Abstract
Rationale: Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are common, associated with acute inflammation, and may increase subsequent cardiovascular disease (CVD) risk.
Objectives: Determine whether AECOPD events are associated with increased risk of subsequent CVD.
Methods: We performed a secondary cohort analysis of the SUMMIT (Study to Understand Mortality and Morbidity) trial, a convenience sample of current/former smokers with moderate COPD from 1,368 centers in 43 countries. All had CVD or increased CVD risk. AECOPD was defined as an increase in respiratory symptoms requiring treatment with antibiotics, systemic corticosteroids, and/or hospitalization. CVD events were a composite outcome of cardiovascular death, myocardial infarction, stroke, unstable angina, and transient ischemic attack. All CVD events were adjudicated. Cox proportional hazards models compared the hazard for a CVD event before AECOPD versus after AECOPD.
Measurements and Main Results: Among 16,485 participants in SUMMIT, 4,704 participants had at least one AECOPD and 688 had at least one CVD event. The hazard ratio (HR) for CVD events after AECOPD was increased, particularly in the first 30 days after AECOPD (HR, 3.8; 95% confidence interval, 2.7–5.5) and was elevated up to 1 year after AECOPD. The 30-day HR after hospitalized AECOPD was more than twofold greater (HR, 9.9; 95% confidence interval, 6.6–14.9).
Conclusions: In patients with COPD with CVD or risk factors for CVD, exacerbations confer an increased risk of subsequent CVD events, especially in hospitalized patients and within the first 30 days after exacerbation. Patients and clinicians should have heightened vigilance for early CVD events after AECOPD.
Clinical trial registered with www.clinicaltrials.gov (NCT 01313676).
Keywords: chronic obstructive pulmonary disease, cardiovascular diseases, cohort study
At a Glance Commentary
Scientific Knowledge on the Subject
1) Patients with chronic obstructive pulmonary disease (COPD) frequently experience cardiovascular disease (CVD); 2) COPD exacerbations are associated with increased systemic inflammation, which is a risk factor for CVD; and 3) preliminary data suggest that acute exacerbations of COPD are associated with an increased risk of subsequent CVD events, but studies have relied on administrative data or nonadjudicated CVD event data.
What This Study Adds to the Field
In this cohort of 16,485 patients with COPD and CVD or multiple CVD risk factors, exacerbations were followed by an increased risk of adjudicated CVD events, especially in hospitalized patients with COPD and in the first 30 days after an acute exacerbation of COPD.
Ischemic heart disease, stroke, and chronic obstructive pulmonary disease (COPD) are three leading causes of death globally (1). These diseases share common risk factors such as older age and cigarette smoking, yet data suggest that COPD and lower lung function are independent risk factors for cardiovascular disease (CVD), even after adjustment for traditional CVD risk factors (2–4).
The mechanisms by which COPD increases CVD risk are not clear, but patients with COPD often display abnormally high concentrations of circulating systemic inflammatory biomarkers such as C-reactive protein, IL-6, and fibrinogen (5)—biomarkers that predict CVD risk in the general population (6, 7) and in COPD (8). Acute exacerbations of COPD (AECOPD) are often associated with particularly high concentrations of these biomarkers (9), which can be slow to return to baseline (10).
In addition, many AECOPD events are triggered by infections (11), and data have shown that infections (mostly respiratory, but also urinary and gastrointestinal) are associated with an increased risk for subsequent CVD events (12–16). The reasons for this are not clear, but hypotheses have focused on infections as inducers of systemic inflammation and procoagulant pathways that subsequently lead to cardiovascular events.
Two previous studies have suggested that AECOPD increases risk for subsequent CVD, but both had significant methodologic limitations including use of administrative data to define COPD, AECOPD, and CVD events (17) or use of nonadjudicated adverse event reporting data (18). The SUMMIT (Study to Understand Mortality and Morbidity) trial was an international, multicenter trial of patients with COPD and either a history of CVD or heightened risk for CVD. SUMMIT assessed the impact of inhaler treatments on mortality and rigorously adjudicated CVD events, therefore reducing the risk of ascertainment bias and providing more accurate estimates of risk. We hypothesized that time periods after AECOPD would be associated with higher risk for CVD events compared with time periods free of AECOPD.
