Abstract
Purpose
Most pediatric spinal tumors are low-grade gliomas (LGGs). Characterization of these tumors has been difficult given their heterogeneity and rare incidence. The objective was to characterize such tumors diagnosed at our institution.
Methods
Spinal tumors diagnosed in our pediatric patients between 1984 and 2014 were reviewed retrospectively. Demographics, presentation, pathology, imaging, management, and sequelae were examined.
Results
Forty patients had spinal LGG tumors, 24 (62%) of which were pilocytic astrocytomas. The most common initial presentations were pain (n=15), partial extremity paralysis (n=13), and ataxia (n=11), with the diagnosis frequently delayed by months (median=5.9 months, range=4 days-6.2 years). Twenty-nine patients had some tumor resection, and 8 required adjuvant therapy with chemotherapy (n=4) or radiation (n=4) post-resection. Ten other patients received only biopsy for histologic diagnosis, who were treated with chemotherapy (n=4) or radiation (n=5) post biopsy. Tumor progression was noted in 16 patients (2 after gross-total resection; 10, partial resection; and 4, biopsy). During the evaluation period, 3 patients died secondary to tumor progression. BRAF status could have shortened progression-free survival: patients with BRAFV600E mutations (n=3) all experienced progression within 10 months. Long-term sequelae of the disease/treatment were mostly residual neurologic deficits (paresthesia, paralysis), chemotherapy-induced hearing loss, and scoliosis.
Conclusions
Spinal LGG is a rare entity with significant long-term effects. Although surgery is the most common initial treatment option, more in-depth analysis of molecular biomarkers may improve stratification and prognostication.
Keywords: Low grade glioma, pediatric, spinal, treatment, biomarkers, BRAF
Introduction
Central nervous system (CNS) malignancies are the second most common cancer among children, surpassed only by leukemias. Low-grade glioma (LGG), a group of heterogenous slow-growing tumors, accounting for almost 40% of pediatric CNS tumors [1,2]. A small subset of these tumors develop in the spine and comprise 4–10% of CNS tumors [3]. Unlike other CNS tumors, intramedullary spinal tumors have a good prognosis (approaching 90% overall survival) but can be associated with significant morbidity secondary to the tumor and its treatment [3,4]. Furthermore, the presentation of intramedullary tumors of the spine is quite variable and is based on the tumor’s origination point. Because of these tumors’ insidious nature and vague, generalized symptoms, their diagnosis can be delayed until the intensity of the symptoms becomes significant or debilitating [5].
Due to the low incidence of these tumors in the pediatric population, controversy about their management has developed, specifically the required extent of the initial surgical resection, treatment with adjuvant chemotherapy versus radiation therapy (RT), and optimal salvage therapies [6–8]. Given the lack of specific guidelines about managing pediatric intramedullary spinal cord LGGs, we evaluated 30 years’ data from our institution’s large population of patients with intramedullary LGGs of the spine to better characterize this disease. By using a larger case series, we hoped to identify patients with worse prognosis who are less likely to respond to more conservative therapy and would require additional more intensive therapy, such as radiation therapy.
Methods
Patient characteristics
Pediatric patients with low-grade gliomas of the spine/cervicomedullary junction diagnosed at St. Jude Children’s Research Hospital (Memphis, TN, USA) between January 1, 1984, and December 31, 2014, were eligible for this retrospective single-institution study. The institutional review board (IRB) at St. Jude reviewed and approved this study before beginning chart review. Imaging by MRI or CT was used for initial diagnosis and pathologic confirmation was performed in 39 patients (29 with tumor resection and 10 with only biopsy). One patient did not undergo any surgery, and that patient’s tumor was diagnosed by imaging alone.
Duration of symptoms
The duration of symptoms was difficult to fully determine because of the variability of description of the patients’ histories: although some descriptions were specific, others were general, with descriptions of symptoms being first noted over years or parts of a given year. Therefore, similar to a previous study performed by our group [5], a conservative approach was used. If only a year was provided, then the last day of that year was used. If a month was provided, then the last day of that month was used. For descriptions that included “spring”, “summer”, “fall”, or “winter” of a specific year, the last day of either April, August, November, or February was used, respectively. Therefore, pre-diagnosis symptom interval (PSI) was defined as the time between onset of symptoms and the initial imaging report that led to the diagnosis.
Tumor site
Tumor location was separated into 5 sites in the spine: cervicomedullary junction, cervical, cervicothoracic, thoracic, and holocord. Although lumbar and sacral tumors were considered, no such LGGs were identified in these patients.
Extent of resection
Extent of resection was determined based on a combination of surgical operative reports and postoperative imaging. Gross-total resection (GTR) indicated 100% resection of the tumor; near-total resection (NTR), 90–99% resection; subtotal resection (STR), 50–89% resection; and debulking/partial, <50% resection. The resection extent of “biopsy” was used for patients who received a biopsy only for diagnostic purposes and no further initial resection or debulking.
BRAF gene status
In a subset of patients, BRAF cytogenetic testing was performed. This testing was done by fluorescence in-situ hybridization (FISH) evaluation using a St. Jude laboratory–developed target probe for locus 7q34 or 7q35 and a control probe for locus 7p11.2, except for one sample that was tested via Sanger sequencing of BRAF exon 15. A PDGFA probe was used for FISH evaluation of locus 4p12 [9].
