Abstract
Purpose of review:
Approximately 10% of patients become blind despite using evidence-based guidelines developed from clinical trials and epidemiology studies. Our purpose is to review opportunities to decrease glaucoma-related blindness using the emerging principles of precision medicine.
Recent findings:
This review focuses on three topics: 1) candidate biomarkers for angle-based surgeries, 2) head-mounted display (HMD) technology for vision and testing, and 3) glaucoma risk alleles discovered by genome-wide association studies (GWAS). First, in angle-based surgeries, tracers injected into the anterior chamber or Schlemm’s canal (SC) have allowed visualization of aqueous veins. We describe an innovative use of optical coherence tomography angiography (OCTA) to visualize aqueous veins in a case with 6-year successful outcome following catheter-based trabeculotomy. Second, HMD technology can augment perceived vision and can be used for perimetry testing. Third, developing genetic risk scores that characterize patients who are at highest risk for blindness is a priority. Such biomarker risk scores will integrate GWAS-based risk alleles for glaucoma along with well-known demographic and clinical risk factors.
Summary:
As we gain more knowledge, precision medicine will enable clinicians to decrease glaucoma-related blindness by providing more timely interventions to those patients who are at highest risk for progression to blindness.
Keywords: aqueous veins, biomarkers, glaucoma, head-mounted display (HMD) technology, precision medicine
Introduction
Glaucoma remains a major cause of blindness with estimates of 13.5%−42% of patients blind unilaterally and 4%−16% blind bilaterally.(1–8) The 39th Shaffer Lecture focused on applying precision medicine for individualized care to patients with the goals of enhancing vision and preventing glaucoma-related blindness.(9) The intent of health service systems is to address reasons for glaucoma-related blindness that include poor access to care,(10) failure to adhere to medications and follow-up care,(11) and lack of application of best practices.(12–15) In spite of access and adherence to care, the current event-based approach based on progressive visual field loss(16, 17) reveals a major gap in the ability to identify patients at risk for blindness.
Glaucoma-related blindness is an unfortunate consequence of progression with a clear phenotype of large cup-to-disc ratio with profound retinal nerve fiber layer thinning. Quigley highlighted future glaucoma care needs to prevent glaucoma-related blindness and vision impairment.(18) First, there is a need to improve the definition of glaucoma to enhance ability for comparing research studies. Second, there is a need to advance evidence-based diagnostic and therapeutic approaches, that will involve biomarkers. Third, there is a need to address the current office-based intraocular pressure (IOP) data collection as this approach because of inadequate sampling of IOPs to assess variance. Fourth, there is a need to adopt systems to improve medication adherence and follow-up care, and shift to sustained drug delivery. Fifth, there is a need to develop and validate new technology for more patient-friendly visual field testing.
According to the NIH Precision Medicine Initiative, precision medicine is described as “an emerging approach for disease treatment and prevention that takes into account individual variability in genes, environment, and lifestyle for each person” (https://ghr.nlm.nih.gov/primer/precisionmedicine/definition accessed 12/8/2018). We categorize three principles of precision medicine relevant to glaucoma care: (A) phenotyping with evolving glaucoma disease definitions using newest technology for earlier diagnosis and detecting disease progression(18); (B) discovery, validation, and understanding of biomarkers for glaucoma, risk factors, disease progression, drug response and surgical outcome; and (C) revamping epidemiology studies and clinical trials to discover and validate environmental risk factors and lifestyle behaviors that affect disease onset and progression.
An integral concept for precision medicine is the role of biomarkers to assess the disease phenotypes, disease stage, risk factors, and response to treatments. The US Food and Drug Administration (FDA) has defined a biomarker “as an indicator of normal biological processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions.” (https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/ucm533161.pdf accessed 12/8/2018). The types of biomarkers include molecular, histologic, radiographic or other imaging, and physiologic. At this time, the role of biomarkers for glaucoma care is limited to the physiologic parameter of IOP and functional testing using visual fields.(19) At present, there is insufficient evidence for structural endpoints of optical coherence tomography (OCT) as a predictor for diagnosis or progression. This review focuses on three topics that represent principles of precision medicine.
Text of review
Candidate Imaging Biomarkers of Schlemm’s Canal-based Surgeries.
The intent of glaucoma surgery is to achieve low IOP with minimal IOP fluctuation and with minimal risk of complications, minimal impact on quality of life, and decrease medication burden. Various microinvasive glaucoma surgeries (MIGS)(20–22) have been introduced, but the long-term efficacy and cost-effectiveness of MIGS compared to trabeculectomy remain to be determined. The mechanisms for MIGS include targeting Schlemm’s canal (SC) to increase aqueous humor outflow into aqueous veins, increase uveoscleral outflow, or create a small fistula to bypass the angle and divert aqueous humor into the subconjunctival space.
The SC-based MIGS builds on Grant’s observation that complete trabeculotomy eliminated 49% of outflow resistance at normal IOP in enucleated healthy human eyes.(23) At higher IOP, 71% of outflow resistance was eliminated.(24) A replication study that titrated trabecular meshwork (TM) tissue ablation with an excimer laser showed that 35% of outflow resistance was eliminated at normal IOP.(25) These studies suggest that a third-to-half of the outflow resistance remains distal to SC, depending upon the IOP. Thus, the successful IOP reduction after eliminating some of the TM assumes that the aqueous humor is able to drain though the downstream pathways beyond SC.
It is believed that this residual outflow resistance beyond SC resides in the aqueous humor pathways of the collector channels to the intrascleral venous plexus and aqueous veins.(26) The precise mechanisms that determine this residual resistance are not fully understood and need to integrate the observations of segmental aqueous humor outflow,(27) peri-limbal tissue biomechanics,(28) and the aqueous veins.(26)
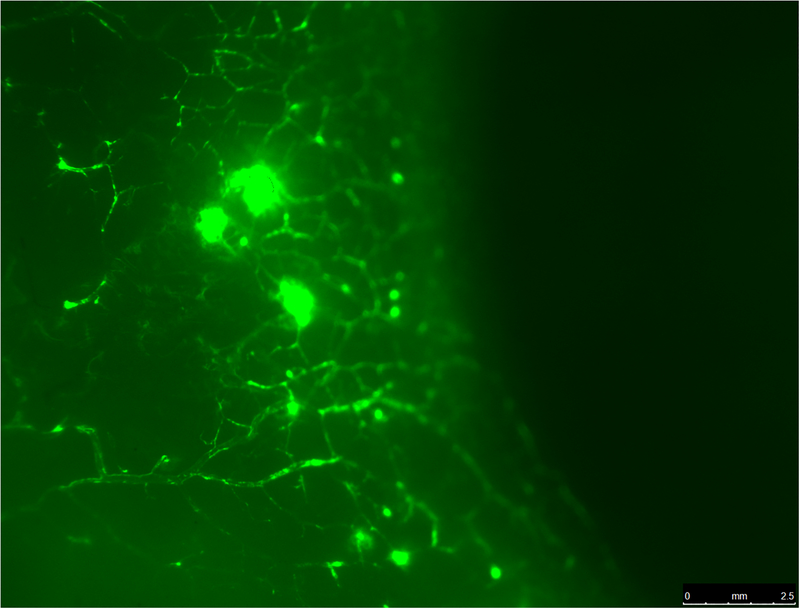
There are three to six large aqueous veins that are predominantly located in the nasal region of the eye.(29) These aqueous “super highways” are visible at the slit lamp under high magnification. These large aqueous veins have been visualized intraoperatively after increasing IOP with physiological solutions. The high IOP causes a “fluid wave” as the pressurized gradient of physiological solution blanches the aqueous veins.(30) Recently, various tracers have been used to visualize the aqueous humor outflow pathway using fluorescein,(31–33) indocyanine green,(34) and trypan blue.(35) These tracers and the use of fluorescent microspheres have confirmed the presence of the large aqueous veins, but more importantly provide additional evidence to visualize the intrascleral venous plexus within the outer peri-limbal scleral tissue (Figure 1).
