Abstract
We assessed the safety and immunogenicity of HIV-DNA priming using Zetajet™, a needle-free device intradermally followed by intramuscular HIV-MVA boosts, in 24 healthy Mozambicans. Volunteers were randomized to receive three immunizations of 600 μg (n = 10; 2 × 0.1 ml) or 1,200 μg (n = 10; 2 × 0.2 ml) of HIV-DNA (3 mg/ml), followed by two boosts of 108 pfu HIV-MVA. Four subjects received placebo saline injections. Vaccines and injections were safe and well tolerated with no difference between the two priming groups. After three HIV-DNA immunizations, IFN-γ ELISpot responses to Gag were detected in 9/17 (53%) vaccinees, while none responded to Envelope (Env). After the first HIV-MVA, the overall response rate to Gag and/or Env increased to 14/15 (93%); 14/15 (93%) to Gag and 13/15 (87%) to Env. There were no significant differences between the immunization groups in frequency of response to Gag and Env or magnitude of Gag responses. Env responses were significantly higher in the higher dose group (median 420 vs. 157.5 SFC/million peripheral blood mononuclear cell, p = .014). HIV-specific antibodies to subtype C gp140 and subtype B gp160 were elicited in all vaccinees after the second HIV-MVA, without differences in titers between the groups. Neutralizing antibody responses were not detected. Two (13%) of 16 vaccinees, one in each of the priming groups, exhibited antibodies mediating antibody-dependent cellular cytotoxicity to CRF01_AE. In conclusion, HIV-DNA vaccine delivered intradermally in volumes of 0.1–0.2 ml using Zetajet was safe and well tolerated. Priming with the 1,200 μg dose of HIV-DNA generated higher magnitudes of ELISpot responses to Env.
Keywords: : HIV, vaccine, Mozambique, HIV-DNA, HIV-MVA
Introduction
According to UNAIDS, there were a total of 36.7 million people living with HIV and 2.1 million new infections in 2015. Eastern and Southern Africa continues to be most severely affected, accounting for 51.7% of the total global infections. Antiretroviral therapy has contributed to slowing the HIV epidemic. However, the low coverage and strict treatment adherence requirement remain significant challenges.1,2 Pre- and postexposure prophylaxes have been used to prevent HIV infection.3,4 Yet, the effectiveness of pre-exposure prophylaxis in high-incidence heterosexual populations has been poorly achieved and is again highly dependent on drug adherence.5 Although a vaginal microbicide containing tenofovir reduced HIV acquisition by 39%,6 a risk for low treatment adherence was demonstrated by the VOICE trial,7 compromising the protective effect previously reported. Therefore, a safe and effective prophylactic HIV vaccine remains the best long-term solution for controlling the HIV pandemic.
After 30 years of research, there have been over 200 phase I to phase III clinical HIV vaccine trials of different vaccine candidates,8,9 but only one of the six HIV vaccine efficacy trials, the RV144 study, has demonstrated a modest efficacy (31.2%) against HIV acquisition at 42 months.10 The analysis of immune correlates of the RV144 trial revealed that IgG antibodies against the V1/V2 region of HIV-1 Envelope (Env) were inversely correlated with the risk of HIV infection, while the presence of IgA Env-binding antibodies was directly correlated with risk of infection.11–13 In addition, antibody-dependent cellular cytotoxicity (ADCC)-mediating antibodies and antibodies to the V3 region correlated with reduced risk of HIV infection in vaccinees with low IgA Env binding antibody titers.14 Analysis of the T cell responses confirmed HIV gp120 V2 specificity and revealed CD4+ T cells exhibiting polyfunctionality and cytolytic capacity.15 Contrary to other infectious disease vaccines where the development of neutralizing antibodies (NAbs) plays a central role in immunity, protection from HIV may require both functional antibody- and cell-mediated immune responses.8
Previous studies conducted in Sweden16,17 and Tanzania18 assessed the safety and immunogenicity of a multigene multiclade HIV-1 DNA vaccine candidate (HIV-DNA) boosted with heterologous HIV-1 modified vaccinia virus Ankara-Chiang Mai double recombinant vaccine (HIV-MVA). These trials explored different modes of delivery, the use of needle-free administration and dosing of the HIV-DNA vaccine, and demonstrated that intradermal (ID) priming using a needle-free device for HIV-DNA delivery (Bioject®) elicited higher IFN-γ ELISpot responses to Env when compared to the intramuscular (IM) route of delivery, and the majority of subjects developed HIV-specific antibodies after two HIV-MVA vaccinations.18 However, five injections with two pools of HIV-DNA plasmids at separate sites were required to achieve the desired 1,000 μg dose of HIV-DNA in a maximum injectable volume of 0.1 ml, using the Bioject®. Munseri et al. showed that HIV-DNA ID priming could be simplified, and two injections of a total of 600 μg administered as combined plasmid pools primed cellular immune responses as efficiently as the standard regimen.19
In the present phase I trial, we explored the safety, tolerability, and immunogenicity of delivering HIV-DNA at three priming doses, each of 600 μg or 1,200 μg ID using the needle-free Zetajet™ injection device that allows up to 0.2 ml ID injections, followed by two HIV-MVA boosts.
Materials and Methods
Ethics and regulatory statement
Approvals to conduct the study were granted by the National Health Bioethics Committee of Mozambique (ref. 76/CNBS/11 and 142/CNBS/11) and by the national regulatory authority, the Pharmaceutical Department (ref. 1554/054.3/DF). The trial was also approved by the Regional Ethics Committee, Stockholm, Sweden (2011/1684-31-4). Written informed consent was obtained before any study activity.
Study design and population
This was a phase I randomized, placebo-controlled, double-blinded trial conducted at the Polana Caniço Health Research and Training Center in Maputo city, Mozambique, from August 2011 to March 2013. Study participants were recruited from a cohort of young adults extensively counseled on HIV and prevention of sexually transmitted infections, described elsewhere.20 Subjects aged 18–26 years, at low risk for HIV infection, not planning to conceive a child for the duration of the study, residing in Maputo city, and in good general health as determined by medical history, physical examination, and laboratory tests were eligible to participate. Subjects with abnormal electrocardiogram (ECG) findings; diagnosed with HIV, syphilis, or hepatitis B; and pregnant and breastfeeding women were excluded at screening. All participants were required to practice effective birth control and avoid pregnancy throughout the study.
Twenty-four participants were randomized to two treatment groups (Table 1). Within each group, subjects were block randomized to receive vaccine or placebo in a ratio of 5:1. The study team and participants were blinded to vaccine or placebo administration but not to the treatment arms. Both groups received three immunizations with HIV-DNA/placebo ID, using the Zetajet (Bioject Medical Technologies, Inc., Tualatin, OR) at weeks 0, 4, and 12, followed by two HIV-MVA/placebo injections, IM, using a 23-gauge syringe at weeks 24 and 36. Groups I and II received HIV-DNA/placebo in volumes of 0.1 ml and 0.2 ml per injection, respectively, in both the left and right deltoid regions. Participants were followed for 12 weeks after the last injection.
Table 1.
