Abstract
Despite traditionally treating autologous and allogeneic transplantation and emerging tissue engineering (TE)-based therapies, which have commonly performed in clinic for skeletal diseases, as the “gold standard” for care, undesirably low efficacy and other complications remain. Therefore, exploring new strategies with better therapeutic outcomes and lower incidences of unfavorable side effect is imperative. Recently, exosomes, secreted microvesicles of endocytic origin, have caught researcher’s eyes in tissue regeneration fields, especially in cartilage and bone-related regeneration. Multiple researchers have demonstrated the crucial roles of exosomes throughout every developing stage of cartilage and bone tissue regeneration, indicating that there may be a potential therapeutic application of exosomes in future clinical use. Herein, we summarize the function of exosomes derived from the primary cells functioning in skeletal diseases and their restoration processes, therapeutic exosomes used to promote cartilage and bone repairing in recent research, and applications of exosomes within the setting of the TE matrix.
Keywords: exosomes, cartilage regeneration, bone regeneration, tissue engineering
Introduction
Cartilage and bone are specific forms of connective tissue, which greatly supports and protects other tissues and organs in the body. With the significant increase of cartilage diseases and bone defects caused by an aging population, trauma, and congenital abnormalities, the demand for efficacious therapies to regenerate these tissues is growing.1,2 Currently, the “gold standard” clinical treatment for both cartilage and bone restoration is autologous and allogeneic transplantation. However, secondary surgery, limited harvesting sources, and complications often render tissue transplantation powerless as a clinical treatment, especially for articular cartilage damage and critical-sized bone defects.3,4 In addition, in cases in which patients received successful grafts, the recovery time is prolonged, resulting in higher risk of complications. Since the initial definition by Vacanti,5 the concept of tissue engineering has been extensively leveraged to design a combination of artificial transplantable substrates with cells and bioactive agents, which are able to accelerate the recovery procedure, in order to provide a promising alternative to further improve cartilage and bone regeneration.6-8 Despite a series of merits and successes in preclinic therapies based on the tissue engineering, major complications remain. Ethical concerns about cell sources, high expenses for both autologous and allogeneic cell-involved treatment, and adverse effects such as immune dysfunction and tumors triggered by indelicate incorporation of exogenous biological factors all represent challenges in the field.9-12 As a result, improved strategies to enhance cartilage and bone regeneration in a manner ensuring safety and efficacy are urgently needed.
In recent years, exosomes, one type of extracellular vesicles (EVs) secreted by cells and that range in size from 50 to 130 nm, have been studied in-depth for their diverse origins and releasing mechanisms. They were found to play emerging roles in many biological processes, such as intercellular communication, immune system regulation, and life cycles of cellular communities.13-16 As an endosome-derived small EV secreted by most of cells, exosomes are also effective transport vesicles that can carry protein content in their cores, as well as on their surface lipid layer, and also contain diverse types of genetic information, including DNA, messenger RNA, microRNAs (miRNAs), and some small noncoding RNAs (long non-coding RNA (lncRNA), circular RNA (cirRNA)).17-20 In virtue of their capacity to carry abundant biological signals, exosomes have been investigated and utilized to effectively facilitate restoration in other tissue or organ defects, including heart, liver, and kidney.21,22 For instance, the transfer of nucleic RNAs in exosomes could regulate a series of behaviors such as reperfusion of glycerol in the kidney,23 ischemia in cardiovascular disease,24 or hepatic fibrosis,21 all of which contribute to the reconstitution and functional recovery of these organs. Similarly, such small vesicles have also been reported to play a crucial role in the repair activities related to bone and cartilage. With the in-depth study of intracellular communication in bone and cartilage regeneration, the effect of exosomes on stimulating the function of cells, including chondrocytes, osteoblasts, osteoclasts, precursor cells, as well as immune cells, has been increasingly appreciated. Compared with current cell-based therapies in cartilage and bone tissue repair, sources of exosomes with diverse functions are much more abundant, resolving the limited availability of transplanted cells to great extent. Most importantly, due to the naturally derived ingredients, exosomes have fewer safety concerns, lower toxicity, and fewer immunogenicity problems than living cell transplantation or synthetic implantation. Therefore, treatment with exosomes has been deemed as a promising strategy to improve cartilage repair and bone growth in the clinical setting.
In this review, we will summarize the role of exosomes in the procedure of both cartilage and bone regeneration. Particularly, treatment with exosomes involved in cartilage defects, bone defects, associated vascular reconstitution, and immune regulation will be introduced in detail with recent research. Based on the current trend, we will give a comprehensive assessment of exosomes as therapeutic agents, bring forward suggestions for improved design using the principles of tissue engineering, and provide future outlooks of exosome-based therapy for cartilage and bone tissue regeneration.
The Role of Exosomes in the Cartilage and Bone Tissue Regeneration
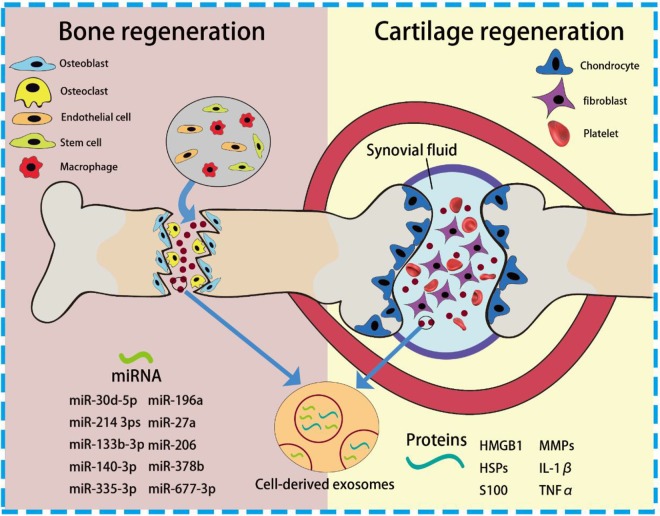
Harmonious cartilage and bone tissue regeneration rely on multicellular activities, which range from immunogenicity, functional cell recruiting, differentiation, remodeling, and angiogenesis. Up until now, a large amount of literature has been focused on the stimulation and responses of the specific cells which dominate the restoration, such as phenotype of macrophages, differentiation of stem cells (SCs), and formation or activation of chondrocytes, osteoblasts, and osteoclasts.25-28 Because of the highly intertwined nature of these regeneration processes, cell-to-cell communication is supposed to greatly affect the progression of repair. As a primary element in cell signaling, exosomes that can convey bioactive lipids, nucleic acids, and proteins are currently a hot spot in the regeneration field. In this section, roles of exosomes in the cartilage and bone tissue regeneration will be discussed in detail (Figure 1).Exosomes in the Cartilage Repair
Figure. 1.
