Abstract
BACKGROUND:
Studies of sex-based differences in older adults with AMI have yielded mixed results. We therefore sought to evaluate sex-based differences in presentation characteristics, treatments, functional impairments and in-hospital complications in a large, well-characterized population of older adults (≥75 years) hospitalized with AMI.
METHODS:
We analyzed data from participants enrolled in SILVER-AMI, a prospective observational study consisting of 3041 older patients (44% women) hospitalized for AMI. Participants were stratified by AMI subtype (STEMI and NSTEMI) and subsequently evaluated for sex-based differences in clinical presentation, functional impairments, management, and in-hospital complications.
RESULTS:
Among the study sample, women were slightly older than men (NSTEMI: 82.1 vs. 81.3, p<0.001; STEMI: 82.2 vs. 80.6, p<0.001) and had lower rates of prior coronary disease. Women in the NSTEMI subgroup presented less frequently with chest pain as their primary symptom. Age-associated functional impairments at baseline were more common in women in both AMI subgroups (cognitive impairment, NSTEMI: 20.6% vs. 14.3%, p<0.001, STEMI: 20.6% vs. 12.4%, p=0.001; ADL disability, NSTEMI: 19.7% vs. 11.4%, p<0.001, STEMI: 14.8% vs. 6.4%, p<0.001; impaired functional mobility, NSTEMI: 44.5% vs. 30.7%, p<0.001, STEMI: 39.4% vs. 22.0%, p<0.001). Women with AMI had lower rates of obstructive coronary disease (NSTEMI: p<0.001; STEMI: p=0.02), driven by lower rates of 3-vessel or left main disease than men (STEMI: 38.8% vs. 58.7%; STEMI: 24.3% vs. 32.1%), and underwent revascularization less commonly (NSTEMI: 55.6% vs. 63.6%, p<0.001; STEMI 87.3% vs. 93.3%, p=0.01). Rates of bleeding were higher among women with STEMI (26.2% vs. 15.6% p<0.001), but not NSTEMI (17.8% vs. 15.7%, p=0.21). Women had a higher frequency of bleeding following PCI with both NSTEMI (11.0% vs. 7.8%, p=0.04) and STEMI (22.6% vs. 14.8%, p=0.02).
CONCLUSIONS:
Among older adults hospitalized with AMI, women had a higher prevalence of age-related functional impairments and, among the STEMI subgroup, a higher incidence of overall bleeding events, which was driven by higher rates of non-major bleeding events and bleeding following PCI. These differences may have important implications for in-hospital and post-hospitalization needs.
Keywords: Myocardial infarction, older adults, functional impairments, sex differences
INTRODUCTION
Sex-based differences in the clinical presentation and outcomes of patients hospitalized for an acute myocardial infarction (AMI) have been documented for decades and confirmed in recent years.1–3 Women generally have a greater burden of comorbidity and are more likely than men to have atypical symptoms.4, 5 They also experience longer delays to reperfusion, more adverse events such as major bleeding and vascular access-related complications,2, 6–9 and are less likely to undergo cardiac catheterization.10 Many of the previously observed differences in management, complications, and outcomes have been attributed to the older average age of female patients presenting with AMI.1–3, 6, 11–14
To date, most studies have failed to describe sex-based differences among older adult (≥75 years) AMI patients, especially in the context of relevant functional impairments that may differ between men and women and may influence treatment decisions and outcomes.15, 16 The ComprehenSIVe Evaluation of Risk Factors in Older Patients with Acute Myocardial Infarction (SILVER-AMI) study is a multi-center prospective observational cohort study of older adults hospitalized with AMI that included a detailed functional assessment, thereby providing an opportunity to investigate sex differences among a broad sample of older patients in contemporary practice. This study evaluated sex-based differences among participants in SILVER-AMI regarding baseline presentation characteristics, prevalence of functional impairments, receipt of in-hospital treatment strategies, and in-hospital complications.
METHODS
DATA SOURCE.