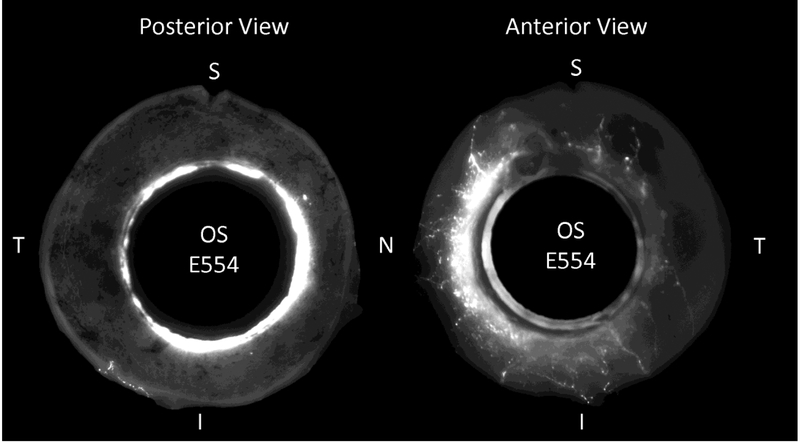
Figure 1.
A. Fluorescent microscopy shows aqueous veins in perilimbal sclera in the nasal region from a normal human donor eye. Schlemm’s canal was perfused with 0.2% fluorescent microsphere solution (0.50 μm Fluoresbrite® YG Carboxylate Microspheres, Polysciences, Inc., Warrington, PA) using a catheter (iTrack™ 250A Microcatheter, iScience Interventional™, Menlo Park, CA). B. Global fluorescent imaging of an anterior segment of a normal human donor eye. After perfusion with the fluorescent tracer, the eye was fixed in 10% buffered formalin. The globe was cut along a coronal plane approximately 6 mm posterior to the limbus. The intraocular tissues of the anterior segment were removed. The left panel shows the internal posterior view with high fluorescence in the trabecular meshwork. The right panel shows the external anterior view showing segmental distribution of aqueous veins in the nasal region. (S=superior, T=temporal, I=inferior, N=nasal).
Recently, spectral domain OCT has been used to visualize these aqueous veins.(36, 37) We present an innovative application of OCT angiography (OCTA) to visualize aqueous veins. We propose that these aqueous veins are structural biomarkers that determine a successful outcome for SC-based MIGS. We present imaging evidence of aqueous veins by fluorescein during surgery and by OCTA five years after surgery that explain the sustained six-year IOP reduction in a case after catheter-based trabeculotomy.
In October 2011, a 14-year old male presented with vision of 20/20 in each eye, and IOP of 14 mmHg right and 50 mmHg, left. After treatment with timolol, brimonidine, brinzolamide, latanoprost, and laser iridotomy, he presented to University of Michigan in March 2012 (he provided assent and his parents provided consent in an approved IRBMED project for this case report). His corrected vision was 20/15 in each eye. Gonioscopy showed open angles without developmental anomalies. Biomicroscropy showed no signs of anterior segment dysgenesis, and an iridotomy in the left eye. He had severe visual field loss that matched his diffuse retinal nerve fiber loss and 0.99 cup-to-disc ratio in his left eye, and healthy right eye (Figure 2). Medical management was continued to minimize disruption with school, and in June 2012, IOPs were 11 mmHg right and 28 mmHg left.
Figure 2.
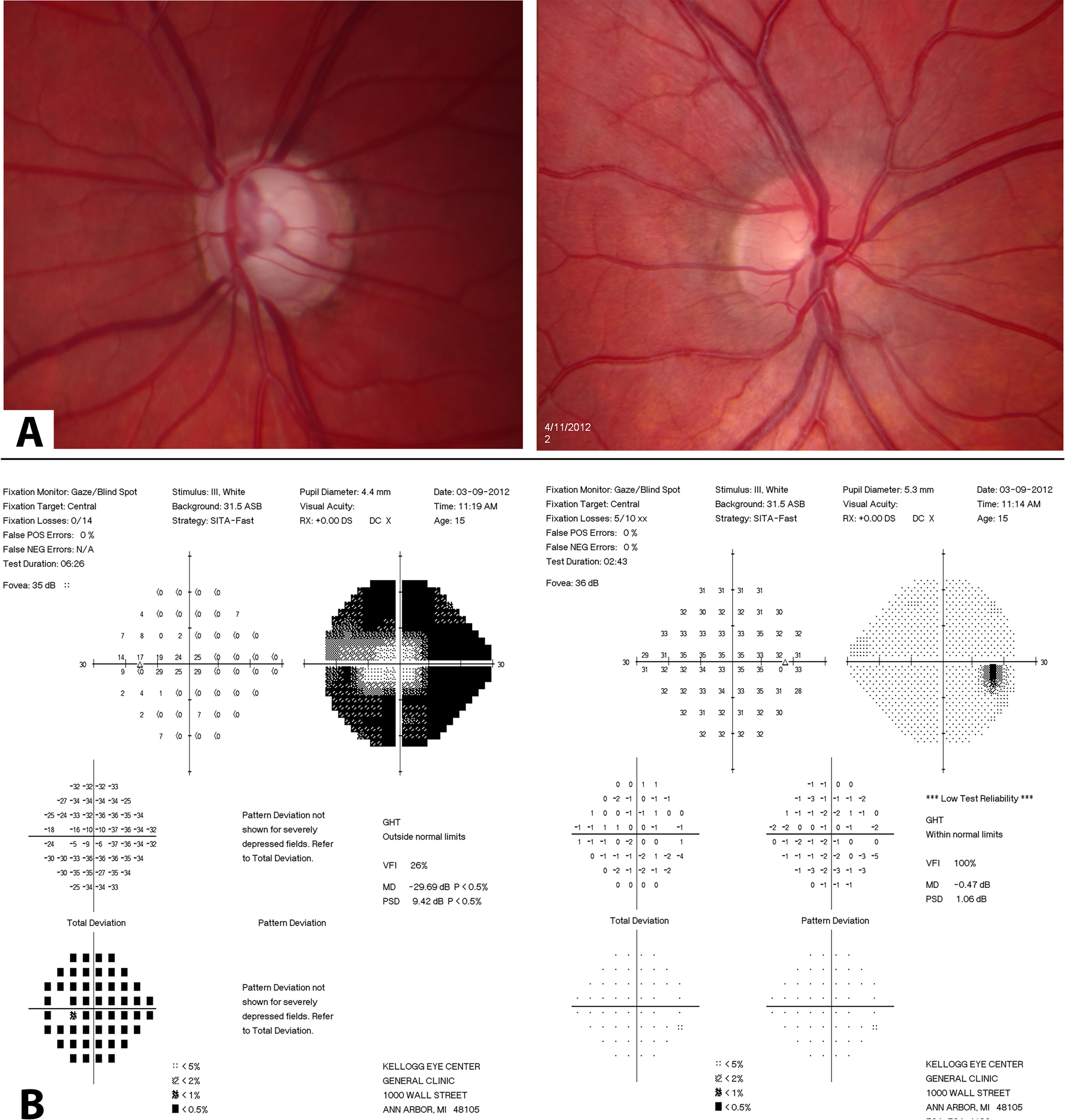
Clinical testing composite of asymmetric glaucomatous in left optic disc compared to right (A; top panel), and corresponding severe visual field loss left eye compared to right (B; bottom panel).
