Abstract
Purpose of review
This review integrates the new thinking about relationships between gastric cancer and intestinal metaplasia/pseudopyloric metaplasia (SPEM). We address whether recent studies have closed or widened the knowledge gap regarding gastric cancer pathogenesis in mice or humans.
Recent findings
Recent studies in mouse models have provided a variety of new insights into the cellular origin and progression of events resulting in gastric cancer. Many suggest a direct transformation from intestinal metaplasia/pseudopyloric metaplasia/SPEM to gastric cancer. However, results from different investigator and models are conflicting and often describe events not present in studies in humans.
Summary
Both Helicobacter pylori-associated and autoimmune gastritis may produce gastric atrophy with extensive intestinal metaplasia and an abnormal gastric microbiome. However, only H. pylori gastritis carries a risk for adenocarcinoma. The differences reported with mouse models can best be explained as the results of different models of regeneration and repair rather than as models of gastric cancer. Overall, the data remains consistent with the original hypothesis that gastric cancer results from increased genetic instability of gastric stem cells rather than a direct transition from metaplasia to cancer. Intestinal metaplasia, pseudopyloric metaplasia, and SPEM have all been falsely accused based on guilt by association.
Keywords: autoimmune gastritis, gastric cancer, Helicobacter pylori, intestinal metaplasia, pseudopyloric metaplasia, risk assessment, spasmolytic polypeptide-expressing metaplasia, stem cells, transdifferentiation
INTRODUCTION
There has been a flurry of new observations and reviews regarding the origin and significance of intestinal metaplasia and its relation to gastric cancer [1■,2■■,3■■,4■,5■,6,7■■,8■,9■■,10,11■,12■]. The majority of new exciting information has come from studies involving experimental animals [1■,2■■,3■■,4■,5■,7■■,8■,9■■,10,11■,12■,13■■]. Observations are also starting to appear using gastric organoids [10,14]. Although these new findings have overall advanced knowledge, their interpretation has often widened the gap between existing concepts regarding the pathogenesis of gastric adenocarcinoma and intestinal metaplasia in humans and the observations from studies in mice. Both mice and humans share a common reparative process for glandular injury, which results in what has been termed pseudopyloric or mucus metaplasia in man and spasmolytic polypeptide-expressing metaplasia (SPEM) in mice. This reparative response is present throughout the intestine [15–17] and provides a niche allowing Helicobacter pylori to colonize the duodenum and cause duodenal ulcers [18,19].
Pseudopyloric metaplasia was first used by Stoerk to describe the appearance of histological regeneration of the stomach after diphtheria-associated gastric injury [20]. Early pathologists, who worked with resected or autopsy specimens used the Swiss-roll technique to clearly visualize the progression of atrophic gastritis into the proximal stomach via the expansion of pseudopyloric metaplasia. The phenomena of the antral–corpus junction moving proximally to replace the fundic gland mucosa was repeatedly described by early investigators [21,22] and also formed the basis of the Kimura-Takemoto endoscopic classification of the extent of gastric atrophy, which remains in common use today [23,24].
Pseudopyloric metaplasia was also identified in experimental models and in gastric remnants of humans [25,26]. In the stomach, it was identified clinically by its location that is, antral-appearing mucosa in the anatomic location of the corpus. This became more difficult when endoscopists placed all biopsy specimens in the same bottle making the origin of any specific biopsy unknown. The corpus location of antral-appearing mucosa could, however, be identified by the presence of pepsinogen I-containing cells on immunohistochemical staining as these cells are not present in the antrum [27]. The discovery that pseudopyloric mucosa exhibited positive staining for SPEM simplified the task of pathologists in identifying pseudopyloric metaplasia and has also become the basis for a proposed new cancer risk stratification system that is fundamentally a histologic correlate of the Kimura-Takemoto endoscopic classification system [28]. However, as cells staining for SPEM are not restricted to pseudopyloric metaplasia, the pathologist remains dependent on the endoscopist to confirm that the biopsy was obtained from the gastric corpus. Although SPEM is the currently preferred nomenclature for studies in experimental animals, we prefer pyloric or pseudo- pyloric metaplasia for studies in humans; pseudopyloric mucosa is unambiguous in relation to appearance and location. Spasmolytic polypeptide-expressing metaplasia or mucosa can occur anywhere in the gastrointestinal tract, whereas pseudopyloric mucosa unambiguously denotes a mucosa that appears similar to that of normal gastric antrum presenting in the corpus.