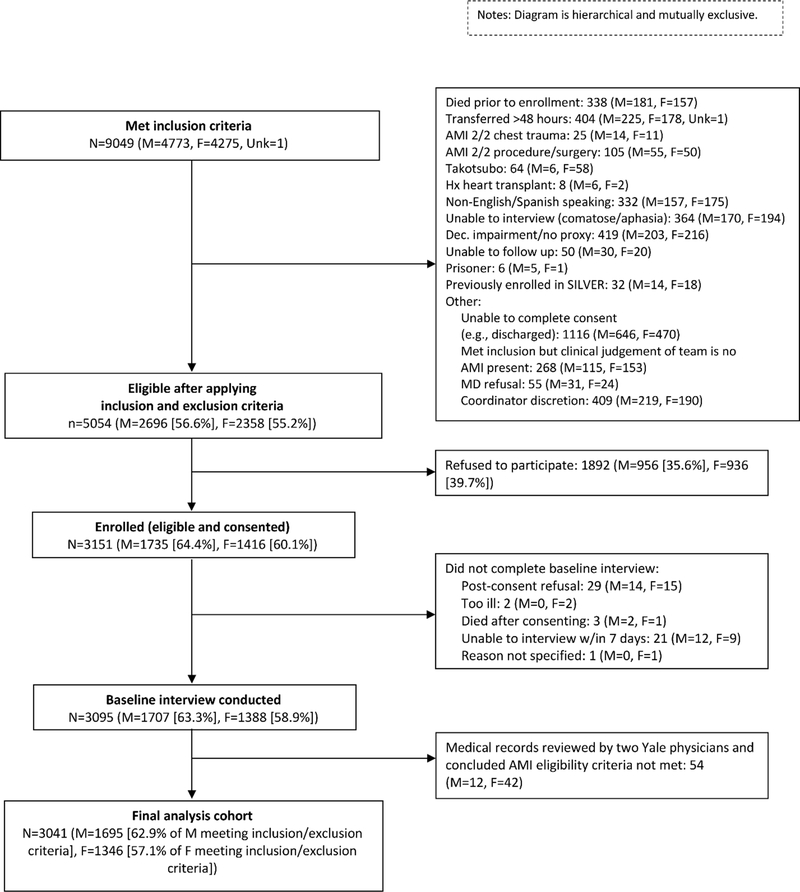
The methods for SILVER-AMI have been previously described.17 Briefly, among 5054 eligible patients, 3041participants (44% women) from 94 hospitals across the United States who met criteria for the Third Universal Definition of AMI18 were enrolled at the time of hospitalization and underwent a comprehensive structured interview and baseline physical assessment (Figure 1). Enrollment rates between eligible men and women were relatively similar (64.4% of eligible men were enrolled in the study, 60.1% of eligible women were enrolled). The assessment included ascertainment of demographics, symptoms, comorbidities, and age-associated functional impairments (cognitive function, vision and hearing impairment, functional mobility, activities of daily living, and fall history). Timing of the baseline assessment was similar between men and women (eTable 1). The in-hospital assessment (baseline interview) was complemented with a detailed medical record abstraction performed by a site research coordinator that included details of initial presentation (blood pressure, heart rate), comorbidities, laboratory results, and in-hospital adverse events. Medical records were provided to the Yale Coordinating Center where a research nurse performed an in-depth chart review to collect information about medications, cardiac procedures, and adverse events. The data, analytic methods, and study materials will not be made available to other researchers for the purpose of reproducing the results or replicating the procedure. All SILVER-AMI sites obtained institutional review board approval and all participants provided written informed consent.
Figure 1. SILVER-AMI Flow Diagram Stratified by Sex.
This figure presents a flow diagram of patients evaluated for enrollment in the SILVER-AMI study.
COVARIATES.
Covariates were collected via the baseline in-hospital assessment and medical record abstraction. Demographic data including race and marital status were self-reported during the baseline interview. Clinical variables including age, sex, medical history, presentation characteristics, medical therapies on admission, interventions, coronary angiography results, and in-hospital complications were collected via abstraction of medical records.
FUNCTIONAL IMPAIRMENTS.
Functional impairments including mobility impairment quantified using the Timed Up and Go test (TUG), pre-admission activities of daily living (ADLs), hearing, vision, cognitive function, fall history and depression were evaluated via self-report or structured objective assessments. The TUG test was used to evaluate mobility impairment, with >25 seconds or unable to complete due to functional limitations used as a cutoff for slow gait speed.19, 20 Vision was evaluated using the Visual Functioning Questionnaire (VFQ-25).21, 22 Patients responded to the question “At the present time, would you say your eyesight using both eyes is excellent, good, fair, poor, or very poor or are you completely blind?” Patients responding “poor” and “very poor or blind” were considered vision impaired. Hearing was assessed using a single question: “How much does your hearing interfere with your activities?” with response options of “not at all”, “a little”, “a moderate amount”, and “a lot”. We considered a participant to be hearing impaired when they responded either “a moderate amount” or “a lot”. ADL disability was defined as impairment in any ADL domain. Cognitive function was assessed using the Telephone Interview of Cognitive Status (TICS),23–25 with TICS scores <27 indicating impairment.