Schematic of cell-derived exosomes and incorporated cargos during cartilage and bone tissue regeneration.
Cartilage, a connective tissue found primarily in joints, has limited capacity for intrinsic regeneration upon injury or lesions. These injuries would be further aggravated by several joint diseases, especially osteoarthritis (OA) and rheumatoid arthritis (RA). As synovial fluid (SF) and incorporated synovial fibroblasts (SFBs) could induce inflammatory propagation and degeneration of cartilage, SF or SFBs-derived EVs are the primary candidates for study in order to elucidate the mechanisms of joint diseases and cartilage damage. As previous research has demonstrated, SF or SFBs-derived EVs can trigger inflammation and further contribute to inflammatory propagation by carrying damage-associated molecular patterns and associated cytokines and miRNAs, thereby influencing downstream cells and causing cartilage degeneration.29 When tissues face stress or injury, high-mobility group protein B1 are highly concentrated within EVs, with heat-shock proteins and S100 proteins also aggregating, giving rise to the inflammatory activation of macrophages. For instance, Domenis’ group reported the immunogenic regulation of SF-derived exosomes from advanced OA patients on M1 macrophages.30 After intaking the SF-derived exosomes, M1 macrophages were found to produce a series of typical proinflammatory cytokines and chemokines, including most members of matrix metalloproteinases (MMPs), interleukin (IL)-1β, IL-16, CXCL1, and CCL15. Compared with OA-induced cartilage degeneration, RA, an autoimmune disease, could contribute more severe and complex inflammatory responses which in turn cause cartilage damage and other morbidities. Importantly, exosomes from SFBs obtained from RA patients were supposed to contain several proinflammatory factors on the surface membrane, such as MMPs, IL-1β, and tumor necrosis factor α (TNFα), rendering T-cells activated and resistant to apoptosis, and further triggering the pathogenic process of RA and irreversible cartilage erosion. Furthermore, platelet-derived microvesicles (PMVs), another active player in patients with RA, were found to be abundant in the SF, contributing to the severity of a series of diseases. Because of their procoagulant features, the abundance of PMVs in patients with RA was found to inhibit the protective function of endothelial cells (ECs) as well as angiogenesis, resulting in cardiovascular comorbidities.31
Human SCs such as mesenchymal SCs (MSCs) and adipose tissue-derived SCs currently dominate clinical trials for treatment of cartilage injuries induced by RA and OA. It is well acknowledged that coculture of SCs with injured cartilage tissue can promote regeneration through a mass of secreted factors from SCs including IL-6, fibroblast growth factor 2, and insulin-like growth factor, which are verified to promote proliferation and matrix synthesis in chondrocytes.32,33 However, as with all cell-based therapies, SCs-based therapy comes with concerns related to their limited source, safety, ethical issues, and high operational costs for harvest, expansion, and storage.34 Inspired by the outcome that the secretion of SCs can effectively promote tissue regeneration, exosomes from SCs, as the principal agents causing the therapeutic efficacy, have been extensively investigated and identified as a promising therapy, especially for diseases associated with cartilage damage. Such therapeutic function of exosomes for cartilage regeneration will be introduced in the following chapters in detail.
Exosomes in the Bone Defect Repair
Bone fractures resulting from severe trauma, malignancy resection, infections, and an aged population have raised significant clinical challenges all over the world. Although great progress has been made in bone tissue engineering, such as engineering scaffolds, hydrogels, growth factors, and cell-based therapies, undesirable efficacy and safety issues remain unaddressed.35-37 Moreover, current research on bone regeneration often focuses on the specific osteogenic stage and affiliated cell behaviors, impeding clear understanding of bone biogenesis and novel approaches to stimulate bone regeneration.25 Exosomes provide not merely a new version to disentangle the complicated bone healing processes but a novel strategy to improve bone repair.
Bone regeneration can be classified into 3 primary stages: immunomodulation, osteogenesis, and bone remodeling. In these overlapping stages, immune cells, osteoblasts, osteoclasts, chondrocytes, and ECs have been reported to be major participants. As essential mediators of intercellular communication and carriers for functional cargo delivery, exosomes were recently found to be fundamental elements in the immune stage, as well as in bone healing, bone metabolism, and mineralization of remodeling. As for osteoimmunomodulation, monocytes and macrophages are known to play a crucial role in the innate immune response and subsequent osteogenic function. They secrete osteoinductive molecules after cellular polarization into proinflammatory (M1) and anti-inflammatory (M2) phenotypes.38 Recently, emerging results demonstrated that macrophage-derived EVs could increase the release of EVs and induce cellular differentiation in naive monocytes. According to Ismail’s group, RNA molecules incorporated in macrophage-derived microvesicles could be transported to target cells, especially monocytes, thereby increasing microvesicles production and driving associated differentiation of macrophages.39 With diverse external stimulus on macrophages such as growth factors, macrophage-derived exosomes tend to exhibit beneficial effects on new bone formation. A recent research article reported that exosomes from BMP2-stimulated macrophages could improve osteogenic differentiation and bioactivity of titanium-based implants.40 Other than immune cells, many investigators have also focused on the function of EVs from nonimmune cells in the osteoimmunomodulatory