Study Schema
| HIVIS DNA prime (week 0, 4, 12) | HIV-MVA boosting (week 24, 36) | ||
|---|---|---|---|
| Treatment group | Left arm (pool 1, Env A, B, C RevB) | Right arm (pool 2, Gag A, B RTmut B) | Left arm (HIV-MVA) |
| IA (n = 10) | 1 i.d. injection (3 mg/ml), 0.1 ml | 1 i.d. injection (3 mg/ml), 0.1 ml | 1 i.m. injection of 108 pfu, 1 ml |
| IB (n = 2) | 1 i.d. injection of saline, 0.1 ml | 1 i.d. injection of saline, 0.1 ml | 1 i.m. injection of saline, 1 ml |
| IIA (n = 10) | 1 i.d. injection (3 mg/ml), 0.2 ml | 1 i.d. injection (3 mg/ml), 0.2 ml | 1 i.m. injection of 108 pfu, 1 ml |
| IIB (n = 2) | 1 i.d. injection of saline, 0.2 ml | 1 i.d. injection of saline, 0.2 ml | 1 i.m. injection of saline, 1 ml |
Wk., week.
Vaccines
HIV-DNA (Lot No. 110524-24:3/42-45) is a DNA vaccine based on seven plasmids carrying HIV-1 genes: Pool 1 encoding Env subtypes A, B, and C and Rev subtype B; and Pool 2 encoding Gag subtypes A and B and RTmut subtype B.16,21 HIV-DNA was manufactured by Vecura (Huddinge, Stockholm, Sweden) and was formulated in physiological saline. The vaccine was presented in liquid form, at a concentration of 3 mg/ml of total DNA, and stored in vials of 0.15 ml at −20°C until use.
The HIV-MVA (Lot No. 0965) is a live recombinant nonreplicating poxvirus vector-based vaccine that had been genetically engineered to express HIV-1 gp150 (subtype E, isolate CM235) and Gag and Pol (integrase deleted and reverse transcriptase nonfunctional, subtype A, isolate CM240).22 HIV-MVA was manufactured for the Walter Reed Army Institute of Research by ABL, Inc. (Rockville, MD). The vaccine was formulated in a sterile PBS buffer (without Ca2+ and Mg2+), 7.5% lactose, pH 7.4, and presented in liquid form in a concentration of 108 pfu/ml. HIV-MVA was stored in 1 ml vials at a temperature of −80°C (±10°C), until use.
The vaccines were thawed at the pharmacy and dispensed into syringes labeled with the study code. They were kept under refrigeration (+2°C to 8°C) and administered within 4 h after being dispensed.
Sterile commercially available normal saline for humans was used as the placebo.
Safety assessment
Safety was assessed clinically and by standard chemistry and hematology tests. All subjects were observed for vital signs and local and systemic reactogenicity, 30 min postvaccination. The ID injection sites were inspected immediately after the injections and wheal diameter measured using a ruler. Subjects were instructed to report any reaction in the postvaccination diary card during 7 days after each immunization (including the night of vaccination), and to contact the clinic if any moderate or severe reaction occurred. Solicited local reactions included pain, itching, warmth, swelling, erythema, and induration. Solicited systemic reactions included fever (axillar temperature >37.5°C), malaise, chills, arthralgia, myalgia, headache, nausea, and vomiting. Safety assessment visits were performed 2 and 4 weeks after each immunization. A 12 lead ECG was performed at screening and 2 weeks after each HIV-MVA immunization as per the U.S. FDA requirements at the time.
All adverse events (AEs) occurring after the first injection up to the last study visit were recorded. AEs were graded as mild (grade 1), moderate (grade 2), severe (grade 3), and potentially life threatening (grade 4), according to the DAIDS Toxicity Table (version 1.0, December 2004, clarification August 2009) and categorized according to the Common Terminology Criteria for Adverse Events (CTCAE, version 4.0), System Organ Class terminology.
Urinalysis and pregnancy tests were performed before each vaccination. Participants with a positive pregnancy test after enrollment were considered ineligible for vaccination but were followed throughout the study for safety assessments.
HIV testing and referrals
HIV testing was performed at screening, at each vaccination visit, at the last study visit (week 48), and whenever the volunteer had the need to establish his/her HIV status. HIV-infected subjects were referred for clinical follow-up at a health facility of their convenience and did not receive further immunizations but continued their safety follow-up at the study site, until protocol completion.
Two concurrent commercial enzyme-linked immunosorbent assay (ELISA) kits, Murex HIV Ag/Ab (Abbott Murex, Dartford, United Kingdom) or GenScreen™ HIV-1/2 version 2 (Bio-Rad, Hercules, CA) and Enzygnost anti-HIV-1/2 Plus (Dade Behring, Marburg, Germany) were used for HIV screening. For eligibility purposes, both ELISA results were required to be nonreactive. Discordant results on the ELISA were resolved using HIV-DNA PCR (Roche Amplicor HIV-1 DNA test, version 1.5; Roche Molecular Diagnostics, Branchburg, NJ). Reactive results were then confirmed by an HIV-RNA PCR assay (COBAS® TaqMan®48 analyzer; Roche Molecular Diagnostics, Mannheim, Germany).
Immunogenicity assessment
The ELISpot assay was performed on freshly isolated peripheral blood mononuclear cell (PBMC) 2 weeks after the third, fourth, and fifth immunization using the h-IFN-γ ELISpot PLUS kit in a two-step detection system (Mabtech, Nacka, Sweden) as previously described.23 Phyto-hemagglutinin (PHA, positive control) and a peptide pool (CEF) composed of a panel of 23 peptides from cytomegalovirus (CMV), Epstein–Barr and influenza viruses were used at a final concentration of 5 μg/ml. A peptide pool of 138 peptides spanning the pp65 protein of human CMV was used at 1 μg/ml (PepMix; JPT, Berlin, Germany). CEF- and CMV-specific peptide pools were used as controls in the ELISpot assay. HIV-1-specific peptide pools representing the DNA vaccine subtypes A and B Gag (Gag DNA, pool of 117 peptides), and HIV-MVA CRF01_AE Gag (Gag CMDR, pool of 95 peptides), envelope (Env CMDR, pool of 138 peptides), and viral polymerase (Pol CMDR, pool of 115 peptides) were used at 1 μg/ml (purity >80%; JPT).
The frequencies of antigen-specific spot-forming cells (SFCs) were measured in an automated ImmunoSpot analyzer (CTL Europe, Bonn, Germany). Results were expressed as SFCs per million PBMCs. ELISpot responses were considered positive if the number of SFCs was >4 times the background and baseline value, and >55 SFCs/106 PBMCs. Data were excluded from analyses if the background responses in medium wells exceeded 60 SFCs/106 PBMCs.
Binding antibodies to recombinant HIV-1 CN54 subtype C gp140 (Centre for AIDS Reagents, NIBSC Potter Bar, United Kingdom) and to native subtype B gp160 (HIV-1IIIB; Advanced Biotechnologies, Inc., Columbia, MD) were measured using standardized ELISAs as previously reported.23
NAbs were measured using the TZM-bl and PBMC neutralization assay platforms as described elsewhere.24 In the TZM-bl assay, SF162.LS (subtype B) and 93MW965.23 (subtype C) pseudoviruses were used. The criteria for a positive result were a reduction of luminescence units (RLU) by 50% in the test sample compared to virus control wells, after subtraction of background (cell alone) RLU. In the PBMC assay, NAbs were measured using SF162.LS (subtype B) and CM244 (CRF01_AE) infectious molecular clone (IMC). The harvested culture supernatants were analyzed in an in-house HIV-1 p24-antigen ELISA. The neutralizing titer was defined as the reciprocal of the highest serum dilution giving a 90% reduction of HIV-1 p24 antigen compared to virus control wells.
ADCC activity was measured using Env.IMC.LucR virus (CRF01_AE HIV-CM235-2-LucR.T2A.ecto/293T, GenBank accession No. AF259954.1)-infected cells as targets.25 ADCC activity was measured as the percent of loss of luciferase activity observed in the presence of serum. The ADCC-mediating antibody titer was defined as the reciprocal of the highest dilution indicating a positive specific killing (>15% specific killing activity) after background subtraction.