INTESTINAL METAPLASIA
The advance of antral–corpus border produces a lawn of pseudopyloric mucosa onto which islands or patches of intestinal metaplasia may appear and over time expand and even coalesce [27]; both are also potentially reversible. Morphologically, intestinal metaplasia appears similar to normal small intestinal mucosa replete with goblet, Paneth, and absorptive as well as displacement of the proliferative zone to the base of the crypts. It is considered as a tissue rather than a cellular transdifferentiation [29]. Regression of intestinal metaplasia has been demonstrated in experimental animals using adenosine diphosphate ribosylation inhibitors, such as olaparib and with prostaglandin E2 [30,31]. Regression has also been demonstrated in humans with H. pylori-induced atrophy-receiving tamoxifen [32]. Striking regression has also been observed in pernicious anemia patients receiving steroids [33–37] and following spontaneous loss of parietal cell auto-anti-bodies [38].
SUBTYPES OF INTESTINAL METAPLASIA
In H. pylori-associated gastritis, the extent and severity of damage correlates with cancer risk [39]. It has also been suggested that the type of intestinal metaplasia also may serve as a biomarker for risk [40]. There are three types of intestinal metaplasia: Type 1 (complete) is characterized by small intestinal type epithelium, Type II (incomplete) is characterized by colonic type epithelium with sialomucins, and Type III (incomplete) is characterized by colonic type epithelium with sulphomucins [40,41]. Type I intestinal metaplasia is usually the first to appear and in autoimmune gastritis may be the primary and only type present; Type II and Type III may appear late in the process when mucosal atrophy is extensive [40]. Type II and Type III likely reflect the presence of increasing genetic instability such as in methylation [41].
PYLORIC METAPLASIA, INTESTINAL METAPLASIA, AND GASTRIC CANCER
Both pyloric metaplasia and intestinal metaplasia are frequently described as precancerous conditions with an arrow drawn between intestinal metaplasia and intraepithelial neoplasia (previously termed dysplasia). However, gastric cancers can occur in areas without intestinal metaplasia [27,42] and detailed mapping of the mucosa in patients with gastric cancer demonstrates multifocal intraepithelial neoplasia in areas without intestinal metaplasia distant from the original tumor [27]. Intestinal metaplasia may also be present at the edges of gastric ulcers [21] and in nonatrophic mucosa at sites of prior mucosal damage; the latter is probably the basis of small intestinal patches observed in what otherwise would be considered normal stomachs. It may also be present in patients with duodenal ulcer disease, a disorder rarely associated with development of gastric cancer [43,44]. Duodenal ulcer disease is characterized by a normal or nearly normal corpus mucosa, and slow or lack of spread of atrophic changes from the antrum into the corpus. This protection is mediated by high-acid secretion and can be abolished by anything that reduces acid secretion such as vagotomy, antisecretory drugs, vitamin deficiency, fever, and so forth [45–48]. Even temporary suppression of the acid pump allows H. pylori access to the proliferative zone, resulting in inflammation and secretion of IL1β, which can further reduce acid secretion and can potentially become self perpetrating [47,48].
NEITHER INTESTINAL METAPLASIA NOR PSEUDOPYLORIC METAPLASIA PROGRESSES TO CANCER
One exception will falsify a hypothesis. The most important and serious challenge to the hypothesis that pseudopyloric metaplasia or intestinal metaplasia leads to adenocarcinoma is exemplified in the natural history of autoimmune gastritis (Table 1) [49]. Table 1 shows that both H. pylori-associated gastritis and autoimmune gastritis may eventually develop corpus atrophy with extensive pseudopyloric and intestinal metaplasia and achlorhydria. In autoimmune gastritis, antral atrophy is absent unless the disease is complicated by an H. pylori gastritis [49]. In addition, achlorhydria in either condition results in acquisition of a new diverse gastric microbiome containing intestinal bacteria in which carcinogens are produced [50–52]. However, the two conditions differ markedly in terms of cancer risk producing adenocarcinoma in H. pylori gastritis versus neuroendocrine tumors in autoimmune gastritis [49]. Data in autoimmune gastritis confirms that neither intestinal metaplasia nor pseudopyloric metaplasis alone carry a significant risk of gastric adenocarcinoma. However, in H. pylori and probably other infectious causes of atrophic gastritis [e.g. Epstein–Barr virus (EBV) infection], the extent and severity of metaplasia serve as a surrogate risk marker as reflected in the OLGA gastric cancer staging system [53].
Table 1.
