Abstract
Purpose: Percutaneous radiofrequency ablation and cryoablation are accepted alternative treatments for small renal cell carcinomas (RCC) in high-risk patients. The recent development of high-powered microwave (MW) ablation offers theoretical advantages over existing ablation systems, including higher tissue temperatures, more reproducible ablation zones, and shorter procedural times. The purpose of this study is to review the feasibility, safety, and early efficacy of a novel high-powered percutaneous MW ablation system to treat RCC.
Methods: An institutional database identified 53 consecutive patients with biopsy-proven RCC ≤4 cm (55 tumors) who were treated with percutaneous MW ablation using a novel MW ablation system. All patients had percutaneous renal mass biopsy, which identified RCC before ablation. Postprocedure follow-up imaging was performed by contrast-enhanced computed tomography or magnetic resonance imaging.
Results: Mean patient age was 66 years and 81% of patients were male. RCC subtypes included clear cell (n=25), papillary (n=12), and unspecified (n=18) and Fuhrman grades 1, 2, 3, and ungraded in 15, 25, 1, and 14 patients, respectively. The mean tumor diameter was 2.6 cm (range 0.8–4.0 cm). Six low-grade complications were recorded during 53 (11.3%) procedures: five Clavien Grade 1 (urine retention, fluid overload, and atrial fibrillation) and one Grade 2 (hemorrhage requiring transfusion). The postprocedure estimated glomerular filtration rate was not significantly changed from preprocedure levels (median: −1.1%, p=0.10). Median follow-up was 8 months (interquartile range [IQR] 5–18.25) with 0/38 (0%) patients demonstrating evidence of local recurrence or metastasis during surveillance imaging.
Conclusions: Use of a high-powered MW ablation system for the treatment of T1a RCC is feasible, safe, and efficacious with short-term follow-up. A longer follow-up is warranted to evaluate oncologic outcomes.
Introduction
Ablative therapies are increasingly utilized for the treatment of renal tumors.1 In the American Urologic Association (AUA) Guideline for Management of the small renal masses (2010), radiofrequency ablation (RFA) and cryoablation are cited as alternative therapies for patients with renal cell carcinoma (RCC) who are poor surgical candidates.2 Long-term recurrence-free survival has been demonstrated for many patients with small RCC who are treated with RFA or cryoablation.3 However, several series have also demonstrated higher rates of local recurrence using ablation compared with surgical series.4 In addition, treatment of larger tumors has been limited by higher complication rates and worse outcomes compared with smaller tumors.3,5
Using microwave (MW) energy for ablation has theoretical advantages compared with RFA or cryoablation. In contrast to RFA, the energy generated during MW ablation penetrates all biologic materials, including charred and desiccated tissue. Higher tissue temperatures can be achieved in less time in larger tumors compared with RFA systems.6 In addition, MW ablation can support synchronous energy delivery from multiple probes to maximize the impact of thermal synergy.7 Despite the advantages of MW energy, early MW ablation devices were underpowered due to heating of the antenna shaft while delivering energy to the target. As a result, early MW devices produced inconsistent ablation zones resulting in incomplete ablation and poor outcomes.8
Higher power MW systems have recently become available that utilize active shaft cooling with water, saline, or carbon dioxide gas, allowing more energy to be delivered to target tissues without unintentional injury to surrounding tissues along the antenna shaft.9 The purpose of this study is to evaluate the feasibility, safety, and early efficacy of patients with small RCC treated with a high-powered, gas-cooled MW ablation system.
Methods
Patient selection
After the Institutional Review Board (IRB) approval, an institutional database identified all consecutive patients who underwent percutaneous MW ablation, with curative intent for RCC ≤4 cm, between January 2011 and February 2014. RCC was diagnosed in all patients by percutaneous biopsy at least 2 weeks before ablation. The decision to offer ablation for RCC treatment was made by a multidisciplinary team of urologists and radiologists.