Some of the results of this study have been previously reported in the form of an abstract (19).
Methods
A detailed description of our methods is included with the online supplement. In brief, we performed a post hoc cohort analysis using data in SUMMIT, a double-blind, parallel group, placebo-controlled, randomized trial conducted at 1,368 centers in 43 countries between 2011 and 2015. Details of the study design and main results have been published (20, 21). Participants (n = 16,485) were randomly assigned to receive either inhaled placebo, fluticasone furoate, vilanterol, or a combination of fluticasone furoate and vilanterol. The study showed no significant differences in risk of death or cardiovascular events between the four arms of the trial.
Participants were current or former smokers with at least a 10-pack-year smoking history, aged 40–80 years with an FEV1/FVC ratio not greater than 70%, FEV1 50–70% of predicted, and a modified Medical Research Council dyspnea scale score equal to or exceeding 2. Participants 40–59 years old were required to have a history of CVD, defined as coronary artery disease, peripheral arterial disease, stroke, myocardial infarction, or diabetes mellitus with target organ disease. Participants 60–80 years old could have either a history of CVD or increased risk for CVD, defined as receiving medication for two or more of the following: hypercholesterolemia, hypertension, diabetes mellitus, or peripheral arterial disease.
Exclusion criteria included respiratory disorders other than COPD, lung reduction surgery, receiving long-term oxygen, chronic oral corticosteroid therapy, severe heart failure (New York Heart Association Class IV or ejection fraction < 30%), life expectancy less than 3 years, and end-stage chronic renal disease.
Participants were seen every 3 months, at which time data relating to AECOPD and CVD were assessed.
Institutional review board approval was obtained at each of the 1,368 participating study centers before study initiation.
Statistical Analysis
We used Cox proportional hazards models with time-dependent “period” covariates, where the hazard for a CVD event was compared between the period before AECOPD (“baseline” in our tables) and after AECOPD (Figure 1). AECOPD was defined as an increase in respiratory symptoms requiring treatment with antibiotics, systemic corticosteroids, and/or hospitalization. Our primary outcome was a composite CVD outcome that included cardiovascular death, myocardial infarction, stroke, unstable angina, and transient ischemic attack. A clinical endpoint committee used data from medical records, witness interviews, autopsy reports, and death certificates to adjudicate all CVD events, using standardized guidelines (22, 23).
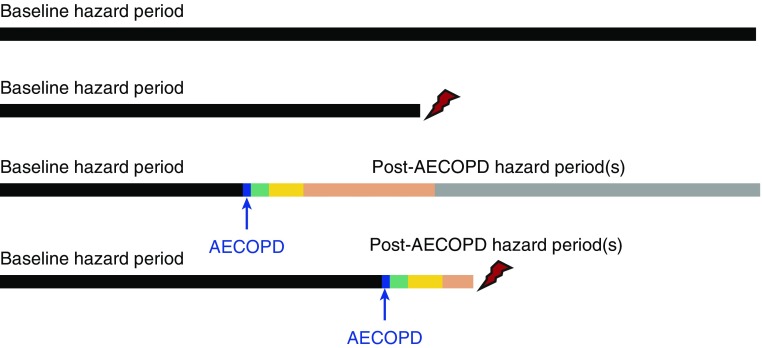
Figure 1.
Graphic representation of analytic method. All study participants with any follow-up time contribute to the analysis. Participants can have one of four possible patterns, as graphically shown, from the top down: 1) No acute exacerbation of chronic obstructive pulmonary disease (AECOPD) and no cardiovascular disease (CVD) events; 2) no AECOPD, but with CVD event (as depicted by red lightning bolt); 3) AECOPD (as indicated by blue arrow/bar), but with no CVD event; and 4) AECOPD and CVD event. Participants with AECOPD events contribute hazard data for baseline, exacerbation-free periods (black bars), and comparison data regarding post-AECOPD hazard data at 1 to 30 days after AECOPD (green bars), 31 to 90 days after AECOPD (yellow bars), 91 days to 1 year after AECOPD (orange bars), and more than 1 year after AECOPD (gray bars). Data are censored at the time of a CVD event. Secondary analyses included 1) only hospitalized AECOPD events, where participants who had a nonhospitalized AECOPD remained in the baseline period (Table 2), and 2) restriction to only the last two groups who experienced an AECOPD during the study (Table 3).