Comparison between the histological features of autoimmune and Helicobacter pylori-associated gastritis and their risk of cancer development
| Autoimmune gastritis |
H. pylori-associated gastritis |
|
|---|---|---|
| Achlorhydria | Yes | Yes |
| Atrophy | Yes | Yes |
| Intestinal metaplasia | Yes | Yes |
| Microbiome change | Yes | Yes |
| Cancer type | Carcinoid | Adenocarcinoma |
HUMAN HELICOBACTER PYLORI- ASSOCIATED GASTRIC ADENOCARCINOMA
H. pylori-associated gastric cancer is an inflammation-associated cancer occurring as a complication of chronic H. pylori infection [54,55] (Figs. 1 and 2) [56■]. The population risk of gastric cancer correlates with the ability of H. pylori to increase the inflammatory response (e.g. virulence) [55]. The cancer risk with the most virulent H. pylori strain (e.g. cagA, vacA s1m1-positive) is only about double that of the least virulent strain [55,56■]. The risk is further modulated by environmental factors, especially diet [47,56■,57]. The traditional paradigm is that malignancy results from progressive genetic instability of stem cells, which eventually develop into cancer stem cells. H. pylori infection contributes indirectly to carcinogenesis by inducing inflammation and eliminating the acid barrier, which prevents growth of most bacteria in the stomach. H. pylori also contributes directly by a variety of mechanisms including breakage of double stranded DNA and promoting abnormal methylation, and so forth (Fig. 2) [54]. The immune system interacts with potential cancer stem cells to govern whether the putative cancer stem cells persist and multiply or are eliminated [58]. The importance of the H. pylori–host interaction is best exemplified by the marked fall in metachronous cancers after cure of the H. pylori infection [59,60]. It remains unclear whether the risk reduction is primarily related to the reduction in inflammation, the elimination of H. pylori–host interactions, or both.
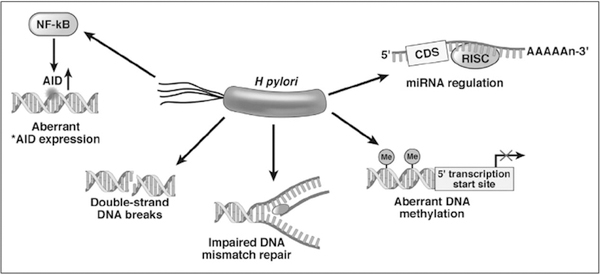
FIGURE 1.
Role of Helicobacter pylori in development of gastric stem cell genetic instability. Reproduced with permission from [54].
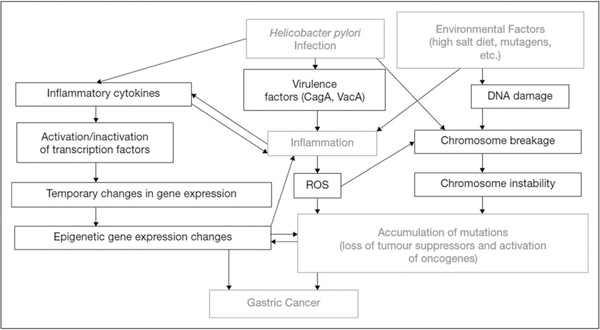
FIGURE 2.
Role of inflammation in the pathogenesis of Helicobacter pylori-associated gastric carcinogenesis. Reproduced with permission from [56■].
THE CELLS OF ORIGIN OF GASTRIC CANCER
Modern lineage tracing methods and unbiased transcriptome sequencing have allowed the identification of gastric cancer stem cell markers. Lgr5+ stem cells have been identified as the active antral stem cells capable of multilineage differentiation [61], whereas the Villin+ population represents the quiescent stem cells that specifically reside within the lesser curvature of the antrum [62]. In mice, deletion of Apc transforms the Lgr5+ stem cells into gastric cancer stem cells and the mice develop microscopic adenomas within 5 weeks [61,63–65].
Analysis of human malignant corpus tumors have also shown high levels of Lgr5 expression [66■]. Similarly, Klf4 deletion in Villin+ gastric stem cells results in pronounced hypertrophy within 35–50 weeks and spontaneous gastric adenoma formation by 80 weeks [67]. In addition, recent studies have also identified the transdifferentiation ability of chief cells, marked by Mist1, in the corpus, in which both gastric-type as well as intestinal-type cancers can be directly driven via genetic alterations in the chief cells [5■,68]. Knockout of Cdh1 gene in Mist1 cells, in combination of Helicobacter felis infection can generate diffuse-type gastric cancer, and aberrant Notch activation can yield intestinal-type cancers [68]. Together, these studies are consistent with the traditional concept that the tumor-initiation potential of gastric stem cell populations is acquired via genetic mutations and that gastric stem and progenitor cell types can likely serve as direct sources of cancer transformation without the intermediate step of SPEM or intestinal metaplasia.