Procedure
All procedures were performed under general anesthesia utilizing ultrasound and computed tomography (CT) guidance for percutaneous antenna placement and confirmation. A multidisciplinary team, consisting of a radiologist and a urologist experienced in tumor ablation, performed each procedure. Depending on the size and location of the tumor, one to three antennas (Certus 140; NeuWave Medical, Madison, WI) were used in each procedure, similar to a recent series of 311 patients where an average of three probes (range 1–12) were used for renal mass cryoablation.5 The MW system used for this study is the Certus 140 (NeuWave Medical, Inc., Madison, WI). The system is an FDA-approved, high-powered (140 W in up to three channels) third-generation MW device that uses CO2 gas cooling to prevent shaft heating. The gas cooling also allows the probes to be stuck into tissue by creating a small ice ball at the tip using the Joule–Thomson method, similar to the tissue cooling mechanism of cryoablation systems. The probes are 17-gauge, and several different ablation zone configurations are available depending on which probe is selected. The expected ablation diameter in ex vivo tissue is available on the manufacturer's website (www.neuwave.com), but varies somewhat depending on the tissue type and tumor vascularity.
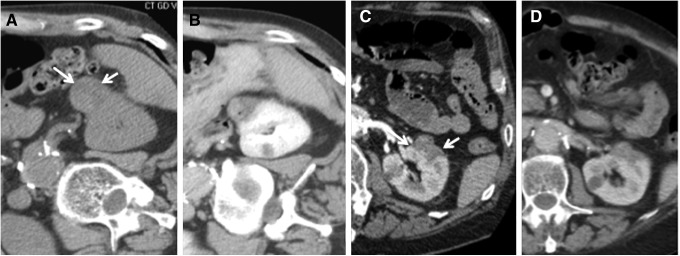
When the tumor was in close proximity to adjacent structures, injectable grade dextrose 5% sterile water was instilled through a spinal needle to increase the distance from the treatment zone (hydrodisplacement), as has been described previously.10 For hydrodisplacement, the treatment team considered the subjective proximity of structures as well as the expected ablation zone, which varies according to the manufacturer's specifications for the individual probe types. An ablation protocol utilizing the 65W power setting for 5 minutes was used in the majority of patients. However, this protocol was modified in selected patients based upon the number of probes, tumor size, tumor location, and use of hydrodisplacement. In general, tumors <2 cm are ablated with a single probe, 2 to 3 cm with two probes, and >3 cm with three probes depending on the location. Ultrasound was used for real-time monitoring of the extent of ablation to achieve a 5 mm margin beyond the tumor, and immediate postprocedure imaging was performed using contrast-enhanced CT (CECT) for all patients with adequate renal function (Fig. 1). All patients were admitted overnight for observation and CECT or magnetic resonance imaging to evaluate for local tumor progression at routine target intervals of 6, 12, and 24 months postablation.
FIG. 1.
RCC treated with MW ablation under CT and US guidance. Axial (A) and coronal (B) preablation CECT images used for procedure planning demonstrate a 2.7 cm RCC (arrows). US visualization of the tumor (C, arrow) and placement of two MW antennas under US guidance (D, tumor indicated by gray arrow, antenna by white arrows). Unenhanced CT confirmation of antenna placement (E) followed by US visualization of evolving ablation zone (F) with bubbles encompassing and obscuring the tumor during a 5-minute 65W ablation. Postablation CECT (G) demonstrates ablation zone (white arrows) encompassing the tumor (gray arrow), which is without any residual enhancement. CECT, contrast-enhanced CT; CT, computed tomography; MW, microwave; RCC, renal cell carcinomas.
Data collection and analysis
Clinical, pathologic, and procedure details were recorded and retrospectively analyzed for each patient. The RENAL nephrometry score was calculated for each tumor,11 and complications occurring during ablation and within 30 days afterward were classified with the Clavien-Dindo system.12 The estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI formula.13 The paired t-test was used to compare pre- and postprocedure eGFRs. The Kaplan–Meier method was used for survival analyses.
Results
Patient/procedure data
A total of 55 biopsy-proven RCC ≤4 cm were treated in 53 patients during 53 treatment sessions. Clinical and pathological characteristics are shown in Table 1. Median eGFR before ablation was 79.4 (interquartile range [IQR] 64.3–87.8). The postprocedure eGFR was not significantly changed from preprocedure levels (median: −1.1%, IQR −13.8%–6.2%), p=0.10.