We excluded events where AECOPD and CVD were reported on the same day, as we were unable to determine which event happened first. We analyzed the hazard of post-AECOPD CVD events at 1 to 30 days, 31 to 90 days, 91 days to 1 year, and more than 1 year after AECOPD events. Covariates are detailed in our table legends. When participants experienced more than one AECOPD, only the first was used. Data were censored after the first CVD event.
Secondary analyses focused on 1) only hospitalized AECOPDs, 2) only myocardial infarctions, 3) comparison of those with established CVD versus those with only increased CVD risk, 4) restriction to each of the four arms of the trial, and 5) restriction to only those who experienced an AECOPD event during the study.
Results
Among the 16,485 participants in SUMMIT, 75% were male, 47% were current smokers, mean body mass index was 28 kg/m2, and 39% had a history of one or more AECOPD events in the year before enrollment (Table 1).
Table 1.
Study Participant Characteristics
| Age, yr | 65 (8) |
| Female | 4,196 (25%) |
| Race | |
| White | 13,357 (81%) |
| Asian | 2,724 (17%) |
| Other | 404 (2%) |
| Body mass index, kg/m2 | 28 (6) |
| Current smokers | 7,678 (47%) |
| Smoking history, pack-years | 41 (24) |
| Systolic blood pressure, mm Hg | 135 (15) |
| Diastolic blood pressure, mm Hg | 80 (10) |
| Cardiac comorbidities | |
| Coronary artery disease | 8,379 (51%) |
| Previous myocardial infarction | 2,774 (17%) |
| Previous stroke | 1,595 (10%) |
| Hypercholesterolemia | 11,518 (70%) |
| Hypertension | 14,851 (90%) |
| Diabetes mellitus | 4,997 (30%) |
| Cardiac medications | |
| Antiplatelet | 8,517 (52%) |
| Statin | 10,721 (65%) |
| β-Blocker | 5,667 (34%) |
| Diuretic | 6,148 (37%) |
| Post-bronchodilator FEV1, L | 1.70 (0.40) |
| % predicted post-bronchodilator FEV1 | 59.7 (6.1) |
| Prestudy COPD inhaler therapy | |
| Long-acting β-agonist | 5,769 (35%) |
| Long-acting muscarinic antagonist | 2,550 (15%) |
| Inhaled corticosteroid | 5,486 (33%) |
| Prestudy exacerbations in 12 mo before study | |
| 0 | 10,021 (61%) |
| 1 | 4,020 (24%) |
| ≥2 | 2,444 (15%) |
Definition of abbreviation: COPD = chronic obstructive pulmonary disease.
Data are reported as mean (SD) or n (%). Total N = 16,485.
Median participant on-treatment follow-up time was 1.5 years with a total of 26,946 patient years of follow-up. During follow-up, 4,704 participants had at least one AECOPD and 688 had at least one adjudicated CVD event. The first CVD event was CV death in 271, myocardial infarction in 173, stroke in 127, unstable angina in 83, and transient ischemic attack in 34. Depending on the particular analysis, between zero and nine participants were excluded because CVD and AECOPD were reported on the same day.