OUTCOMES.
We defined in-hospital bleeding as any clinically apparent bleeding event (identified in the medical record) that occurred during hospitalization, with the exclusion of bleeding related to CABG given the different clinical context of post-CABG bleeding (and differential CABG rates between women and men in our sample). We further classified bleeding severity as either major bleeding, adapted from the Thrombolysis in Myocardial Infarction (TIMI) definition (any intracranial bleed, clinically overt bleeding with hemoglobin drop ≥5g/dL or hematocrit drop ≥15%, or fatal bleeding)26 or non-major bleeding, inclusive of all bleeding events that did not meet major bleeding criteria. These events were recorded by the site coordinator who had all medical records available at the time of enrollment. Subsequently, a research nurse at the Yale Coordinating Center performed a central quality review to ensure that no bleeding events were missed. Acute kidney injury was based on laboratory values entered at the time of hospitalization, and defined using the Kidney Disease: Improving Global Outcomes (KDIGO) criteria27 which included an increase in serum creatinine of either ≥0.3 mg/dL from baseline or ≥1.5 times baseline (baseline being creatinine at hospital admission).
STATISTICAL ANALYSIS.
Given inherent differences regarding presentation, consensus treatment recommendations, and potential for in-hospital complications among the two AMI subtypes (ST-segment elevation MI [STEMI] and non-ST-segment elevation AMI [NSTEMI]), we stratified all analyses by AMI subtype. With the exception of TUG, where missingness was approximately 14%, missingness in our data ranged from none to approximately 3%. TUG was dichotomized in our analysis as abnormal vs. normal (>25 seconds to complete or unable vs. completion in ≤25 seconds). The remaining missing values for TUG and the small amount of missing data in the other candidate variables were multiply imputed based on an assumption of missing-at-random, as previously described.28
Frailty has been linked to adverse outcomes including a higher risk of major bleeding in patients with AMI.29 Mobility impairment has been previously identified as a surrogate for frailty status.20 Given the potential influence of frailty on the outcomes of interest, we also stratified analyses of interventions and bleeding complications by mobility impairment (defined as TUG>25 seconds or unable to complete) in the NSTEMI and STEMI subgroups. This was to assess whether observed sex differences in certain outcomes would be attenuated in evaluations of men and women with comparable mobility status. We chose TUG because it represents an objective and reproducible measure that represents a sensitive and specific proxy for frailty, including among patients with cardiovascular disease.20, 30, 31
We reported categorical variables as percentages and continuous variables as means. To compare differences between men and women (baseline characteristics, treatment patterns, in-hospital complications), we used the chi-squared or Fisher’s Exact test for categorical variables and the T-test or Wilcoxon rank sum test for continuous variables. Adjustment for multiple comparisons was conducted using the Benjamini-Hochberg approach to multiple testing for the three bleeding sub-groups (major bleeding events, non-major bleeding events, and bleeding events following PCI), in both the NSTEMI & STEMI strata.32 P values <0.001 were rounded to p=0.001 for this adjustment. A two-sided p value <0.05 was considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
BASELINE CHARACTERISTICS.
Overall, 3041 participants were enrolled and included in the final analysis cohort, with relatively similar enrollment rates among those eligible between men and women (63.3% of eligible men, 58.9% of eligible women) (Figure 1). Among patients with NSTEMI, women were slightly older (82.1 vs. 81.3 years old, p<0.001), more frequently nonwhite (15.3% vs. 7.9%, p<0.001), with less prior known coronary disease (53.6% vs. 63.8%, P<0.001), and more hypertension (90.5% vs. 86.0%, p=0.001) (Table 1). Women had lower household income (p<0.001) and lower overall education levels compared with men (p<0.001). Women were less likely to live with a partner (26.3% vs. 67.6%, p<0.001), have a history of tobacco use (56.1% vs. 64.3%, p<0.001), or have a prior percutaneous coronary intervention (PCI) (28.4% vs. 37.9%, p<0.001). Regarding AMI presentation, 44% of AMI patients did not report chest pain as their primary symptom, including 40% of patients presenting with STEMI. Women presenting with NSTEMI were less likely to have chest pain as a primary symptom (50.0% vs. 58.6%, p<0.001) and had slightly lower TIMI scores than men (4.5 vs. 4.7, p<0.001). Several differences observed among the NSTEMI cohort were also present between men and women presenting with STEMI, although differences in history of hypertension and chest pain as a primary symptom were no longer statistically significant between men and women (Table 1). Women with STEMI did more frequently have troponin values >3x the upper limit of normal (96.3% vs. 92.4%, p<0.001) and higher TIMI scores (6.4 vs. 5.9, p<0.001) than men despite similar GRACE scores.
