Clostridium difficile infection (CDI) is the most common cause of nosocomial diarrhea. Residents of long-term care facilities (LTCFs) are an especially vulnerable population. Translating infection control measures successfully applied at hospitals to LTCF poses challenges [1-3]. C. difficile spores may survive on surfaces for months and are difficult to eradicate with routine disinfectants. Environmental cleaning with a 10% bleach solution reduces the incidence of CDI [4] but requires manual application and is thus, operator dependent [5]. Automated disinfection methods have been proposed, though their impact on clinical outcomes has not been established. We used ultraviolet radiation to prevent further CDI episodes in two LTCF roommates with two coincident CDI recurrences.
Patient 1 is a 65-year-old male who, during his 1-month stay in acute care, had multiple IV antibiotic exposures. He was transferred to the LTCF for rehabilitation and, 7 weeks later, developed CDI that was treated with oral vancomycin (Figure). Patient 2 is a 56-year-old male who received several courses of antibiotics and developed severe CDI while in the acute care setting. Patient 2 was then transferred to the LTCF where he was cohorted with Patient 1.
Figure.
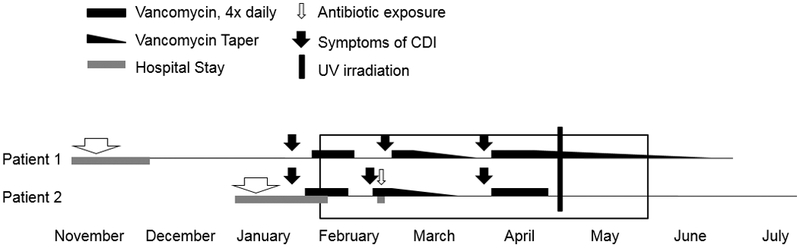
Timeline of recurrent C. difficile infection (CDI). Patient 1 was admitted to the LTCF in December and developed CDI in late January. Patient 2 was diagnosed with C. difficile infection during his initial hospitalization. Upon admission to the LTCF, he became Patient 1’s roommate for 4 months (box). Both patients had two recurrences of C. difficile infection which were temporally coincident. After irradiating their room with ultraviolet light in late April, neither patient has had a recurrence.
Within days of completing oral vancomycin, Patient 2 developed recurrent CDI. Other medical issues prompted Patient 2 to be transferred back to acute care where he had additional antibiotic exposure. He returned to the LTCF on an oral vancomycin taper. Two days later, Patient 1 also developed recurrent CDI and started oral vancomycin. Both patients recovered from their CDI yet days after Patient 1 finished his vancomycin, they simultaneously developed diarrhea.
Both patients were largely bed-bound and required assistance with bathing, dressing and toileting. Routine environmental cleaning measures were employed, including 10% bleach disinfectant between each C. difficile infection episode. Despite these measures, the coincident timing of the patients’ CDI recurrences suggested a heavy environmental burden of C. difficile spores. We surveyed multiple surfaces in the patients’ room for C. difficile spores using sterile swabs [5]. Within 5 hours, the swabs were used to inoculate pre-reduced agar with media selective for C. difficile (cycloserine-cefoxitin-bruclla agar containing 0.1% taurocholic acid and lysozyme 5mg/ml) [6]. C. difficile was detected on bed rails (1 colony, each bed), a bedside table (2 colonies), shower handrail (1 colony) and the trash can (25 colonies). It was not found on the mattresses, sinks, call buttons, night tables, shower chair or toilet.
In an effort to prevent further CDI recurrences, we used an automated ultraviolet radiation device (Tru-D™ Rapid Room Disinfection device, TRUDefence, Inc., Lexington SC ) which has been shown to reduce the environmental burden of C. difficile [7]. Tru-D was run for a full cycle in both the bathroom and patient room (22,000 μWs/cm2 for ~90 minutes) at a time when both patients were absent. A second set of environmental swabs was obtained afterward; C. difficile was detected only on a bed rail (1 colony). Five weeks following ultraviolet radiation, rectal swabs taken from both patients were negative for C. difficile. In the subsequent three months, neither patient had further episodes of CDI.
The coincident timing of the patients’ CDI recurrences suggests the possibility of cross-infection. McFarland et al. described an increase risk for C. difficile acquisition in the first 48 hours of becoming a roommate of a patient with a positive culture; [8]. Among 23 patients whose CDI was thought to have developed after exposure to a roommate, 87% of the isolates were identical to their roommate’s [8]. While our patients clearly both acquired their initial infection prior to becoming roommates, cohorting them may have resulted in each patient becoming exposed to a new C. difficile strain, increasing their risk for recurrent CDI.