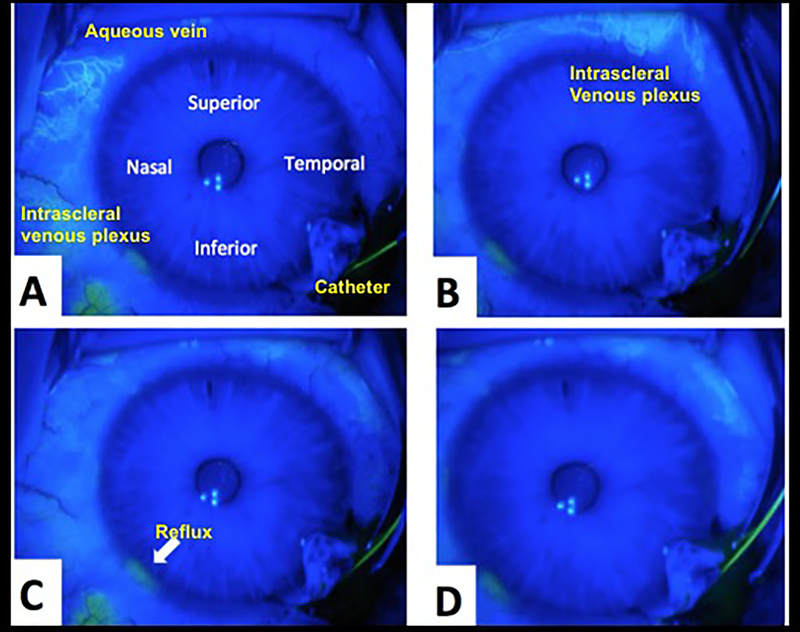
In July 2012, a left trabeculotomy was performed using a microcatheter (iTrack™ 250A Microcatheter, iScience Interventional™, Menlo Park, CA) through a temporal approach under a scleral flap. A fluorescein angiogram was performed(33) by injecting fluorescein from the microcatheter into SC (see supplemental video). His angiogram revealed several tracer patterns (Figure 3): (a) large aqueous veins with rapid transit and clearance of fluorescein without leakage, (b) small aqueous veins with slower transit and fluorescein leakage, (c) virtually no reflux of fluorescein from SC across the TM and into the anterior chamber, and (d) regional variation of fluorescein tracer outflow.
Figure 3.
Still frames from intraoperative fluorescein aqueous angiogram during catheter-based trabeculotomy with catheter inserted under scleral flap (see supplemental video). (A) shows large aqueous veins with rapid transit and clearance of fluorescein without leakage, (B) demonstrates small, intricate branching aqueous veins with slower transit of fluorescein with leakage and staining of surrounding sclera tissue, (C) shows virtually no reflux of fluorescein from SC across the TM and into the anterior chamber, and (D) regional variation of fluorescein outflow.
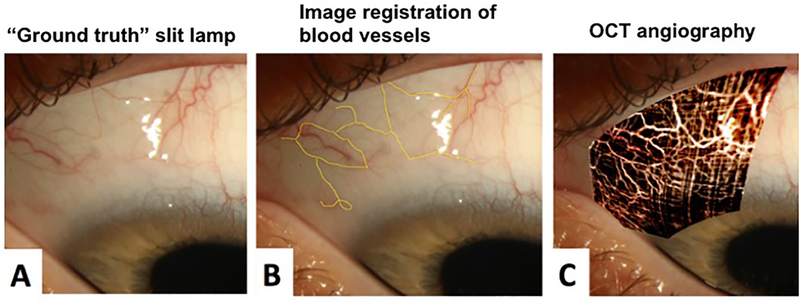
In August 2017, slit lamp photos and anterior segment OCTA (Optovue Inc., Fremont, CA, USA) imaging were performed. We established the following protocol for OCTA imaging of the perilimbal vessels with the anterior segment lens: (i) retina 3 mm by 3 mm scan setting, (ii) F setting −15 D, (iii) Z setting +15 D, (iv) cautious initial focus anterior to lens capsule as the anterior segment lens is very close to the cornea, and then (v) instruct the patient to look in the proper direction to scan the aqueous veins of interest with orientation perpendicular to the OCTA lens. All OCTA scans attempted to capture a portion of the angle and distinct conjunctival and episcleral vessels for image registration. Surface conjunctival and episcleral vessels were used as ground truth for image registration to overlay the OCTA image (Figure 4).
Figure 4.
Slit lamp photography with OCT angiography (OCTA) overlay. (A) Slit lamp photograph. (B) Reference vessels (yellow tracing). Surface conjunctival and episcleral vessels were used as reference markers for image registration to overlay the OCTA image. (C) OCTA Overlay. We used Adobe Photoshop® software version 14.2.1 ×32 (San Jose, CA) to create an overlay the flat 2-dimensional OCTA image in the orientation of the curved 2-dimensional slit lamp image while maintaining the true anatomic orientation of the vascular bed. This was executed using the “transformation” tool. The aqueous flow was then revealed using the “layer” tool of Adobe Photoshop® on the newly oriented OCTA image.
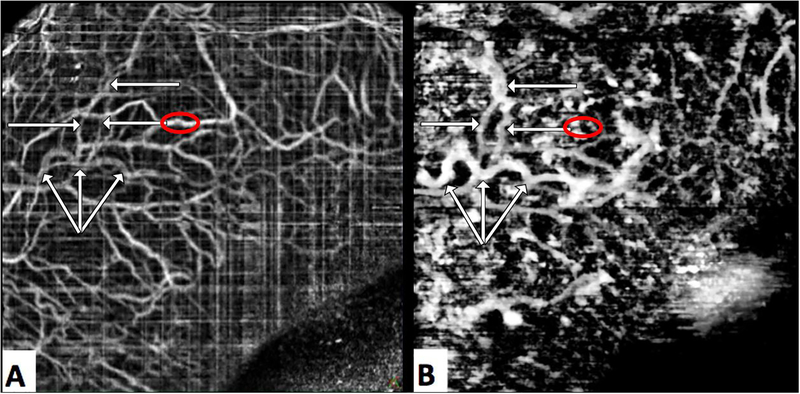
The OCTA images (Figure 5A) were processed from the raw scan data using an image browser supplied by the manufacturer (Avanti RTVue XR Image Viewing Software, ReVue Version 2016.2.0.35, Optovue). These files were imported into ImageJ (FIJI v1.51s).(36, 38) Scan volumes were aligned using the StackReg Plugin for ImageJ.(39) The aligned volumetric data set was averaged three times, yielding B-scans displaying dark aqueous humor-filled vasculature, which appeared as “scleral voids”. Contrast was locally enhanced and images were black-white inverted, yielding white aqueous humor filled vasculature on a dark background. The “subtract background” plug-in isolated the white aqueous humor filled vasculature. The image stack was then re-sliced to position the stack into an en-face orientation, and a z-projection used to create an en-face map of scleral voids, presumably the aqueous veins (Figure 5B).