stage. Extracellular vesicles from MSCs have been extensively reported to polarize monocytes toward corresponding phenotypes (primarily toward an anti-inflammatory M2 phenotype). Zhang et al demonstrated that MSC-derived exosomes exhibited high expression of anti-inflammatory IL-10 and TGFβ1 transcripts, while proinflammatory expression of IL-1β, IL-6, and TNF-α transcripts was significantly decreased, thus activating regulatory T cells (Tregs) and enhancing the healing effects of allogenic skin grafts in mice.41 Similar results were reported in the work of Sicco et al, in which EVs generated under both normoxic and hypoxic conditions exhibited anti-inflammatory function and were able to shift the balance of monocytes toward M2 phenotype.42 Moreover, EVs produced by ECs are able to trigger osteoimmune responses and thus regulate new formation of blood vessels which coordinate nutrient and oxygen transfer. This kind of angiogenesis is deemed as a synergetic behavior in bone regeneration. Deregibus et al found that the treatment of endothelial progenitor cell (EPC)-EVs could induce human umbilical vein ECs (HUVECs) and human microvascular ECs (HMECs) to form vessel-like structures both in vivo and in vitro via the activation of the PI3/AKT and endothelial nitric oxide synthase (eNOS) pathways.43
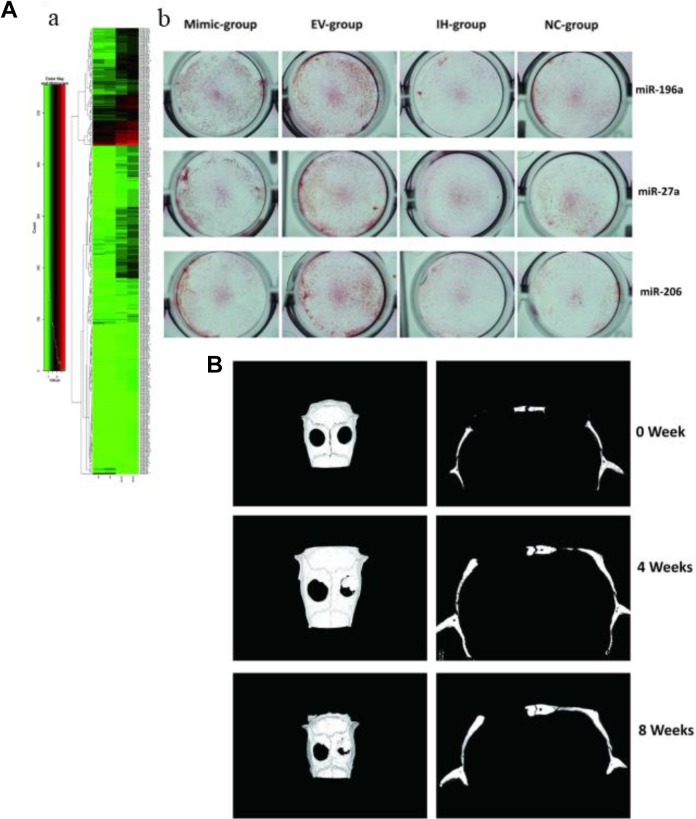
When it comes to the ossification stage, osteoblasts, whose major function are bone formation, have been extensively investigated. Also, exosomes derived from osteoblasts were found to stimulate bone regeneration by regulating the differentiation behaviors of precursor cells and thereby improving osteoblasts’ proliferation. In the work of the Cui et al group, exosomes derived from mineralizing osteoblast cells (MOBs) were evidenced to promote the differentiation of precursor cells from bone marrow to mature osteoblasts through regulating miRNA profiles (miR-30d-5p, miR-133b-3p miR-140-3p, miR-335-3p, miR-378b, and miR-677-3p) in the recipient cells and consequently activating downstream signaling pathways, especially Wnt signaling insulin signaling, TGF-β signaling, and calcium signaling.44 Exosomes from other sources can also stimulate bone formation by directly regulating osteoblast proliferation and thereby improving matrix mineralization. Recently, tumor-derived EVs were also found to be able to affect osteoblast behavior in bone regeneration. In a study conducted by Inder et al, prostate cancer cell-derived exosomes significantly increased human osteoblast proliferation, as well as osteoclast activation and osteoclastogenesis.45 Other than the striking in vitro influence, the obvious in vivo influence of exosomes on osteoblasts further confirmed the function of exosomes on osteogenesis. As illustrated in Figure 2, Qin et al showed that EVs had a significant effect on the expression of osteogenic genes (miR-196a, miR-27a, and miR-206) and osteoblastic differentiation without a negative influence on osteoblastic proliferation. Most importantly, the in vivo calvarial defects model when treated with bone marrow-derived SC (BMSC)-derived exosomes (Figure 2B), exhibited significantly earlier healing of defects. This indicated the potent therapeutic effect of exosomes on bone fracture.46In addition to the stage of osteogenesis, bone remodeling involving osteoblasts and osteoclasts is an essential part of reconstruction that occurs throughout the healing process.25 As is well known, coordinated bone regeneration relies on orchestrated resorption and new bone formation. It involves multicellular activities and complex cellular interplay. Exosomes that mediate the function of intracellular communication play an essential role in this mechanism. Previously, interplay between osteoblasts and osteoclasts and crosstalk between the related signaling pathways have been widely reported to regulate bone remodeling during regeneration. In light of these interaction, exosome-mediated intercellular communication between osteoblasts and osteoclasts can provide a new vision for bone regeneration. Moreover, exosomes derived from osteoclasts themselves or osteoclast precursors could be the regulators of osteoclastogenesis by stimulating the differentiation of osteoclasts into their mature phenotypes. But notably, many research teams have reported that osteoclast-derived exosomal miRNAs, especially osteoclastic miR-214-3ps, represent a novel class of osteoclast-released coupling factors that could suppress osteoblastic differentiation and reduce bone formation.47 Such negative regulation is consistent with previous results on the cellular level that the proliferation and differentiation of osteoblasts or their precursor cells could be impaired by coculture with osteoclasts. Conversely, exosomes derived from osteoblasts were recently demonstrated to be able to induce differentiation and maturation of osteoclasts through the RANKL protein secreted by osteoblasts and through corresponding receptor ligand (RANKL-RANK) signaling in osteoclasts.48 Based on the aforementioned discussion of diverse functions and interactions between participant cells and their exosomes, a clear understanding of the role of exosomes in each stage for bone regeneration is still uncovered and requires to be perfected in order to maximize the therapeutic applications of exosomes in this field.
Figure. 2.
A-a, The RNA sequencing indicated that miR-196a, miR27a, and miR-206 were highly increased in EVs from BMSCs. A-b, Alizarin Red staining of osteoblasts treated with miR-196a, miR-27a, and miR-206 at 3 days. EV’s group indicated significant increase of calcium compounds after treatment of miR-196a, miR-27a, and miR-206. B, Micro-CT from 0 to 8 weeks after surgery showed that the EV delivery system (right defect) improved bone repair compared with gel group (left defect) (Reprinted with permission from46. Copyright © 2016, Springer Nature). BMSCs indicates bone marrow-derived stem cells; CT, computed tomography; EV, extracellular vesicle.