Table 1.
Patient and Disease Characteristics
| Characteristic | Value |
|---|---|
| Median age (years) | 65 (IQR 60–74) |
| Gender, n (%) | |
| Male | 43 (81.1) |
| Female | 10 (18.9) |
| Median Charlson Comorbidity Index | 3 (IQR 2–4) |
| Median RENAL nephrometry score | 6 (IQR 5–7) |
| Tumor side, n (%) | |
| Right | 32 (58.2) |
| Left | 23 (41.8) |
| Median tumor diameter (cm) | 2.7 (IQR 1.9–3.2) |
| Histologic RCC subtype, n (%) | |
| Clear cell | 25 (45.5) |
| Papillary | 12 (21.8) |
| Unspecified | 18 (32.7) |
| Grade, n (%) | |
| 1 | 15 (27.3) |
| 2 | 25 (45.5) |
| 3 | 1 (1.8) |
| No grade assigned | 14 (25.4) |
IQR=interquartile range; RCC=renal cell carcinomas.
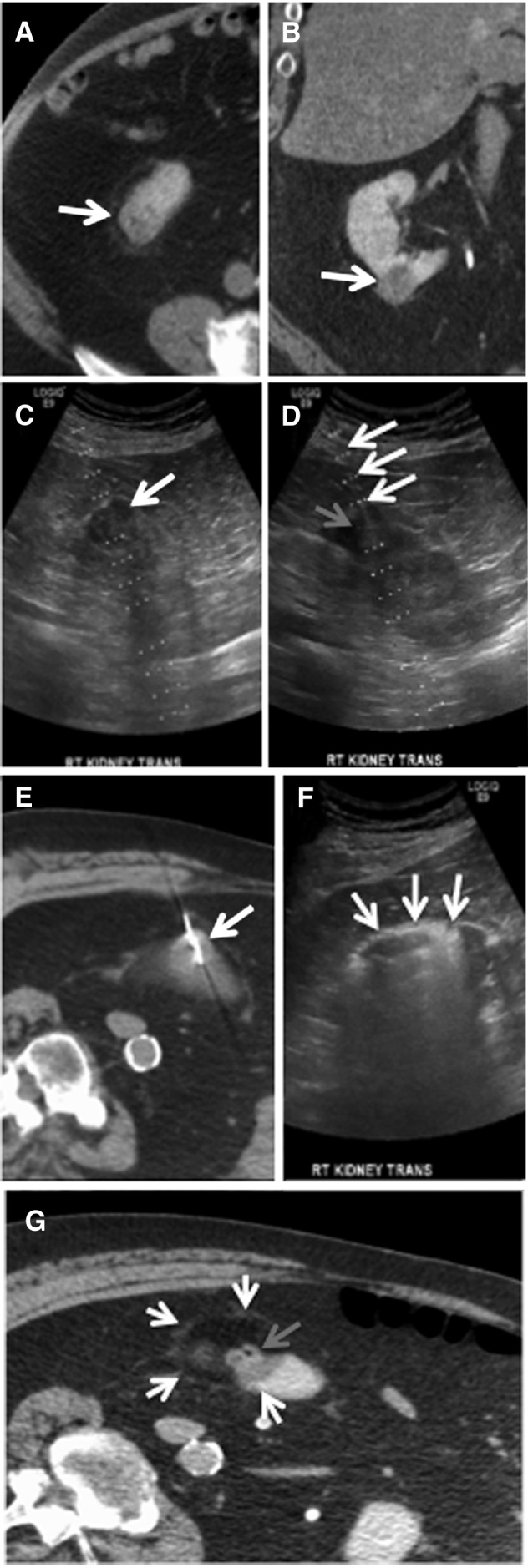
The median pretreatment tumor diameter was 2.7 cm (IQR 1.9–3.2). The tumor diameter decreased by a median of 22% (IQR 14%–31%), and tumor volume decreased by a median of 52% (IQR 36%–67%) on immediate postablation CT (Fig. 2). One, two, or three probes were used in 18, 24, and 13 tumors, respectively. The median duration of power application was 5 minutes (IQR 5–7 minutes), and median generator power was 65 W (IQR 65–65).