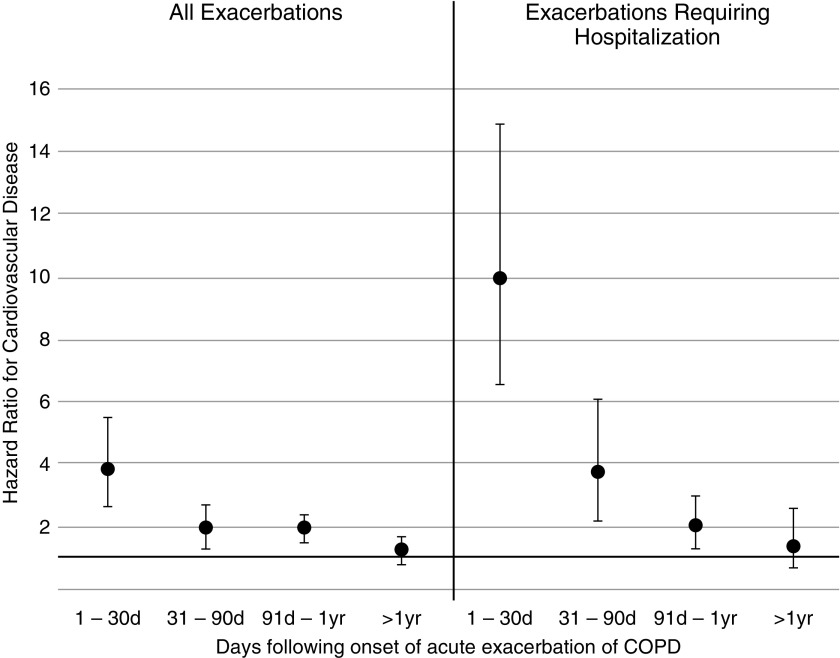
A total of 487 participants experienced a CVD event during the baseline period (487 events in 21,624 patient-years is 2.3 per 100 patient-years). Between Days 1 and 30 after AECOPD, 32 participants experienced a CVD event (8.8 per 100 patient-years); 29 participants had a CVD event between Days 31 and 90 (4.4 per 100 patient-years), 91 participants had a CVD event between Day 91 and 1 year (4.0 per 100 patient-years), and 41 participants had a CVD event after 1 year (2.4 per 100 patient-years). Compared with pre-AECOPD baseline periods, the hazard of CVD events after AECOPD was increased, particularly in the first 30 days after AECOPD (hazard ratio [HR], 3.8; 95% confidence interval [CI], 2.7–5.5), although it remained increased between 31 and 90 days and between 91 days and 1 year, and was no longer increased beyond 1 year after AECOPD (Table 2 and Figure 2).
Table 2.
Hazard Ratios for Cardiovascular Disease Event (Cardiovascular Death, Myocardial Infarction, Stroke, Unstable Angina, and Transient Ischemic Attack) after an Acute Exacerbation of Chronic Obstructive Pulmonary Disease
| Period | Number of Participants in Period | Observed Follow-up in Period (Patient-Years) | Number of Participants with Adjudicated CVD Event | Hazard Ratio (95% CI) |
|---|---|---|---|---|
| All exacerbations* | ||||
| Baseline, AECOPD-free | 16,477 | 21,624 | 487 | Reference |
| 1 to 30 d | 4,639 | 363 | 32 | 3.8 (2.7–5.5) |
| 31 d to 90 d | 4,235 | 658 | 29 | 1.9 (1.3–2.7) |
| 91 d to 1 yr | 3,779 | 2,267 | 91 | 1.9 (1.5–2.4) |
| >1 yr | 2,179 | 1,744 | 41 | 1.2 (0.8–1.7) |
| Exacerbations requiring hospitalization | ||||
| Baseline, AECOPD-free | 16,476 | 25,595 | 605 | Reference |
| 1 to 30 d | 1,243 | 90 | 24 | 9.9 (6.6–14.9) |
| 31 to 90 d | 998 | 152 | 15 | 3.7 (2.2–6.1) |
| 91 d to 1 yr | 862 | 487 | 24 | 2.0 (1.3–3.0) |
| >1 yr | 447 | 330 | 11 | 1.3 (0.7–2.6) |
Definition of abbreviations: AECOPD = acute exacerbation of chronic obstructive pulmonary disease; CI = confidence interval; CVD = cardiovascular disease.
Analysis is shown for all exacerbations (top) and for exacerbations requiring hospitalization (bottom). Covariates included AECOPD period (baseline free of AECOPD or other post-AECOPD periods as in Figure 1), treatment assignment arm, age, sex, body mass index, region, race, ethnicity, ischemic and vascular indicators (e.g., previous treatment of coronary or vascular disease), cardiovascular disease/risk indicators (with CVD; with CVD risk), smoking status, previous exacerbation history, and percent predicted post-bronchodilator FEV1.
Eight participants were excluded from the All Exacerbations analysis because they experienced an AECOPD and CVD event on the same day; nine were excluded from the Exacerbations Requiring Hospitalization analysis because they experienced an AECOPD and CVD event on the same day. One hundred eighty-three participants were excluded from the calculation of hazard ratios in both analyses because they did not have all model covariates.
Figure 2.
