To the Editor:
Over time, patients with longstanding pulmonary arterial hypertension (PAH) experience pressure overload in the right ventricle (RV), leading to reduced contractile force and chamber dilation. Present strategies to treat PAH consist of pulmonary arterial vasodilators by way of the prostacyclin, endothelin, or nitric oxide pathways. It is now appreciated that maladaptive inflammatory signaling is a key contributor to the development of obliterative pulmonary arteriolar lesions and RV failure in PAH (1, 2), and that IL-1 and IL-6 levels correlate with the degree of RV failure (3).
With experimental animal data suggesting anakinra (recombinant IL-1 receptor antagonist) protects against development of PAH (4), we designed a single-group, open-label phase IB/II pilot study (clinicaltrials.gov identifier: NCT03057028) to evaluate the feasibility and safety of treatment with anakinra as add-on therapy to standard of care in patients with stable PAH and RV failure. In addition to the preclinical data, anakinra was chosen on the basis of multiple favorable clinical trials in left-sided systolic dysfunction by our group (5, 6).
Here we report the findings from our pilot study. From October 2017 to May 2018, patients at the Virginia Commonwealth University treated for pulmonary hypertension were screened for eligibility. Inclusion criteria included group 1 PAH (7) (not associated with connective tissue disease, human immunodeficiency virus, portal hypertension, or schistosomiasis), age >18 years, and symptomatic RV failure (objective findings of RV dysfunction by echocardiography [RV diastolic diameter >4.3 cm, fractional area change <35%, or tricuspid annular plane systolic excursion ≤1.5 cm] with New York Heart Association class II or III heart failure symptoms) despite optimal PAH therapy. Exclusion criteria included autoimmune/autoinflammatory diseases, anti-inflammatory medications, recent malignancy or infection, and severe renal dysfunction. Of the 39 patients who met inclusion criteria, 10 were excluded (chronic inflammatory disorders [n = 4], infection [n = 1], severe renal dysfunction [n = 2], non–English speaking [n = 1], unable to consent [n = 1], pregnant [n = 1]). Sixteen patients were approached for the study: 6 declined to participate, and 10 agreed to enroll, which was the sample size chosen to gather preliminary data. However, scheduling conflicts prevented two patients from completing baseline testing, and one patient was hospitalized for urosepsis before enrollment. Thus, seven patients were enrolled in the study, in which they provided signed consent (approved by the institutional review board) and received anakinra 100 mg subcutaneously daily for 14 days, which is the dose approved by the U.S. Food and Drug Administration for the treatment of rheumatoid arthritis. Baseline testing before treatment included biomarkers (high-sensitivity C-reactive protein [hsCRP], IL-6, and N-terminal pro B-type natriuretic peptide [NT-proBNP]), quality-of-life questionnaires (Duke Activity Severity Index and Minnesota Living with Heart Failure Questionnaire), transthoracic echocardiography, and cardiopulmonary exercise testing for measurement of peak oxygen consumption and ventilatory efficiency (e:co2 slope) (8). All tests were repeated at Day 14 of treatment, in addition to a safety assessment. This was intended to be a safety and feasibility study, but for the purposes of designing future studies, two co–primary endpoints were specified: change in peak o2 and change in e:co2 slope, which were chosen because of their established reproducibility, prognostic value, and placebo-insensitive nature resulting from the open-label design of the study (9). Statistical analysis was performed with SPSS 24.0 (IBM), using the Wilcoxon test for paired data or the Spearman rank test for correlations.
Six patients completed the protocol and returned for repeat testing; they were included in the main analysis. Baseline characteristics are presented in Table 1. One patient was unwilling to return for repeat testing, and thus was not included in the main analysis but was included in the safety analysis.
Table 1.