Statistical analysis
All statistical analysis was performed with the open-source statistics software R (Bell Laboratories, version 3.5.0). Survival distribution was determined by using Kaplan-Meier curves, with subgroup comparison performed with log-rank test. Fisher’s exact test was used to determine correlation between categorical variables (pre-diagnosis symptom [PSI] ≤ or > 6 months versus presence or absence of morbidities) and Wilcoxon rank sum test for comparison of means between groups (PSI for spinal tumors with and without cervicomedullary involvement, and for patients with and without progression).
Results
Patient characteristics
A total of 714 pediatric patients had low-grade gliomas diagnosed at our institution during the study period. Of these patients, 40 (5.6%) had tumors that were localized to the spinal cord or the cervicomedullary junction and were included in this study. Patient characteristics, tumor location, histology, treatment, and progression (when applicable) are presented in Table 1. Table 2 summarizes the characteristics of the cohort. The treatment course and outcome of each patient is summarized in Supplementary Figure 1. The patients were evenly distributed by sex but were mostly white (73%). Median age at diagnosis was 7.0 years, ranging from 0.6 years to 18.3 years. Patient 27 with the stigmata of neurofibromatosis (NF) (café-au-lait macules, fibromas, etc.) was evaluated by our genetics team and underwent NF1 germline testing and café-au-lait biopsy, which were negative; however, segmental/mosaic NF was suspected.
Table 1.
Characteristics of the 40 patients with intramedullary spinal low-grade gliomas
| Patient no. | Sex/race | Age at diagnosis (y) | Tumor location | Symptoms/signs | PSI (months) | Extent of primary surgery | Tumor histology | BRAF Status | Post-operative adjuvant treatment | No. of progressions | Follow-up (y) | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/W | 2.5 | Cervicomedullary | L hemiparesis, torticollis, ataxia | 0.1 | STR | AstrocytomaNOS | Not tested | 26.6 | SD | ||
| 2 | M/B | 9.5 | Thoracic | Ataxia, low back/abdominal pain | 0.5 | GTR | AstrocytomaNOS | Not tested | Radiation | 24.8 | NED | |
| 3 | F/B | 10.7 | Cervicomedullary | L hemiparesis | 2.1 | STR | AstrocytomaNOS | Not tested | Radiation | 23.9 | NED | |
| 4 | F/W | 0.8 | Holocord | Hydrocephalus, ataxia | 2.4 | GTR† | Fibrillary astrocytoma | Not tested | 1 | 21.6 | NED | |
| 5 | M/W | 13.8 | Cervicomedullary | Neck pain | 6.1 | GTR | Oligoastrocytoma | Not tested | 20.7 | NED | ||
| 6 | F/W | 2.3 | Cervicothoracic | Ataxia, Develop. delay | 14.5 | STR | PA | Not tested | 2 | 20.2 | NED | |
| 7 | M/W | 11.9 | Thoracic | Neck pain | 0.2 | NTR | PA | Not tested | 1 | 18.4 | SD | |
| 8 | F/W | 0.9 | Cervical | Torticollis, upper extremity weakness, loss of milestones | 11.3 | STR | AstrocytomaNOS | Not tested | Chemotherapy | 1 | 18.2 | NED |
| 9 | M/W | 2.1 | Thoracic | Ataxia, constipation, scoliosis | 10.0 | GTR | PA | Not tested | 1 | 17.6 | NED | |
| 10 | F/W | 8.9 | Thoracic | Leg/back pain, scoliosis | 0.8 | GTR | PA | Not tested | 15.8 | NED | ||
| 11 | M/W | 17.0 | Cervicothoracic | Dysphagia | 2.0 | STR | PA | Not tested | 1 | 14.0 | SD | |
| 12 | F/W | 16.2 | Cervicothoracic | Scoliosis | 29.6 | NTR | Ganglioglioma | Not tested | 12.3 | SD | ||
| 13 | F/W | 2.3 | Cervicomedullary | Torticollis, night terrors, lower back pain | 0.6 | GTR | PA | Not tested | Chemotherapy | 11.5 | NED | |
| 14 | M/W | 14.3 | Cervical | L hemiatrophy | 20.6 | STR | PA | Not tested | 10.9 | SD | ||
| 15 | F/W | 15.7 | Cervicothoracic | Scoliosis | 6.1 | STR | PA | Not tested | 9.9 | SD | ||
| 16 | F/W | 10.4 | Cervicothoracic | Scoliosis | 2.9 | STR | AstrocytomaNOS | Not tested | 9.9 | SD | ||
| 17 | F/W | 1.1 | Cervicomedullary | Lethargic, torticollis, upper weakness | 0.6 | Partial resection | PA | V600E | Chemotherapy | 3 | 9.6 | Died |
| 18 | F/W | 2.2 | Cervicomedullary | Emesis, HA, FTT | 11.8 | Partial resection | PA | Duplication | 2 | 9.2 | PR | |
| 19 | M/W | 1.7 | Cervical | L arm weakness, back pain | 1.9 | Partial resection | Fibrillary astrocytoma | Not tested | 1 | 8.9 | SD | |
| 20 | M/MR | 1.7 | Thoracic | Ataxia, leg pain | 7.7 | GTR | PA | Not tested | 8.9 | NED | ||
| 21 | F/W | 8.9 | Cervicomedullary | Emesis, ataxia, blurry vision | 9.2 | Biopsy | Ganglioglioma | Not tested | Radiation | 8.4 | SD | |
| 22 | M/W | 5.4 | Cervicomedullary | Vocal cord paralysis, torticollis | 4.7 | Biopsy | Ganglioglioma | V600E | Radiation | 2 | 7.9 | SD |
| 23 | F/W | 6.8 | Cervicomedullary | L weakness, ataxia | 5.3 | STR | PA | Duplication | Radiation | 1 | 7.6 | PD |