Study endpoints
The safety endpoint was defined as any grade 3 or 4 clinical or laboratory (if clinically significant) AE that occurred after the first immunization.
The primary immunogenicity endpoint was assessed as the frequency of IFN-γ ELISpot responses 2 weeks after the HIV-MVA vaccinations. Secondary immunogenicity endpoints were evaluated as (1) the magnitude of the IFN-γ ELISpot responses determined 2 weeks after the HIV-MVA vaccinations; (2) the antibody responses to HIV-1 subtype C gp140 and subtype B gp160; and (3) NAbs and antibodies exhibiting ADCC determined 4 weeks after the second HIV-MVA vaccination.
Statistical analysis
Clinical and safety laboratory data were recorded in case report forms and double entered in an SQL Server 2008 Express edition database (Microsoft®, Redmond, WA). Immunological data were entered into Microsoft Office Excel 2007 (Microsoft). Data were exported and analyzed in Stata 14 (StataCorp. 2015. Stata: Release 14. Statistical Software: StataCorp LP, College Station, TX). Descriptive statistics were used to summarize baseline characteristics. Categorical variables were expressed in percentages and continuous data as means with standard deviations, and medians with respective interquartile ranges. Most immunological data were presented without statistical analysis as this was an exploratory study. Fischer's exact test was used for comparison of frequencies of responses between groups. The magnitude of IFN-γ ELISpot responses and antibody titers were compared using the Mann–Whitney U-test. Pairwise analysis of IFN-γ ELISpot responses was performed using the Wilcoxon matched-pair signed rank test. Significance level was set at 5%.
Results
Screening, enrollment, and participant characteristics
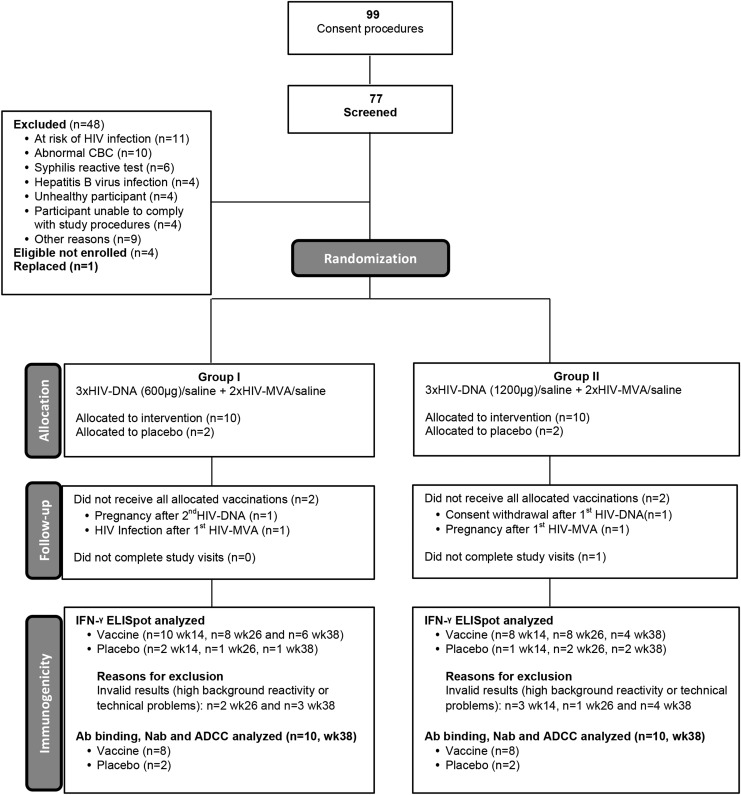
Seventy-seven volunteers were screened over a period of 7 months. Forty-eight (62%) did not fulfill the inclusion criteria and four, although eligible, were not enrolled due to completion of enrollment (Fig. 1). Baseline characteristics of enrolled subjects are presented in Table 2. The cohort was predominantly female (14/24; 58%). The median age at enrollment was 21.7 years (interquartile ranges: 20.9–22.9). All participants had formal education with half attending or completing postsecondary education degrees. All participants, except one, were single. No vaccinia scars were found. The two study arms had similar baseline demographic, clinical, and clinical laboratory profiles.
FIG. 1.
Consort diagram.
Table 2.
Baseline Demographic, Clinical, and Laboratory Characteristics
| Characteristics | Group 1–2 injections HIV-DNA, 600 μg (n = 12) | Group 2–2 injections HIV-DNA, 1,200 μg (n = 12) | Total (n = 24) |
|---|---|---|---|
| Female | 6 (50) | 8 (67) | 14 (58) |
| Age (years)a | 22.2 (21.5–22.9) | 21.2 (20.7–22.8) | 21.7 (20.9–22.9) |
| Educationb | |||
| Secondary | 6 (50) | 6 (50) | 12 (50) |
| Postsecondary | 6 (50) | 6 (50) | 12 (50) |
| Singleb | 12 (100) | 11 (92) | 23 (96) |
| Body mass index (kg/m2)a | 21.6 (2.19) | 20.1 (2.42) | 20.8 (2.37) |
| White blood cells (109cells/liter)a | 4.1 (3.3–4.7) | 4.4 (4.2–5.1) | 4.3 (3.8–4.7) |
| CD4+ T cells (cells/μl)an = 23 | 873 (696–1,078) | 637 (542–884) | 784 (564–1,014) |
| Lymphocytes (109cells/liter)a | 1.8 (1.5–2.0) | 1.6 (1.4–1.8) | 1.7 (1.4–1.9 |
| Neutrophils (109cells/liter)a | 2.0 (1.5–2.1) | 2.7 (2.2–3.4) | 2.1 (2.0–2.8) |
| Hemoglobin (g/dl)a | 12.3 (11.0–13.9) | 11.9 (11.0–13.1) | 12.2 (11.0–13.7) |
| Platelets (109 cells/liter)a | 210 (187–273) | 224 (200–256) | 214 (198–267) |
| Creatinine (mg/dl)a | 0.57 (0.45–0.73) | 0.58 (0.53–0.66) | 0.58 (0.47–0.70) |
| ALT (U/liter)a | 18.0 (14.5–18.5) | 13.5 (12.0–21.0) | 16.0 (12.5–18.5) |
| Total bilirubin (μM)a | 0.63 (0.46–0.82) | 0.53 (0.31–0.68) | 0.62 (0.39–0.80) |
| Random glucose (mM)a | 4.2 (3.8–4.4) | 4.3 (3.8–4.7) | 4.2 (3.8–4.5) |
Values are numbers (%), means (standard deviations), or medians (interquartile ranges).
Data collected at enrollment day, before 1st vaccination.
Data collected at screening.
Withdrawals/termination of vaccination
Twenty-three of 24 participants completed the study and the visit compliance rate (visits observed/visits expected) was 97%. Four participants discontinued vaccinations: (1) one withdrew consent after the first immunization due to incompatible work schedules (Group II); (2) two became pregnant, one after the second HIV-DNA (Group I) and the other after the first HIV-MVA vaccination (Group II); and (3) one became infected with HIV after the first HIV-MVA immunization (Group I) (Fig. 1). All 4 participants were vaccine recipients, thus a total of 16 vaccinees completed all 5 immunizations.