FIG. 2.
RCC contraction with MW ablation. CT images demonstrating immediate contraction of an exophytic RCC preablation; tumor indicated by arrows (A), immediate postablation (B), 3 months postablation; tumor indicated by arrows (C), and 8 months postablation (D).
Postprocedure imaging
Thirty-eight patients have had follow-up imaging (in addition to the immediate postablation scan) at a median of 8 months postablation (IQR 6–9 months). No residual tumor was observed on immediate postprocedure for any tumor, and no patients were found to have local recurrence or metastatic progression in the follow-up period.
Complications/procedural follow-up
There was one Clavien Grade 2 complication (1.9%; n=1/53): retroperitoneal hematoma on postablation day 10. In the year before the procedure, this patient had been treated for a symptomatic recurrent pulmonary embolus with a combination of warfarin and low-molecular-weight heparin, according to her primary physician. The patient was restarted on warfarin on postablation day 5 and low-molecular-weight heparin on postablation day 10, as per her preoperative regimen. The patient presented with flank pain 12 hours after reinitiation of heparin and required readmission and transfusion of 2 U packed red blood cells (Fig. 3). There were five Grade 1 complications in four patients (7.5%; n=4/53): three cases of urinary retention following urinary catheter removal requiring discharge with a urinary catheter, one of these patients additionally was found to have transient periprocedural atrial fibrillation. Another patient readmitted on postoperative day 4 for shortness of breath secondary to fluid overload.
FIG. 3.
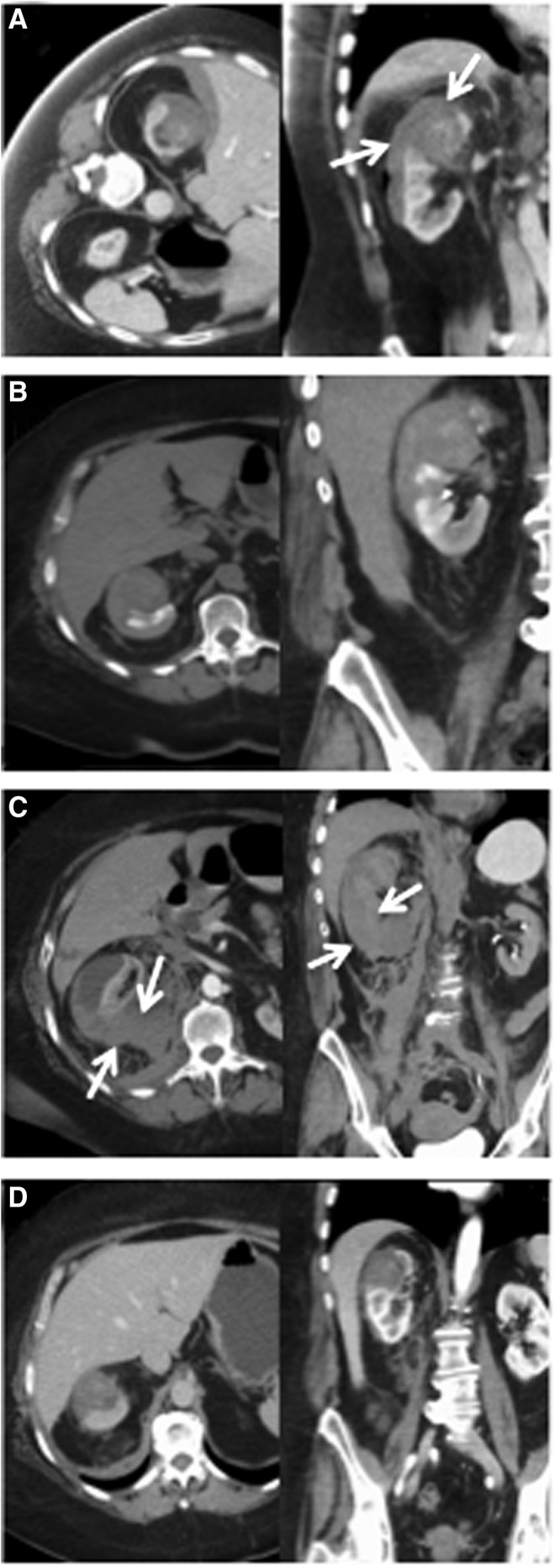
Retroperitoneal hematoma postpercutaneous MW ablation of RCC. CECT images of a large retroperitoneal hematoma developing on postprocedure day 11 after percutaneous MW ablation of a 3.5 cm RCC. Small, stable subcapsular hematoma evident immediately postablation; tumor indicated by arrows (A) and on postablation day 1 (B). (C) Expansion of hematoma into subcapsular and perirenal spaces on postablation day 11. Bleeding began within an hour after restarting enoxaparin (dual prophylaxis with coumadin) in a patient with a history of pulmonary emboli; tumor indicated by arrows. (D) Interim resorption of hematoma without evidence of recurrence on follow-up CECT 3 months postablation.