From epidemiological studies, SPEM and intestinal metaplasia are certainly correlated with chronic gastric atrophy and gastric cancer [69,70]. However, the direct experimental evidence of metaplasia to gastric cancer transition is minimal. Some mouse models have shown invasive submucosal glands, but failed to demonstrate important signs of cancer, such as nuclear atypia and dysplasia [71■■]. Studies using animal models have focused on identifying the stem cells involved in regeneration and repair resulting in erroneous conclusions that a prominent component (e.g. SPEM or intestinal metaplasia) is responsible for cancer transformation. We, in term, align with the traditional belief that cancers directly arise from the genetic instability of stem cells, rather than via metaplasia.
SUMMARY OF ANIMAL AND HUMAN DATA REGARDING SPASMOLYTIC POLYPEPTIDE-EXPRESSING METAPLASIA AND INTESTINAL METAPLASIA
SPEM is a tissue reparative response following glandular injury in intestine and pancreas [2■■]. It is generally reversible and often disappears upon compete healing. Intestinal metaplasia can produce a mucosa replete with a characteristic intestinal brush border containing brush border enzymes. It is unclear, but likely, that intestinal metaplasia is also a reparative response to tissue injury. However, its presence is difficult or impossible to discern outside the stomach as it is nearly indistinguishable from normal intestinal mucosa.
Many experimental animal models, such as Helicobacter spp. infection, DMP-777 injury, or tamoxifen injection, have been developed to study gastric neoplasia. As SPEM or intestinal metaplasia have been considered preneoplastic, the development of metaplasia has often been considered an end-point of cancer studies. However, none of these commonly used preneoplastic mouse models results in what would be considered the equivalent of gastric adenocarcinoma in humans [7■■,54] and metaplasia has not been shown to be a direct precursor of adenocarcinoma transformation. The disease called dysplasia in animals has been described as exuberantly proliferative metaplastic or reactive lesions rather than as intraepithelial neoplasia, which is the definition of dysplasia in humans [7■■]. Further confirmation of its benign nature comes from experiments where organoids were made from three mouse models of gastric cancer and in a xenograft model failed to cause tumors whereas the control of carcinogen-treated gastric mucosa did. It is also not known whether the experimental animal disease resolves after cure of Helicobacter spp. infection [7■■,54].
CONCLUSION
Overall, current preneoplastic mouse models represent models for regeneration and repair rather than for development of gastric cancer. There is no compelling reason to discard the prior conclusions that intestinal metaplasia is not the direct precursor to gastric adenocarcinoma but rather the presence, extent, and possibly the type of intestinal metaplasia in H. pylori gastritis is an easily recognizable biomarker useful for risk-stratification for development of gastric cancer [72].
KEY POINTS.
Gastric cancer remains one of the most common and lethal human cancers.
Experiments using mice models of gastric injury that produce SPEM and intestinal metaplasia have reported conflicting results regarding the origin and fate of metaplastic epithelium and the cell type responsible for producing cancer. The models do not appear to mimic the development of gastric cancer in humans.
Data are insufficient to support the hypothesis that intestinal metaplasia transforms into gastric cancer in humans as only pseudopyloric and intestinal metaplasia associated with H. pylori infection is associated with gastric cancer.
Acknowledgements
Financial support and sponsorship
D.Y.G. is in part by the Research Service Department of Veterans Affairs and by Public Health Service grant DK56338, which funds the Texas Medical Center Digestive Diseases Center. W.Y.Z. is part of the Medical Scientist Training Program at Baylor College of Medicine, supported by F30 DK107173.
Footnotes
Conflicts of interest
D.Y.G. is a consultant for RedHill Biopharma regarding novel H. pylori therapies and has received research support for culture of Helicobacter pylori and is the PI of an international study of the use of antimycobacterial therapy for Crohn’s disease. He is also a consultant for BioGaia in relation to probiotic therapy for H. pylori infection and for Takeda in relation to H. pylori therapies. W.Y.Z. has no interests to declare.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Choi E, Lantz TL, Vlacich G, et al. Lrig1+ gastric isthmal progenitor cells restore normal gastric lineage cells during damage recovery in adult mouse stomach. Gut 2018; 67:1595–1605.■ The identification of Lrig1 as a marker for gastric stem cells and their roles in the development of gastric injury and repair. An example of using DMP-777 treatment as an injury model, contrasting to previous studies that have claimed its use as a preneoplasia inducer.