Table 1:
Baseline Clinical Characteristics among Older Men and Women Hospitalized with AMI (N=3041)
| NSTEMI (n=2244) | STEMI (n=797) | |||||
|---|---|---|---|---|---|---|
| Clinical Characteristics | Men (n=1276) | Women (n=968) | P-value* | Men (n=419) | Women (n=378) | P-value* |
| Demographics | n (%) or mean[SD] | n (%) or mean[SD] | ||||
| Age in years (mean [SD]) | 81.3 [4.84] | 82.1 [5.24] | <0.001 | 80.6 [4.80] | 82.2 [5.17] | <0.001 |
| Nonwhite Race | 101 (7.9%) | 148 (15.3%) | <0.001 | 30 (7.2%) | 46 (12.2%) | 0.02 |
| Married/living as married or with partner (%) | 862 (67.6%) | 255 (26.3%) | <0.001 | 304 (72.6%) | 107 (28.3%) | <0.001 |
| Household income | <0.001 | <0.001 | ||||
| < $10,000 per year | 45 (3.5%) | 77 (8.0%) | 10 (2.4%) | 21 (5.6%) | ||
| $10,000 – $29,999 per year | 273 (21.4%) | 350 (36.2%) | 90 (21.5%) | 115 (30.4%) | ||
| $30,000 – $49,999 per year | 261 (20.5%) | 125 (12.9%) | 90 (21.5%) | 61 (16.1%) | ||
| $50,000 – $69,999 per year | 158 (12.4%) | 63 (6.5%) | 64 (15.3%) | 27 (7.1%) | ||
| $70,000 – $99,999 per year | 115 (9.0%) | 40 (4.1%) | 38 (9.1%) | 13 (3.4%) | ||
| >= $100,000 per year | 138 (10.8%) | 28 (2.9%) | 49 (11.7%) | 8 (2.1%) | ||
| Refused | 204 (16.0%) | 124 (12.8%) | 57 (13.6%) | 68 (18.0%) | ||
| Don’t know | 82 (6.4%) | 161 (16.6%) | 21 (5.0%) | 65 (17.2%) | ||
| Education Level | <0.001 | <0.001 | ||||
| Less than high school | 147 (11.5%) | 144 (14.9%) | 49 (11.7%) | 53 (14.0%) | ||
| High school / GED | 466 (36.5%) | 506 (52.3%) | 170 (40.6%) | 188 (49.7%) | ||
| 2-year or 4-year college degree | 396 (31.0%) | 224 (23.1%) | 130 (31.0%) | 107 (28.3%) | ||
| Graduate or post-graduate degree | 253 (19.8%) | 86 (8.9%) | 69 (16.5%) | 27 (7.1%) | ||
| Did not answer | 14 (1.1%) | 8 (0.8%) | 1 (0.2%) | 3 (0.8%) | ||
| Medical History | ||||||
| Hypertension (%) | 1098 (86.0%) | 876 (90.5%) | 0.001 | 317 (75.7%) | 304 (80.4%) | 0.11 |
| Dyslipidemia (%) | 840 (65.8%) | 631 (65.2%) | 0.75 | 238 (56.8%) | 210 (55.6%) | 0.72 |
| Tobacco Use (% Current or Ever Smoker) | 821 (64.3%) | 446 (56.1%) | <0.001 | 266 (63.5%) | 160 (42.3%) | <0.001 |
| History of CAD | 814 (63.8%) | 519 (53.6%) | <0.001 | 174 (41.5%) | 116 (30.7%) | <0.01 |
| Prior MI | 422 (33.1%) | 267 (27.6%) | <0.01 | 85 (20.3%) | 55 (14.6%) | 0.03 |
| Atrial fibrillation (%) | 174 (13.6%) | 112 (11.6%) | 0.15 | 34 (8.1%) | 28 (7.4%) | 0.71 |