Figure 5.
Frames A and B were derived from a single OCT-A scan. Frame A is the conventional OCT-A image comprised of a map of moving reflective sources presumed to be blood cells. Frame B is a is a map of scleral voids; presumed to be areas in which clear aqueous create dark openings in OCT structural scans. Aqueous humor outflow vasculature (white arrows) produce a strong “signal” map of scleral voids (B), but are faintly visible in the OCT-A image (A) suggesting that the aqueous within contains some scattering media. Prominent blood vessels (red circle, A) present as shadows in the map of scleral voids (red circle, B).
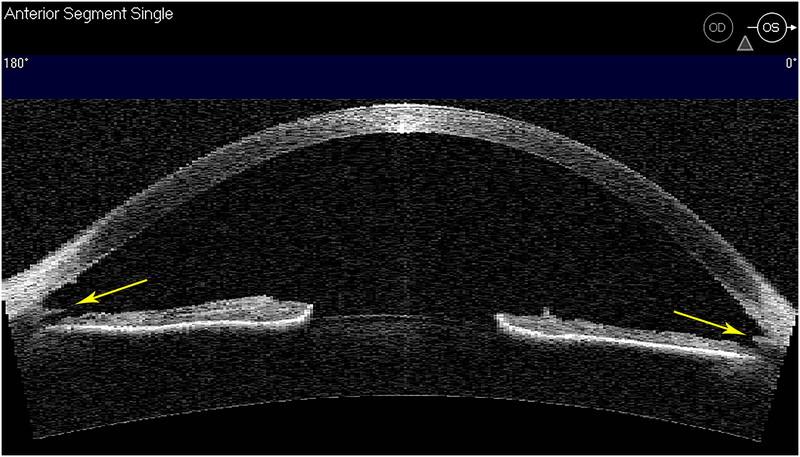
With the combined fluorescein tracer observations at the time of surgery and OCTA map obtained five years later, we propose that this patient had primary pathology of his TM causing elevated IOP. The trabeculotomy tore through his diseased TM and created a cleft into SC (Figure 6). We believe that his successful 6-year outcome with average IOP ± SD of 8.7 ± 1.6 mmHg (range 6–11) without medications nor filtration bleb was due to normal sclera and healthy deep and superficial aqueous veins.
Figure 6.
Anterior segment OCT (Visante™OCT) imaging along horizontal meridian of left eye. Gonioscopy showed a cleft into the back wall of Schlemm’s canal and the torn edges of trabecular meshwork (yellow arrows).
HMD Technology to Augment Vision and to Test Visual Fields.
Augmented reality is an established technology that superimposes a computer-generated image onto a user’s view. Within ophthalmology, such technology has been established as an assistive low vision aid, and a lexicon has been proposed to communicate about the various types of display type and optical design.(40) For patients who have a severely constricted visual field, a commercial off-the-shelf HMD device was successfully used to expand the perceived field of view.(41) The subject of this case report had a remarkable change in his mobility due to an enlarged perceived field of view due to the optical mechanism of projecting a wide-angle video through a prism onto a healthy portion of his retina. The profound impact of HMD on this patient’s mobility and perception of his environment is truly inspirational and was shared in the 39th Shaffer Lecture. This patient’s story generated enthusiasm for a pilot clinical trial to examine the ability of optical HMD to enlarge the visual field of patients who have severe visual field loss due to retinal dystrophy and to improve mobility and patient-reported outcomes (https://clinicaltrials.gov/ct2/show/NCT02983305 accessed 12/12/2018).
Another active area of research is the potential use of augmented reality HMD technology for visual field testing. The potential advantages from a human factor engineering (or “patient-friendly”) perspective include no need of a dark room, no need for an occlusive patch for testing, and no need to maintain head position.
Matsumoto et al designed a portable HMD perimeter called “imo” under similar test conditions as the Humphrey Field Analyzer (HFA).(42) A unique feature is separate right and left optical systems and pupil monitoring. A pilot test in 20 subjects with glaucoma showed similar visual field deficits measured by HFA and HMD “imo.” The sensitivity to detect glaucomatous field loss were highly correlated comparing the two perimetry test methods (right eye r=0.96, left eye r=0.94, P<0.001).
Tsapakis et al used a commercial HMD unit combined with a 6” display smartphone.(43) In a pilot study of 10 subjects with glaucoma, the visual field test results with HFA compared to their off-the-shelf HMD system were highly correlated (r=0.808, P<0.0001).
Nakanishi et al designed a cellphone-based HMD perimetry called “nGoggle” with integrated electroencephalogram and electrooculogram systems in order to assess the electrical brain responses associated with visual field stimulation.(44) This was a robust case-control study design with 33 glaucoma cases and 17 controls. For the visual field parameters, there was no difference between HFA and nGoggle sectoral testing. Test-retest variability was good with intraclass correlation coefficients ranging between 0.74 and 0.92 among test subjects.
The application of HMD technology is an exciting application of augmented reality to enhance vision(41) as a means for low vision rehabilitation(40) and as a platform to test visual fields.(42–44)
Developing Genetic Risk Scores to Identify Patients at Highest Risk for Glaucoma-related Blindness.
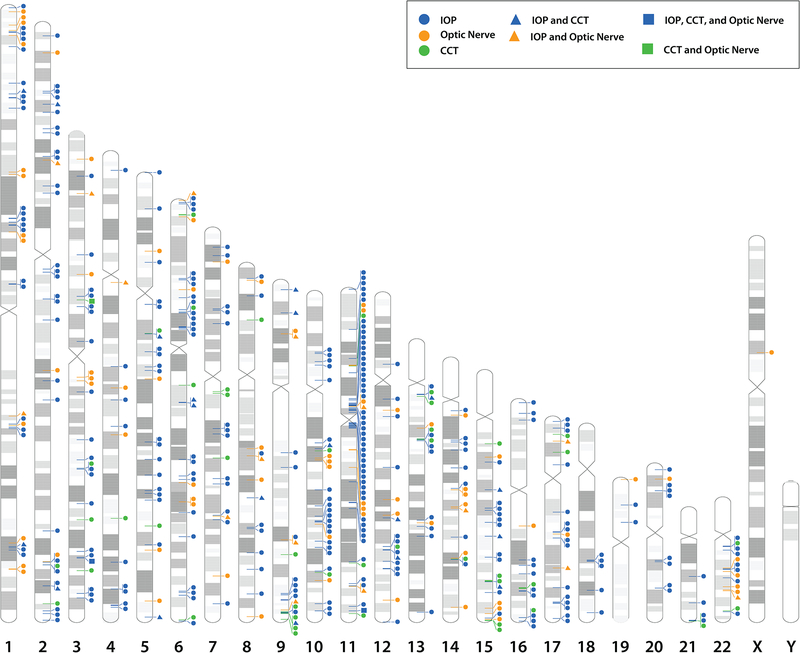
Great discoveries have been made to dissect the genetic architecture of the complex diseases(45) that include primary open-angle glaucoma(46, 47) and the quantitative traits of IOP,(48–50) central cornea thickness (CCT),(51) and optic nerve features.(51) These glaucoma loci, glaucoma genes and risk alleles for the clinical risk factors, that are quantitative traits such as IOP and CCT, are summarized across the human genome (Figure 7).