- 2.Goldenring JR. Pyloric metaplasia, pseudopyloric metaplasia, ulcer-associated cell lineage and spasmolytic polypeptide-expressing metaplasia: reparative lineages in the gastrointestinal mucosa. J Pathol 2018; 245: 132–137.■■ Review of the history of spasmolytic polypeptide-expressing metaplasia (SPEM) and the possible lineages of the cells responsible. The authors favor the hypothesis that SPEM arises from transdifferentiation of chief cells.
- 3.Hayakawa Y, Fox JG, Wang TC. Isthmus stem cells are the origins of metaplasia in the gastric corpus. Cell Mol Gastroenterol Hepatol 2017;4:89–94.■■ Excellent review supporting their mouse data implicating isthmus stem cells as the origin for metaplastic gastric epithelia. The authors present evidence against the trans differentiation hypothesis and support the more traditional view for a direct stem cell pathway to gastric cancer.
- 4.Hayakawa Y, Fox JG, Wang TC. The origins of gastric cancer from gastric stem cells: lessons from mouse models. Cell Mol Gastroenterol Hepatol 2017; 3:331–338.■ Review of recent human and mouse studies that uses traditional approaches requiring mutations that are destined to accumulate and be propagated in progeny to occur in long lived stem cells while recognizing that progenitor cells can interconvert into stem-like cells. Evidence is reviewed supporting the notion that antral and corpus stem cells directly participate in gastric carcinogenesis.
- 5.Kinoshita H, Hayakawa Y, Niu Z, et al. Mature gastric chief cells are not required for the development of metaplasia. Am J Physiol Gastrointest Liver Physiol 2018; 314:G583–G596.■ Further data strengthening the conclusion that gastric chief cells are not required for development of metaplasia.
- 6.Kinoshita H, Hayakawa Y, Koike K. Metaplasia in the stomach-precursor of gastric cancer? Int J Mol Sci 2017; 18: pii: E2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen CP, Mills JC, Goldenring JR. Murine models of gastric corpus preneoplasia. Cell Mol Gastroenterol Hepatol 2017; 3:11–26. ■■ An important comprehensive review of the murine models used to study gastric neoplasia. The most important conclusion is that with one exception (a transgenic mouse that produces a neuroendocrine tumor), none of the models produces a true malignancy comparable with human adenocarcinoma. A better description of the models would be of tissue regeneration and repair and the most dramatic disease being an exuberant expression of regeneration and repair.
- 8.Radyk MD, Burclaff J, Willet SG, Mills JC. Metaplastic cells in the stomach arise, independently of stem cells, via dedifferentiation or transdifferentiation of chief cells. Gastroenterology 2018; 154:839–843.■ Study of the origins of pseudopyloric metaplasia (SPEM) using a nongenetic approach in mice and human gastric adenocarcinoma. They found SPEM first appeared in the crypt region and cells suggesting transformation of chief cells into SPEM supporting the concept of one mechanism of SPEM production being from reprogramming of chief cells.
- 9.Saenz JB, Mills JC. Acid and the basis for cellular plasticity and reprogramming in gastric repair and cancer. Nat Rev Gastroenterol Hepatol 2018;15:257–273.■■ Review of the data regarding gastric mucosal repair based on the long-standing approach of separating superficial injury (restitution) and deeper injury to the glandular epithelium with a focus on cellular plasticity. The usefulness is somewhat diminished by their belief that intestinal metaplasia is definitely a precursor lesion of gastric cancer.
- 10.Shibata W, Sue S, Tsumura S, et al. Hellcobacter-induced gastric inflammation alters the properties of gastric tissue stem/progenitor cells. BMC Gastroenterol 2017; 17:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zavros Y Initiation and maintenance of gastric cancer. A focus on CD44 variant isoforms and cancer stem cells. Cell Mol Gastroenterol Hepatol 2017; 4:55–63.■ One of the first uses of gastric organoids or gastroids to study gastric stem cells in humans.