Hospital stay was 1 day for 52 patients (1 patient stayed for 3 days postprocedure for flank pain without radiographic evidence of complication, 1 patient stayed 3 days for urinary retention, and another patient was discharged the day of the procedure). Two patients required readmission for a 30-day readmission rate of 3.8%.
Discussion
High-powered percutaneous MW ablation is feasible and can be applied safely for the treatment of cT1a RCC. Low-grade complications were reported overall with no Clavien Grade 3 to 5 complications recorded during the procedure or the 30 days postoperatively. No patients have demonstrated local or metastatic recurrence during median follow-up of 8 months. Although oncologic efficacy must be demonstrated in studies with longer follow-up, initial data suggest that MW ablation is safe, feasible, and should continue to be evaluated as an alternative therapy for small RCC.
The rate of major perioperative morbidity is low when using a high-powered system for percutaneous MW ablation, similar to renal mass RFA and cryoablation studies.5 One Clavien Grade 2 complication occurred in this series (1/53, 1.9%), a delayed hemorrhage on postoperative day 10 after restarting combination therapy with low-molecular-weight heparin and warfarin. Bleeding resolved after transfusion and the patient was discharged home without additional morbidity. Hemorrhage is the most common major complication after percutaneous cryoablation, with a significant number of patients receiving blood transfusion in early series of percutaneous cryoablation.14 Unlike RFA and MW ablation, cryoablation has no intrinsic cautery effect and hemorrhage is most common after cryoablation of large tumors using multiple probes.5
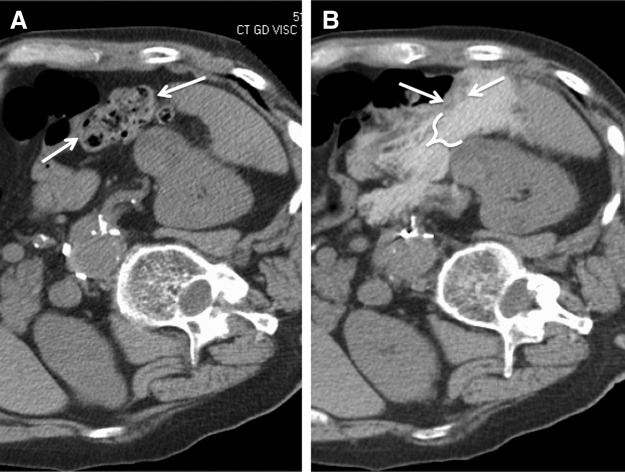
The low overall number of major complications post-MW ablation in this initial study is encouraging, especially considering that most patients in this series had significant comorbidities. It should be noted that our institution has a more than 10 years of experience with percutaneous RFA and cryoablation of renal masses, and it is possible that the rate of complications with MW ablation may be greater at a less experienced center. In addition to morbidity, the majority (98%) of patients were able to be discharged on the day following the procedure with 3.8% requiring readmission similar to other renal ablation series. Although general anesthesia is not required, it has been our practice to use general anesthesia for most ablation patients, allowing better control of respiratory movements during the procedure. However, in selected patients, using sedation with local anesthesia is possible and may allow for shorter hospital stay. Mobilization of adjacent structures such as bowel by injecting fluid between structures was performed in 38%, which is similar to a recent renal cryoablation series where hydrodisplacement was used in 24% of cases.15 The use of hydrodisplacement in the present series was not associated with any complications and subjectively allowed percutaneous ablation to be safely applied in cases where bowel was adjacent to the targeted tumor (Fig. 4). Renal function post-MW ablation remained stable with no significant change in eGFR postprocedure. Preservation of renal function using MW ablation is similar to that observed in RFA and cryoablation series16,17 and may be expected given the consistent and reproducible ablation zones produced by using MW probes.18
FIG. 4.