Figure 7.
Human chromosome ideograms (labeled 1 – 22, X and Y) with glaucoma loci, genes and glaucoma risk factor alleles. The glaucoma loci are indicated by black bars on the left side of the chromosome. The glaucoma genes are indicated by the black triangle on the right side of the chromosome. The genes representing glaucoma risk factors of optic nerve (orange circle), intraocular pressure (IOP, blue circle), and central cornea thickness (CCT, red square) are on the right side of the chromosome.
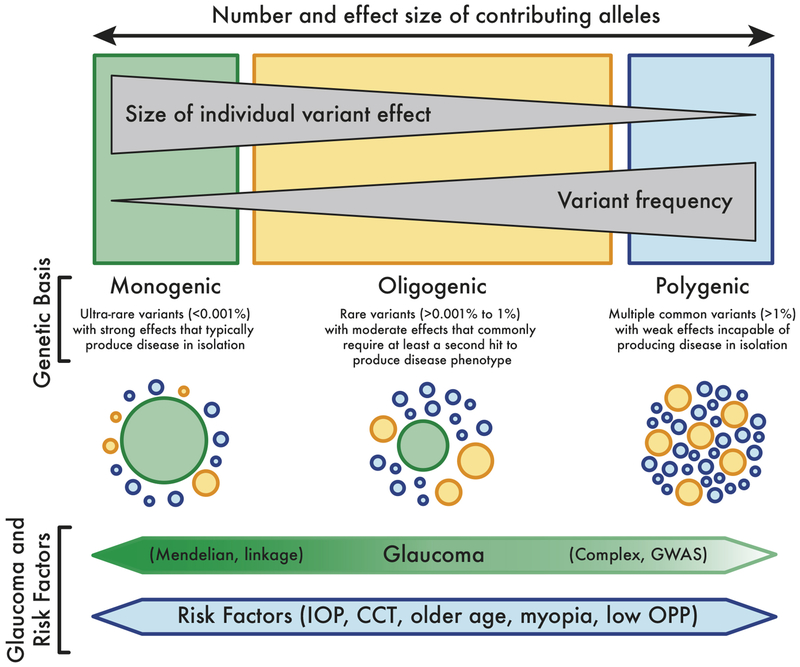
The general strategies for discovering these genes are summarized in Figure 8. In order to understand the success of gene discoveries from these genetic research strategies, it is helpful to understand the clinical context with three different clinical scenarios that represent the monogenic (Figure 8, right side), oligogenic (middle) and polygenic (left side) mechanisms of glaucoma. Three probands, who represent three patients or cases as the first affected family member seeking medical attention, are described below.
Figure 8.
Spectrum of the genetics of glaucoma and glaucoma risk factors with consideration of allele effect size and allele frequency (schematic modified from Giudicessi et al.(63)). For glaucoma genes with a large effect size and rare frequency (large green circle), the clinician observes a strong family history, such as autosomal dominant juvenile glaucoma or autosomal dominant Axenfeld-Rieger syndrome. In these rare glaucoma diagnoses, there is a single gene (or monogenic) that causes the glaucoma. Such monogenic disease genes are typically discovered by linkage methods. There are likely other variants (smaller yellow and blue circles) that may affect this rare monogenic variant. On the opposite spectrum, there are common variants with small effect size (smaller blue and yellow circles) that are associated with glaucoma risk factors. These glaucoma risk alleles are typically discovered by genome-wide association study (GWAS) methods.
Case 1 illustrates a monogenic, simple Mendelian form of glaucoma (Figure 8, left side). Genes for this kind of glaucoma are found through the application of genetic linkage and sequencing strategies to discover rare, single gene variants that cause glaucoma with Mendelian inheritance patterns. A patient presents with elevated IOP, open-angle with a prominent Schwalbe’s line on gonioscopy, hypoplastic iris features, and glaucomatous optic nerve damage with matching nerve fiber layer defects and visual field loss. Systemic findings include flat cheek bones consistent with maxillary hypoplasia, abnormal teeth, and umbilical hernia. There is a strong family history of similar ocular and systemic findings in two other generations. The combined evidence of the proband’s phenotype of ocular examination findings, systemic findings, dental history, and family history is consistent with Axenfeld-Rieger syndrome, and a novel causative PITX2 defect was identified.(52)
This single genetic defect in the PITX2 gene has a large contribution, or effect size, and causes the autosomal dominant Axenfeld-Rieger syndrome. Axenfeld-Rieger has an estimated prevalence of 1 in 200,000 (https://ghr.nlm.nih.gov/condition/axenfeld-rieger-syndrome accessed 2.16.2019). The current tally of Mendelian forms of various ocular and non-ocular disease phenotypes and quantitative traits includes 3,657 genes (https://www.omim.org/statistics/geneMap accessed 2.16.2019). Thus far for glaucoma, there are several dozen “major” glaucoma genes mapped so far with each representing founder effects in which rare gene mutations are passed down from one generation to the next through a family. However, even in these families, there are differences among relatives’ phenotypes due to variable expressivity of the genetic mutation. This variable expressivity is due to the influence of other genes which modify the expression of the genetic mutation.
Case 2 illustrates oligogenic form of glaucoma resulting from the action of a small number of genes. In such cases, the glaucoma disease phenotype results from the additive effects of several genes. In other cases, a proband with a rare Mendelian gene defect causing the glaucoma may also have other genes that modify the characteristics of the rare gene defect. A patient was diagnosed with juvenile-onset glaucoma and positive family history of open-angle glaucoma. Mutation screening showed that affected family members all possessed the same defect in the Mendelian glaucoma gene myocilin. However, even though the primary cause of disease appeared to be the same for everyone in the family, family history demonstrated that some family members showed much earlier onset of glaucoma than others. While two copies of CYP1B1 cause congenital/infantile glaucoma, families have been described in which a single defective copy of CYP1B1 can influence age at onset when glaucoma is cause by a myocilin defect.(53) Family members with both myocilin and CYP1B1 defects had a younger age at onset of glaucoma compared to patients who only had myocilin mutations. Patients with both myocilin and CYP1B1 genes are manifesting a type of digenic inheritance, the simplest version of oligogenic inheritance. In this case, CYP1B1 is not the cause of glaucoma, but it is a modifier, acting along with myocilin to create the total phenotype.