Safety and tolerability
In total, 128 wheal measurements were recorded at the injection sites (left and right deltoids) following the three HIV-DNA immunizations, being 56 in Group I (low-dose HIV-DNA), 50 in Group II (high-dose HIV-DNA), and 22 in placebo recipients. The mean value of the wheal diameters was 0.51 cm (SD: ± 0.32), 0.79 cm (SD: ± 0.49), and 0.69 cm (SD: ± 0.51) for Groups I, II, and placebo, respectively. Six participants had no wheal formation at the injection sites (one in Group I, three in Group II, and two in placebos). There were no differences between the priming groups (p = .092), but a significant difference was seen in the mean wheal diameter per priming injection, with higher diameters seen with the third injection in both vaccination groups and placebos (p = .032).
Table 3 shows the distribution of local and systemic solicited AEs during the 7-day reactogenicity assessment period, in the two vaccination arms and placebo recipients after each injection and Figure 2 shows the distribution of these events after the three HIV-DNA immunizations combined (Fig. 2A) and after the two HIV-MVA immunizations combined (Fig. 2B). Out of the 24 enrolled subjects, 23 (96%) and 21 (88%) reported at least one local and one systemic solicited AE during the study, respectively. There was no difference in reactogenicity between the groups primed with 0.1 ml (600 μg HIV-DNA) and those primed with 0.2 ml (1,200 μg HIV-DNA). The vast majority of events were mild (91%), and the maximum toxicity grade was moderate. Pain was the most common local solicited AE in vaccine recipients (33%) followed by itching (29%). The most common systemic solicited AE in vaccinees was headache (39%) followed by malaise (20%). There were few local solicited AEs reported by placebo recipients but a higher number of systemic AEs were reported by these volunteers. More solicited AEs were seen after HIV-DNA injections compared with HIV-MVA.
Table 3.
Local and Systemic Solicited Adverse Events Reported Within 7 Days After Immunizations
| 1st DNA prime | 2nd DNA prime | 3rd DNA prime | 1st MVA boost | 2nd MVA boost | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-DNA 600 μg (n = 10) | HIV-DNA 1,200 μg (n = 10) | Placebosa(n = 4) | HIV-DNA 600 μg (n = 10) | HIV-DNA 1,200 μg (n = 9) | Placebosa(n = 4) | HIV-DNA 600 μg (n = 10) | HIV-DNA 1,200 μg (n = 8) | Placebosa(n = 4) | Total after the three HIV-DNA combinedb | HIV-DNA 600 μg (n = 10) | HIV-DNA 1,200 μg (n = 8) | Placebosa(n = 4) | HIV-DNA 600 μg (n = 8) | HIV-DNA 1,200 μg (n = 8) | Placebosa(n = 4) | Total after the two HIV-MVA combinedb | Totalb | |
| Local solicited adverse events | ||||||||||||||||||
| Pain | 5 (50) | 6 (60) | 1 (25) | 5 (50) | 5 (56) | 1 (25) | 5 (50) | 1 (13) | 0 | 27 | 8 (80) | 4 (50) | 1 (25) | 7 (88) | 3 (38) | 0 | 22 | 49 |
| Itching | 5 (50) | 5 (50) | 0 | 6 (60) | 3 (33) | 0 | 5 (50) | 5 (63) | 0 | 29 | 2 (20) | 2 (25) | 0 | 2 (25) | 3 (38) | 0 | 9 | 38 |
| Warmth | 1 (10) | 2 (20) | 0 | 0 | 0 | 0 | 2 (20) | 2 (25) | 1 (25) | 7 | 1 (10) | 0 | 1 (25) | 1 (13) | 2 (25) | 0 | 4 | 11 |
| Swelling | 1 (10) | 1 (10) | 1 (25) | 2 (20) | 1 (11) | 1 (25) | 0 | 0 | 0 | 5 | 1 (10) | 0 | 0 | 1 (13) | 0 | 0 | 2 | 7 |
| Erythema | 1 (10) | 1 (10) | 0 | 1 (10) | 1 (11) | 0 | 1 (10) | 1 (13) | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| Induration | 3 (30) | 2 (20) | 0 | 3 (30) | 2 (22) | 0 | 2 (20) | 2 (25) | 0 | 14 | 1 (10) | 1 (13) | 0 | 2 (25) | 0 | 0 | 4 | 18 |
| Clear blister | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Blood blister | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Papule | 6 (60) | 6 (60) | 1 (25) | 3 (30) | 6 (67) | 1 (25) | 6 (60) | 2 (25) | 0 | 29 | 0 | 0 | 0 | 2 (25) | 0 | 0 | 2 | 31 |
| Total | 22 | 23 | 3 | 20 | 18 | 3 | 21 | 13 | 1 (25) | 117 | 13 | 7 | 2 | 15 | 8 | 0 | 43 | 160 |
| Systemic solicited adverse events | ||||||||||||||||||
| Headache | 8 (80) | 3 (30) | 3 (75) | 6 (60) | 5 (56) | 2 (50) | 6 (60) | 3 (38) | 3 (75) | 31 | 5 (50) | 4 (50) | 1 (25) | 4 (50) | 3 (38) | 2 (50) | 16 | 47 |
| Malaise | 5 (50) | 2 (20) | 2 (50) | 3 (30) | 3 (33) | 1 (25) | 1 (10) | 0 | 1 (25) | 14 | 2 (20) | 5 (63) | 2 (50) | 1 (13) | 1 (13) | 0 | 9 | 23 |
| Myalgia | 1 (10) | 2 (20) | 0 | 3 (30) | 1 (11) | 1 (25) | 2 (20) | 0 | 0 | 9 | 1 (10) | 3 (38) | 0 | 4 (50) | 1 (13) | 0 | 9 | 18 |
| Chills | 2 (20) | 1 (10) | 2 (50) | 2 (20) | 0 | 1 (25) | 1 (10) | 0 | 1 (25) | 6 | 2 (20) | 1 (13) | 0 | 4 (50) | 1 (13) | 0 | 8 | 14 |
| Arthralgia | 4 (40) | 0 | 1 (25) | 5 (50) | 1 (11) | 0 | 1 (10) | 1 (13) | 1 (25) | 12 | 1 (10) | 2 (25) | 0 | 0 | 0 | 1 (25) | 3 | 15 |
| Nausea | 3 (30) | 1 (10) | 2 (50) | 1 (10) | 0 | 0 | 1 (10) | 0 | 3 (75) | 6 | 2 (20) | 1 (13) | 0 | 1 (13) | 1 (13) | 1 (25) | 5 | 11 |
| Vomit | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Fever | 1 (10) | 0 | 0 | 1 (10) | 0 | 1 (25) | 0 | 1 (13) | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Total | 24 | 9 | 10 | 21 | 10 | 6 | 12 | 5 | 9 | 81 | 13 | 16 | 3 | 14 | 7 | 4 | 50 | 131 |
Values are number of events. In brackets, proportions of participants.
Data from placebos combined.
Only vaccine recipients.
FIG. 2.
Local and systemic solicited adverse events. Total number of reported events (A) after three HIV-DNA immunizations combined and (B) after two HIV-MVA boosts combined, in the lower dose (600 μg) and higher dose (1,200 μg) HIV-DNA recipients and placebos.
The distribution of unsolicited AEs per vaccination group is presented in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/aid). In total, 169 AEs were reported with 143 (85%) in vaccine recipients. Of these, 65 (46%) were mild, 77 (54%) were moderate, and 1 met the criteria of serious adverse event (SAE) as per protocol. There were no differences in the number of unsolicited AEs reported in the two vaccination groups (600 μg vs. 1,200 μg HIV-DNA). Three events were considered “possibly related” to the investigational products (IPs) and were all graded as mild; two were reported as dizziness occurring within 24 h after the second HIV-DNA and the second HIV-MVA injections; and one was a skin nodule at the site of injection that appeared 18 days after the first HIV-DNA prime. These three AEs resolved spontaneously. One HIV infection was reported as an SAE, per protocol, in a male subject in the lower HIV-DNA dose group (600 μg), after the first HIV-MVA immunization, and was considered “not related” to the IPs.