Bowel displacement by hydrodisplacement before MW ablation of RCC. CT images demonstrating (A) proximity of colon (arrows) to target RCC with (B) subsequent introduction of 700 mL of 5% dextrose in water with 15 mL iohexol per liter (bracket) to displace colon away from RCC in preparation for MW ablation.
Longer term data with both RFA and cryoablation have demonstrated the ability to achieve local tumor control and cancer-free survival using these techniques.3,19 Outcomes are significantly better in patients with smaller tumors20 and less complex tumors.21 In the current series, treatment was limited to tumors ≤4 cm and of low or moderate complexity to generate experience using a new treatment modality. In larger tumors and some small tumors, increased rates of recurrence with initial treatment using RFA or cryoablation have been demonstrated.3,21 A consistent problem in evaluating cancer outcomes after renal mass ablation is the lack of tissue diagnosis before ablation in many series,3 which leads to overestimation of treatment success when nonmalignant tumors are included in analysis. The current study includes only patients with biopsy-proven RCC, which may enable more accurate evaluation of cancer outcomes after a longer term follow-up.
The treatment effect of high-powered MW ablation, as demonstrated on imaging, differs from other ablation modalities. The device used in the current study quickly generates high tissue temperatures (>150°C, significantly above the tumoricidal threshold of 60°C), which result in marked tissue contraction as the tumor is pulled into the zone of active heating.22 Previous ex vivo studies have noted that MW ablation zones contract by 30% to 38% in diameter in the liver, 47% to 52% in the lung, and 4% to 7% in the kidney, proportional to tissue dehydration.22,23 In this study, the tumors contracted 22% in diameter (52% in volume) on immediate postablation images. Posttreatment tissue contraction is not seen with cryoablation and is less marked with RFA due to the less extreme tissue heating and dehydration.22 In the current study, multiple MW antennas were frequently used, which takes advantage of both thermal and electrical synergy and increases the size of the ablation zone. The zone of ablation varies depending on the type of probe according to the manufacturer's specifications. The decision to place multiple probes was made by the treatment team according to the tumor size and location, similar to cryoablation.24
Multiple studies have suggested that achieving a tissue temperature of at least 60°C will cause cell death regardless of the technology used to achieve these tumoricidal temperatures.25,26 The use of heat-based ablation technologies for the treatment of RCC was originally felt to be suboptimal. This was based on reports of skip areas of viable tumor after RFA.27 In addition, early treat-and-resect studies demonstrated residual viable tumor immediately after RFA.28 However, more recent data have suggested that skip areas are only associated with certain multiple prong deployable RFA devices, in which irregular ablation zones allow viable tumor to remain in the interstices.29 Earlier reports of viable tumor after heat-based therapies have been refuted as tumor viability stains such as NADH have become available.30 Because of the higher temperatures produced by gas-cooled MW probes and better tissue penetration creating a larger active heating zone, improved local control of tumors may potentially occur with less chance of suboptimal heating at the edges of tumors. Longer term follow-up and comparative studies will be necessary to evaluate whether potential benefits of MW ablation lead to improved cancer-specific outcomes.