| 24 | M/B | 18.3 | Thoracic | Leg pain | 1.2 | GTR | Ganglioglioma | Not tested | 7.5 | NED | ||
| 25 | F/MR | 0.6 | Cervicomedullary | R weakness, torticollis | 1.8 | Biopsy | PA | Duplication | Chemotherapy | 6.5 | SD | |
| 26 | F/W | 17.2 | Thoracic | Numbness, back pain, scoliosis, ataxia | 40.2 | NTR | PA | Duplication | 6.3 | SD | ||
| 27 | F/MR | 7.2 | Cervicomedullary | Develop. delay, leg weakness, strabismus | 74.5 | Biopsy | Ganglioglioma | Not tested | Radiation | 5.8 | SD | |
| 28 | M/W | 4.8 | Cervicomedullary | Ataxia, torticollis, neck pain | 9.7 | Partial resection | PA | Duplication | Radiation | 5.8 | SD | |
| 29 | F/Alaskan Indian | 5.6 | Cervicomedullary | Vertigo, emesis | 19.7 | None | Not tested | 5.4 | SD | |||
| 30 | M/B | 6.3 | Cervicomedullary | HA, ataxia | 11.4 | GTR | PA | Not tested | 5.0 | NED | ||
| 31 | M/W | 12.1 | Cervicothoracic | Neck/chest pain, upper extremity pain | 2.0 | NTR | PA | Wild-type | 5.0 | SD | ||
| 32 | F/W | 17.5 | Thoracic | Lower back pain | 11.6 | Biopsy | PA | Duplication | 4.9 | SD | ||
| 33 | M/W | 18.0 | Cervical | Back/neck/arm pain, numbness | 22.6 | GTR | PA | Duplication | 4.9 | PD | ||
| 34 | M/W | 2.5 | Cervicomedullary | Arm/leg weakness | 5.6 | STR | PA | Duplication | Chemotherapy | 2 | 4.7 | SD |
| 35 | M/MR | 5.2 | Thoracic | Mild back/leg pain | 6.1 | NTR | PA | Duplication | 2 | 4.3 | PD | |
| 36 | M/W | 7.7 | Cervicomedullary | Ataxia, R weakness, hearing difficulty | 6.6 | Biopsy | PA | V600E | Radiation | 1 | 3.7 | SD |
| 37 | F/W | 0.6 | Cervical | Develop. delay, seizures, L weakness | 3.6 | Biopsy | PA | Not tested | Chemotherapy | 3.2 | SD | |
| 38 | M/B | 3.2 | Thoracic | FTT, nausea/vomiting, fatigue | 0.7 | Biopsy | PA | Duplication | Chemotherapy | 3.0 | PR | |
| 39 | M/W | 13.0 | Thoracic | HA, seizures, increased ICP, diplopia | 23.0 | Biopsy | PXA | Not tested | Radiation | 5 | 1.8 | Died |
| 40 | F/B | 14.9 | Cervicomedullary | Extremity weakness, hydrocephalus | 0.1 | Biopsy | Oligoastrocytoma | Not tested | Chemotherapy | 1 | 0.6 | Died |
M male, F female, W white, B black, MR multiracial, L left, R right, FTT failure to thrive, HA headache, ICP intracranial pressure, PSI pre-diagnosis symptom interval, GTR gross-total resection, NTR near-total resection, STR subtotal resection, PA pilocytic astrocytoma, NOS not otherwise specified, PXA pleomorphic xanthoastrocytoma, NED no evidence of disease, PR partial response, SD stable disease, PD progressive disease
3 separate surgeries were required to achieve GTR
Table 2:
Summary of clinical characteristics of patients
| Variables | |
|---|---|
| Median age (range) | 7.0 (0.6 – 18.3) years |
| Sex M:F | 1:1 |
| Race | |
| White | 29 (72.5%) |
| Black | 6 (15%) |
| Other/Multiracial | 5 (12.5%) |
| Tumor location | |
| Cervicomedullary | 17 (42.5%) |
| Cervical | 5 (12.5%) |
| Thoracic | 11 (27.5%) |
| Cervicothoracic | 6 (15%) |
| Holocord | 1 (2.5%) |
| Extent of initial surgery | |
| GTR | 10 (25%) |
| NTR | 5 (12.5%) |
| STR | 10 (25%) |
| Partial resection/debulking | 5 (12.5%) |
| Biopsy | 9 (22.5%) |
| None | 1 (2.5%) |
| Tumor histology | |
| PA | 24 (60%) |
| FA | 2 (5%) |
| Oligoastrocytoma | 2 (5%) |
| PXA | 1 (2.5%) |
| Ganglioglioma | 5 (12.5%) |
| Astrocytoma NOS | 5 (12.5%) |
| Initial treatment | |
| Observation only | 1 (2.5%) |
| Surgery only | 23 (57.5%) |
| Surgery + radiation | 9 (22.5%) |
| Surgery + chemotherapy | 7 (17.5%) |
| Number of progressions | |
| 0 | 24 (60%) |
| 1 | 9 (22.5%) |
| 2 | 5 (12.5%) |
| 3+ | 2 (5%) |
| Status at last follow-up | |
| NED | 12 (30%) |
| PR | 2 (5%) |
| SD | 20 (50%) |
| PD | 3 (7.5%) |
| Died | 3 (7.5%) |
M male, F female, GTRgross-total resection, NTR near-total resection, STR subtotal resection, PA pilocytic astrocytoma, FA fibrillary astrocytoma, NOS not otherwise specified, PXA pleomorphic xanthoastrocytoma, NED no evidence of disease, PR partial response, SD stable disease, PD progressive disease
Characterization of symptoms and signs
The presenting signs and symptoms were evaluated based on parental descriptions provided during initial clinic intake. A summary of signs and symptoms is in Table 3. The most common presentations were back pain (38%), extremity weakness (33%), and ataxia (28%). Less frequent symptoms included torticollis (18%) and scoliosis (15%). Developmental delay and GI complaints were also noted, more frequently in the infants/toddlers. Three patients had increased intracranial pressure (ICP) upon diagnosis, with 4 others having this condition identified soon after diagnosis, resulting in a total of 7 patients requiring ventriculoperitoneal shunting.
