1.02, 1.11) | 1.00 (Reference) | 0.96 (0.93, 1.00) | 1.12 (1.08, 1.16) |
| Neonatal (0–27 days) | ||||||
| Unadjusted | 1.98 (1.87, 2.09) | 1.26 (1.19, 1.33) | 1.05 (1.00, 1.11) | 1.00 (Reference) | 1.08 (1.03, 1.13) | 1.52 (1.46, 1.59) |
| Adjusteda | 1.60 (1.51, 1.69) | 1.13 (1.07, 1.19) | 1.03 (0.98, 1.08) | 1.00 (Reference) | 1.04 (1.00, 1.09) | 1.40 (1.34, 1.47) |
| Postneonatal (28–365 days) | ||||||
| Unadjusted | 2.32 (2.17, 2.47) | 1.54 (1.45, 1.63) | 1.14 (1.08, 1.21) | 1.00 (Reference) | 0.93 (0.89, 0.98) | 0.92 (0.87, 0.98) |
| Adjusteda | 1.61 (1.51, 1.72) | 1.32 (1.24, 1.40) | 1.11 (1.05, 1.18) | 1.00 (Reference) | 0.86 (0.81, 0.90) | 0.77 (0.73, 0.82) |
| All external causesb | ||||||
| Unadjusted | 2.83 (2.49, 3.22) | 1.72 (1.53, 1.94) | 1.31 (1.16, 1.48) | 1.00 (Reference) | 0.87 (0.78, 0.97) | 0.70 (0.62, 0.79) |
| Adjusteda | 1.77 (1.55, 2.01) | 1.41 (1.25, 1.59) | 1.25 (1.10, 1.41) | 1.00 (Reference) | 0.78 (0.69, 0.87) | 0.55 (0.48, 0.62) |
| External causes excluding W75c | ||||||
| Unadjusted | 3.29 (2.71, 3.99) | 1.81 (1.50, 2.18) | 1.42 (1.18, 1.71) | 1.00 (Reference) | 0.90 (0.76, 1.07) | 0.70 (0.58, 0.86) |
| Adjusteda | 2.11 (1.73, 2.56) | 1.53 (1.27, 1.84) | 1.36 (1.13, 1.64) | 1.00 (Reference) | 0.80 (0.67, 0.95) | 0.54 (0.44, 0.67) |
| External causes and SUIDd | ||||||
| Unadjusted | 2.73 (2.52, 2.95) | 1.69 (1.57, 1.82) | 1.21 (1.13, 1.31) | 1.00 (Reference) | 0.91 (0.85, 1.97) | 0.71 (0.66, 0.77) |
| Adjusteda | 1.73 (1.59, 1.87) | 1.40 (1.30, 1.51) | 1.17 (1.08, 1.26) | 1.00 (Reference) | 0.81 (0.76, 0.86) | 0.56 (0.52, 0.60) |
| SUID onlye | ||||||
| Unadjusted | 2.63 (2.41, 2.87) | 1.67 (1.54, 1.81) | 1.18 (1.08, 1.28) | 1.00 (Reference) | 0.91 (0.84, 0.98) | 0.71 (0.66, 0.77) |
| Adjusteda | 1.66 (1.52, 1.81) | 1.38 (1.27, 1.49) | 1.13 (1.04, 1.23) | 1.00 (Reference) | 0.81 (0.75, 0.87) | 0.56 (0.52, 0.61) |
Abbreviations: HR, hazard ratio; SUID, sudden unexpected infant death.
Models were adjusted for maternal age at prior birth (under 20, 20-24, 25-29, 30-34, 35-39, 40+), birth order (2, 3, and 4+), maternal race/ethnicity (non-Hispanic black, non-Hispanic white, Hispanic, and other race or unknown ethnicity), maternal education (no high school diploma/no General Education Diploma (GED), high school diploma/GED, some college, bachelor's degree, or higher), marital status (currently married or not), and county poverty level (continuous).
ICD-10 codes: U01, V01-Y84.
ICD-10 codes: U01, V01-W74, W76-Y84.