The primary limitation to this study is the short follow-up period. The rate of local recurrence or metastatic progression may increase with further follow-up. However, the purpose of this article was to report on the safety and feasibility of this approach and we do not expect the complication rate to significantly change with a longer follow-up. Other limitations include the retrospective nature of this study and the single type of MW system used in this study. The current results are likely not applicable to all MW systems, particularly single antenna and/or low-power devices, which may require multiple overlapping ablations to adequately treat tumors, and systems that produce different shaped ablation zones. Finally, renal function outcomes were measured as a secondary endpoint and may change with longer systematic follow-up.
In summary, our early clinical results demonstrate that percutaneous MW ablation is associated with a low procedural complication rate and a low rate of local tumor progression at early clinical follow-up. Longer term follow-up studies are warranted to determine if the physical and technical advantages associated with MW ablation translate into lower local recurrence rates and improved survival.
Abbreviations Used
- CECT
contrast-enhanced CT
- CT
computed tomography
- eGFR
estimated glomerular filtration rate
- MW
microwave
- RCC
renal cell carcinomas
- RFA
radiofrequency ablation
Disclosure Statement
Christopher L. Brace is a shareholder and consultant for NeuWave Medical, Inc. and Symple Surgical. J. Louis Hinshaw is a Board of Medical advisor and stock holder for Neuwave Medical, Inc. Fred T. Lee Jr. is a board member and stockholder for NeuWave Medical, Inc. For the remaining authors, no competing financial interests exist.
References
- 1.Venkatesan AM, Wood BJ, Gervais DA. Percutaneous ablation in the kidney. Radiology 2011;261:375–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell SC, Novick AC, Belldegrun A, et al. . Guideline for management of the clinical T1 renal mass. J Urol 2009;182:1271–1279 [DOI] [PubMed] [Google Scholar]
- 3.El Dib R, Touma NJ, Kapoor A. Cryoablation vs radiofrequency ablation for the treatment of renal cell carcinoma: A meta-analysis of case series studies. BJU Int 2012;110:510–516 [DOI] [PubMed] [Google Scholar]
- 4.Guillotreau J, Haber GP, Autorino R, et al. . Robotic partial nephrectomy versus laparoscopic cryoablation for the small renal mass. Eur Urol 2012;61:899–904 [DOI] [PubMed] [Google Scholar]
- 5.Atwell TD, Carter RE, Schmit GD, et al. . Complications following 573 percutaneous renal radiofrequency and cryoablation procedures. J Vasc Interv Radiol 2012;23:48–54 [DOI] [PubMed] [Google Scholar]
- 6.Lubner MG, Hinshaw JL, Andreano A, Sampson L, Lee FT, Jr., Brace CL. High-powered microwave ablation with a small-gauge, gas-cooled antenna: Initial ex vivo and in vivo results. J Vasc Interv Radiol 2012;23:405–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brace CL, Laeseke PF, Sampson LA, Frey TM, van der Weide DW, Lee FT., Jr. Microwave ablation with multiple simultaneously powered small-gauge triaxial antennas: Results from an in vivo swine liver model. Radiology 2007;244:151–156 [DOI] [PubMed] [Google Scholar]
- 8.Castle SM, Salas N, Leveillee RJ. Initial experience using microwave ablation therapy for renal tumor treatment: 18-month follow-up. Urology 2011;77:792–797 [DOI] [PubMed] [Google Scholar]
- 9.Lubner MG, Brace CL, Hinshaw JL, Lee FT., Jr. Microwave tumor ablation: Mechanism of action, clinical results, and devices. J Vasc Interv Radiol 2010;21(8 Suppl):S192–S203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SJ, Choyke LT, Locklin JK, Wood BJ. Use of hydrodissection to prevent nerve and muscular damage during radiofrequency ablation of kidney tumors. J Vasc Interv Radiol 2006;17:1967–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: A comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 2009;182:844–853 [DOI] [PubMed] [Google Scholar]