Table 3.
Summary of symptoms reported
| Symptom | Number of patients reporting symptom | % of patients (N=40) |
|---|---|---|
| Pain | 15 | 37.5 |
| Extremity weakness | 13 | 32.5 |
| Ataxia | 10 | 25 |
| Torticollis | 7 | 17.5 |
| Scoliosis | 6 | 15 |
| Nausea/vomiting/constipation | 4 | 10 |
| Developmental delay | 4 | 10 |
| Vision changes | 3 | 7.5 |
| Increased intracranial pressure | 3 | 7.5 |
| Numbness | 2 | 5 |
| Headaches | 2 | 5 |
| Seizures | 2 | 5 |
| Hearing defect | 1 | 2.5 |
| Hemiatrophy | 1 | 2.5 |
| Vocal cord paralysis | 1 | 2.5 |
| Dysphagia | 1 | 2.5 |
| Vertigo | 1 | 2.5 |
Time to diagnosis
A conservative approach was used to determine PSI in this cohort. The median duration of symptoms was 5.9 months, ranging from 4 days to 6.2 years. The 2 children with the shortest PSI had cervicomedullary tumors: one presented with torticollis, hemiparesis, and ataxia (patient 1), and the other presented with extremity weakness and was found to have hydrocephalus (patient 40). The patient with the most delayed diagnosis was a 7-year-old who presented with extremity weakness, developmental delay, and strabismus (patient 27). Eighty-three percent of the patients received a diagnosis within 12 months of the beginning of symptoms. Comparing subsets of patients by tumor location, only a small difference in PSI was noted between patients with cervicomedullary involvement (mean 10.0 months) and those without (mean 9.6 months) (Wilcoxon test p=0.61).
Distribution of tumors by location
MRI was used initially to identify the intramedullary tumors. Cervicomedullary tumors predominated (43%), and 28% of patients had thoracic tumors. Cervicothoracic tumors made up 15% followed by 13% cervical tumors. One patient was noted to have a tumor spanning the whole cord; and no patients had tumors spanning only the lumbar or sacral spine. No metastatic lesions were noted in any of the patients upon diagnosis.
Extent of initial surgical intervention
Surgery was found to play a substantial role in spinal LGG diagnosis and treatment. All but one patient had neurosurgical intervention (98%). Although surgery was used to histologically confirm the diagnosis, at least 70% of patients received at least a partial resection/debulking of the tumor. Twenty-five percent of patients underwent only a biopsy. GTR and STR was performed in 10 patients each (25%); followed by NTR (5 patients, 13%) and partial resection (4 patients, 10%). One patient (patient 29) received no neurosurgical intervention and was only observed. As previously stated, 7 patients required VP shunt placement, though not all of their procedures were performed in concert with tumor resection.
Pathologic Findings
Histologic confirmation was provided for all patients except 1 (patient 29), who did not have a biopsy. Based on the available technology at the time of diagnosis, more in-depth evaluation was performed on some tissue samples, including cytogenetic studies such as fluorescent in-situ hybridization (FISH). Pilocytic astrocytomas (WHO grade I) were the most frequent tumor identified (24 cases, 60%), followed by ganglioglioma and astrocytoma not otherwise specified (5 cases each, 13%). Patient 29’s tumor was diagnosed by MRI alone which was most consistent with a low-grade glioma, though low-grade glioneuronal tumor and ependymoma were also possible.
Cytogenetic testing was performed in pathologic specimens collected after 2005. One-third of samples (n=14) were analyzed for BRAF status. Of those tested, 10 patients had BRAF duplication, and 3 had a BRAF V600E mutation. One sample was negative for a BRAF alteration. PDGFRA double minute amplification was identified in a patient with BRAF V600E (patient 17).
Post-operative adjuvant treatment
Twenty-two patients in the cohort received surgery followed by observation with serial imaging; 9 received adjuvant RT (total cumulative dose: 45–54 Gy). The RT field was focal to the primary site in all cases except one metastatic case in which the RT field consisted of the whole brain RT plus focal RT to disease in the thoracic and lumbar spine. Eight other patients received adjuvant chemotherapy. Platinum-based regimens were used for all initial chemotherapy regimens with carboplatin/vincristine/temozolomide (n=3), carboplatin/vincristine (n=2), carboplatin alone (n=1), carboplatin/cyclophosphamide/etoposide (n=1), and cisplatin/etoposide (n=1).
In a small subset of patients (n=10) who initially had only a biopsy, 5 received adjuvant RT, and 4 patients continued with a chemotherapy regimen. In the group of non-biopsy patients (at least a partial resection), 21 were only observed, and adjuvant chemotherapy or RT were used in 4 patients each.