Infection control measures aimed at reducing the incidence of CDI are based primarily on successful practices employed in hospitals. Using these same measures in LTCF presents challenges due to the shared dining, bathing, toileting and rehabilitation facilities. Furthermore, the long-term care setting, where patients reside for weeks to years, makes it difficult to implement the same disinfection measures routinely used in hospitals. Although a measurable impact on patient outcomes has not yet been reported, automated disinfection devices are able to reduce the number of organisms in places easily missed or inaccessible to human cleaning. Tru-D requires less than 1 hour per bed for a typical cycle and is easy to use. Routine use of Tru-D and similar devices to decrease the environmental burden of pathogens is a feasible addition to current infection control and housekeeping measures and may ultimately help reduce rates of CDI among patients in hospitals and LTCF.
Acknowledgements
Financial Support: This work was supported by a Merit Review Grant from the Department of Veterans Affairs to C.J.D. R.L.P.J. gratefully acknowledges the T. Franklin Williams Scholarship with funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, the Association of Specialty Professors, the Infectious Diseases Society of America and the National Foundation for Infectious Diseases.
Footnotes
Potential Conflicts of Interest: C.J.D. has received research funding from GOJO Industries, Ortho-McNeil and ViroPharma. R.L.P.J. has been a consultant for GOJO Industries and Pfizer. All other authors have no conflicts of interest. All authors submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest, and the conflicts that the editors consider relevant to this article are disclosed here.
Contributor Information
Brett Sitzlar, Research Service and Geriatric Research, Education and Clinical Center (GRECC) at the Louis Stokes Cleveland Veterans Affairs Medical Center, Cleveland, Ohio.
Ravy K. Vajravelu, Research Service and Geriatric Research, Education and Clinical Center (GRECC) at the Louis Stokes Cleveland Veterans Affairs Medical Center, Cleveland, Ohio.
Lucy Jury, Research Service and Geriatric Research, Education and Clinical Center (GRECC) at the Louis Stokes Cleveland Veterans Affairs Medical Center, Cleveland, Ohio.
Curtis J. Donskey, Research Service and Geriatric Research, Education and Clinical Center (GRECC) at the Louis Stokes Cleveland Veterans Affairs Medical Center, Cleveland, Ohio; Division of Infectious Disease, Department of Medicine, Case Western Reserve University School of Medicine, Cleveland, Ohio.
Robin L.P. Jump, Research Service and Geriatric Research, Education and Clinical Center (GRECC) at the Louis Stokes Cleveland Veterans Affairs Medical Center, Cleveland, Ohio; Division of Infectious Disease, Department of Medicine, Case Western Reserve University School of Medicine, Cleveland, Ohio.
References
- 1.Malani PN, Infection control in long-term care facilities: the need for engagement. J Am Geriatr Soc, 2009. 57(3): p. 569–70. [DOI] [PubMed] [Google Scholar]
- 2.Mylotte JM, Surveillance for Clostridium difficile-associated diarrhea in long-term care facilities: what you get is not what you see. Infect Control Hosp Epidemiol, 2008. 29(8): p. 760–3. [DOI] [PubMed] [Google Scholar]
- 3.Simor AE, et al. , Clostridium difficile in long-term-care facilities for the elderly. Infect Control Hosp Epidemiol, 2002. 23(11): p. 696–703. [DOI] [PubMed] [Google Scholar]
- 4.Mayfield JL, et al. , Environmental control to reduce transmission of Clostridium difficile. Clin Infect Dis, 2000. 31(4): p. 995–1000. [DOI] [PubMed] [Google Scholar]
- 5.Eckstein BC, et al. , Reduction of Clostridium difficile and vancomycin-resistant Enterococcus contamination of environmental surfaces after an intervention to improve cleaning methods. BMC Infect Dis, 2007. 7: p. 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nerandzic MM and Donskey CJ, Effective and reduced-cost modified selective medium for isolation of Clostridium difficile. J Clin Microbiol, 2009. 47(2): p. 397–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nerandzic MM, et al. , Evaluation of an automated ultraviolet radiation device for decontamination of Clostridium difficile and other healthcare-associated pathogens in hospital rooms. BMC Infect Dis, 2010. 10: p. 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McFarland LV, et al. , Nosocomial acquisition of Clostridium difficile infection. N Engl J Med, 1989. 320(4): p. 204–10. [DOI] [PubMed] [Google Scholar]