Hazard ratios (95% confidence intervals) for cardiovascular disease (cardiovascular death, myocardial infarction, stroke, unstable angina, and transient ischemic attack) after an acute exacerbation of chronic obstructive pulmonary disease. COPD = chronic obstructive pulmonary disease.
In a further analysis, we restricted the AECOPD events to only hospitalized AECOPD events and considered participants who had a nonhospitalized AECOPD to remain in the baseline period. A total of 605 participants experienced a CVD event during the baseline period (2.4 per 100 patient-years). Between Days 1 and 30 after hospitalized AECOPD, 24 participants experienced a CVD event (26.7 per 100 patient-years); 15 participants had a CVD event between Days 31 and 90 (9.9 per 100 patient-years); 24 participants had a CVD event between Day 91 and 1 year (4.9 per 100 patient-years), and 11 participants had a CVD event after 1 year (3.3 per 100 patient-years). In this case, the post-AECOPD hazard for CVD events was again particularly increased in the first 30 days after hospitalized AECOPD (HR, 9.9; 95% CI, 6.6–14.9), remained increased between 31 and 90 days and between 91 days and 1 year, but was not increased beyond 1 year after hospitalized AECOPD (Table 2 and Figure 2).
Analyses restricted only to those who experienced an AECOPD event during the study (n = 4,629 with all covariates) showed that the hazard for CVD after AECOPD was again particularly increased in the first 30 days after AECOPD (HR, 6.4; 95% CI, 4.1–10.2). The hazard was attenuated, but still significant, between 31 days and 1 year after AECOPD, and remained slightly elevated more than 1 year after AECOPD (Table 3).
Table 3.
Secondary Analysis Restricted to Only Those Study Participants Who Experienced an Acute Exacerbation of Chronic Obstructive Pulmonary Disease during the Study
| Period | Number of Participants in Period | Observed Follow-up in Period (Patient-Years) | Number of Participants with Adjudicated CVD Event | Hazard Ratio (95% CI) |
|---|---|---|---|---|
| Baseline, AECOPD-free | 4,696 | 3,695 | 55 | Reference |
| 1 to 30 d | 4,639 | 363 | 32 | 6.4 (4.1–10.2) |
| 31 d to 1 yr | 4,235 | 2,926 | 120 | 3.0 (2.1–4.4) |
| >1 yr | 2,179 | 1,744 | 41 | 1.8 (1.1–3.1) |
For definition of abbreviations, see Table 2.
Hazard ratios for CVD events after an AECOPD are shown. Because of small numbers in this restricted analysis, the post-AECOPD periods of 31 to 90 days and 90 days to 1 year were combined. Covariates included AECOPD period (baseline free of AECOPD or other post-AECOPD periods as in Figure 1), treatment assignment arm, age, sex, body mass index, region, race, ethnicity, ischemic and vascular indicators (e.g., previous treatment of coronary or vascular disease), cardiovascular disease/risk indicators (with CVD; with CVD risk), smoking status, previous exacerbation history, and percent predicted post-bronchodilator FEV1. Eight participants were excluded because they experienced an AECOPD and CVD event on the same day. Sixty-seven participants were excluded from the calculation of hazard ratios because they did not have all model covariates.
Analyses restricted to only myocardial infarction events (i.e., excluding other, nonmyocardial infarction CVD events) showed similar results, with a substantially increased risk of myocardial infarction in the first 30 days after AECOPD, a lower but still significant risk between 31 days and 1 year, and no significant increased risk beyond 1 year (see Table E1 in the online supplement).
Analyses stratified by whether participants entered the study with a history of established CVD or CVD risk are shown in Table E2. The hazard ratio for experiencing a CVD event after AECOPD was again most pronounced in the first 30 days after AECOPD, regardless of whether participants entered the study with established CVD or CVD risk. Among those with established CVD, the younger and older age groups had similar 95% CI bounds for the hazard ratios at each time period after AECOPD, but there were very few CVD events, so these estimates may not be reliable.
Last, we analyzed the hazard for CVD after AECOPD separately in each of the four original trial arms of the parent SUMMIT study. Results were again similar to that observed in our other analyses, with each arm demonstrating hazard ratios that were particularly increased in the first 30 days after AECOPD, remained increased between 31 days and 1 year, and were no longer significant beyond 1 year after AECOPD (Table E3).