The third case illustrates common, complex forms of glaucoma discovered through the application of genome-wide association study (GWAS) to discover the many common gene variants that contribute to the common forms of glaucoma or the quantifiable risk factors, such as IOP or CCT. A patient returns for follow-up, repeat visual field testing, retinal nerve fiber layer imaging, and clinical exam for normal tension glaucoma based on maximum office-based IOPs of 19 mmHg. Often, the open-angle forms of glaucoma are complex diseases represented on the right side of Figure 8. These common, complex forms of glaucoma result from the combined effects of common variants in multiple genes, each of which have small effect sizes, such as CDKN2B-AS1, ABCA1, SIX6 and AFAP1.(54) The exact role of these multiple genes, with common variants and small effects on disease onset, progression, and severity, have yet to be determined. The current tally of 507 genes involved in the common complex forms of various ocular and non-ocular disease phenotypes and quantitative traits of the disease such as IOP, is incomplete since all of the genes involved have not yet been identified (https://www.omim.org/statistics/geneMap accessed 2.16.2019).
The next phases of translational research are to integrate the genetic findings with the established clinical risk factors of aging, higher IOP, thin central cornea, optic disc features, visual field loss patterns, low ocular perfusion pressure, and family history.(55–57) These next phases include: (i) adopt the engineering principle of iterative analysis to advance the definition of glaucoma based on validated discoveries using new and established technologies; (ii) develop new clinical care models that enhance data collection to better characterize the clinical parameters, or endophenotypes, such as IOP with newer technologies;(58, 59) (iii) promote “participatory research” which means educating clinicians and their patients on the value and importance to volunteer for research that includes contributing biological samples, clinical data and other data related to behaviors, lifestyle and environmental risk factors; (iv) re-vamp epidemiology studies, clinical studies, and clinical trials to include data capture of lifestyle and health behaviors so that the role of environmental factors can be investigated; and (v) developing genetic risk scores that can be tested for the specificity and sensitivity to stratify risk for glaucoma-related blindness.
Conclusion
Current event-based glaucoma care is centered on progressive visual field and shows a major gap in the ability to identify patients at high risk for progression to blindness. With the large data generated from the translational studies described in the preceding section, machine learning will be applied to tease out biologically relevant pathways from these genetic risk alleles.(60, 61) These pathways will be investigated in the context of candidate biomarkers for diagnosis and prognosis, and potential new therapeutic targets. The discovery and validation of genetic risk scores can be tested as candidate molecular biomarkers to stratify risk for glaucoma progression.
Imaging biomarkers of the aqueous humor outflow pathways will help clinicians decide upon the initial surgical intervention based on a patient’s aqueous vein map. Future studies need to evaluate the relationships among structural data of aqueous veins, functional data of IOP and outflow facility, and the peri-limbal tissue biomechanical properties. Understanding how IOP outcome of SC-based MIGS is related to the density of aqueous veins, outflow facility function, and tissue properties will allow us to model with surgical outcome.
In summary, the discovery and validation of biomarkers have the potential to shift the current model of event-based care based on visual field change toward preventing glaucoma-related vision impairment and blindness. Once new biomarkers are validated, then the clinician will be able to target more aggressive interventions to the patients who are at highest risk for blindness. Such robust biomarkers could ultimately lead to more efficient management with fewer follow-up office visits to assess treatment efficacy, fewer treatment failures, and decrease glaucoma-related blindness.(62)
Supplementary Material
KEY POINTS:
Identifying and validating structural biomarkers of the aqueous humor outflow system in patients with glaucoma is an opportunity for implementing precision-based surgical care
Application of augmented reality technology has the potential to provide practical and transformative wearable HMD that will decrease barriers for mobility caused by constricted visual fields
A future roadmap that integrates risk scores based on genetics of glaucoma and glaucoma traits, clinical risk factors, and clinical outcomes will be critical to developing effective targeted therapies for patients, who are at the highest risk for glaucoma-related blindness.
ACKNOWLEDGEMENTS:
We would like to thank Alon Kahana, MD, PhD, Wonsuk Kim, PhD, Hemant Pawar, PhD, Sarah Garnai, BS, Elliot Cha, MA, and Haiyan Gong, MD, PhD for contributions for Figure 1. We would like to thank Timothy Steffens, MS (Director of Ophthalmic Photography, Kellogg Eye Center, University of Michigan, Ann Arbor, MI) and his photography staff for the clinical photography (Figures 2, 4 and 6). We would like to thank Kirk Hodgson, BS, for helping to establish anterior segment imaging for OCTA and Greg Nguyen, BS, for helping on the OCTA raw image data acquisition (Optovue Inc., Fremont, CA, USA) (Figures 4 and 5). David M Reed, PhD, and David Murrel, MFA, contributed to creation Figure 7. David Murrel, MFA, and Sayoko E. Moroi, MD, PhD generated Figure 8 as modified from Giudicessi et al.(63).
FINANCIAL SUPPORT AND SPONSORSHIP:*Presented as the 39th Shaffer Lecture at American Academy of Ophthalmology 2018, sponsored by Prevent Blindness and the Glaucoma Research Foundation, MCubed (University of Michigan Health System, SM), P30 EY007003 (University of Michigan NEI Core Grant), unrestricted grant from Research to Prevent Blindness, R01 EY022124 (SEM)
Abbreviations:
- CCT
central cornea thickness
- GWAS
genome-wide association
- HMD
head-mounted display
- IOP
intraocular pressure
- OCTA
optical coherence tomography angiography
- OCT
optical coherence tomography
- SC
Schlemm’s canal
Footnotes
CONFLICTS OF INTEREST:
None relevant to the content of this manuscript. A single Bausch and Lomb consulting event by Sayoko Moroi was unrelated to this manuscript.
REFERENCES
*of special interest
**of outstanding interest
- 1.Pleet A, Sulewski M, Salowe RJ, Fertig R, Salinas J, Rhodes A, et al. Risk Factors Associated with Progression to Blindness from Primary Open-Angle Glaucoma in an African-American Population. Ophthalmic epidemiology. 2016;23(4):248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.**.Grant WM, Burke JF Jr. Why do some people go blind from glaucoma? Ophthalmology. 1982;89(9):991–8. [DOI] [PubMed] [Google Scholar]; Dr. Grant describes important observations about clinical course of patients who progress to blindness as the 2nd annual Robert N. Shaffer Lecture.
- 3.Malihi M, Moura Filho ER, Hodge DO, Sit AJ. Long-term trends in glaucoma-related blindness in Olmsted County Minnesota., Ophthalmology. 2014;121(1):134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen PP. Blindness in patients with treated open-angle glaucoma. Ophthalmology. 2003;110(4):726–33. [DOI] [PubMed] [Google Scholar]
- 5.Bourne RR, Taylor HR, Flaxman SR, Keeffe J, Leasher J, Naidoo K, et al. Number of People Blind or Visually Impaired by Glaucoma Worldwide and in World Regions 1990 – 2010: A Meta-Analysis. PloS one. 2016;11(10):e0162229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossetti L, Digiuni M, Montesano G, Centofanti M, Fea AM, Iester M, et al. Blindness and Glaucoma: A Multicenter Data Review from 7 Academic Eye Clinics. PloS one. 2015;10(8):e0136632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forsman E, Kivela T, Vesti E. Lifetime visual disability in open-angle glaucoma and ocular hypertension. Journal of glaucoma. 2007;16(3):313–9. [DOI] [PubMed] [Google Scholar]
- 8.Peters D, Bengtsson B, Heijl A. Lifetime risk of blindness in open-angle glaucoma. American journal of ophthalmology. 2013;156(4):724–30. [DOI] [PubMed] [Google Scholar]
- 9.**.Moroi SE. 39th Robert N. Shaffer Lecture: At the Technological Confluence of Glaucoma Clinical Care and Research American Academy of Ophthalmology 2018; October/28/2018; Chicago, IL, 2018. [Google Scholar]; This lecture summarized rates of glaucoma-related blindness since Dr. Morton Grant’s 2nd lecture and serves as a call to arms to apply principles of precision medicine to prevent blindness.