Patient outcome and survival
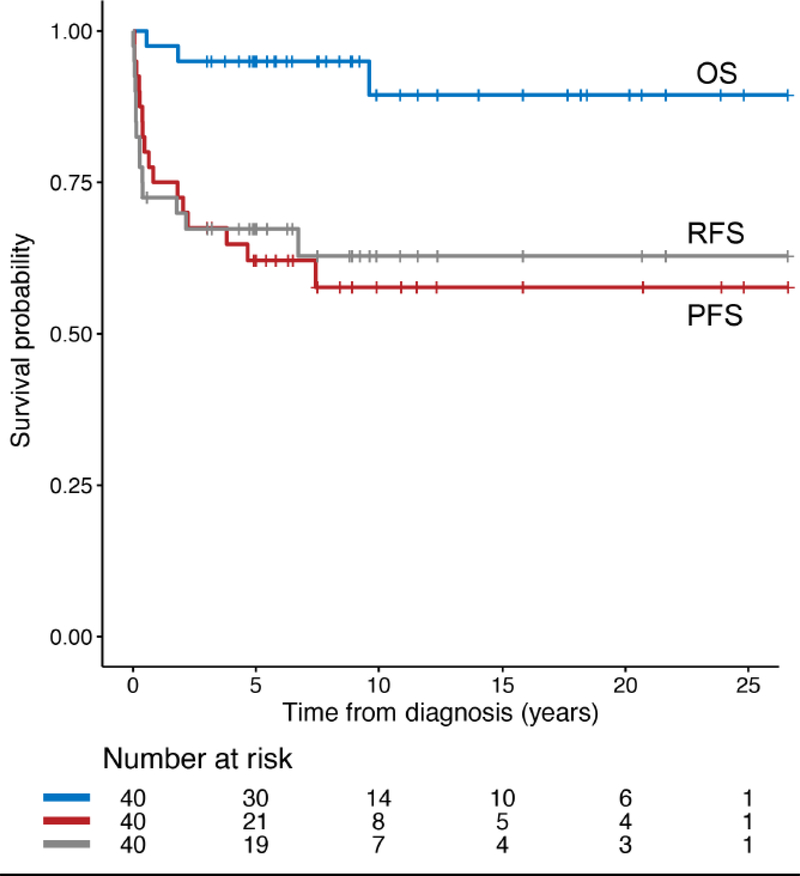
The patients were followed up for a median period of 8.6 years (range: 0.6–26.6 years). The 5- and 10-year progression-free survival rates were 62±8% and 58±8%, respectively. The age at diagnosis did not significantly affect progression-free survival (p = 0.31). The respective 5- and 10-year overall survival rates remained relatively high, at 95±3% and 89±6%. Radiation-free survival, the interval from diagnosis to progression requiring RT, was similar to PFS (Figure 1). At last follow-up, 3 patients had died from disease progression. Two of the patients (patient 17 and 40) with cervicomedullary disease experienced disease progression with leptomeningeal metastases. Metastatic disease in the pons developed in the third patient with thoracic primary disease (patient 39), leading to his death. Although most patients survive their disease, only 12 patients had no evidence of disease on the most recent follow-up MRI, 9 of whom received GTR. Instead, most patients (n=20) had stable disease or experienced partial response (n=3); 3 other patients were noted to have disease progression.
Fig.1.
Survival rates in patient cohort, OS overall survival, PFS progression-free survival, RFS radiation-free survival
Progression was noted in 16 patients within a median 0.73 years (range, 0.1–7.4 years). The tumors of 9 patients progressed only once; 5 and 2 patients’ tumors progressed twice or more than 3 times, respectively. Most progressions were localized (88%), with only 3 patients having distant metastases, one of whom did not experience recurrence at the primary site (patient 40). Progression was noted in only 2 of 10 patients treated at diagnosis with GTR and in 10 of 19 who underwent resection without GTR being achieved (NTR/STR/partial resection); however, this difference was not statistically significant (log-rank test p=0.15) (Figure 2b). Also, no significant difference in PFS (p=0.64) or OS (p=0.39) between spinal (n=40) and non-spinal (n=674) LGG was noted in this cohort.
Fig.2.
The effect of (a) BRAF mutation status and (b) gross-total resection on progression-free survival. V600E BRAF V600E mutation, GTR gross-total resection, PFS progression-free survival
Treatment at recurrence
Sixteen patients experienced recurrence/progression of disease with resection at diagnosis of GTR (n=2), biopsy (n=4), or other resection extent (n=10). At first relapse, these patients received STR (n=1), STR and RT (n=1), RT only (n=4), conventional chemotherapy (n=7), and targeted therapy (n=3). Although 9 patients relapsed only once, some patients (n=7) had additional recurrences (see Supplementary Figure 1).
Five patients with recurrent disease who had been evaluated for BRAF status were treated with agents that target the mitogen-activating protein kinase (MAPK) pathway, with 3 receiving a BRAFV600E inhibitor and 2 patients with BRAF duplication treated with MEK inhibitors. Interestingly, a statistically significant difference in PFS was identified when evaluating the records of patients with BRAF cytogenetic data. Patients with BRAF duplication had a 5-year PFS of 67.5±16%; patients with BRAF V600E tumors all experienced disease progression within 10 months of diagnosis (log-rank test p=0.001, Figure 2a). The patient with a BRAF wild-type tumor (patient 31) had not experienced disease progression and had stable disease at last follow-up. Though all 3 patients with BRAF V600E mutation initially experienced disease progression, one such patient (patient 17) died of progressive disease after being treated with multiple chemotherapeutic regimens, including a BRAFV600E inhibitor. Interestingly, 8 patients with progression (50%) were less than 3 years old at diagnosis (log-rank test p=0.05).
