Introduction
Helicobacter pylori (H. pylori) is an important human pathogen thought to play a causative role in many gastrointestinal diseases including gastritis, peptic ulcer, and gastric cancer. The efficacy of the commonly recommended empirical combination therapies used in eradicating H. pylori has declined to the point that in most regions traditional empiric therapies are no longer effective 1. Although there have been a number of recently published guidelines and recommendations for treating H. pylori 2–5, failures are still common 6. Resistance to clarithromycin, metronidazole and fluoroquinolones are increasingly reported and are strongly associated with treatment failures. Culture-guided treatment is standard for infectious diseases where antimicrobial resistance is common as it allows therapy to be tailored to antimicrobial susceptibilities 7. Susceptibility-based therapy is especially important to guide tailored treatment after failure of standard empirical therapies. However, the effectiveness of culture-guided and sensitivity based treatment of H. pylori in a referral setting in the United States is unknown.
Methods
This was a retrospective cohort study of patients referred following failure to eradicate H. pylori during 2011–2016 at the Baylor Clinic in Houston, Texas. We evaluated all patients with an esophagogastroduodenoscopy with gastric biopsies for histology and culture with susceptibility testing. One to two biopsy specimens were transported in saline containing tubes on ice to the H. pylori culture laboratory at the Michael E. DeBakey VA Medical Center. H. pylori infection was diagnosed by histology and confirmed by immunohistochemical staining. H. pylori was identified by culture when the oxidase, catalase, and urease reactions were positive. Susceptibility using E-test to clarithromycin, metronidazole, amoxicillin, and levofloxacin was assessed for all samples and tetracycline susceptible was assessed for a subset of samples. H. pylori eradication was evaluated by urea breath test (UBT) or fecal antigen test (HpSAg) while off of proton pump inhibitors (PPI) for a minimum of two weeks. We evaluated patients and their outcomes as of May 20, 2017.
Results
We evaluated 49 referred patients who on average had 3.3 previous H. pylori treatments (range 1 to 12). Their mean age was 51 years, 41 (84%) were women, and their racial groups were 41% White, 31% Hispanic or Latino, 10% Black or African American, 6% Asian, 6% South Asian, and 6% declined to answer. No allergies to the antibiotics used in the treatment for H. pylori were reported in 30 (61%); 8 (16%) reported allergy to penicillin, 2 (4%) to clarithromycin, 1 (2%) to metronidazole, 1 (2%) to tetracycline and 7 (15%) to a combination of these antibiotics. The culture positivity of these 49 patients was only 82% (40 patients). Of these, 93% (n=37) were resistant to metronidazole, 88% (n=35) to clarithromycin, and 73% (n=29) to levofloxacin. No strain was resistant to amoxicillin. Tetracycline sensitivity was tested in 19 samples and only 1 strain (5%) obtained from a subject from the United States was resistant.
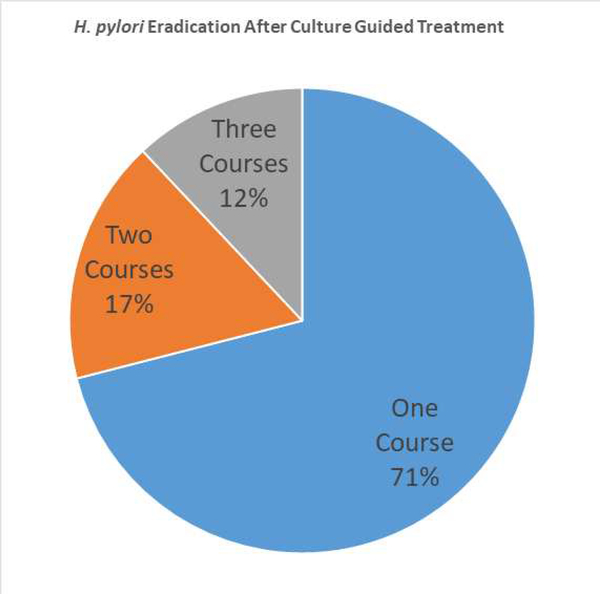
Following culture and allergy guided treatment; 24 patients (60%) were confirmed to achieve H. pylori eradication. Of the 24 participants, 17 (71%) received one course of treatment, 4 (17%) received two treatments and 3 (12%) received three treatments post culture ([dummy]Figure 1). The remaining 16 patients with positive H. pylori cultures, 5 (12%) failed the first course of treatment and are pending repeat testing after another course, while 11 (28%) were lost to follow up.
Figure 1.
The distribution of number of culture guided H pylori treatments required to achieve cure in 24 patients with multiple previous failed empiric treatments.
Discussion
Failure to eradicate H. pylori infection particularly after several attempts presents a difficult clinical scenario especially in the presence of patient refusal or drug allergy, which limits use of one or more antimicrobials. Clearly, the approach used here provided some cures (42.5% with the first regimen used) but overall the results were not very satisfactory. Lessons learned included the need to better coordinate with the culture laboratory to ensure availability of special transport media or the ability to freeze the sample in transport media until transport to the off site laboratory. Ideally, culture should be available on site. While it is challenging to separate the difficult infection (multiple resistances) from poorly adherent patient, it is difficult to achieve reliably high cure rates even in the presence of susceptibility testing. We suggest that the result of all H. pylori treatments are logged to provide feedback so that failure of therapy is identified early. After treatment failures, patients should probably be managed either by experts in the field or within a system that provides reliable feedback so that problems can be identified and corrected or the poorly performing regimen can be abandoned.
Acknowledgments
Support: Drs El-Serag and Graham are supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grant DK56338 which funds the Texas Medical Center Digestive Diseases Center.
Footnotes
Disclosures:
El-Serag, Dimpal and Chan: none
Dr. Graham is a consultant for RedHill Biopharma regarding novel H. pylori therapies and has received research support for culture of Helicobacter pylori and is the PI of an international study of the use of antimycobacterial therapy for Crohn’s disease. He is also a consultant for BioGaia in relation to probiotic therapy for H. pylori infection and for Takeda in relation to H. pylori therapies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Rimbara E, Fischbach LA, Graham DY. Optimal therapy for Helicobacter pylori infections. Nat Rev Gastroenterol Hepatol 2011;8:79–88. [DOI] [PubMed] [Google Scholar]
- 2.Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori infection. Am J Gastroenterol 2017;112:212–239. [DOI] [PubMed] [Google Scholar]
- 3.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017;66:6–30. [DOI] [PubMed] [Google Scholar]
- 4.Fallone CA, Chiba N, van Zanten SV, et al. The Toronto Consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 2016;151:51–69. [DOI] [PubMed] [Google Scholar]
- 5.Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015;64:1353–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiotani A, Lu H, Dore MP, Graham DY. Treating Helicobacter pylori effectively while minimizing misuse of antibiotics. Cleve Clin J Med 2017;84:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leekha S, Terrell CL, Edson RS. General principles of antimicrobial therapy. Mayo Clin Proc 2011;86:156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]