Individual Patient Demographics and Baseline Characteristics
| Patient No. | Age (yr) | BMI (kg/m2) | Sex | Ethnicity | Coexisting Conditions | Classification of PAH | Qualifying Right Heart Catheterization | Current PAH/RV Failure Treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | 64 | 16 | F | W | None | Idiopathic | PA mean, 47 mm Hg | Selixipag |
| PCWP, 7 mm Hg | Riociguat | |||||||
| PVR, 13.3 WU | Ambrisentan | |||||||
| Carvedilol | ||||||||
| Furosemide | ||||||||
| 2 | 75 | 34.5 | F | B | Paroxysmal atrial fibrillation | Idiopathic | PA mean, 30 mm Hg | Sildenafil |
| PCWP, 7 mm Hg | Oxygen | |||||||
| Hypertension | PVR, 6.6 WU | |||||||
| Diabetes mellitus | ||||||||
| Amlodipine | ||||||||
| Bumetanide | ||||||||
| 3 | 30 | 31.8 | F | W | Secondary Graves’ disease | Idiopathic | PA mean, 49 mm Hg | Tadalafil |
| PCWP, 3 mm Hg | Epoprostenol | |||||||
| Supraventricular tachycardia | PVR, 17.7 WU | Macitentan | ||||||
| Spironolactone | ||||||||
| Torsemide | ||||||||
| 4 | 83 | 34.4 | M | B | Atrial flutter | Idiopathic | PA mean, 46 mm Hg | Sildenafil |
| PCWP, 9 mm Hg | Selixipag | |||||||
| Diabetes mellitus | PVR, 5.8 WU | Oxygen | ||||||
| Metoprolol | ||||||||
| Amlodipine | ||||||||
| Furosemide | ||||||||
| 5 | 71 | 33.8 | M | B | Hypertension | Idiopathic | PA mean, 45 mm Hg | Treprostinil |
| Diabetes mellitus | PCWP, 15 mm Hg | Oxygen | ||||||
| Atrial fibrillation and flutter | PVR, 4.7 WU | Metolazone | ||||||
| Carvedilol | ||||||||
| Furosemide | ||||||||
| 6 | 61 | 30.8 | F | W | Hypertension | Idiopathic | PA mean, 39 mm Hg | Sildenafil |
| Diabetes mellitus | PCWP, 13 mm Hg | Macitentan | ||||||
| PVR, 6.7 WU |
Definition of abbreviations: B = black; BMI = body mass index; PA = pulmonary artery; PAH = pulmonary arterial hypertension; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; RV = right ventricle; W = white; WU = Wood units.
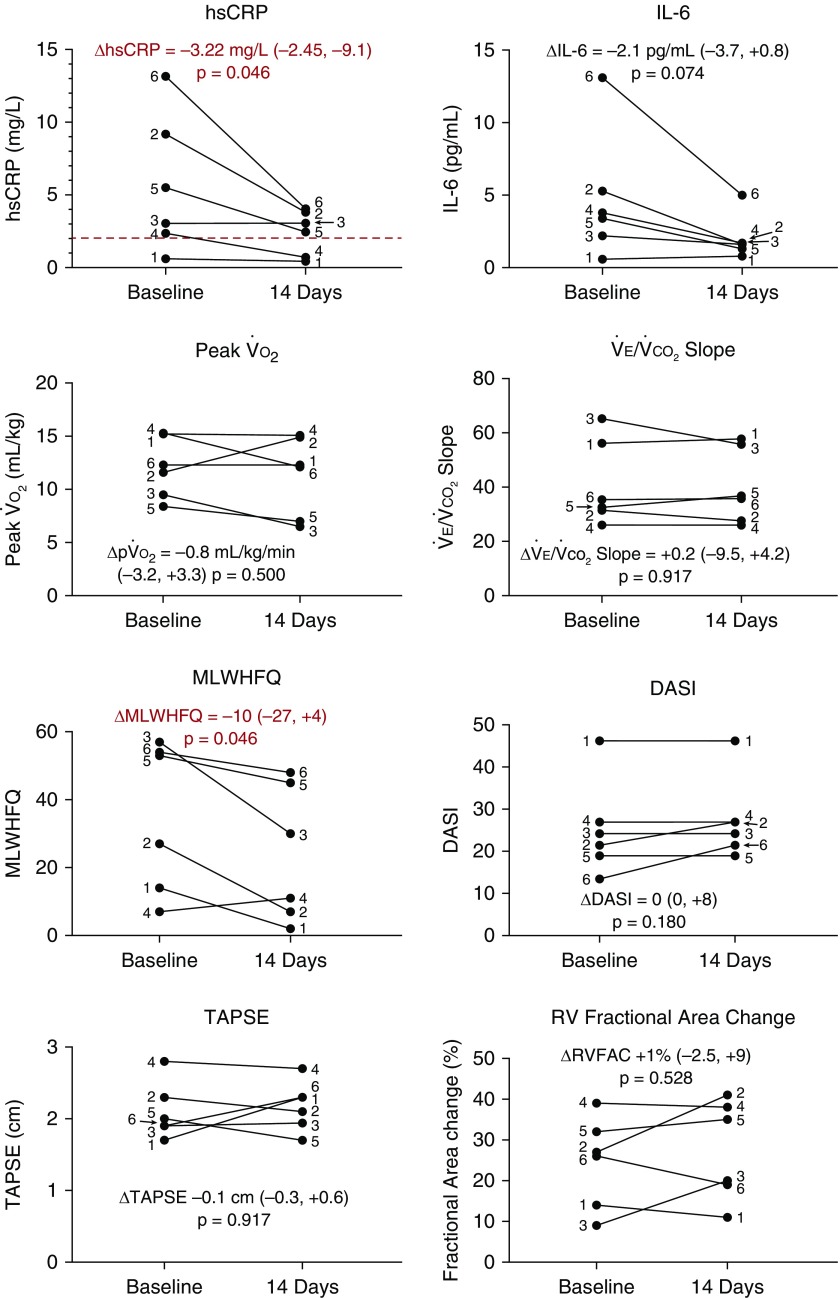
No major safety events were observed during the study period. After 14 days of treatment, hsCRP levels were significantly reduced compared with baseline, with a trend toward IL-6 reduction (Figure 1). There were no significant changes from baseline in peak o2, e:co2 slope, exercise time, NT-proBNP, RV fractional area change, or tricuspid annular plane systolic excursion (Figure 1; all P ≥ 0.463). There was a significant improvement in heart failure symptoms as assessed by the Minnesota Living with Heart Failure Questionnaire and a trend toward improvement in the Duke Activity Severity Index scores (Figure 1). Changes in hsCRP inversely correlated with changes in peak oxygen consumption (R = −0.829; P = 0.046) and Duke Activity Severity Index scores (R = −0.845; P = 0.034).
Figure 1.
Study results. DASI = Duke Activity Severity Index; hsCRP = high-sensitivity C-reactive protein; MLWHFQ = Minnesota Living with Heart Failure Questionnaire; po2 = peak o2; RV = right ventricular; RVFAC = RV fractional area change; TAPSE = tricuspid annular plane systolic excursion.