- 12.Zhang Y, Chen JN, Dong M, et al. Clinical significance of spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia in Epstein-Barr virus-associated and Epstein-Barr virus-negative gastric cancer. Hum Pathol 2017; 63:128–138.■ An important addition to the literature allowing generalizing the data regarding inflammation-associated gastric cancerto include EBV-associated gastric cancer.
- 13.Meyer AR, Goldenring JR. Injury, repair, inflammation and metaplasia in the stomach. J Physiol 2018; doi: 10.1113/JP275512. [Epub ahead of print]■■ An excellent review of the mechanism of SPEM development following injury. The authors hypothesize the SPEM development increases the risk of dysplasia but give no current studies that supports the hypothesis.
- 14.Engevik AC, Feng R, Choi E, et al. The development of spasmolytic poly-peptide/TFF2-expressing metaplasia (SPEM) during gastric repair is absent in the aged stomach. Cell Mol. Gastroenterol Hepatol 2016; 2:605–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee FD. Pyloric metaplasia in the small intestine. J Pathol 1964; 87:267–277. [DOI] [PubMed] [Google Scholar]
- 16.Yokoyama I, Kozuka S, Ito K, et al. Gastric gland metaplasia in the small and large intestine. Gut 1977; 18:214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liber AF. Aberrant pyloric glands in regional ileitis. AMA Arch Pathol 1951; 51:205–212. [PubMed] [Google Scholar]
- 18.El-Zimaity HM, Wu J, Akamatsu T, Graham DY. A reliable method for the simultaneous identification of H. pylori and gastric metaplasia in the duodenum. J Clin Pathol 1999; 52:914–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiotani A, Graham DY. Pathogenesis and therapy of gastric and duodenal ulcer disease. Med Clin North Am 2002; 86:1447–1466; viii. [DOI] [PubMed] [Google Scholar]
- 20.Faber K. Gastritis and its consequences. Paris: Oxford University Press; 1935 [Google Scholar]
- 21.Hebbel R The topography of chronic gastritis in otherwise normal stomachs. Am J Pathol 1949; 25:125–141. [PMC free article] [PubMed] [Google Scholar]
- 22.Oi M, Oshida K, Sugimura S. The location of gastric ulcer. Gastroenterology 1959; 36:45–56. [PubMed] [Google Scholar]
- 23.Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy 1969; 1:87–97. [Google Scholar]
- 24.Graham DY, Kato M, Asaka M. Gastric endoscopy in the 21st century: appropriate use of an invasive procedure in the era of noninvasive testing. Dig Liver Dis 2008; 40:497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miwa K, Kamata T, Miyazaki I, Hattori T. Kinetic changes and experimental carcinogenesis after Billroth I and II gastrectomy. Br J Surg 1993; 80: 893–896. [DOI] [PubMed] [Google Scholar]
- 26.Savage A, Jones S. Histological appearances of the gastric mucosa 15–27 years after partial gastrectomy. J Clin Pathol 1979; 32:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Zimaity HM, Ota H, Graham DY, et al. Patterns of gastric atrophy in intestinal type gastric carcinoma. Cancer 2002; 94:1428–1436. [DOI] [PubMed] [Google Scholar]
- 28.Tsai YC, Hsiao WH, Yang HB, et al. The corpus-predominant gastritis index may serve as an early marker of Helicobacter pylori-infected patients at risk of gastric cancer. Aliment Pharmacol Ther 2013; 37:969–978. [DOI] [PubMed] [Google Scholar]
- 29.Mesquita P, Raquel A, Nuno L, Reis CA, et al. Metaplasia-a transdifferentiation process that facilitates cancer development: the model of gastric intestinal metaplasia. Crit Rev Oncog 2006; 12:3–26. [DOI] [PubMed] [Google Scholar]
- 30.Toller IM, Altmeyer M, Kohler E, et al. Inhibition of ADP ribosylation prevents and cures Hellcobacter-induced gastric preneoplasia. Cancer Res 2010; 70:5912–5922. [DOI] [PubMed] [Google Scholar]
- 31.Toller IM, Hitzler I, Sayi A, Mueller A. Prostaglandin E2 prevents Hellcobacter-induced gastric preneoplasia and facilitates persistent infection in a mouse model. Gastroenterology 2010; 138:1455–1467; 1467. [DOI] [PubMed] [Google Scholar]
- 32.Moon CM, Kim SH, Lee SK, et al. Chronic tamoxifen use is associated with a decreased risk of intestinal metaplasia in human gastric epithelium. Dig Dis Sci 2014; 59:1244–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wall AJ, Whittingham S, Mackay IR, Ungar B. Prednisolone and gastric atrophy. Clin Exp Immunol 1968; 3:359–366. [PMC free article] [PubMed] [Google Scholar]
- 34.Fahrenkrug J, Schaffalitzky de Muckadell OB, et al. The mechanism of hypergastrinemia in achlorhydria. Effect of food, acid, and calcitonin on serum gastrin concentrations and component pattern in pernicious anemia, with correlation to endogenous secretin concentrations in plasma. Gastroenterology 1976; 71:33–37. [PubMed] [Google Scholar]