ICD-10 codes: U01, VO1-Y84, R95, R99.
ICD-10 codes: R95, R99, W75.
Further adjustment for proxy variables of SES (health insurance, WIC use) and pre-pregnancy health (smoking, BMI) had minimal impact on the hazard ratio estimates (Table S1). Adjustment for preterm birth attenuated the HR estimates. This attenuation was considerably greater for overall infant mortality and neonatal mortality than for postneonatal, external causes, or SUID deaths. For external causes of death, there were no significant interactions between IPI and study year or SES indicators (all P-values >.12). Imputation of missing data resulted in attenuation of HRs for IPI of less than 6 or 6-11 months, but inferences remained similar (Table S2). After imputation, the percentage in each IPI category changed to 5.4, 11.1, 13.7, 12.0, 36.4, and 21.4 for less than 6 months through 60+ months, respectively.
4 |. COMMENT
4.1 |. Principal findings
To our knowledge, this is the first manuscript to examine the relationship between IPI and injury-related causes of infant mortality in the United States.34 We found a decreasing risk of infant death due to external causes, postneonatal mortality, and each alternative grouping of external cause of death as the time between pregnancies increased. This is in contrast to the observed J-shaped relationship between IPI and overall and neonatal infant mortality found in our study and previous studies.9 In the United States, the neonatal mortality rate is almost twofold higher than postneonatal mortality, encompassing a larger proportion of overall infant deaths.35 Leading causes of neonatal mortality are more likely due to factors related to the pregnancy or birth (eg low birthweight or maternal complications during pregnancy) compared to leading causes of postneonatal mortality (eg SIDS and unintentional injury).35 Accordingly, our findings suggest a different relationship between IPI and injury-related mortality as compared with overall and neonatal infant mortality, highlighting that research on alternative outcomes beyond the perinatal or neonatal period could contribute to additional information when considering birth spacing guidelines.
Previous research using 1991 US birth certificate data showed an elevated association between short IPI (defined as <19 months) only and infant mortality.33 Similarly, more recent state-based studies using 2003 revised birth certificate data also found similar associations between only short IPI (defined as either <18 months or <12 months) and infant mortality.30,36 In contrast to these studies, we observed a significant association between long IPI and increased risk of overall infant mortality. This discrepancy may be explained by our larger sample size or differences between our cohort and a given state or time period.
4.2 |. Strengths of the study
We expand on previous studies using cross-sectional vital statistics data by examining the impact of residual confounding from SES. Estimates were attenuated after initial adjustment for SES and other confounders in our models, but there was minimal change when county-level poverty was added to the model or after adjustment for additional SES proxy variables (Table S1). Moreover, the underlying risk profiles for both short (<6 months) and long (60+ months) IPI were similar, which would not explain why we would observe a continued decline in risk of injury-related mortality. The consistency of our findings with the proposed mechanism and prior studies on birth order and plurality and injury-related infant mortality underscore the need for further investigation on this relationship and other postneonatal infant health outcomes. Additional data sources are needed to further explore the association between short IPI and non-fatal injuries, including hospitalisations due to injury.