Three patients (43%) experienced minor injection site reactions. No reactions required treatment discontinuation, and all resolved spontaneously. One patient with a history of symptomatic atrial ectopy perceived a higher burden of palpitations and prematurely discontinued the treatment after 4 days; she was not willing to return for repeat testing. Another patient with a history of secondary Graves’ disease had symptomatic improvement of preexisting proptosis during anakinra treatment, but subsequent progression after drug discontinuation for which she was seen by rheumatology and started on methotrexate therapy with a single dose of rituximab, with good clinical response; this was considered by the investigators to be a moderate event, and possibly related to anakinra treatment. There were no infections, hospitalizations, emergency department visits, or unplanned escalation of medical care during the study period.
The data obtained from this pilot study suggest that IL-1 blockade with anakinra in patients with PAH and RV failure is feasible and safe. Further studies are needed to validate these findings and explore the therapeutic potential of this treatment strategy. Limitations include the open-label treatment design without placebo comparison, conduct at a single center, small sample size, and the use of only one dose of anakinra tested over a short duration. In addition, although every patient had invasive hemodynamic measurements consistent with PAH, the majority of our patients were older than 60 years with multiple risk factors for left-sided heart disease. Nonetheless, we observed a significant reduction in hsCRP in this study cohort, suggesting that the inflammatory burden in this population may be alleviated by IL-1 blockade. This reduction correlated with improvements in peak oxygen consumption during maximal effort cardiopulmonary exercise testing, as well as improved symptom burden of heart failure.
Of note, although there was a significant reduction in hsCRP levels, only 2 patients achieved on-treatment hsCRP <2 mg/L (which is considered the upper limit of normal). In CANTOS (Canakinumab Antiinflammatory Thrombosis Outcome Study), a recently published trial of IL-1 blockade in patients after myocardial infarction, overall rates of composite cardiovascular events were reduced with canakinumab, a monoclonal antibody to IL-1β (10), especially in those patients who achieved hsCRP levels <2.0 mg/L (11). Thus, in addition to being hindered by a small sample size, this study may have been underpowered to detect changes in surrogate markers of improved clinical status (cardiopulmonary exercise testing parameters) because of a failure to achieve the desired degree of inflammatory suppression. Whether this could be solved by longer treatment, higher doses of anakinra, or an alternative agent is uncertain. A larger study, ideally one that is longer in treatment duration as well as randomized and placebo controlled, is needed to further expand on these findings and explore the potential role of IL-1 blockade in PAH with RV failure.