Discussion
This analysis of prospectively collected data from a multicenter, international study of patients with moderately severe COPD and rigorously adjudicated CVD events supports the notion that AECOPD increases the risk for subsequent CVD events, especially in the first 30 days after an AECOPD. Moreover, the observed effect size was substantial, with a fourfold increased hazard for CVD events after AECOPD, and a 10-fold increase in those hospitalized with AECOPD. These results suggest that clinicians and patients need to be vigilant for the occurrence of CVD events after AECOPD, especially in those hospitalized with AECOPD.
Our findings are notable for remarkable consistency among the primary analysis and multiple secondary analyses regarding the particularly high CVD risk in the first 30 days after AECOPD, whether we analyzed all AECOPD events, hospitalized AECOPD events, myocardial infarctions only, or stratified by age and established CVD versus CVD risk. Our sample consisted of more than 16,000 study participants enrolled from multiples sites and countries, thus increasing generalizability to patients seen in varying clinical settings. Our findings are further strengthened by the blinded adjudication of CVD events. This adjudication provides us with a high degree of confidence regarding the validity of the CVD events.
Our findings validate preliminary observations in the UPLIFT (Understanding Potential Long-term Impacts on Function with Tiotropium) trial, where AECOPD was associated with a higher risk of cardiovascular serious adverse events in both the first 30 days and first 180 days after AECOPD, with higher risk in the first 30 days (18). CVD event data in UPLIFT consisted of only serious adverse event reporting data without detailed adjudication, and the analysis did not include adjustment for multiple potential confounders. Unlike SUMMIT, UPLIFT did not specifically select for patients with COPD at risk for CVD, but in both UPLIFT and our SUMMIT results, associations between AECOPD and CVD were present whether patients entered the studies with a history of previously diagnosed CVD or not.
Our findings also build on a previous study of AECOPD and CVD relationships, using administrative data in England and Wales. Among those with administrative codes for physician-diagnosed COPD (not necessarily confirmed by spirometry), prescriptions for oral antibiotics and corticosteroids (considered a surrogate marker of AECOPD) were associated with a higher risk for subsequent myocardial infarctions and stroke (17). These associations were dependent on the outcomes and time period examined. For example, the increased risk for myocardial infarction was only observed for 5 days after a prescription for both antibiotics and steroids—there was no association with antibiotics alone, steroids alone, or beyond 5 days of the combination prescription. However, for stroke, the association was significant up to 49 days after a prescription for a steroid or an antibiotic, but not the combination steroid plus antibiotic. These complex observations may reflect the limitations of administrative data, as compared with our study’s strict criteria for spirometry confirmation of COPD, prospective collection of predefined AECOPD and CVD data, and detailed adjudication of CVD events.
AECOPD events are associated with elevated concentrations of circulating proinflammatory biomarkers (24) that can be slow to return to baseline (10). The high initial concentrations with slow recovery might help explain why we observed the most risk for CVD in the first 30 days after AECOPD, but we continued to observe a statistically significant, albeit much smaller, risk up to 1 year after AECOPD. The prolonged duration of increased CVD risk is consistent with studies that have shown that respiratory events such as pneumonia (13) and other respiratory infections (14) are associated with prolonged CVD risk.
Inflammation might also explain why hospitalized patients with AECOPD had a 30-day CVD risk more than double that seen in those with less severe AECOPD. Hospitalized AECOPD episodes are often associated with higher concentrations of circulating proinflammatory biomarkers compared with AECOPD events treated outside of the hospital (25). We did not measure biomarkers in this study, so we were unable to determine the contribution of inflammation to post-AECOPD CVD risk. We were also unable to test other potential mechanisms such as AECOPD leading to hypoxemia, increased respiratory muscle work diverting perfusion from the coronary circulation, induction of a prothrombotic state, increases in blood pressure, or worsening adherence to nonrespiratory medications.
From a therapeutic standpoint, our data suggest that the immediate post-AECOPD period is a window of heightened CVD susceptibility, and therefore future studies should test interventions in this period to reduce CVD risk. Possible interventions to test might include established CVD therapies (e.g., antiplatelet agents, statins, and/or β-blockers) and/or experimental CVD interventions (e.g., antiinflammatory drugs).