4.3 |. Limitations of the data
While this study is unique in exploring injury-related infant mortality, it has limitations. In the absence of maternally linked records, we could not conduct a within-mother analysis for comparison. Recent studies, which rely on a sibling comparison design, have questioned the causal association between IPI and birth outcomes, suggesting that these associations may be attributable to unmeasured confounding, primarily due to SES.12,13,37 However, it is unclear whether these new studies represent a better analytic approach or introduce both bias and lack of generalisability.38 For example, estimates from these studies are based on women with 3 or more births who are discordant on both exposure and outcome status. Furthermore, these study designs have the potential to introduce greater bias in cases where unmeasured confounders, such as SES, vary between pregnancies within women—a plausible scenario in the context of increasing family size and short IPI.39–41
4.4 |. Interpretation
While mechanisms for the relationship between IPI and infant mortality are likely multifactorial, one proposed mechanism postulates that closely spaced births may be at higher risk of death due to sibling competition for resources and parental care and attention.11 A prior study also found a higher likelihood of maternal depression and neglectful parenting behaviours towards children born after closely spaced births.42 In addition, epidemiologic studies have found a relationship between short IPI and postneonatal mortality, which may be served as a proxy for injury-related infant mortality.11,30 Our study supports and expands these findings by specifically examining external causes of death. In combination with our other recent studies that have observed increased risk of injury-related death for plural and higher parity births,6,7 our findings are consistent with the hypothesis that parental time and resources may be more divided among children who are closely spaced (ie short IPI, plural births) or who have older siblings. In line with our findings, a study from New Zealand found that children living in families with high parenting demand were at higher risk of injury-related death and SUID.43
4.5 |. Conclusions
In sum, short birth spacing may be a marker for increased risk of mortality due to injury in infants. While injury-related infant mortality is rare in the United States, relative associations with IPI were consistent across multiple analyses. These findings have implications for understanding potential mechanisms for the relationship between IPI and infant mortality beyond the maternal depletion hypothesis and for targeting prevention strategies accordingly—as both short IPI and injury-related deaths are preventable. The first year postpartum is an important time for the delivery of evidence-based interventions to ensure optimal birth spacing and prevent injury.
Supplementary Material
Synopsis.
Study question
Is interpregnancy interval (IPI) associated with injury-related infant mortality?
What is already known
IPI has been associated with increased risk of adverse birth outcomes and infant mortality, but little is known on its association with other postnatal outcomes, including external (injury-related) causes of infant death.
What this study adds
Unlike the J-shaped association with overall infant mortality, there is a decreasing risk of infant death due to injury-related causes with increasing IPI. This finding underscores the need to look beyond the perinatal or neonatal period in order to better inform birth spacing guidelines.
Acknowledgments
Funding information
This work was performed under a subcontract with Atlas Research, LLC for US Department of Health and Human Services, Office of Population Affairs (HHSP-233201450040A). Atlas Research, LLC had no role in the collection, analysis, or interpretation of the data. The role of co-authors from the Office of Population Affairs is specified below. Federal employees performed this work under the employment of the US federal government and did not receive any outside funding. We also gratefully acknowledge support from the Eunice Kennedy Shriver National Center for Child Health and Human Development grant P2C-HD041041, Maryland Population Research Center.
Footnotes
COMPETING INTEREST STATEMENT
All authors have completed the ICMJE uniform disclosure form and declare: Marie E. Thoma received financial support from Atlas Research, LLC for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
DISCLAIMER
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the National Center for Health Statistics, Centers for Disease Control and Prevention, ECHO Program Office, Office of the Director, National Institutes of Health, or the Office of Population Affairs.
CONFLICT OF INTEREST
All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Mathews TJ, Driscoll AK. Trends in Infant Mortality in the United States, 2005–2014. NCHS Data Brief. 2017;279:1–8. [PubMed] [Google Scholar]