Eighty-three laboratory events were registered in vaccine recipients, 72 (87%) reported as mild, 10 (12%) as moderate, and 1 (1%) as grade 4 according to the DAIDS criteria. The grade 4 event was an asymptomatic hypoglycemia on the day of the first HIV-MVA vaccination, which resolved spontaneously. Of the laboratory events occurring in vaccine recipients within 4 weeks postimmunizations (n = 75), hypoglycemia was the most commonly found (27, 36%) in 15 of 20 vaccinees followed by low hemoglobin count (20, 27%) in 8 of 20 vaccinees, all females, and neutropenia (20, 27%) in 12 of 20 volunteers. All volunteers were asymptomatic. All laboratory AEs were considered “not related” or “probably not related” to the IPs. No ECG abnormalities were seen after the two HIV-MVA boosts (data not shown).
Cell-mediated immune responses
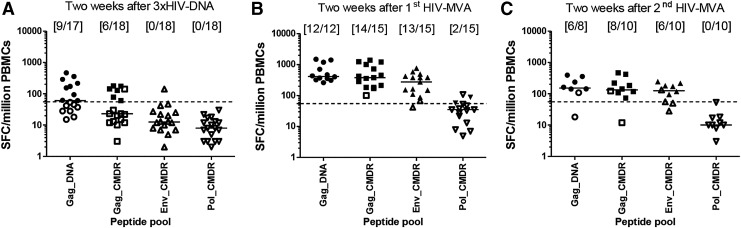
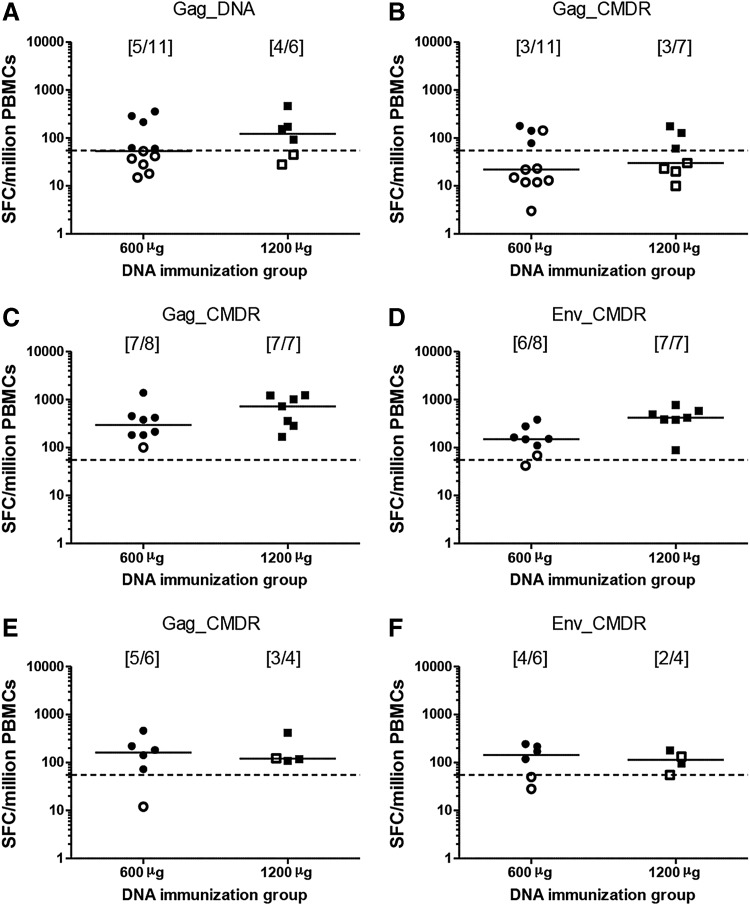
After the third HIV-DNA immunization, 9/17 (53%) vaccinees had IFN-γ responses to at least one Gag peptide pool but no response to Env. After the first HIV-MVA boost, the overall response rate to Gag and/or Env increased to 14/15 (93%); 14/15 (93%), 13/15 (87%), and 2/15 (13%) to Gag CMDR, Env, and Pol peptide pools, respectively. After the second HIV-MVA boost, the overall response rate to Gag and/or Env was 8/10 (80%) (Fig. 3). There was no significant difference in response rates to Gag and Env between the HIV-DNA dose groups after the HIV-MVA immunizations, p = 1.00 and p = .47, respectively (Fig. 4).
FIG. 3.
HIV-specific IFN-γ ELISpot responses. IFN-γ ELISpot responses in vaccinees after stimulation with Gag DNA, Gag CMDR, Env CMDR, and Pol CMDR peptide pools (A) 2 weeks after the third HIV-DNA immunization, (B) 2 weeks after the first HIV-MVA vaccination, and (C) 2 weeks after the second HIV-MVA vaccination. The number of responders per number of evaluable vaccinees is given in brackets. The bars show median values. ELISpot responses were considered positive if the number of SFCs was >55 spots/million PBMCs and four times the background value. The dashed line is at 55 SFCs/million PBMCs. Responders are shown by filled symbols and nonresponders are shown by open symbols.
FIG. 4.
IFN-γ ELISpot responses by HIV-DNA priming groups. The magnitude of IFN-γ ELISpot responses to (A) Gag DNA and (B) Gag CMDR 2 weeks after the third HIV-DNA immunization, to (C) Gag and (D) Env 2 weeks after the first HIV-MVA vaccination, and to (E) Gag and (F) Env 2 weeks after the second HIV-MVA vaccination. Data are presented for each of the HIV-DNA priming groups. The number of responders per number of evaluable vaccinees is given in brackets. Median values are given by the bars. ELISpot responses were considered positive if the number of SFCs was >55 spots/million PBMCs and four times the background value. The dashed line is at 55 SFCs/million PBMCs. Responders are shown by filled symbols and nonresponders are shown by open symbols.
The magnitude of Gag responses was modest after three HIV-DNA immunizations (Fig. 3). After the first HIV-MVA immunization, the response in Gag CMDR responders was 380 (range 182–1,390) SFCs/million PBMCs in the low HIV-DNA dose group and 722 (167–1,285) SFCs/million PBMCs in the high HIV-DNA dose group, p = .530. Importantly, the responses to Env were significantly higher in high-dose recipients compared with low-dose recipients when comparing all vaccinees (median 420, range 88–765 vs. 157.5, range 42–383 SFCs/million PBMCs), p = .014, and a trend toward a difference was seen when only responders were compared (median 420 vs. 150 SFCs/million PBMCs, p = .051) (Fig. 4).
Pairwise analysis of IFN-γ ELISpot data showed that Gag responses were significantly higher after the first than after the second HIV-MVA vaccinations with a median of 416 versus 198 SFCs/million PBMCs, p = .0313, to Gag DNA and 360 versus 142 SFCs/million PBMCs to Gag CMDR, p = .0391. The Env responses did not differ significantly between the two time points (median 148 vs. 118 SFCs/million PBMCs), p = .6523 (data not shown).