- 35.Jeffries GH. Recovery of gastric mucosal structure and function in pernicious anemia during prednisolone therapy. Gastroenterology 1965; 48:371–378. [PubMed] [Google Scholar]
- 36.Jeffries GH, Todd JE, Sleisenger MH. The effect of prednisolone on gastric mucosal histology, gastric secretion, and vitamin B 12 absorption in patients with pernicious anemia. J Clin Invest 1966; 45:803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodbro P, Dige-Petersen H, Schwartz M, Dalgaard OZ. Effect of steroids on gastric mucosal structure and function in pernicious anemia. Acta Med Scand 1967; 181:445–452. [DOI] [PubMed] [Google Scholar]
- 38.Span J, Koopmans P, Jansen J. A reversible case of pernicious anemia. Am J Gastroenterol 1993; 88:1277–1278. [PubMed] [Google Scholar]
- 39.Rugge M, Meggio A, Pennelli G, et al. Gastritis staging in clinical practice: the OLGA staging system. Gut 2007; 56:631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Filipe MI, Potet F, Bogomoletz WV, et al. Incomplete sulphomucin-secreting intestinal metaplasia for gastric cancer. Preliminary data from a prospective study from three centres. Gut 1985; 26:1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mesquita P, Jonckheere N, Almeida R, et al. Human MUC2 mucin gene is transcriptionally regulated by Cdx homeodomain proteins in gastrointestinal carcinoma cell lines. J Biol Chem 2003; 278:51549–51556. [DOI] [PubMed] [Google Scholar]
- 42.Kakinoki R, Kushima R, Matsubara A, et al. Re-evaluation of histogenesis of gastric carcinomas: a comparative histopathological study between Helicobacter pylori-negative and H. plyori-positive cases. Dig Dis Sci 2009; 54:614–620. [DOI] [PubMed] [Google Scholar]
- 43.El-Zimaity HMT, Gutierrez O, Kim JG, et al. Geographic differences in the distribution of intestinal metaplasia in duodenal ulcer patients. Am J Gastroenterol 2001; 96:666–672. [DOI] [PubMed] [Google Scholar]
- 44.Hansson LE, Nyren O, Hsing AW, et al. The risk of stomach cancer in patients with gastric or duodenal ulcer disease. N Engl J Med 1996; 335:242–249. [DOI] [PubMed] [Google Scholar]
- 45.Graham DY. Campylobacter pylori and peptic ulcer disease. Gastroenterology 1989; 96:615–625. [DOI] [PubMed] [Google Scholar]
- 46.Graham DY. Helicobacter pylori infection in the pathogenesis of duodenal ulcer and gastric cancer: a model. Gastroenterology 1997;113:1983–1991. [DOI] [PubMed] [Google Scholar]
- 47.Graham DY. History of Helicobacter pylori infection duodenal ulcer, gastric ulcer and gastric cancer. World J Gastroenterol 2014; 20:5191–5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graham DY, Opekun AR, Yamaoka Y, et al. Early events in proton pump inhibitor-associated exacerbation of corpus gastritis. Aliment Pharmacol Ther 2003; 17:193–200. [DOI] [PubMed] [Google Scholar]
- 49.Coati I, Fassan M, Farinati F, et al. Autoimmune gastritis: Pathologist’s viewpoint. World J Gastroenterol 2015; 21:12179–12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bartholomew BA, Hill MJ, Hudson MJ, et al. Gastric bacteria, nitrate, nitrite and nitrosamines in patients with pernicious anaemia and in patients treated with cimetidine. IARC Sci Publ 1980; 595–608. [PubMed] [Google Scholar]
- 51.Borriello SP, Reed PJ, Dolby JM, et al. Microbial and metabolic profile of achlorhydric stomach: comparison of pernicious anaemia and hypogammaglobulinaemia. J Clin Pathol 1985; 38:946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Forsythe SJ, Dolby JM, Webster AD, Cole JA. Nitrate- and nitrite-reducing bacteria in the achlorhydric stomach. J Med Microbiol 1988; 25:253–259. [DOI] [PubMed] [Google Scholar]
- 53.Rugge M, Correa P, Di Mario F, et al. OLGA staging for gastritis: a tutorial. Dig Liver Dis 2008; 40:650–658. [DOI] [PubMed] [Google Scholar]
- 54.Graham DY. Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology 2015; 148:719.e3–731.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanada K, Graham DY. Helicobacter pylori and the molecular pathogenesis of intestinal-type gastric carcinoma. Expert Rev Anticancer Ther 2014; 14:947–954. [DOI] [PubMed] [Google Scholar]
- 56.Miftahussurur M, Yamaoka Y, Graham DY. Helicobacter pylori as an oncogenic pathogen, revisited. Expert Rev Mol Med 2017; 19:e4.■ Review of the role and mechanism of H. pylori in gastric carcinogenesis including virulence factors as well as changes in genetic instability and gastric microbiome.