- 2.Moon RY, Syndrome TF on SID. SIDS and Other Sleep-Related Infant Deaths: Evidence Base for 2016 Updated Recommendations for a Safe Infant Sleeping Environment. Pediatrics. 2016;138(5): e20162940. [DOI] [PubMed] [Google Scholar]
- 3.Lambert A, Parks SE, Shapiro-Mendoza CK. National and State Trends in Sudden Unexpected Infant Death: 1990–2015. Pediatrics. 2018;141:e20173519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy SL, Xu J, Kochanek KD, Curtin SC, Arias E. Deaths: final data for 2015. Natl Vital Stat Rep Cent Dis Control Prev Natl Cent Health Stat Natl Vital Stat Syst. 2017;66(6):1–75. [PubMed] [Google Scholar]
- 5.Kochanek KD, Murphy S, Xu J, Arias E. Mortality in the United States, 2016. NCHS Data Brief. 2017;293:1–8. [PubMed] [Google Scholar]
- 6.Ahrens KA, Rossen LM, Thoma ME, Warner M, Simon AE. Birth order and injury-related infant mortality in the U.S. Am J Prev Med. 2017;53(4):412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahrens KA, Thoma ME, Rossen LM, Warner M, Simon AE. Plurality of birth and infant mortality due to external causes in the United States, 2000–2010. Am J Epidemiol. 2017;185(5):335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wendt A, Gibbs CM, Peters S, Hogue CJ. Impact of increasing interpregnancy interval on maternal and infant health. Paediatr Perinat Epidemiol. 2012;26:239–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295(15):1809–1823. [DOI] [PubMed] [Google Scholar]
- 10.Ahrens KA, Nelson H, Stidd RL, Moskosky S, Hutcheon JA. Short interpregnancy intervals and adverse perinatal outcomes in highresource settings: An updated systematic review. Paediatr Perinat Epidemiol. 2019;33(1):O25–O47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conde-Agudelo A, Rosas-Bermudez A, Castaño F, Norton MH. Effects of birth spacing on maternal, perinatal, infant, and child health: a systematic review of causal mechanisms. Stud Fam Plann. 2012;43(2):93–114. [DOI] [PubMed] [Google Scholar]
- 12.Hanley GE, Hutcheon JA, Kinniburgh BA, Lee L. Interpregnancy interval and adverse pregnancy outcomes: an analysis of successive pregnancies. Obstet Gynecol. 2017;129(3):408–415. [DOI] [PubMed] [Google Scholar]
- 13.Klebanoff MA. Interpregnancy interval and pregnancy outcomes: causal or not? Obstet Gynecol. 2017;129(3):405–407. [DOI] [PubMed] [Google Scholar]
- 14.Regan AK, Ball SJ, Warren JL, et al. A population-based matched sibling analysis estimating the association between first interpregnancy interval and birth outcomes. Am J Epidemiol. 2019;188:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahrens KA, Hutcheon JA. Birth spacing in the United States— Towards evidence-based recommendations. Paediatr Perinat Epidemiol. 2019;33(1):O1–O4. [DOI] [PubMed] [Google Scholar]
- 16.Ahrens KA, Hutcheon JA, Ananth CV, et al. Report of the Office of Population Affairs’ expert work group meeting on short birth spacing and adverse pregnancy outcomes: methodological quality of existing studies and future directions for research. Paediatr Perinat Epidemiol. 2019;33(1):O5–O14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shelley J Spacing babies. BMJ. 2014;349:g4717. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention, National Center for Health Statistics. Vital statistics data available online. May 2017. [Google Scholar]
- 19.Ventura SJ. The U.S. National Vital Statistics System: Transitioning into the 21st century, 1990-2017. Vital Health Stat 1 2018;(62):1–84. [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention, National Center for Health Statistics. User Guide to the 2011 Period Linked Birth/Infant Death Public Use File. https://www.cdc.gov/nchs/data_access/vitalstatsonline.htm. Accessed January 5, 2018.
- 21.Thoma ME, De Silva DA, MacDorman MF. Examining interpregnancy intervals and maternal and perinatal health outcomes using U.S. vital records: Important considerations for analysis and interpretation. Paediatr Perinat Epidemiol. 2019;33(1):O60–O72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Institute of Minority Health and Health Disparities. HDPulse Data Portal. https://hdpulse.nimhd.nih.gov/data/index.html. Accessed January 5, 2018.
- 23.Copen CE, Thoma ME, Kirmeyer S. Interpregnancy Intervals in the United States: Data From the Birth Certificate and the National Survey of Family Growth. Natl Vital Stat Rep. 2015;64(3):1–10. [PubMed] [Google Scholar]
- 24.Gregory E, Martin JA, Argov EL, Osterman M. Assessing the quality of medical and health data from the 2003 birth certificate revision: results from New York City. Natl Vital Stat Rep. 2019;68(8):1–20. [PubMed] [Google Scholar]
- 25.Thoma ME, Copen CE, Kirmeyer SE. Short interpregnancy intervals in 2014: differences by maternal demographic characteristics. NCHS Data Brief. 2016;240:1–8. [PubMed] [Google Scholar]
- 26.Office of Management and Budget. Revisions to the Standards for the Classification of Federal Data on Race and Ethnicity. 1997:58782-58790. https://www.whitehouse.gov/node/15626 Accessed October 8, 2016.