- 10.Sapru S, Berktold J, Crews JE, Katz LJ, Hark L, Girkin CA, et al. Applying RE-AIM to evaluate two community-based programs designed to improve access to eye care for those at high-risk for glaucoma. Eval Program Plann. 2017;65:40–6. [DOI] [PubMed] [Google Scholar]
- 11.Newman-Casey PA, Niziol LM, Mackenzie CK, Resnicow K, Lee PP, Musch DC, et al. Personalized behavior change program for glaucoma patients with poor adherence: a pilot interventional cohort study with a pre-post design. Pilot Feasibility Stud. 2018;4:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prum BE Jr., Herndon LW Jr., Moroi SE, Mansberger SL, Stein JD, Lim MC, et al. Primary Angle Closure Preferred Practice Pattern((R)) Guidelines. Ophthalmology. 2016;123(1):P1–P40. [DOI] [PubMed] [Google Scholar]
- 13.Prum BE Jr., Rosenberg LF, Gedde SJ, Mansberger SL, Stein JD, Moroi SE, et al. Primary Open-Angle Glaucoma Preferred Practice Pattern((R)) Guidelines. Ophthalmology. 2016;123(1):P41–P111. [DOI] [PubMed] [Google Scholar]
- 14.Stanley J, Huisingh CE, Swain TA, McGwin G Jr., Owsley C, Girkin CA, et al. Compliance With Primary Open-angle Glaucoma and Primary Open-angle Glaucoma Suspect Preferred Practice Patterns in a Retail-based Eye Clinic. Journal of glaucoma. 2018;27(12):1068–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prum BE Jr., Lim MC, Mansberger SL, Stein JD, Moroi SE, Gedde SJ, et al. Primary Open-Angle Glaucoma Suspect Preferred Practice Pattern((R)) Guidelines. Ophthalmology. 2016;123(1):P112–51. [DOI] [PubMed] [Google Scholar]
- 16.**.Kazemian P, Lavieri MS, Van Oyen MP, Andrews C, Stein JD. Personalized Prediction of Glaucoma Progression Under Different Target Intraocular Pressure Levels Using Filtered Forecasting Methods. Ophthalmology. 2018;125(4):569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using longitudinal visual field and IOP data from two glaucoma clinical trials (i.e., Collaborative Initial Glaucoma Treatment Study, known as CIGTS, and Advanced Glaucoma Intervention Study, known as AGIS), a decision-making tool to determine target IOP was developed to forecast visual field progression.
- 17.Aref AA, Budenz DL. Detecting Visual Field Progression. Ophthalmology. 2017;124(12S):S51–S6. [DOI] [PubMed] [Google Scholar]
- 18.*.Quigley HA. 21st century glaucoma careEye. 2018. [Google Scholar]; Dr. Quigley shares his perspectives on the evolution of glaucoma care.
- 19.Medeiros FA. Biomarkers and Surrogate Endpoints: Lessons Learned From Glaucoma. Investigative ophthalmology & visual science. 2017;58(6):BIO20–BIO6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.*.Saheb H, Ahmed, II. Micro-invasive glaucoma surgery: current perspectives and future directions. Current opinion in ophthalmology. 2012;23(2):96–104. [DOI] [PubMed] [Google Scholar]; Initial description of micro-invasive glaucoma surgery or MIGS.
- 21.Schehlein EM, Kaleem MA, Swamy R, Saeedi OJ. Microinvasive Glaucoma Surgery: An Evidence-Based Assessment. Expert review of ophthalmology. 2017;12(4):331–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agrawal P, Bradshaw SE. Systematic Literature Review of Clinical and Economic Outcomes of Micro-Invasive Glaucoma Surgery (MIGS) in Primary Open-Angle Glaucoma. Ophthalmology and therapy. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.GRANT WM. Experimental aqueous perfusion in enucleated human eyes. Archives of ophthalmology. 1963;69(6):783–801. [DOI] [PubMed] [Google Scholar]
- 24.Rosenquist R, Epstein D, Melamed S, Johnson M, Grant WM. Outflow resistance of enucleated human eyes at two different perfusion pressures and different extents of trabeculotomy. Current eye research. 1989;8(12):1233–40. [DOI] [PubMed] [Google Scholar]
- 25.Schuman JS, Chang W, Wang N, de Kater AW, Allingham RR. Excimer laser effects on outflow facility and outflow pathway morphology. Investigative ophthalmology & visual science. 1999;40(8):1676–80. [PubMed] [Google Scholar]
- 26.**.Carreon T, van der Merwe E, Fellman RL, Johnstone M, Bhattacharya SK. Aqueous outflow - A continuum from trabecular meshwork to episcleral veins. Progress in retinal and eye research. 2017;57:108–33. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors review current understanding of aqueous humor outflow.
- 27.Cha ED, Xu J, Gong L, Gong H. Variations in active outflow along the trabecular outflow pathway. Experimental eye research. 2016;146:354–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.**.Man X, Arroyo E, Dunbar M, et al. Perilimbal sclera mechanical properties: Impact on intraocular pressure in porcine eyes. PloS one. 2018;13(5):e0195882. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper provides evidence that the biomechanics of the perilimbal scleral tissue influences IOP. The study design combines whole globe perfusion and mechanical stress-strain and relaxation testing in tissue specimens from the same perfused porcine eyes.
- 29.Johnstone M, Jamil A, Martin J. Aqueous Veins and Open Angle Glaucoma In: Schacknow PN, Samples JR, editors. The Glaucoma Book. New York: Springer; 2010. [Google Scholar]
- 30.Fellman RL, Feuer WJ, Grover DS. Episcleral Venous Fluid Wave Correlates with Trabectome Outcomes: Intraoperative Evaluation of the Trabecular Outflow Pathway. Ophthalmology. 2015;122(12):2385–91 e1. [DOI] [PubMed] [Google Scholar]
- 31.Loewen RT, Brown EN, Roy P, Schuman JS, Sigal IA, Loewen NA. Regionally Discrete Aqueous Humor Outflow Quantification Using Fluorescein Canalograms. PloS one. 2016;11(3):e0151754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.*.Huang AS, Penteado RC, Saha SK, Do JL, Ngai P, Hu Z, et al. Fluorescein Aqueous Angiography in Live Normal Human Eyes. Journal of glaucoma. 2018;27(11):957–64. [DOI] [PMC free article] [PubMed] [Google Scholar]; Intraoperative aqueous angiography with two different sequential tracers was detected in patients without glaucoma and were undergoing cataract surgery.