Supplementary Material
Footnotes
This study was supported by a grant from the VCU Johnson Center for Critical Care and Pulmonary Research and a Clinical and Translational Science Award (UL1TR000058 from the National Center for Research Resources) to the Virginia Commonwealth University Center for Clinical and Translational Research. Swedish Orphan Biovitrum LLC (Stockholm, Sweden) provided the study medication (anakinra) free of cost, but had no role in the study design, conduct, analysis, or reporting.
Author Contributions: Conception and design: C.R.T., J.M.C., A.F., B.W.V.T., A.A., and D.G.; data acquisition: C.R.T., J.M.C., D.K., R.M., H.M.D.C., J.P., A.F., B.W.V.T., A.A., and D.G.; interpretation of data: C.R.T., J.M.C., D.K., R.M., H.M.D.C., J.P., A.F., B.W.V.T, A.A., and D.G.; intellectual contributions to the manuscript: C.R.T., J.M.C., D.K., R.M., H.M.D.C., J.P., A.F., B.W.V.T., A.A., and D.G.
Originally Published in Press as DOI: 10.1164/rccm.201809-1631LE on November 12, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Huertas A, Perros F, Tu L, Cohen-Kaminsky S, Montani D, Dorfmüller P, et al. Immune dysregulation and endothelial dysfunction in pulmonary arterial hypertension: a complex interplay. Circulation. 2014;129:1332–1340. doi: 10.1161/CIRCULATIONAHA.113.004555. [DOI] [PubMed] [Google Scholar]
- 2.Pickworth J, Rothman A, Iremonger J, Casbolt H, Hopkinson K, Hickey PM, et al. Differential IL-1 signaling induced by BMPR2 deficiency drives pulmonary vascular remodeling. Pulm Circ. 2017;7:768–776. doi: 10.1177/2045893217729096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soon E, Holmes AM, Treacy CM, Doughty NJ, Southgate L, Machado RD, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation. 2010;122:920–927. doi: 10.1161/CIRCULATIONAHA.109.933762. [DOI] [PubMed] [Google Scholar]
- 4.Voelkel NF, Tuder RM, Bridges J, Arend WP. Interleukin-1 receptor antagonist treatment reduces pulmonary hypertension generated in rats by monocrotaline. Am J Respir Cell Mol Biol. 1994;11:664–675. doi: 10.1165/ajrcmb.11.6.7946395. [DOI] [PubMed] [Google Scholar]
- 5.Van Tassell BW, Abouzaki NA, Oddi Erdle C, Carbone S, Trankle CR, Melchior RD, et al. Interleukin-1 blockade in acute decompensated heart failure: a randomized, double-blinded, placebo-controlled pilot study. J Cardiovasc Pharmacol. 2016;67:544–551. doi: 10.1097/FJC.0000000000000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Tassell BW, Canada J, Carbone S, Trankle C, Buckley L, Oddi Erdle C, et al. Interleukin-1 blockade in recently decompensated systolic heart failure: results From REDHART (Recently Decompensated Heart Failure Anakinra Response Trial) Circ Heart Fail. 2017;10:e004373. doi: 10.1161/CIRCHEARTFAILURE.117.004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25) Suppl:D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 8.Arena R, Humphrey R, Peberdy MA, Madigan M. Predicting peak oxygen consumption during a conservative ramping protocol: implications for the heart failure population. J Cardiopulm Rehabil. 2003;23:183–189. doi: 10.1097/00008483-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Arena R, Lavie CJ, Milani RV, Myers J, Guazzi M. Cardiopulmonary exercise testing in patients with pulmonary arterial hypertension: an evidence-based review. J Heart Lung Transplant. 2010;29:159–173. doi: 10.1016/j.healun.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ CANTOS Trial Group. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391:319–328. doi: 10.1016/S0140-6736(17)32814-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.