- 57.Kamada T, Haruma K, Ito M, et al. Time trends in Helicobacter pylori infection and atrophic gastritis over 40 years in Japan. Helicobacter 2015; 20: 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsueda S, Graham DY. Immunotherapy in gastric cancer. World J Gastroenterol 2014; 20:1657–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoon SB, Park JM, Lim CH, et al. Effect of Helicobacter pylori eradication on metachronous gastric cancer after endoscopic resection of gastric tumors: a meta-analysis. Helicobacter 2014; 19:243–248. [DOI] [PubMed] [Google Scholar]
- 60.Graham DY, Matsueda S, Shiotani A. Changing the natural history of metachronous gastric cancer after H. pylori eradication. Jpn J Helicobacter Res 2015; 16:42–50. [PMC free article] [PubMed] [Google Scholar]
- 61.Barker N, Huch M, Kujala P, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 2010; 6:25–36. [DOI] [PubMed] [Google Scholar]
- 62.Qiao XT, Ziel JW, McKimpson W, et al. Prospective identification of a multilineage progenitor in murine stomach epithelium. Gastroenterology 2007; 133:1989–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barker N, Ridgway RA, van Es JH, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009; 457:608–611. [DOI] [PubMed] [Google Scholar]
- 64.Zheng ZX, Sun Y, Bu ZD, et al. Intestinal stem cell marker LGR5 expression during gastric carcinogenesis. World J Gastroenterol 2013; 19:8714–8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li XB, Yang G, Zhu L, et al. Gastric Lgr5(+) stem cells are the cellular origin of invasive intestinal-type gastric cancer in mice. Cell Res 2016; 26:838–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leushacke M, Tan SH, Wong A, et al. Lgr5-expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat Cell Biol 2017; 19:774–786.■ In addition to previous studies showing its expression in the antrum, authors here show that Lgr5 is also expressed in chief cells of the corpus. In studying the role of Lgr5+ chief cells in tumorigenesis, this study is an example of using SPEM, not dysplasia or neoplasia, as an end-point. The authors, however, did add studies in human gastric tumor specimen that supported their conclusions.
- 67.Li Q, Jia Z, Wang L, Kong X, et al. Disruption of Klf4 in villin-positive gastric progenitor cells promotes formation and progression of tumors of the antrum in mice. Gastroenterology 2012; 142:531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hayakawa Y, Ariyama H, Stancikova J, et al. Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell 2015; 28:800–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Halldorsdottir AM, Sigurdardottrir M, Jonasson JG, et al. Spasmolytic poly-peptide-expressing metaplasia (SPEM) associated with gastric cancer in Iceland. Dig Dis Sci 2003; 48:431–441. [DOI] [PubMed] [Google Scholar]
- 70.Schmidt PH, Lee JR, Joshi V, et al. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest 1999; 79: 639–646. [PMC free article] [PubMed] [Google Scholar]
- 71.Demitrack ES, Samuelson LC. Notch as a driver of gastric epithelial cell proliferation. Cell Mol Gastroenterol Hepatol 2017; 3:323–330. ■■ An excellent review on Notch-signaling pathway in the gastric epithelium. Also reviewed are various mouse models of gastric stem cells. The authors give examples of genetic manipulation of the Notch pathway in gastric stem cells that led to gastric tumorigenesis.
- 72.Shiotani A, Cen P, Graham DY. Eradication of gastric cancer is now both possible and practical. Semin Cancer Biol 2013; 23:492–501. [DOI] [PubMed] [Google Scholar]