- 33.Grieshaber MC, Pienaar A, Olivier J, Stegmann R. Channelography: imaging of the aqueous outflow pathway with flexible microcatheter and fluorescein in canaloplasty. Klin Monbl Augenheilkd. 2009;226(4):245–8. [DOI] [PubMed] [Google Scholar]
- 34.Huang AS, Saraswathy S, Dastiridou A, Begian A, Legaspi H, Mohindroo C, et al. Aqueous Angiography with Fluorescein and Indocyanine Green in Bovine Eyes. Translational vision science & technology. 2016;5(6):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.*.Laroche D, Nortey A, Ng C. A Novel Use of Trypan Blue During Canalicular Glaucoma Surgery to Identify Aqueous Outflow to Episcleral and Intrascleral Veins. Journal of glaucoma 2018;27(10):e158–e61. [DOI] [PubMed] [Google Scholar]; Intracameral trypan blue was used as a tracer to assess the distal aqueous humor outflow after goniotomy in four patients with glaucoma.
- 36.Kagemann L, Wollstein G, Ishikawa H, Sigal IA, Folio LS, Xu J, et al. 3D visualization of aqueous humor outflow structures in-situ in humans. Experimental eye research. 2011;93(3):308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xin C, Wang RK, Song S, Shen T, Wen J, Martin E, et al. Aqueous outflow regulation: Optical coherence tomography implicates pressure-dependent tissue motion. Experimental eye research. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abramoff MD, Magalhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11(7):36–42. [Google Scholar]
- 39.Thevenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE transactions on image processing : a publication of the IEEE Signal Processing Society. 1998;7(1):27–41. [DOI] [PubMed] [Google Scholar]
- 40.Ehrlich JR, Ojeda LV, Wicker D, Day S, Howson A, Lakshminarayanan V, et al. Head-Mounted Display Technology for Low Vision Rehabilitation and Vision Enhancement. American journal of ophthalmology. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trese MG, Khan NW, Branham K, Conroy EB, Moroi SE. Expansion of Severely Constricted Visual Field Using Google Glass. Ophthalmic surgery, lasers & imaging retina. 2016;47(5):486–9. [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto C, Yamao S, Nomoto H, Takada S, Okuyama S, Kimura S, et al. Visual Field Testing with Head-Mounted Perimeter ‘imo’. PloS one. 2016;11(8):e0161974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsapakis S, Papaconstantinou D, Diagourtas A, Droutsas K, Andreanos K, Moschos MM, et al. Visual field examination method using virtual reality glasses compared with the Humphrey perimeter. Clinical ophthalmology. 2017;11:1431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakanishi M, Wang YT, Jung TP, Zao JK, Chien YY, Diniz-Filho A, et al. Detecting Glaucoma With a Portable Brain-Computer Interface for Objective Assessment of Visual Function Loss. JAMA ophthalmology. 2017;135(6):550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.**.McCarthy MI, Abecasis GR, Cardon LR, Goldstein DB, Little J, Ioannidis JP, et al. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat Rev Genet. 2008;9(5):356–69. [DOI] [PubMed] [Google Scholar]; These all star genetic experts define the basic concepts of GWAS to study complex traits and diseases. The tables, figures, and side bar boxes provide excellent teaching points of this genetic approach to work toward determing the genetic architecture of traits and diseases.
- 46.Wiggs JL, Pasquale LR. Genetics of glaucoma. Human molecular genetics. 2017;26(R1):R21–R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Allingham RR. Major review: Molecular genetics of primary open-angle glaucoma. Experimental eye research. 2017;160:62–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hysi PG, Cheng CY, Springelkamp H, Macgregor S, Bailey JN, Wojciechowski R, et al. Genome-wide analysis of multi-ancestry cohorts identifies new loci influencing intraocular pressure and susceptibility to glaucoma. Nature genetics. 2014;46(10):1126–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ozel AB, Moroi SE, Reed DM, Nika M, Schmidt CM, Akbari S, et al. Genome-wide association study and meta-analysis of intraocular pressure. Human genetics. 2014;133(1):41–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.**.Gao XR, Huang H, Nannini DR, Fan F, Kim H. Genome-wide association analyses identify new loci influencing intraocular pressure. Human molecular genetics. 2018;27(12):2205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper demonstrates the value of access to public biorespositories, e.g., UK Biobank. Gao and colleagues replicate known IOP risk alleles, and identify new variants. Gao uses other bioinformatic resources to show the context of these IOP risk alleles shared with other GWAS results of different phenotypes and pathways.
- 51.Asefa NG, Neustaeter A, Jansonius NM, Snieder H. Heritability of glaucoma and glaucoma-related endophenotypes: systematic review and meta-analysis protocol. BMJ open. 2018;8(2):e019049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brooks BP, Moroi SE, Downs CA, Wiltse S, Othman MI, Semina EV, et al. A novel mutation in the PITX2 gene in a family with Axenfeld-Rieger syndrome. Ophthalmic genetics. 2004;25(1):57–62. [DOI] [PubMed] [Google Scholar]
- 53.Vincent AL, Billingsley G, Buys Y, Levin AV, Priston M, Trope G, et al. Digenic inheritance of early-onset glaucoma: CYP1B1, a potential modifier gene. American journal of human genetics. 2002;70(2):448–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shiga Y, Akiyama M, Nishiguchi KM, Sato K, Shimozawa N, Takahashi A, et al. Genome-wide association study identifies seven novel susceptibility loci for primary open-angle glaucoma. Human molecular genetics. 2018;27(8):1486–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi J, Kook MS. Systemic and Ocular Hemodynamic Risk Factors in Glaucoma. BioMed research international. 2015;2015:141905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leske MC. Ocular perfusion pressure and glaucoma: clinical trial and epidemiologic findings. Current opinion in ophthalmology. 2009;20(2):73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Archives of ophthalmology. 2002;120(6):714–20; discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 58.**.Kim JH, Caprioli J. Intraocular Pressure Fluctuation: Is It Important? Journal of ophthalmic & vision research. 2018;13(2):170–4. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper proposes a data dictionary to characterize IOP fluctuation based on time frame. Such definitions are important as they will facilitate comparison of clinical studies.
- 59.Ittoop SM, SooHoo JR, Seibold LK, Mansouri K, Kahook MY. Systematic Review of Current Devices for 24-h Intraocular Pressure Monitoring. Advances in therapy. 2016;33(10):1679–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.**.Lopez C, Tucker S, Salameh T, Tucker C. An unsupervised machine learning method for discovering patient clusters based on genetic signatures. J Biomed Inform. 2018;85:30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]; These authors describe basic analytical tools used on big data to characterize clusters of patients based on genetic signatures. The relationships of these genetic signatures to clinical presentation, disease progression, and response to treatment will be valuable to develop prediction models to stratify risk.
- 61.Ai C, Kong L. CGPS: A machine learning-based approach integrating multiple gene set analysis tools for better prioritization of biologically relevant pathways. J Genet Genomics. 2018;45(9):489–504. [DOI] [PubMed] [Google Scholar]
- 62.Moroi SE, Raoof DA, Reed DM, Zollner S, Qin Z, Richards JE. Progress toward personalized medicine for glaucoma. Expert review of ophthalmology. 2009;4(2):145–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giudicessi JR, Wilde AAM, Ackerman MJ. The genetic architecture of long QT syndrome: A critical reappraisal. Trends Cardiovasc Med. 2018;28(7):453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.