Antibody-mediated immune responses
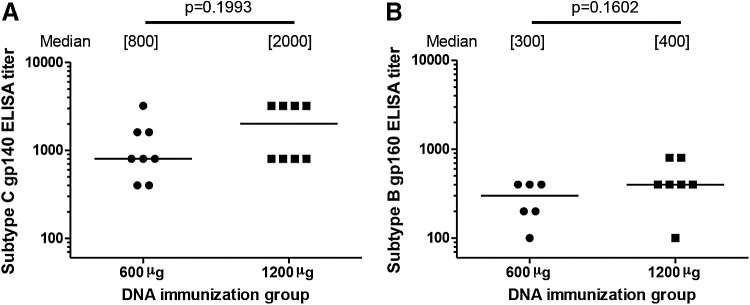
Four weeks after the first HIV-MVA boost, 1 (6%) of 16 vaccinees had detectable antibodies to recombinant CN54 subtype C gp140 and to native subtype B gp160. The reactive volunteer was a high HIV-DNA dose recipient with a titer of 400 in both assays. Four weeks after the second HIV-MVA boost, anti-Env antibodies were elicited in all (100%) of 16 vaccinees with a median antibody ELISA titer to subtype C gp140 of 800 (range 400–3,200) and to subtype B gp160 of 400 (range 200–800). There were no significant differences between low and high HIV-DNA dose vaccine recipients with regards to antibody titers to subtype C gp140 or subtype B gp160, p = .1993 and p = .1602, respectively (Fig. 5).
FIG. 5.
Binding antibody titers by HIV-DNA priming group. Binding antibody titers to (A) recombinant HIV-1 CN54 subtype C gp140 and to (B) native HIV-1IIIB subtype B gp160 4 weeks after the second HIV-MVA vaccination. All evaluable vaccinees were reactive. The magnitude of the responses in the lower and higher HIV-DNA dose recipients is shown. The median titers are given in brackets. The Mann–Whitney U-test was used for comparisons.
NAbs to subtype B SF162:LS or subtype C 93MW965.23 pseudovirus were not demonstrated in any of the vaccinees using the TZM-bl assay nor were NAbs to subtype B SF162.LS or CRF01_AE CM244 IMC detected in the PBMC assay using an HIV p24 readout (data not shown).
Two of 16 vaccinees, 1 in each of the HIV-DNA immunization groups, exhibited antibodies mediating ADCC to CRF01_AE CM235 with a titer of 55 and >156,250, respectively, 4 weeks after the second HIV-MVA boost (data not shown).
Discussion
In the present trial, we assessed the safety, tolerability, and immunogenicity of ID HIV-DNA priming at a low dose (600 μg) and a high dose (1,200 μg) in combination with HIV-MVA boost IM. This was the first study to assess HIV-DNA delivery using the Zetajet, a needle-free device, with which a volume of 0.2 ml can reproducibly be injected intradermally. The HIV-DNA/HIV-MVA vaccination regimen was safe and well tolerated, independently of the volume and dose used in this trial. Furthermore, this vaccine regimen was immunogenic in Mozambican vaccinees, with the higher HIV-DNA dose inducing higher cellular immune responses to Env than the low HIV-DNA dose.
Safety reports from this study are similar to the ones in previous trials using the same vaccine candidates.16,18,19 Other clinical studies have also reported a good safety profile with the use of the plasmid HIV-DNA/HIV-MVA prime-boost vaccination strategy.26,27 The solicited AEs reported here were mainly mild. Pain and headache were the most common local and systemic solicited AEs, respectively; similar findings have been described by Bakari et al. and Munseri et al.18,19 Placebo recipients also reported a high frequency of headache events in this study. No increase in reactogenicity was seen in those exposed to a higher volume and HIV-DNA dose. Nearly all unsolicited AEs were mild or moderate and no differences were seen between the two vaccination groups. Three events were considered possibly related to the study products and included dizziness and a skin nodule at the site of injection. One HIV infection was diagnosed in a volunteer who had not completed the vaccination schedule and was reported as an SAE, as per protocol, and considered unrelated to the study products.
After three priming HIV-DNA ID injections, overall IFN-γ response rates to Gag were modest (53%) and none of the vaccinees had a response to Env. There was no significant difference between the low- and high-dose recipients. Notably, 2 weeks after the first HIV-MVA boost, the proportion of IFN-γ ELISpot responders to Gag and Env increased significantly to 93% and 87%, respectively. The IFN-γ ELISpot response rates did not increase following the second HIV-MVA boost. After one immunization with HIV-MVA, the median magnitude in IFN-γ ELISpot responders was 380 and 157 SFCs/million PBMCs to Gag and Env, respectively. The levels were somewhat lower than those reported in a similar trial in Sweden, where the median values in responders was 540 and 246 SFCs/million PBMCs to Gag and Env, respectively.28 The differences between the trials may be due to genetic and environmental factors that have been shown to influence immune responses.29 The magnitude of the responses to Gag was significantly higher after the first HIV-MVA compared with the second HIV-MVA boosts. These findings mirror those reported previously in Tanzanian,18,19 Swedish,28 and Indian30 trials exploring HIV-DNA prime HIV-MVA boost vaccine strategies. The failure to boost the cellular immune responses by the second HIV-MVA may be attributed to pre-existent immunity against the vector proteins.27 In this trial, after the first HIV-MVA vaccination, IFN-γ responses to Env were significantly higher in vaccinees primed using the higher HIV-DNA dose compared with the lower dose (median 420 vs. 157.5 SFCs/million PBMCs, p = .014) and a trend toward a difference was noted when only responders were compared (median 420 vs. 150 SFCs/million PBMCs, p = .0513), suggesting that the higher HIV-DNA dose (1,200 μg) may be beneficial for inducing an Env-specific IFN-γ response.
We previously performed a small pilot study where a single HIV-MVA vaccination was administered to ten healthy Swedish vaccinees. Two weeks after the vaccination, three (33%) of nine evaluable vaccinees had Gag- and/or Env-specific IFN-γ ELISpot response. The magnitude of Gag-specific responses in the three responders was 85, 130, and 75 SFCs/106 PBMCs, respectively. The same vaccinees also had Env-specific responses of 95, 75, and 60 SFCs/106 PBMCs, respectively (Biberfeld, Earl, Hejdeman, Nilsson, Robb, Sandström, unpublished data). Collectively, the IFN-γ ELISpot data presented here highlight the importance of DNA priming immunizations.