Morbidity associated with disease or treatment
Although most patients with intramedullary LGG survive their disease, sequelae that are associated with the disease or its management develop in many. At last follow-up, residual deficits were noted in 31 patients. Residual motor deficits were noted in 15 patients, while sensory deficits in 8 patients. Most of these deficits were mild and did not significantly affect the patients’ quality of life. Scoliosis/kyphosis was noted in 13 patients, 3 of whom received subsequent surgical repair in severe cases. Chemotherapy-induced hearing loss was identified in 5 patients with 3 patients at least Chang 2b bilaterally, seizures in 4 patients, and ataxia/dysmetria in 4 patients. Five patients had endocrinopathies, namely hypothyroidism (4 patients) and precocious puberty (1 patient). A secondary malignancy (thyroid papillary carcinoma) developed in the radiation field of 1 patient (patient 7) approximately 12 years after receiving RT; and he required total thyroidectomy with neck dissection and is alive, living with stable disease. No correlation was noted between delayed diagnosis of greater than 6 months and increased frequency of morbidities (scoliosis, sensory deficit, motor deficit, endocrinopathies, hearing loss, seizure, and any morbidity, all p>0.05 by Fisher’s exact test).
Discussion
Our study confirmed previously published works, namely age of diagnosis and histopathology of intramedullary LGG. Our median age of diagnosis agrees with some institution-based studies [7,10] while a large SEER-based study found that almost a third of the patients to be in the 10–14-year age bracket [11]. Pilocytic astrocytomas are frequently reported [3,4,6,7,12–16] as the most common spinal LGG histology, as in this study.
Intramedullary LGGs have a relatively good prognosis [2,8,10] which was confirmed in this study. However, approximately 30% of LGGs have been shown to relapse or progress [17], requiring further management. Certain risk factors have been identified which can help predict which tumors are more likely to relapse following initial treatment. For example, the extent of initial surgical resection has shown prognostic value [12,18]. In our cohort, only 20% of the patients who received GTR relapsed compared to 53% without GTR. Though not statistically significant due to our limited numbers, this trend is suggestive of better PFS with complete tumor resection. However, GTR of spinal astrocytomas can be technically difficult due to its anatomy and frequent lack of a true surgical plane, leading to GTR in only 70–80% of such cases [19]. Fortunately, though 36% of our patients who initially received only surgical intervention (to any extent) experienced relapse, no mortality was noted in these patients, and many have stable disease, which is comparable to a near 90% overall survival in similar studies [3,10,18]. Therefore, extent of tumor resection does not negatively affect OS in this patient population.
Recent advances in cytogenetic analysis have illustrated worse prognosis in certain molecular subgroups, including tumors with mutations in BRAFV600E mutation, and better PFS and OS in patients with KIAA1549-BRAF fusions [17,20]. Results of a large study from the University of Toronto Sick Kids cohort also indicated the prognostic importance of BRAFV600E, with 17% of their cohort harboring the mutation and having a poor 10-year PFS of 27% compared to 60% in patients with wild-type BRAF. CDKN2A deletion also was a biomarker of worse outcome in pediatric LGG patients with BRAFV600E mutations [20]. Our study demonstrated the same findings (Figure 2a). Given that KIAA1549-BRAF fusions and BRAFV600E mutations are frequently noted in pediatric spinal LGG, especially pilocytic astrocytomas [17,20,21], molecular analysis at diagnosis would be invaluable for both risk stratification and treatment plan development [22,23], especially in cases of relapse. Younger age also has been identified as a negative prognostic factor in pediatric spinal LGGs [24,25]. Significantly, in our study, 8 (50%) of the 16 patients with progressive disease were 3 years-old or less at diagnosis (Table 1).
Frequently, the diagnosis of spinal LGGs has been delayed due to multiple factors, including their relative rarity and vague presenting symptoms [5,26,27], leading to providers placing a neoplastic diagnosis low on their differential diagnosis. In patients who have tumors in the distal spinal cord, scoliosis, back pain, ataxia, and weakness are the most common symptoms [11]. However, patients who have cervicomedullary tumors, especially involving the fourth ventricle, also can present with more rare symptoms, such as torticollis, vocal cord defects, headaches, and emesis [26,28], as in our study. In fact, acquired torticollis can be the first symptom noted in approximately 10% of spinal tumors in children [26,28,29], leading to diagnosis delay until other concerning neurologic findings are noted. Therefore, it is not surprising that the PSI of our patients averaged approximately 6 months which is consistent with that of our previous study of 16 spinal LGGs [5]. Although diagnosis delay was apparent, our patients who relapsed had approximately the same PSI as those who did not relapse (5.5 versus 6.1 months, respectively, Wilcoxon test p=0.97). This lack of negative impact of delayed diagnosis on PFS could be due to small numbers and the retrospective nature of the study. Others showed that time to diagnosis could impact functional outcome in children with spinal LGGs [18] but did not reach statistical significance. This issue needs to be further investigated in a prospective fashion at a larger scale.
Not surprisingly, the long-term morbidity in our patient population was kyphoscoliosis and residual motor deficits. Endocrinopathies, mainly hypothyroidism, were also noted. Some studies have suggested that preoperative neurological status [18], presence of kyphoscoliosis, laminectomy/laminoplasty spanning more than 4 vertebrae [3], and tumor location, specifically thoracic [30], significantly contribute to the long-term sequelae in this patient population.