Our study has several important limitations. SUMMIT participants were selected on the basis of being at high CVD risk, due to either preexisting disease or having multiple risk factors for CVD. Although estimates of CVD prevalence in patients with COPD have ranged from 28% to 70% (3), our findings may not apply to COPD patients without CVD or CVD risk factors. SUMMIT participation was also restricted to those with FEV1 between 50% and 70% of predicted, so we cannot generalize our findings to those with milder or more severe airflow limitation. Our follow-up time was also relatively short, at a median of 1.5 years. Although data from our study and other studies suggest that most of the excess CVD risk occurs within the first year after AECOPD, we had limited power to study long-term event risk beyond 1 year. Last, although CVD events were adjudicated, AECOPD events were self-reported and not adjudicated. Therefore, we cannot exclude the possibility that some AECOPD events were CVD events to begin with. However, our AECOPD definition is that used by nearly every contemporary COPD trial and is a definition that has proven to be modifiable by treatments such as inhalers and oral medications (26). Moreover, we found even stronger associations in hospitalized patients, who typically undergo more detailed assessments for other clinical etiologies of acute-onset dyspnea (e.g., myocardial infarction, pulmonary embolism) compared with outpatients. Therefore, we think misclassification of AECOPD is not likely.
Conclusions
In patients with COPD and with CVD or risk factors for CVD, exacerbations confer an increased risk of subsequent CVD events, especially in hospitalized patients and within the first 30 days after exacerbation. Patients and clinicians should have heightened vigilance for early CVD events in this patient group after AECOPD.
Acknowledgments
Acknowledgment
The authors thank each of the SUMMIT study participants.
Footnotes
Supported by GlaxoSmithKline, which provided funding for the original SUMMIT trial (NCT01313676) (GSK113782). GlaxoSmithKline employees performed the statistical analysis and participated in the writing group team, but GlaxoSmithKline did not direct or make final decisions regarding study conception, analysis of results, manuscript writing, or the decision to submit for publication. The views expressed in this article are those of the authors and do not reflect the views of the U.S. government, the Department of Veterans Affairs, the funders, the sponsors, or any of the authors’ affiliated academic institutions.
Author Contributions: Conceived the current analysis: K.M.K. and D. E. Niewoehner; designed the analysis: K.M.K., M.T.D., J.A.A., J.C.Y., B.F.H., and D. E. Niewoehner; acquired the data: J.A.A., J.C.Y., and C.C.; performed the primary statistical analyses: B.F.H. and J.A.A.; drafted the manuscript: K.M.K.; provided critical input and revised the manuscript for important intellectual content and approved the final manuscript: all authors; take responsibility for the integrity of the data and the accuracy of the data analysis: all authors.
Originally Published in Press as DOI: 10.1164/rccm.201711-2239OC on February 14, 2018
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: on behalf of the SUMMIT Investigators
References
- 1.GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sin DD, Man SFP. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003;107:1514–1519. doi: 10.1161/01.cir.0000056767.69054.b3. [DOI] [PubMed] [Google Scholar]
- 3.Müllerova H, Agusti A, Erqou S, Mapel DW. Cardiovascular comorbidity in COPD: systematic literature review. Chest. 2013;144:1163–1178. doi: 10.1378/chest.12-2847. [DOI] [PubMed] [Google Scholar]
- 4.Shibata Y, Inoue S, Igarashi A, Yamauchi K, Abe S, Aida Y, et al. A lower level of forced expiratory volume in 1 second is a risk factor for all-cause and cardiovascular mortality in a Japanese population: the Takahata study. PLoS One. 2013;8:e83725. doi: 10.1371/journal.pone.0083725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agustí A, Edwards LD, Rennard SI, MacNee W, Tal-Singer R, Miller BE, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 2012;7:e37483. doi: 10.1371/journal.pone.0037483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 8.Thomsen M, Dahl M, Lange P, Vestbo J, Nordestgaard BG. Inflammatory biomarkers and comorbidities in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:982–988. doi: 10.1164/rccm.201206-1113OC. [DOI] [PubMed] [Google Scholar]