In our study, there was a balance between Gag and Env IFN-γ responses (93% and 87%, respectively) similar to what we have reported in our previous HIV-DNA/HIV-MVA vaccine trials.16,18,19 Others have reported similar findings using a multiclade HIV-DNA and rAd5 boost, where 54.7% of participants exhibited IFN-γ responses to Gag and 54.2% to Env, but the frequency of responses was lower in that study.31 Goepfert et al. reported balanced Gag and Env CD4+ and CD8+ T cell responses after two GeoVax pGA2/JS7 DNA and two MVA/HIV62 immunizations.32 Predominant Env-specific responses have been reported after vaccinations with multigenic HIV-DNA vaccines and rAd5 boost,33–36 DNA and poxvirus (NYVAC) boost,37 as well as ADVAX DNA and MVA boost.30
In the present trial, 4 weeks after the first HIV-MVA vaccination, only 6% of the vaccinees mounted antibodies to subtype C gp140 and subtype B gp160, whereas 4 weeks after the second HIV-MVA boost, all (100%) of the vaccinees exhibited binding antibodies to subtype B and subtype C Env antigens. These findings confirm those reported in our previous trials where after immunizing three times with HIV-DNA and twice with HIV-MVA, anti-Env antibody responses were frequently detected (response rates 90%–100%) and anti-Env antibodies targeted multiple subtypes.18,19,23,28,38 The same HIV-MVA construct used in Mozambique has also been evaluated in a phase I safety and immunogenicity trial in the United States and Thailand, where 2 weeks after three immunizations of 108 pfu HIV-MVA, 90% of volunteers had binding antibodies to CM243 gp120.39 A study by Mehendale et al. that used a subtype C HIV-DNA prime and HIV-MVA boost vaccination strategy reported a somewhat lower frequency of binding antibody response (75%) 2 weeks after the last vaccination compared with the present trial.30
In the present trial, ADCC-mediating antibodies to CM235 IMC were rare and only detected in 13% of vaccinees. In the recently completed TaMoVac I trial conducted in Tanzania, we reported that 29% of the vaccinees developed ADCC responses to CM235 after three HIV-DNA and two HIV-MVA.40 No neutralization activity was seen in the present trial when testing was performed using either the TZM-bl or PBMC/p24 assay. This confirms our previous findings.17–19,28,38 In the HIVIS03 trial, NAb responses were detected in 83% of the vaccinees by using a PBMC-based assay to CM235 subtype CRF01_AE IMC.18,38 The PBMC/IMC assay differs from the assays used here in that the serum/plasma is present during the incubation period and the assay was shown to be NK cell dependent.38 The PBMC/IMC assay thus allows for detection of antibodies with Fc-γ receptor interactions. Subsequently, high ADCC-mediating antibody titers against CRF01_AE and/or subtype B were noted in 97% of vaccinees' sera.38 Up to 62% of the vaccinees exhibited NAb responses by the PBMC/IMC assay depending on the route and/or dose of HIV-MVA immunizations in the extended HIVIS01/02 trial10 in Sweden. The trial conducted by Mehendale et al. using a similar HIV-DNA/MVA prime-boost strategy showed the presence of NAbs against Tier-1 subtype C in TZM-bl assay in 83% of the vaccinees.30
Here HIV-DNA immunizations were delivered at weeks 0, 4, 12 and HIV-MVA at weeks 24 and 36. This was the shortest immunization schedule used in the series of HIVIS/TaMoVac trials. HIV-DNA was given at 0, 4, or 6 weeks and 12 weeks in all trials.18,19,23 HIV-MVA boost vaccinations were given at weeks 30 and 46 in the Tanzanian TaMoVac I trial.13 A longer interval between the first and second HIV-MVA was used in the HIVIS03 trial where HIV-MVA immunizations were given at weeks 36 and 84.12 In the extended HIVIS01/02 trial, the second HIV-MVA boost was delivered at an even later time point, ∼3 years after the first HIV-MVA.23 Although different modes of delivery and dosing were explored in the four trials, the use of vaccination schedules with long intervals between vaccinations may have positively influenced the induction of functional antibodies.
The present trial had several limitations. It was a small phase I trial and a limited number of samples were therefore collected and analyzed. Originally, we planned for additional testing of cell-mediated immune responses using cryopreserved cells. However, due to the low viability of frozen, stored, and thawed PBMCs, we could not perform the intracellular cytokine staining assay and a flow cytometric lymphoproliferation assay as planned. A number of IFN-γ ELISpot results 2 weeks after the second HIV-MVA boost were invalid due to technical difficulties experienced in the laboratory. Nevertheless, the comparison between proportions and magnitudes of IFN-γ ELISpot responses after the first and second HIV-MVA boost was not significantly affected. HIV infections in Mozambique are predominantly subtype C41–44 and we limited binding antibody assessment and NAb testing using the TZM-bl/pseudovirus assay to detection of subtype C- and subtype B-specific antibodies despite HIV-MVA being CRF01_AE CM235 specific. The use of a broader panel of antigens/pseudoviruses that include CRF01_AE and other subtypes would be useful to probe antibody breadth in the future. A study of antibody responses to Env variable region 2 (V2) and 3 (V3) using microarray epitope mapping will be presented in a separate article.
This was the first HIV vaccine clinical trial to be conducted in Mozambique. This trial was grounded in the previous successful establishment of a cohort of young adults20 from where volunteers have been recruited. Retention and visit compliance rates were remarkable. The capacity built during the preparation and execution of this study paved the way for future HIV prevention trials to be conducted in the country.
Conclusion
This was the first HIV vaccine trial conducted in Mozambique. The trial demonstrated that the delivery of HIV-DNA vaccine ID at a concentration of 3 mg/ml, in volumes of 0.1–0.2 ml using Zetajet intradermally, was safe and highly tolerable in healthy Mozambican volunteers. Furthermore, we have shown the superiority of priming with the higher dose (1,200 μg) of HIV-DNA to generate cell-mediated immune responses. Our findings support the use of the Zetajet for ID delivery of HIV-DNA and we recommend a larger study to assess the use of the higher dose (1,200 μg) with this HIV-DNA prime HIV-MVA boost HIV vaccine candidate.
Supplementary Material
Contributor Information
Collaborators: the TaMoVac Study Group
Acknowledgments
We are especially thankful to all study participants, to the staff at the Polana Caniço Health Research and Training Center and at the National Institute of Health of Mozambique Laboratories, to the collaborators at the Public Health Agency of Sweden, at the IRCCS San Raffaele Scientific Institute, and at the Department of Surgery and Molecular Genetics and Microbiology, Duke University. We thank the CFAR Immunology Core support to ADCC testing and analysis work by Guido Ferrari (NIH grant number AI064518). We thank Pontus Blomberg at Vecura for producing the HIV-DNA plasmid vaccines. We are grateful to Kátia Cossa for her outstanding contribution on the trial coordination and Jorge Ribeiro for the trial data management.
This study was supported by the European and Developing Countries Clinical Trials Partnership (CT.200633111.007). Additional support was provided by the Regional HIV/AIDS Team for Africa, Embassy of Sweden, Lusaka, jointly funded by Sweden and Norway (Sida contribution number 2150012801). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. This work was supported by a cooperative agreement (W81XWH-07–2-0067 and W81XWH-11-0174) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense (DOD). The views expressed are those of the authors and should not be construed to represent the positions of the Departments of the Army.
The trial is registered at the U.S. National Institutes of Health (NCT01407497) and at the Pan African Clinical Trials Registry (PACTR201106000304583).
TaMoVac Study Group: Igor Capitine, Norma Mabota, Nádia Sitoe, Raquel Chissumba, Victória Cumbane, Instituto Nacional de Saúde, Mozambique; Eulália Macovela, Emília Gonçalves, Hospital Central de Maputo, Mozambique.
Data from this study were presented, in part, at the 2014 HIV Research for Prevention conference, Cape Town, South Africa. Abstract P26.16.
Author Disclosure Statement
Richard Stout was an officer, employee and shareholder of Bioject, Inc., during the conduct of this trial. All other authors have no conflicts of interest.