Exosomes as Therapeutic Agents for Cartilage and Bone Regeneration
Tissue engineering-based biomaterials and cell therapy are the major fields in preclinical research into cartilage and bone regeneration. Although these strategies partially solve the problems that clinics are facing with autologous/allogeneic transplantation, some drawbacks remain. Suboptimal biocompatibility or bioactivity of the implant, and side effects related with tumor and emboli formation, often cause undesirable regenerative effects. With therapeutic exosome technology emerging in the context of tissue and organ recovery, their capacity for containing functional cargos and their specific immune-privileged properties caught the attention of researchers in the field of cartilage and bone repair. In the following section, recent studies of therapeutic exosomes for cartilage and bone regeneration will be introduced, with additional discussion of potential role of exosomes as a clinical therapeutic.
Therapeutic Exosomes for Cartilage Repair and Debilitating Cartilage Diseases
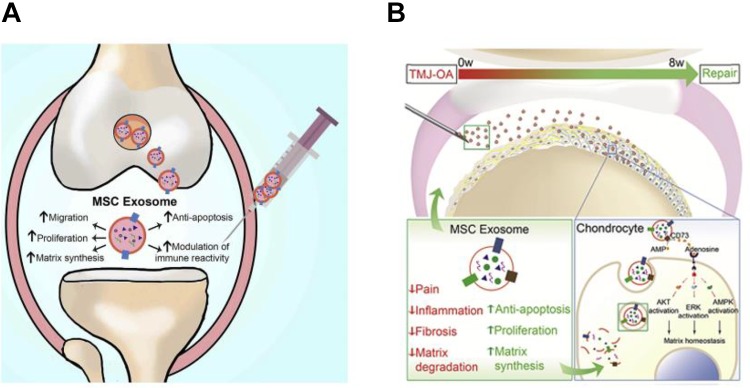
Current clinical treatment modalities for cartilage injuries are primary cell-based therapies. Among these, MSCs transplantation has been widely used in the clinical setting of cartilage repair due to its paracrine effects and extensive capacities for proliferation and differentiation into various cell lineages. However, as with all cell-based therapies, high operational costs and other challenges impede its further application. Cell-based medicine often requires stringently monitored manufacturing and storage procedures to ensure optimal viability of the cells before final delivery to the patient.34,49 In this case, therapy based on SC-derived exosomes seems to be an ideal alternative because of their stability, easy accessibility, and extensive source supply.Given that the efficacy of MSC-based therapies for joint diseases is ascribed to paracrine secretion, exosomes, as one of the products of MSC secretion, which also have a mass of biologically active factors, have been proposed as an effective treatment for the restoration of osteochondral defects. The work of Cosenza et al proved exosomes from MSCs could exert a chondroprotective effects in joint diseases. Intra-articular injection of MSC-derived exosomes into a collagenase-induced OA model was found to significantly induce the expression of chondrocyte markers (type II collagen, aggrecan) while inhibiting catabolic (MMP-13, ADAMTS5) and inflammatory (iNOS) markers which could give rise to the apoptosis of chondrocytes as well as the aggravation of inflammation through the activation of monocytes.50 Similarly, Zhang et al used in vivo critical-sized osteochondral defects to further verify the function and efficiency of human embryonic MSC-derived exosomes for cartilage repair. In this case, exosome-treated defects exhibited complete restoration of cartilage and subchondral bone with moderate surface regularity and good integration with the host cartilage.51 To systematically elucidate the therapeutic role of exosomes, Zhang’s group clarified the function of MSC-derived exosomes in the cartilage through a multi-faceted assessment of chondrocyte behaviors (proliferation and apoptosis) and immune responses.49 As shown in Figure 3A, MSC exosomes were found to initiate or motivate the migration and proliferation of chondrocytes, and simultaneously attenuate apoptosis in chondrocytes, consequently promoting chondral matrix synthesis through the activation of the AKT and/or ERK signaling pathway. Other than the beneficial impact of MSC-derived exosomes on chondrocyte activation, a significant immune component also cannot be ignored. In this study, higher amounts of CD163+ regenerative M2 macrophages were observed at the defect site with a significantly lower amount of proinflammatory synovial cytokines, including IL-1β and TNF-α. This is in accordance with the results shown in recent studies for MSC-derived exosomes on osteoimmunological regulation. For a more specific cartilage-related disease, temporomandibular joint-OA (TMJ-OA), the regulation of MSC exosomes on early immune responses, was further investigated by Zhang and his group.52 In agreement with the research on common OA treatment reported before, MSC exosomes caused an early suppression of local inflammation through a significantly reduced expression of inflammatory genes especially IL-1β, thus disactivating inflammatory IL-β+ and iNOS+ cells.53,54 Similar with the previous report, chondral matrix production was induced through the activation of AKT and/or ERK signaling pathway and remarkable restoration of condylar structure, matrix deposition, and subchondral bone integrity could be achieved, which closely resembled the intact TMJ condyle (Figure 3B).
Figure. 3.
A, Therapeutic function of MSC exosomes on cartilage regeneration through multiple facets including the increasing the viability of chondrocytes and modulating immune responses. Reprinted with permission from49. Copyright © 2018 Elsevier Ltd. B, Underlying mechanism of MSC exosomes promoting TMJ repair and regeneration in OA (Reprinted with permission from52. Copyright © 2019 Elsevier Ltd). MSC indicates mesenchymal stem cell; OA, osteoarthritis; TMJ, temporomandibular joint.