- 12.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, et al. . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finley DS, Beck S, Box G, et al. . Percutaneous and laparoscopic cryoablation of small renal masses. J Urol 2008;180:492–498; discussion 8. [DOI] [PubMed] [Google Scholar]
- 15.Bodily KD, Atwell TD, Mandrekar JN, et al. . Hydrodisplacement in the percutaneous cryoablation of 50 renal tumors. AJR Am J Roentgenol 2010;194:779–783 [DOI] [PubMed] [Google Scholar]
- 16.Hegarty NJ, Gill IS, Desai MM, Remer EM, O'Malley CM, Kaouk JH. Probe-ablative nephron-sparing surgery: Cryoablation versus radiofrequency ablation. Urology 2006;68(1 Suppl):7–13 [DOI] [PubMed] [Google Scholar]
- 17.Johnson DB, Taylor GD, Lotan Y, Sagalowsky AI, Koenemann KS, Cadeddu JA. The effects of radio frequency ablation on renal function and blood pressure. J Urol 2003;170(6 Pt 1):2234–2236 [DOI] [PubMed] [Google Scholar]
- 18.Fan W, Li X, Zhang L, Jiang H, Zhang J. Comparison of microwave ablation and multipolar radiofrequency ablation in vivo using two internally cooled probes. AJR Am J Roentgenol 2012;198:W46–W50 [DOI] [PubMed] [Google Scholar]
- 19.Psutka SP, Feldman AS, McDougal WS, McGovern FJ, Mueller P, Gervais DA. Long-term oncologic outcomes after radiofrequency ablation for T1 renal cell carcinoma. Eur Urol 2013;63:486–492 [DOI] [PubMed] [Google Scholar]
- 20.Best SL, Park SK, Youssef RF, et al. . Long-term outcomes of renal tumor radio frequency ablation stratified by tumor diameter: Size matters. J Urol 2012;187:1183–1189 [DOI] [PubMed] [Google Scholar]
- 21.Schmit GD, Thompson RH, Kurup AN, et al. . Usefulness of R.E.N.A.L. nephrometry scoring system for predicting outcomes and complications of percutaneous ablation of 751 renal tumors. J Urol 2013;189:30–35 [DOI] [PubMed] [Google Scholar]
- 22.Brace CL, Diaz TA, Hinshaw JL, Lee FT., Jr. Tissue contraction caused by radiofrequency and microwave ablation: A laboratory study in liver and lung. J Vasc Interv Radiol 2010;21:1280–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sommer CM, Sommer SA, Mokry T, et al. . Quantification of tissue shrinkage and dehydration caused by microwave ablation: Experimental study in kidneys for the estimation of effective coagulation volume. J Vasc Interv Radiol 2013;24:1241–1248 [DOI] [PubMed] [Google Scholar]
- 24.Young JL, McCormick DW, Kolla SB, et al. . Are multiple cryoprobes additive or synergistic in renal cryotherapy? Urology 2012;79:484.e1–e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: A unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol 2000;174:323–331 [DOI] [PubMed] [Google Scholar]
- 26.Lui KW, Gervais DA, Arellano RA, Mueller PR. Radiofrequency ablation of renal cell carcinoma. Clin Radiol 2003;58:905–913 [DOI] [PubMed] [Google Scholar]
- 27.Pavlovich CP, Walther M, Choyke PL, et al. . Percutaneous radio frequency ablation of small renal tumors: Initial results. J Urol 2002;167:10–15 [PMC free article] [PubMed] [Google Scholar]
- 28.Rendon RA, Kachura JR, Sweet JM, et al. . The uncertainty of radio frequency treatment of renal cell carcinoma: Findings at immediate and delayed nephrectomy. J Urol 2002;167:1587–1592 [PubMed] [Google Scholar]
- 29.Pereira PL, Trubenbach J, Schenk M, et al. . Radiofrequency ablation: In vivo comparison of four commercially available devices in pig livers. Radiology 2004;232:482–490 [DOI] [PubMed] [Google Scholar]
- 30.Anderson JK, Baker M, Jaffers O, Pearle MS, Lindberg GL, Cadeddu JA. Time course of nicotinamide adenine dinucleotide diaphorase staining after renal radiofrequency ablation influences viability assessment. J Endourol 2007;21:223–227 [DOI] [PubMed] [Google Scholar]