One of the strengths of our study is it being one of the larger studies of intramedullary spinal LGGs [3,10]. Also, our hospital is a large, international referral center with advanced facilities, allowing for molecular testing of tumor samples and long follow-up (>20 years in some patients); however, there also can be an institutional bias. Also, the strong neurosurgical program at our institution allows for histologic confirmation in almost all cases, as noted in this study. Another limitation is our study is retrospective and did not allow for power calculations. Though we evaluated patients’ long-term functional outcome, standardized functional testing, such as use of the Modified McCormick Scale [31,32], was not available but would be a significant addition to future studies of this disease. Furthermore, as treatment techniques have become more advanced, the extent of surgical resection and incidence of treatment sequelae have improved, which may skew the results when comparing our data to those of older studies.
Conclusion
Pediatric intramedullary spinal tumors are a very rare subset of tumors that continue to have a favorable OS, though progression is common and associated treatment sequelae affecting quality of life have been noted. Specific recommendations for management of these spinal lesions are not well-defined and are frequently combined with the management of other LGGs. However, given this data, we recommend that one have a high suspicion for a spinal tumor in pediatric patients with persistent back pain, scoliosis, or neurological deficits, including less common presentations, such as unexplained torticollis. GTR should be performed when possible to decrease progression; however, salvage therapy is effective when necessary. Finally, molecular cytogenetics should be incorporated into the standard of care when available to help with risk stratification and salvage therapy selection.
Supplementary Material
Fig.Suppl.1 Swimmer’s plot depicting treatment course from diagnosis to last follow-up/death in patients of the cohort. GTR gross-total resection, NTR near-total resection, STR subtotal resection, NED no evidence of disease, PR partial response, SD stable disease, PD progressive disease
Acknowledgements
We would like to thank Cherise Guess, PhD, ELS, for scientific editing of the final manuscript, and Laura Patterson, AuD, for help with audiogram interpretation.
Funding: This work was supported, in part, by Cancer Center Support Grant CA21765 from the National Cancer Institute and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Compliance with Ethical Standards
Conflict of Interest:
All authors report no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Our institutional review board reviewed and approved this retrospective chart review.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Ostrom QT, Gittleman H, Xu J et al. (2016) CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009–2013. Neuro Oncol 18 (suppl_5):v1–v75. doi: 10.1093/neuonc/now207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qaddoumi I, Sultan I, Gajjar A (2009) Outcome and prognostic features in pediatric gliomas: a review of 6212 cases from the Surveillance, Epidemiology, and End Results database. Cancer 115 (24):5761–5770. doi: 10.1002/cncr.24663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed R, Menezes AH, Awe OO et al. (2014) Long-term incidence and risk factors for development of spinal deformity following resection of pediatric intramedullary spinal cord tumors. J Neurosurg Pediatr 13 (6):613–621. doi: 10.3171/2014.1.PEDS13317 [DOI] [PubMed] [Google Scholar]
- 4.Schneider C, Hidalgo ET, Schmitt-Mechelke T et al. (2014) Quality of life after surgical treatment of primary intramedullary spinal cord tumors in children. J Neurosurg Pediatr 13 (2):170–177. doi: 10.3171/2013.11.PEDS13346 [DOI] [PubMed] [Google Scholar]
- 5.Arnautovic A, Billups C, Broniscer A et al. (2015) Delayed diagnosis of childhood low-grade glioma: causes, consequences, and potential solutions. Childs Nerv Syst 31 (7):1067–1077. doi: 10.1007/s00381-015-2670-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong GT, Conklin HM, Huang S et al. (2011) Survival and long-term health and cognitive outcomes after low-grade glioma. Neuro Oncol 13 (2):223–234. doi: 10.1093/neuonc/noq178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khalil J, Chuanying Z, Qing Z et al. (2017) Primary spinal glioma in children: Results from a referral pediatric institution in Shanghai. Cancer Radiother 21 (4):261–266. doi: 10.1016/j.canrad.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 8.Merchant TE, Kiehna EN, Thompson SJ et al. (2000) Pediatric low-grade and ependymal spinal cord tumors. Pediatr Neurosurg 32 (1):30–36. doi: 10.1159/000028894 [DOI] [PubMed] [Google Scholar]
- 9.Broniscer A, Baker SD, Wetmore C et al. (2013) Phase I trial, pharmacokinetics, and pharmacodynamics of vandetanib and dasatinib in children with newly diagnosed diffuse intrinsic pontine glioma. Clin Cancer Res 19 (11):3050–3058. doi: 10.1158/1078-0432.CCR-13-0306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouffet E, Pierre-Kahn A, Marchal JC et al. (1998) Prognostic factors in pediatric spinal cord astrocytoma. Cancer 83 (11):2391–2399 [DOI] [PubMed] [Google Scholar]