Therapeutic Exosomes for Bone Defect Repair and Angiogenesis
There is growing evidence to show that exosomes can regulate and guide differentiation of MSCs into the osteoblastic lineage and thus directly promote bone regeneration. Among diverse sources of exosomes, MSC-derived exosomes have been widely identified as the most effective to induce osteodifferentiational activity. Profiling of the MSC-derived exosomes has verified the function of osteodifferentiation through a series assessment of the regulation of miRNAs expression.55 For example, let-7 was reported to be a positive regulator of osteogenesis and new bone formation through suppressing the expression of high-mobility group AT-hook 2.56 Another miRNA, miR-199b was involved in the regulation of Runx 2, which can control the differentiation of MSCs into osteoblasts and osteocytes.55 Moreover, upregulation of miR-135b expression was found to significantly suppress osteogenic differentiation of MSCs derived from multiple myeloma patients, while downregulation of miR-221 induced osteogenic differentiation in both human unrestricted somatic SCs and human MSCs.57,58 All these investigations of miRNAs’ function represent the influence of bioinformatic MSC-derived exosomes on osteogenic differentiation and other osteoactivities. This provides a foundation for using MSC-derived exosomes and miRNA-based therapies in clinical applications. Aside from the exosomes originating from MSCs, exosomes from other sources such as osteoblasts could also establish a positive feedback loop to promote new bone growth. As mentioned above, exosomes derived from MOBs could directly regulate osteoblast proliferation and simultaneously promote the differentiation of precursor cells from bone marrow to mature osteoblasts through mediating miRNA profiles. This could in turn activate downstream signaling pathways related to osteogenesis and improving matrix mineralization.44
As is well known, vascularization is extremely important for bone regeneration since new vessel formation improves diffusion of oxygen and nutrition, which are essential to new bone formation.59 It has been widely demonstrated that exosomes can stimulate the activities of ECs including proliferation, migration, and tube formation. In previous discussion, we mentioned a report that EPCs-EVs can induce HUVECs and HMECs to form vessel-like structures both in vivo and in vitro through the activation of the PI3/AKT and eNOS pathways.43 In addition, multiple experiments have shown that MSC-derived exosomes can successfully improve angiogenesis both in vitro and in vivo.60,61 Although there have been many studies on MSC-derived exosomes stimulating angiogenesis, only one experiment was closely associated with the effect of exosomes on both osteogenesis and angiogenesis. The result showed that incorporation of MSC-derived EVs into a TE-based matrix induced pro-angiogenic and pro-osteogenic activities. This indicated that synergistic bone regeneration with simultaneously enhanced angiogenesis and osteogenesis can be regulated by exosomes.62 The underlying mechanism of the coupling of angiogenesis and osteogenesis in the presence of exosomes is still unknown, and exploring the roles of exosomes in bone and vessel formation is expected to aid the development of novel treatment for bone regeneration.
Exosome/Matrix Combination for Cartilage and Bone Tissue Engineering
Although conventional therapies such as autografts and allografts are considered as the “gold standard” for treating cartilage and bone diseases, limited sources and severe morbidities render these strategies dissatisfactory for the treatment of many serious skeletal defects.3 With the introduction of tissue engineering and the development of artificial transplantable materials, biological knowledge combined with artificial matrices provide a promising alternative for cartilage and bone restoration.5 In recent decades, hydrogels (high water content and high cartilage matrix mimetics) and biomimetic scaffolds (with their natural composition and hierarchically porous structure) have been widely used as carriers or shields of cells and factors for both cartilage and bone regeneration. These materials better support the mechanical requirements of initial implantation and they also provide desirable releasing profiles of bioactive factors.63-66 According to recent studies, exosomes have been identified as a novel and effective therapy for skeletal tissue repair. Unfortunately, the common mode of administration for therapeutic exosomes was primarily through injection, in which therapeutic exosomes were unable to be retained at the desired site because they were inevitably rapidly cleared.60 Therefore, immobilization of therapeutic exosomes in engineered hydrogels and scaffold matrices is a very promising option for tissue regeneration (See Table 1).
Table 1.
Recent Progress of Therapeutic Exosomes/Engineered Hydrogels for Cartilage and Bone Tissue Regeneration.
| Type | Origin of Exosomes | TE Matrix | In Vitro and In Vivo Effect | Reference |
|---|---|---|---|---|
| Cartilage | hiPS-MSC-exosomes | Acellular tissue patch composed of photo-induced imine crosslinking (PIC) hydrogel | Good integration with native cartilage matrix and enhanced cell deposition at cartilage defect sites | 67 |
| MSC-derived Exosomes | 3D printed cartilage extracellular matrix (ECM)/gelatin methacrylate hybrid scaffold | Improved chondrocyte migration. Recovery of degraded cartilage caused by mitochondrial dysfunction and oxidative stress. Excellent integration of the hybrid scaffold with surrounding tissue | 68 | |
| hiPS-MSC-Exos | β-TCP | Remarkable enhancement of osteogenic differentiation of hBMSCs through PI3K/Akt signaling pathway | 69 | |
| Bone | hASC-derived exosomes | PLGA/pDA scaffolds | Controlled release of hASC-derived exosomes and accelerated bone healing in in vivo critical-sized defects | 70 |
| MSC-derived exosomes | DBM scaffolds | Enhance bone regeneration by proangiogenic activity | 62 | |
| Macrophage-derived exosomes | Titanium oxide nanotubes | Increased expression of early osteoblastic differentiation markers, such as ALP and BMP2 | 40 |
Abbreviations: DBM, decalcified bone matrix; hASC, human adipose stem cell; hBMSC, human bone marrow stem cell; hiPS-MSC-Exos, human-induced pluripotent SC-derived exosomes; MSC, mesenchymal stem cells; PLGA/pDA, polydopamine-coating poly(lactic-co-glycolic acid); TE, tissue engineering; β-TCP, β-tricalcium phosphate.