- 9.Bathoorn E, Liesker JJ, Postma DS, Koëter GH, van der Toorn M, van der Heide S, et al. Change in inflammation in out-patient COPD patients from stable phase to a subsequent exacerbation. Int J Chron Obstruct Pulmon Dis. 2009;4:101–109. doi: 10.2147/copd.s4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koutsokera A, Kiropoulos TS, Nikoulis DJ, Daniil ZD, Tsolaki V, Tanou K, et al. Clinical, functional and biochemical changes during recovery from COPD exacerbations. Respir Med. 2009;103:919–926. doi: 10.1016/j.rmed.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Dickson RP, Martinez FJ, Huffnagle GB. The role of the microbiome in exacerbations of chronic lung diseases. Lancet. 2014;384:691–702. doi: 10.1016/S0140-6736(14)61136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corrales-Medina VF, Madjid M, Musher DM. Role of acute infection in triggering acute coronary syndromes. Lancet Infect Dis. 2010;10:83–92. doi: 10.1016/S1473-3099(09)70331-7. [DOI] [PubMed] [Google Scholar]
- 13.Corrales-Medina VF, Alvarez KN, Weissfeld LA, Angus DC, Chirinos JA, Chang CC, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015;313:264–274. doi: 10.1001/jama.2014.18229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 15.Barnes M, Heywood AE, Mahimbo A, Rahman B, Newall AT, Macintyre CR. Acute myocardial infarction and influenza: a meta-analysis of case-control studies. Heart. 2015;101:1738–1747. doi: 10.1136/heartjnl-2015-307691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwong JC, Schwartz KL, Campitelli MA, Chung H, Crowcroft NS, Karnauchow T, et al. Acute Myocardial Infarction after Laboratory-Confirmed Influenza Infection. N Engl J Med. 2018;378:345–353. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 17.Donaldson GC, Hurst JR, Smith CJ, Hubbard RB, Wedzicha JA. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest. 2010;137:1091–1097. doi: 10.1378/chest.09-2029. [DOI] [PubMed] [Google Scholar]
- 18.Halpin DM, Decramer M, Celli B, Kesten S, Leimer I, Tashkin DP. Risk of nonlower respiratory serious adverse events following COPD exacerbations in the 4-year UPLIFT® trial. Hai. 2011;189:261–268. doi: 10.1007/s00408-011-9301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunisaki KM, Dransfield M, Anderson JA, Brook RD, Calverley PMA, Celli BR, et al. Acute exacerbations of chronic obstructive pulmonary disease increase subsequent cardiovascular event risk: A secondary analysis of adjudicated SUMMIT study data [abstract] Am J Respir Crit Care Med. 2017;195:A1014. [Google Scholar]
- 20.Vestbo J, Anderson J, Brook RD, Calverley PM, Celli BR, Crim C, et al. The Study to Understand Mortality and Morbidity in COPD (SUMMIT) study protocol. Eur Respir J. 2013;41:1017–1022. doi: 10.1183/09031936.00087312. [DOI] [PubMed] [Google Scholar]
- 21.Vestbo J, Anderson JA, Brook RD, Calverley PM, Celli BR, Crim C, et al. SUMMIT Investigators. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387:1817–1826. doi: 10.1016/S0140-6736(16)30069-1. [DOI] [PubMed] [Google Scholar]
- 22.Hicks KA, Hung HMJ, Mahaffey KW, Mehran R, Nissen SE, Stockbridge NL, et al. Standardized Data Collection for Cardiovascular Trials Initiative. Standardized definitions for end point events in cardiovascular trials. Austin, TX: Clinical Data Interchange Standards Consortium; 2010. [Google Scholar]
- 23.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–1598. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Pinto-Plata VM, Livnat G, Girish M, Cabral H, Masdin P, Linacre P, et al. Systemic cytokines, clinical and physiological changes in patients hospitalized for exacerbation of COPD. Chest. 2007;131:37–43. doi: 10.1378/chest.06-0668. [DOI] [PubMed] [Google Scholar]
- 25.Bozinovski S, Hutchinson A, Thompson M, Macgregor L, Black J, Giannakis E, et al. Serum amyloid A is a biomarker of acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:269–278. doi: 10.1164/rccm.200705-678OC. [DOI] [PubMed] [Google Scholar]
- 26.Wedzicha JA, Calverley PM, Albert RK, Anzueto A, Criner GJ, Hurst JR, et al. Prevention of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2017;50:1602265. doi: 10.1183/13993003.02265-2016. [DOI] [PubMed] [Google Scholar]