- 11.Hayden Gephart MG, Lober RM, Arrigo RT et al. (2012) Trends in the diagnosis and treatment of pediatric primary spinal cord tumors. J Neurosurg Pediatr 10 (6):555–559. doi: 10.3171/2012.9.PEDS1272 [DOI] [PubMed] [Google Scholar]
- 12.Kutluk T, Varan A, Kafali C et al. (2015) Pediatric intramedullary spinal cord tumors: a single center experience. Eur J Paediatr Neurol 19 (1):41–47. doi: 10.1016/j.ejpn.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 13.Constantini S, Houten J, Miller DC et al. (1996) Intramedullary spinal cord tumors in children under the age of 3 years. J Neurosurg 85 (6):1036–1043. doi: 10.3171/jns.1996.85.6.1036 [DOI] [PubMed] [Google Scholar]
- 14.McAbee JH, Modica J, Thompson CJ et al. (2015) Cervicomedullary tumors in children. J Neurosurg Pediatr 16 (4):357–366. doi: 10.3171/2015.5.PEDS14638 [DOI] [PubMed] [Google Scholar]
- 15.Nadkarni TD, Rekate HL (1999) Pediatric intramedullary spinal cord tumors. Critical review of the literature. Childs Nerv Syst 15 (1):17–28 [DOI] [PubMed] [Google Scholar]
- 16.Reimer R, Onofrio BM (1985) Astrocytomas of the spinal cord in children and adolescents. J Neurosurg 63 (5):669–675. doi: 10.3171/jns.1985.63.5.0669 [DOI] [PubMed] [Google Scholar]
- 17.Yang RR, Aibaidula A, Wang WW et al. (2018) Pediatric low-grade gliomas can be molecularly stratified for risk. Acta Neuropathol 136 (4):641–655. doi: 10.1007/s00401-018-1874-3 [DOI] [PubMed] [Google Scholar]
- 18.Choi GH, Oh JK, Kim TY et al. (2012) The clinical features and surgical outcomes of pediatric patients with primary spinal cord tumor. Childs Nerv Syst 28 (6):897–904. doi: 10.1007/s00381-012-1718-8 [DOI] [PubMed] [Google Scholar]
- 19.Kothbauer KF (2007) Neurosurgical management of intramedullary spinal cord tumors in children. Pediatr Neurosurg 43 (3):222–235. doi: 10.1159/000098835 [DOI] [PubMed] [Google Scholar]
- 20.Lassaletta A, Zapotocky M, Mistry M et al. (2017) Therapeutic and Prognostic Implications of BRAF V600E in Pediatric Low-Grade Gliomas. J Clin Oncol 35 (25):2934–2941. doi: 10.1200/JCO.2016.71.8726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cruz GR, Dias Oliveira I, Moraes L et al. (2014) Analysis of KIAA1549-BRAF fusion gene expression and IDH1/IDH2 mutations in low grade pediatric astrocytomas. J Neurooncol 117 (2):235–242. doi: 10.1007/s11060-014-1398-1 [DOI] [PubMed] [Google Scholar]
- 22.Banerjee A, Jakacki RI, Onar-Thomas A et al. (2017) A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: a Pediatric Brain Tumor Consortium (PBTC) study. Neuro Oncol 19 (8):1135–1144. doi: 10.1093/neuonc/now282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Upadhyaya SA, Robinson GW, Harreld JH et al. (2018) Marked functional recovery and imaging response of refractory optic pathway glioma to BRAFV600E inhibitor therapy: a report of two cases. Childs Nerv Syst 34 (4):605–610. doi: 10.1007/s00381-018-3739-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ater JL, Zhou T, Holmes E et al. (2012) Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J Clin Oncol 30 (21):2641–2647. doi: 10.1200/JCO.2011.36.6054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gajjar A, Sanford RA, Heideman R et al. (1997) Low-grade astrocytoma: a decade of experience at St. Jude Children’s Research Hospital. J Clin Oncol 15 (8):2792–2799. doi: 10.1200/JCO.1997.15.8.2792 [DOI] [PubMed] [Google Scholar]
- 26.Wilne S, Collier J, Kennedy C et al. (2007) Presentation of childhood CNS tumours: a systematic review and meta-analysis. Lancet Oncol 8 (8):685–695. doi: 10.1016/S1470-2045(07)70207-3 [DOI] [PubMed] [Google Scholar]
- 27.Wilne S, Koller K, Collier J et al. (2010) The diagnosis of brain tumours in children: a guideline to assist healthcare professionals in the assessment of children who may have a brain tumour. Arch Dis Child 95 (7):534–539. doi: 10.1136/adc.2009.162057 [DOI] [PubMed] [Google Scholar]
- 28.Kumandas S, Per H, Gumus H et al. (2006) Torticollis secondary to posterior fossa and cervical spinal cord tumors: report of five cases and literature review. Neurosurg Rev 29 (4):333–338; discussion 338. doi: 10.1007/s10143-006-0034-8 [DOI] [PubMed] [Google Scholar]
- 29.Fafara-Les A, Kwiatkowski S, Marynczak L et al. (2014) Torticollis as a first sign of posterior fossa and cervical spinal cord tumors in children. Childs Nerv Syst 30 (3):425–430. doi: 10.1007/s00381-013-2255-9 [DOI] [PubMed] [Google Scholar]
- 30.Scheinemann K, Bartels U, Huang A et al. (2009) Survival and functional outcome of childhood spinal cord low-grade gliomas. Clinical article. J Neurosurg Pediatr 4 (3):254–261. doi: 10.3171/2009.4.PEDS08411 [DOI] [PubMed] [Google Scholar]
- 31.McCormick PC, Stein BM (1990) Intramedullary tumors in adults. Neurosurg Clin N Am 1 (3):609–630 [PubMed] [Google Scholar]
- 32.McCormick PC, Torres R, Post KD et al. (1990) Intramedullary ependymoma of the spinal cord. J Neurosurg 72 (4):523–532. doi: 10.3171/jns.1990.72.4.0523 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig.Suppl.1 Swimmer’s plot depicting treatment course from diagnosis to last follow-up/death in patients of the cohort. GTR gross-total resection, NTR near-total resection, STR subtotal resection, NED no evidence of disease, PR partial response, SD stable disease, PD progressive disease