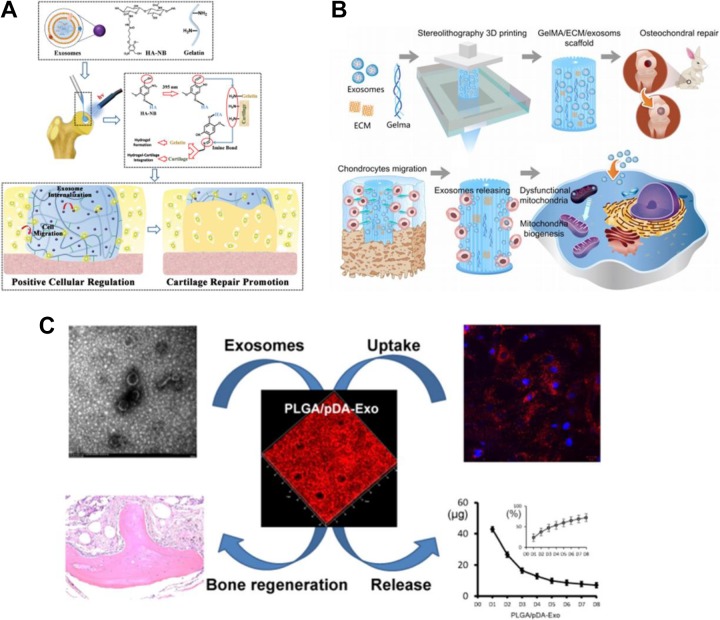
Regarding bone tissue regeneration, scaffolds or other supporting matrices are appealing for bone repair applications because of the high mechanical demand when bone defects occur. However, inadequate osteoactivities of synthetic materials limit their healing effects after surgical implantation, leading to long recovery periods and a series of complications. To address this drawback, incorporation of bioactive compounds has been widely used to modify the implant for more desirable osteoactivities that promote bone healing. When compared to traditional bioactive compounds such as growth factors and cell-based therapies, exosome-related therapies have a far lower probability of adverse effects and they also have adequate parent cell sources which ensure sufficient production. Therefore, many studies have identified exosomes as a potentially useful bioactive factor and incorporated it into TE scaffolds. Zhang et al combined human-induced pluripotent SC-derived exosomes (hiPS-MSC-Exos) with tricalcium phosphate (β-TCP) to promote bone regeneration.70 Compared to bare β-TCP scaffolds, hiPS-MSC-Exos/β-TCP scaffolds showed a remarkable enhancement of osteogenic differentiation of human bone marrow stem cells through PI3K/AKT signaling pathway. Similarly, poly(lactic-co-glycolic acid), PLGA, scaffolds, another loading matrix commonly used in bone tissue engineering, was used by the Li et al group to immobilize human adipose SC (hASC)-derived exosomes for improved bone regeneration.69 As shown in Figure. 4C, by virtue of the controlled release of hASC-derived exosomes from the polydopamine-coating PLGA scaffolds, significantly accelerated bone healing could be accomplished in in vivo critical-sized defects. In addition to the osteogenesis, vascularization is also a crucial factor for bone regeneration as discussed before. Many reports have demonstrated that MSC-derived exosomes are potently proangiogenic both in vitro and in vivo. In Xie et al’s study, the synergistic effect of EVs on osteogenesis and angiogenesis, with the support of decalcified bone matrix (DBM) scaffolds, was investigated for enhanced proangiogenic and probone regenerative effects.62 Through in vitro and in vivo assessment, MSCs-derived EVs functionalized DBM scaffolds were proved to significantly enhance bone regeneration by proangiogenic activity. This positive outcome provided a new horizon for exosome-based synergetic therapy in bone regeneration.
Figure. 4.
A, Synthesis of acellular tissue patch and exosomes for cartilage regeneration (Reprinted with permission from67 Copyright © 2017 Royal Society of Chemistry). B, Combination of extracellular matrix/MSC-derived exosomes through desktop-stereolithography 3D printing technology for osteochondral defect regeneration. (Reprinted with permission from68 Copyright © Ivyspring International Publisher). C, hASC-derived exosomes loading on tissue-engineered PLGA/pDA scaffolds for promotion of bone tissue regeneration (Reprinted with permission from69, Copyright © 2018 American Chemical Society). MSC indicates mesenchymal stem cell; hASC, human adipose stem cell.
Conclusion and Perspective
As an emerging factor for intracellular communication, exosomes have been evidenced to play a crucial role in many skeletal activities and are a highly promising treatment for patients with cartilage and bone defects. Compared to conventional therapies in clinical use, exosomes, with bioactive cargos loaded inside and biomarkers on their surface lipid layer, show excellent stability, favorable maneuverability, and have an extensive source supply. In combination with a TE-matrix such as a biomimetic hydrogel or a scaffold, therapeutic exosomes could sustainably exert their function throughout the entire process of bone regeneration. Although the merits, strength, and positive outcomes of exosomes, especially SCs-derived exosomes, have been presented by mass of preclinical studies, therapeutic exosomes for cartilage and bone tissue regeneration are still in preliminary stages. Countless problems remain to be solved. One of the most important challenges is clearly understanding the composition of the exosomes. Although extensive investigation has been conducted into the influence and application of exosomes, few studies show in-depth analysis of their specific function and biodistribution after intake. Profiling and establishing a clear understanding of exosomes are the top priority before their further application in clinic. Moreover, optimal purification technique for exosome isolation has not been established and remain a future challenge. Currently, physical isolation techniques are still mainstream. However, relatively low quantity of exosomes can be recovered from the mix of other microvesicles. Moreover, obstacles associated with large scale production for clinical studies are hard to overcome.71,72 Therefore, a distinct optimal purification technique for isolation of exosomes with high recovery and purity is absolutely necessary. In summary, exosome-based therapy has represented great potential in the field of cartilage and bone regeneration with a mass of inspiring preclinical results. But notably, there is still a long way to go before therapeutic exosomes can reach their full clinical potential. The exact mechanism of exosomes during regeneration remains elusive, and techniques of exosome collection must be improved for large-scale production.
Acknowledgments
We would also like to thank Emily Park (Carnegie Mellon University, Departments of Chemical and Biomedical Engineering) for her careful and critical reading of this manuscript.
Authors’ Note: Yanxin Liu and Yifan Ma contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors wish to express their gratitude for the financial support from the National Natural Science Foundation of China for Innovative Research Groups (No.51621002), National Natural Science Foundation of China (No. 31771040), National Key R&D Program of China (2018YFC1105700) and Leading talents in Shanghai in 2017.