References
- 1.UNAIDS: AIDS by the numbers 2015. Geneva, Switzerland, 2015 [Google Scholar]
- 2.Glass T, Cavassini M: Asking about adherence—from flipping the coin to strong evidence. Swiss Med Wkly 2014;144:w14016. [DOI] [PubMed] [Google Scholar]
- 3.Grant RM, Lama JR, Anderson PL, et al. : Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010;363:2587–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baggaley R, Doherty M, Ball A, Ford N, Hirnschall G: The strategic use of antiretrovirals to prevent HIV infection: A converging agenda. Clin Infect Dis 2015;60 Suppl 3:S159–S160 [DOI] [PubMed] [Google Scholar]
- 5.Van Damme L, Corneli A, Ahmed K, et al. : Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012;367:411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. : Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010;329:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marrazzo JM, Ramjee G, Richardson BA, et al. : Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015;372:509–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esparza J: A brief history of the global effort to develop a preventive HIV vaccine. Vaccine 2013;31:3502–3518 [DOI] [PubMed] [Google Scholar]
- 9.Excler JL, Tomaras GD, Russell ND: Novel directions in HIV-1 vaccines revealed from clinical trials. Curr Opin HIV AIDS 2013;8:421–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. : Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009;361:2209–2220 [DOI] [PubMed] [Google Scholar]
- 11.Haynes BF, Gilbert PB, McElrath MJ, et al. : Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 2012;366:1275–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yates NL, Liao H-X, Fong Y, et al. : Vaccine-induced Env V1–V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med 2014;6:228ra39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zolla-Pazner S, deCamp A, Gilbert PB, et al. : Vaccine-induced IgG antibodies to V1 V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection. PLoS One 2014;9:e87572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottardo R, Bailer RT, Korber BT, et al. : Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PLoS One 2013;8:e75665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Souza MS, Ratto-Kim S, Chuenarom W, et al. : The Thai phase III trial (RV144) vaccine regimen induces T cell responses that preferentially target epitopes within the V2 region of HIV-1 envelope. J Immunol 2012;188:5166–5176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandstrom E, Nilsson C, Hejdeman B, et al. : Broad immunogenicity of a multigene, multiclade HIV-1 DNA vaccine boosted with heterologous HIV-1 recombinant modified vaccinia virus Ankara. J Infect Dis 2008;198:1482–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nilsson C, Godoy-Ramirez K, Hejdeman B, et al. : Broad and potent cellular and humoral immune responses after a second late HIV-modified vaccinia virus ankara vaccination in HIV-DNA-primed and HIV-modified vaccinia virus Ankara-boosted Swedish vaccinees. AIDS Res Hum Retroviruses 2014;30:299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakari M, Aboud S, Nilsson C, et al. : Broad and potent immune responses to a low dose intradermal HIV-1 DNA boosted with HIV-1 recombinant MVA among healthy adults in Tanzania. Vaccine 2011;29:8417–8428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munseri PJ, Kroidl A, Nilsson C, et al. : Priming with a simplified intradermal HIV-1 DNA vaccine regimen followed by boosting with recombinant HIV-1 MVA vaccine is safe and immunogenic: A phase IIa randomized clinical trial. PLoS One 2015;10:e0119629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viegas EO, Tembe N, Macovela E, et al. : Incidence of HIV and the prevalence of HIV, hepatitis B and syphilis among youths in Maputo, Mozambique: A cohort study. PLoS One 2015;10:e0121452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brave A, Ljungberg K, Boberg A, et al. : Multigene/multisubtype HIV-1 vaccine induces potent cellular and humoral immune responses by needle-free intradermal delivery. Mol Ther 2005;12:1197–1205 [DOI] [PubMed] [Google Scholar]
- 22.Earl PL, Cotter C, Moss B, et al. : Design and evaluation of multi-gene, multi-clade HIV-1 MVA vaccines. Vaccine 2009;27:5885–5895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nilsson C, Aboud S, Karlen K, Hejdeman B, Urassa W, Biberfeld G: Optimal blood mononuclear cell isolation procedures for gamma interferon enzyme-linked immunospot testing of healthy Swedish and Tanzanian subjects. Clin Vaccine Immunol 2008;15:585–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edmonds TG, Ding H, Yuan X, et al. : Replication competent molecular clones of HIV-1 expressing Renilla luciferase facilitate the analysis of antibody inhibition in PBMC. Virology 2010;408:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonsignori M, Pollara J, Moody MA, et al. : Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol 2012;86:11521–11532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goonetilleke N, Moore S, Dally L, et al. : Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J Virol 2006;80:4717–4728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gudmundsdotter L, Nilsson C, Brave A, et al. : Recombinant Modified Vaccinia Ankara (MVA) effectively boosts DNA-primed HIV-specific immune responses in humans despite pre-existing vaccinia immunity. Vaccine 2009;27:4468–4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nilsson C, Hejdeman B, Godoy-Ramirez K, et al. : HIV-DNA given with or without intradermal electroporation is safe and highly immunogenic in healthy swedish HIV-1 DNA/MVA vaccinees: A phase I randomized trial. PLoS One 2015;10:e0131748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimman TG, Vandebriel RJ, Hoebee B: Genetic variation in the response to vaccination. Community Genet 2007;10:201–217 [DOI] [PubMed] [Google Scholar]
- 30.Mehendale S, Thakar M, Sahay S, et al. : Safety and immunogenicity of DNA and MVA HIV-1 subtype C vaccine prime-boost regimens: A phase I randomised Trial in HIV-uninfected Indian volunteers. PLoS One 2013;8:e55831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Churchyard GJ, Morgan C, Adams E, et al. : A phase IIA randomized clinical trial of a multiclade HIV-1 DNA prime followed by a multiclade rAd5 HIV-1 vaccine boost in healthy adults (HVTN204). PLoS One 2011;6:e21225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goepfert PA, Elizaga ML, Sato A, et al. : Phase 1 safety and immunogenicity testing of DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J Infect Dis 2011;203:610–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koup RA, Roederer M, Lamoreaux L, et al. : Priming immunization with DNA augments immunogenicity of recombinant adenoviral vectors for both HIV-1 specific antibody and T-cell responses. PLoS One 2010;5:e9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kibuuka H, Kimutai R, Maboko L, et al. : A phase 1/2 study of a multiclade HIV-1 DNA plasmid prime and recombinant adenovirus serotype 5 boost vaccine in HIV-Uninfected East Africans (RV 172). J Infect Dis 2010;201:600–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaoko W, Karita E, Kayitenkore K, et al. : Safety and immunogenicity study of Multiclade HIV-1 adenoviral vector vaccine alone or as boost following a multiclade HIV-1 DNA vaccine in Africa. PLoS One 2010;5:e12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koblin BA, Casapia M, Morgan C, et al. : Safety and immunogenicity of an HIV adenoviral vector boost after DNA plasmid vaccine prime by route of administration: A randomized clinical trial. PLoS One 2011;6:e24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harari A, Bart PA, Stohr W, et al. : An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J Exp Med 2008;205:63–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joachim A, Nilsson C, Aboud S, et al. : Potent functional antibody responses elicited by HIV-I DNA priming and boosting with heterologous HIV-1 recombinant MVA in healthy Tanzanian adults. PLoS One 2015;10:e0118486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Currier JR, Ngauy V, de Souza MS, et al. : Phase I safety and immunogenicity evaluation of MVA-CMDR, a multigenic, recombinant modified vaccinia Ankara-HIV-1 vaccine candidate. PLoS One 2010;5:e13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joachim A, Bauer A, Joseph S, et al. : Boosting with subtype C CN54rgp140 protein adjuvanted with glucopyranosyl lipid adjuvant after priming with HIV-DNA and HIV-MVA is safe and enhances immune responses: A phase I trial. PLoS One 2016;11:e0155702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abreu CM, Brindeiro PA, Martins AN, et al. : Genotypic and phenotypic characterization of human immunodeficiency virus type 1 isolates circulating in pregnant women from Mozambique. Arch Virol 2008;153:2013–2017 [DOI] [PubMed] [Google Scholar]
- 42.Bila DC, Young P, Merks H, et al. : Evolution of primary HIV drug resistance in a subtype C dominated epidemic in Mozambique. PLoS One 2013;8:e68213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ismael N, Bila D, Mariani D, et al. : Genetic analysis and natural polymorphisms in HIV-1 gp41 isolates from Maputo City, Mozambique. AIDS Res Hum Retroviruses 2014;30:603–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parreira R, Piedade J, Domingues A, et al. : Genetic characterization of human immunodeficiency virus type 1 from Beira, Mozambique. Microbes Infect 2006;8:2442–2451 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.