- 27.Shapiro-Mendoza CK, Parks SE, Brustrom J, et al. Variations in cause-of-death determination for sudden unexpected infant deaths. Pediatrics. 2017;140(1):e20170087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malloy MH, MacDorman M. Changes in the classification of sudden unexpected infant deaths: United States, 1992–2001. Pediatrics. 2005;115(5):1247–1253. [DOI] [PubMed] [Google Scholar]
- 29.Shachar BZ, Mayo JA, Lyell DJ, et al. Interpregnancy interval after live birth or pregnancy termination and estimated risk of preterm birth: a retrospective cohort study. BJOG Int J Obstet Gynaecol. 2016;123(12):2009–2017. [DOI] [PubMed] [Google Scholar]
- 30.Hussaini KS, Ritenour D, Coonrod DV. Interpregnancy intervals and the risk for infant mortality: a case control study of arizona infants 2003–2007. Matern Child Health J. 2013;17(4):646–653. [DOI] [PubMed] [Google Scholar]
- 31.Cole SR, Hudgens MG, Brookhart MA, Westreich D. Risk. Am J Epidemiol. 2015;181(4):246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hutcheon JA, Moskosky S, Ananth CV, et al. Good practices for the design, analysis, and interpretation of observational studies on birth spacing and perinatal health outcomes. Paediatr Perinat Epidemiol. 2019;33(1):O15–O24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kallan JE. Reexamination of interpregnancy intervals and subsequent birth outcomes: evidence from U.S. linked birth/infant death records. Soc Biol. 1997;44(3–4):205–212. [PubMed] [Google Scholar]
- 34.Khoshnood B, Wall SN, Ranganathan D, Hsieh H- L, Singh JK, Lee K. Effect of short (%3c 12 months) interpregnancy intervals on the risk of cause-specific infant mortality rates in the US. Pediatr Res. 1999;45(4, Part 2 of 2):103A. [Google Scholar]
- 35.Ely DM, Driscoll AK, Matthews TJ. Infant mortality by age at death in the United States, 2016. NCHS Data Brief. 2018;326:1–8. [PubMed] [Google Scholar]
- 36.McKinney D, House M, Chen A, Muglia L, DeFranco E. The influence of interpregnancy interval on infant mortality. Am J Obstet Gynecol. 2017;216(3):316.e1-316.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ball SJ, Pereira G, Jacoby P, de Klerk N, Stanley FJ. Re-evaluation of link between interpregnancy interval and adverse birth outcomes: retrospective cohort study matching two intervals per mother. BMJ. 2014;349:g4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahrens KA, Thoma ME, Rossen LM. Interpregnancy interval and adverse pregnancy outcomes: an analysis of successive pregnancies. Obstet Gynecol. 2017;130(2):464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keyes KM, Smith GD, Susser E. On sibling designs. Epidemiology. 2013;24(3):473–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frisell T, Öberg S, Kuja-Halkola R, Sjölander A. Sibling comparison designs: bias from non-shared confounders and measurement error. Epidemiology. 2012;23(5):713–720. [DOI] [PubMed] [Google Scholar]
- 41.Sjölander A, Frisell T, Kuja-Halkola R, Öberg S, Zetterqvist J. Carryover effects in sibling comparison designs. Epidemiology. 2016;27(6):852–858. [DOI] [PubMed] [Google Scholar]
- 42.El-Kamary SS, Higman SM, Fuddy L, McFarlane E, Sia C, Duggan AK. Hawaii’s healthy start home visiting program: determinants and impact of rapid repeat birth. Pediatrics. 2004;114(3):e317–e326. [DOI] [PubMed] [Google Scholar]
- 43.Vaithianathan R, Rouland B, Putnam-Hornstein E. Injury and Mortality Among Children Identified as at High Risk of Maltreatment. Pediatrics. 2018;141(2):e20172882. 10.1542/peds.2017-2882 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.