ORCID iD: Yifan Ma  https://orcid.org/0000-0002-9371-4365
https://orcid.org/0000-0002-9371-4365
References
- 1. Yang K, Zhang J, Ma X, et al. Beta-Tricalcium phosphate/poly(glycerol sebacate) scaffolds with robust mechanical property for bone tissue engineering. Mater Sci Eng C Mater Bio Appl. 2015;56:37–47. [DOI] [PubMed] [Google Scholar]
- 2. Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(18):3413–3431. [DOI] [PubMed] [Google Scholar]
- 3. Kolambkar YM, Boerckel JD, Dupont KM, et al. Spatiotemporal delivery of bone morphogenetic protein enhances functional repair of segmental bone defects. Bone. 2011;49(3):485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Medvedeva EV, Grebenik EA, Gornostaeva SN, et al. Repair of damaged articular cartilage: current approaches and future directions. Int J Mol Sci. 2018;19(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cao YL, Vacanti JP, Paige KT, Upton J, Vacanti CA. Transplantation of chondrocytes utilizing a polymer-cell construct to produce tissue-engineered cartilage in the shape of a human ear. Plast Reconstr Surg. 1997;100(2):297–302. [DOI] [PubMed] [Google Scholar]
- 6. Wu S, Liu X, Yeung KWK, Liu C, Yang X. Biomimetic porous scaffolds for bone tissue engineering. Mater Sci Eng R. 2014;80:1–36. [Google Scholar]
- 7. Ma Y, Zhang W, Wang Z, et al. PEGylated poly(glycerol sebacate)-modified calcium phosphate scaffolds with desirable mechanical behavior and enhanced osteogenic capacity. Acta Biomater. 2016;44:110–124. [DOI] [PubMed] [Google Scholar]
- 8. Moutos FT, Guilak F. Composite scaffolds for cartilage tissue engineering. Biorheology. 2008;45(3-4):501–512. [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang W, Tian Y, He H, et al. Strontium attenuates rhBMP-2-induced osteogenic differentiation via formation of Sr-rhBMP-2 complex and suppression of Smad-dependent signaling pathway. Acta Biomater. 2016;33:290–300. [DOI] [PubMed] [Google Scholar]
- 10. Ogino S, Nowak JA, Hamada T, et al. Integrative analysis of exogenous, endogenous, tumour and immune factors for precision medicine. Gut. 2018;67(6):1168–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ma Y, Huang B, Lin D, Yuan Y, Liu C. Bioactivation of calcium phosphate cement by growth factors and their applications In: Liu C, He H, eds. Developments and Applications of Calcium Phosphate Bone Cements. Singapore: Springer; 2018: pp 257–298. [Google Scholar]
- 12. Wang Z, Ma Y, Wang Y, et al. Urethane-based low-temperature curing, highly-customized and multifunctional poly(glycerol sebacate)-co-poly(ethylene glycol) copolymers. Acta Biomater. 2018;71:279–292. [DOI] [PubMed] [Google Scholar]
- 13. Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75(2):193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. [DOI] [PubMed] [Google Scholar]
- 15. Rogers LK, Robbins M, Dakhlallah D, et al. Attenuation of miR-17 approximately 92 Cluster in Bronchopulmonary Dysplasia. Ann Am Thorac Soc. 2015;12(10):1506–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee LJ, Yang Z, Rahman M, et al. Extracellular mRNA detected by tethered lipoplex nanoparticle biochip for lung adenocarcinoma detection. Am J Respir Crit Care Med. 2016;193(12):1431–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang Z, Ma Y, Zhao H, Yuan Y, Kim BYS. Nanotechnology platforms for cancer immunotherapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019:e1590. [DOI] [PubMed] [Google Scholar]
- 18. Luan X, Sansanaphongpricha K, Myers I, Chen HW, Yuan HB, Sun DX. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacologica Sinica. 2017;38(6):754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang Z, Xie J, Zhu J, et al. Functional exosome-mimic for delivery of siRNA to cancer: in vitro and in vivo evaluation. J Control Release. 2016;243:160–171. [DOI] [PubMed] [Google Scholar]
- 20. Shi JF, Ma YF, Zhu J, et al. A review on electroporation-based intracellular delivery. Molecules. 2018;23(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tan CY, Lai RC, Wong W, Dan YY, Lim SK, Ho HK. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther. 2014;5(3):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lou G, Chen Z, Zheng M, Liu Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med. 2017;49(6):e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cantaluppi V, Gatti S, Medica D, et al. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 2012;82(4):412–427. [DOI] [PubMed] [Google Scholar]
- 24. Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011;6(4):481–492. [DOI] [PubMed] [Google Scholar]
- 25. Niu H, Ma Y, Wu G, et al. Multicellularity-interweaved bone regeneration of BMP-2-loaded scaffold with orchestrated kinetics of resorption and osteogenesis. Biomaterials. 2019;216:119216. [DOI] [PubMed] [Google Scholar]
- 26. Lin D, Chai Y, Ma Y, Duan B, Yuan Y, Liu C. Rapid initiation of guided bone regeneration driven by spatiotemporal delivery of IL-8 and BMP-2 from hierarchical MBG-based scaffold. Biomaterials. 2019;196:122–137. [DOI] [PubMed] [Google Scholar]
- 27. Liu Y, Ma Y, Zhang J, et al. MBG-Modified beta-TCP scaffold promotes mesenchymal stem cells adhesion and osteogenic differentiation via a FAK/MAPK signaling pathway. ACS Appl Mater Interfaces. 2017;9(36):30283–30296. [DOI] [PubMed] [Google Scholar]
- 28. Duan B, Niu H, Zhang W, Ma Y, Yuan Y, Liu C. Microporous density-mediated response of MSCs on 3D trimodal macro/micro/nano-porous scaffolds via fibronectin/integrin and FAK/MAPK signaling pathways. J Mater Chem B. 2017;5(19):3586–3599. [DOI] [PubMed] [Google Scholar]
- 29. Buzas EI, Gyorgy B, Nagy G, Falus A, Gay S. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol. 2014;10(6):356–364. [DOI] [PubMed] [Google Scholar]
- 30. Domenis R, Zanutel R, Caponnetto F, et al. Characterization of the Proinflammatory profile of synovial fluid-derived exosomes of patients with osteoarthritis. Mediators Inflamm. 2017;2017:4814987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rautou PE, Vion AC, Amabile N, et al. Microparticles, vascular function, and atherothrombosis. Circ Res. 2011;109(5):593–606. [DOI] [PubMed] [Google Scholar]
- 32. Wu L, Leijten JCH, Georgi N, Post JN, van Blitterswijk CA, Karperien M. Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Eng Part A. 2011;17(9-10):1425–1436. [DOI] [PubMed] [Google Scholar]
- 33. Lai JH, Kajiyama G, Smith RL, Maloney W, Yang F. Stem cells catalyze cartilage formation by neonatal articular chondrocytes in 3D biomimetic hydrogels. Sci Rep. 2013;3:3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jiang YZ, Zhang SF, Qi YY, Wang LL, Ouyang HW. Cell transplantation for articular cartilage defects: principles of past, present, and future practice. Cell Transplant. 2011;20(5):593–607. [DOI] [PubMed] [Google Scholar]
- 35. Chai Y, Lin D, Ma Y, Yuan Y, Liu C. RhBMP-2 loaded MBG/PEGylated poly(glycerol sebacate) composite scaffolds for rapid bone regeneration. J Mater Chem B. 2017;5(24):4633–4647. [DOI] [PubMed] [Google Scholar]
- 36. Huang B, Tian Y, Zhang W, Ma Y, Yuan Y, Liu C. Strontium doping promotes bioactivity of rhBMP-2 upon calcium phosphate cement via elevated recognition and expression of BMPR-IA. Colloids Surf B Biointerfaces. 2017;159:684–695. [DOI] [PubMed] [Google Scholar]
- 37. Kuang L, Ma X, Ma Y, et al. Self-assembled injectable nanocomposite hydrogels coordinated by in situ generated CaP nanoparticles for bone regeneration. ACS Appl Mater Inter. 2019;11(19):17234–17246. [DOI] [PubMed] [Google Scholar]
- 38. Honda Y, Anada T, Kamakura S, Nakamura M, Sugawara S, Suzuki O. Elevated extracellular calcium stimulates secretion of bone morphogenetic protein 2 by a macrophage cell line. Biochem Biophys Res Commun. 2006;345(3):1155–1160. [DOI] [PubMed] [Google Scholar]
- 39. Ismail N, Wang Y, Dakhlallah D, et al. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood. 2013;121(6):984–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wei F, Li M, Crawford R, Zhou Y, Xiao Y. Exosome-integrated titanium oxide nanotubes for targeted bone regeneration. Acta Biomater. 2019;86:480–492. [DOI] [PubMed] [Google Scholar]
- 41. Zhang B, Yin YJ, Lai RC, Tan SS, Choo ABH, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23(11):1233–1244. [DOI] [PubMed] [Google Scholar]
- 42. Lo Sicco C, Reverberi D, Balbi C, et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: endorsement of macrophage polarization. Stem Cells Transl Med. 2017;6(3):1018–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Deregibus MC, Cantaluppi V, Calogero R, et al. Endothelial progenitor cell-derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110(7):2440–2448. [DOI] [PubMed] [Google Scholar]
- 44. Cui YZ, Luan J, Li HY, Zhou XY, Han JX. Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Lett. 2016;590(1):185–192. [DOI] [PubMed] [Google Scholar]
- 45. Inder KL, Ruelcke JE, Petelin L, et al. Cavin-1/PTRF alters prostate cancer cell-derived extracellular vesicle content and internalization to attenuate extracellular vesicle-mediated osteoclastogenesis and osteoblast proliferation. J Extracell Vesicles. 2014;3(1):23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Qin Y, Wang L, Gao Z, Chen G, Zhang C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci Rep. 2016;6:21961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li DF, Liu J, Guo BS, et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat Commun. 2016;7:10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yuan FL, Wu QY, Miao ZN, et al. Osteoclast-derived extracellular vesicles: novel regulators of osteoclastogenesis and osteoclast-osteoblasts communication in bone remodeling. Front Physiol. 2018;9:628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh WS. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. [DOI] [PubMed] [Google Scholar]
- 50. Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noël D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017;7(1):16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang S, Chu WC, Lai RC, Lim SK, Hui JHP, Toh WS. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24(12):2135–2140. [DOI] [PubMed] [Google Scholar]
- 52. Zhang S, Teo KYW, Chuah SJ, Lai RC, Lim SK, Toh WS. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35–47. [DOI] [PubMed] [Google Scholar]
- 53. Tao SC, Yuan T, Zhang YL, Yin WJ, Shang-Chun G, Zhang CQ. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7(1):180–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang YF, Yu DS, Liu ZM, et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther. 2017;8(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xu JF, Yang GH, Pan XH, et al. Altered MicroRNA expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. Plos One. 2014;9(12):e114627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wei J, Li H, Wang S, et al. Let-7 enhances osteogenesis and bone formation while repressing adipogenesis of human stromal/mesenchymal stem cells by regulating HMGA2. Stem Cells Dev. 2014;23(13):1452–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xu S, Santini GC, De Veirman K, et al. Upregulation of miR-135b Is Involved in the impaired osteogenic differentiation of mesenchymal stem cells derived from multiple myeloma patients. Plos One. 2013;8(11):e79752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bakhshandeh B, Hafizi M, Ghaemi N, Soleimani M. Down-regulation of miRNA-221 triggers osteogenic differentiation in human stem cells. Biotechnol Lett. 2012;34(8):1579–1587. [DOI] [PubMed] [Google Scholar]
- 59. Zhang WJ, Wray LS, Rnjak-Kovacina J, et al. Vascularization of hollow channel-modified porous silk scaffolds with endothelial cells for tissue regeneration. Biomaterials. 2015;56:68–77. [DOI] [PubMed] [Google Scholar]
- 60. Bian SY, Zhang LP, Duan LF, Wang X, Min Y, Yu HP. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl). 2014;92(4):387–397. [DOI] [PubMed] [Google Scholar]
- 61. Zhang HC, Liu XB, Huang S, et al. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev. 2012;21(18):3289–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xie H, Wang ZX, Zhang LM, et al. Extracellular vesicle-functionalized decalcified bone matrix scaffolds with enhanced pro-angiogenic and pro-bone regeneration activities. Sci Rep. 2017;7:45622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Niu H, Lin D, Tang W, ET AL. Surface topography regulates osteogenic differentiation of MSCs via crosstalk between FAK/MAPK and ILK/β-Catenin pathways in a hierarchically porous environment. ACS Biomater Sci Eng. 2017;3(12):3161–3175. [DOI] [PubMed] [Google Scholar]
- 64. Liu M, Zeng X, Ma C, et al. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017;5:17014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang Y, Wu H, Wang Z, et al. Optimized synthesis of biodegradable elastomer PEGylated Poly(glycerol sebacate) and their biomedical application. Polymers(Basel). 2019;11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shi H, Gan Q, Liu X, et al. Poly(glycerol sebacate)-modified polylactic acid scaffolds with improved hydrophilicity, mechanical strength and bioactivity for bone tissue regeneration. RSC Adv. 2015;5(97):79703–79714. [Google Scholar]
- 67. Liu X, Yang Y, Li Y, et al. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale. 2017;9(13):4430–4438. [DOI] [PubMed] [Google Scholar]
- 68. Chen P, Zheng L, Wang Y, et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics. 2019;9(9):2439–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li WY, Liu YS, Zhang P, et al. Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS App Mater Interfaces. 2018;10(6):5240–5254. [DOI] [PubMed] [Google Scholar]
- 70. Zhang J, Liu X, Li H, et al. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res Ther. 2016;7(1)136–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Walters N, Nguyen LTH, Zhang J, Shankaran A, Reátegui E. Extracellular vesicles as mediators of in vitro neutrophil swarming on a large-scale microparticle array. Lab on a Chip. 2019;19(17):2874–2884. [DOI] [PubMed] [Google Scholar]
- 72. Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7(3):789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]