| Heart Failure (%) | 278 (21.8%) | 236 (24.4%) | 0.15 | 27 (6.4%) | 31 (8.2%) | 0.34 |
| Peripheral Vascular Disease (%) | 187 (14.7%) | 123 (12.7%) | 0.19 | 27 (6.4%) | 29 (7.7%) | 0.50 |
| Stroke (%) | 149 (11.7%) | 123 (12.7%) | 0.42 | 28 (6.7%) | 34 (9.0%) | 0.45 |
| Diabetes Mellitus (%) | 507 (39.7%) | 384 (39.7%) | 0.98 | 125 (29.8%) | 112 (29.6%) | 0.95 |
| COPD (%) | 192 (15.1%) | 164 (16.9%) | 0.22 | 38 (9.1%) | 40 (10.6%) | 0.47 |
| Chronic Kidney Disease (%) | 782 (61.3%) | 621 (64.2%) | 0.17 | 215 (51.3%) | 213 (56.4%) | 0.16 |
| Prior PCI (%) | 484 (37.9%) | 275 (28.4%) | <0.001 | 107 (25.5%) | 65 (17.2%) | <0.01 |
| Presentation Characteristics | ||||||
| Any chest pain | 991 (77.7%) | 694 (71.7%) | 0.001 | 335 (80.0%) | 286 (75.7%) | 0.14 |
| Chest pain as primary symptom | 748 (58.6%) | 484 (50.0%) | <0.001 | 265 (63.2%) | 217 (57.4%) | 0.09 |
| Any other discomfort | 598 (46.9%) | 550 (56.8%) | <0.001 | 251 (59.9%) | 260 (68.8%) | <0.01 |
| Any other discomfort as primary | 104 (8.2%) | 117 (12.1%) | <0.01 | 52 (12.4%) | 68 (18.0%) | 0.03 |
| Any Respiratory symptoms | 635 (49.8%) | 517 (53.4%) | 0.09 | 157 (37.5%) | 139 (36.8%) | 0.84 |
| Any respiratory as primary | 185 (14.5%) | 163 (16.8%) | 0.13 | 26 (6.2%) | 16 (4.2%) | 0.21 |
| Any GI symptoms | 409 (32.1%) | 405 (41.8%) | <0.001 | 165 (39.4%) | 192 (50.8%) | 0.001 |
| Any GI as primary | 71 (5.6%) | 68 (7.0%) | 0.16 | 28 (6.7%) | 34 (9.0%) | 0.22 |
| Weakness or fatigue | 350 (27.4%) | 277 (28.6%) | 0.54 | 102 (24.3%) | 112 (29.6%) | 0.09 |
| Weakness or fatigue as primary symptom | 46 (3.6%) | 33 (3.4%) | 0.80 | 7 (1.7%) | 9 (2.4%) | 0.48 |
| Any symptom at presentation (%) | 1236 (96.9%) | 935 (96.6%) | 0.72 | 402 (95.9%) | 365 (96.6%) | 0.65 |
| BMI (mean ± SD) | 27.5 (4.65) | 27.7 (6.44) | 0.39 | 27.1 (4.26) | 27.1 (5.45) | 0.84 |
| Decompensated CHF (%) | 179 (14.0%) | 147 (15.2%) | 0.44 | 38 (9.1%) | 34 (9.0%) | 1.00 |
| Killip Class III/IV (%) | 52 (4.1%) | 53 (5.5%) | 0.12 | 23 (5.5%) | 19 (5.0%) | 0.87 |
| First Systolic BP, mmHg (mean ± SD) | 145.6 (29.87) | 149.2 (30.62) | <0.01 | 140.1 (32.54) | 143.2 (31.99) | 0.18 |
| First Diastolic BP, mmHg (mean ± SD) | 78.5 (17.60) | 77.3 (17.62) | 0.11 | 78.1 (17.44) | 78.1 (18.51) | 0.97 |
| First Heart Rate, bpm (mean ± SD) | 84.7 (22.68) | 85.7 (21.36) | 0.28 | 77.9 (24.63) | 81.2 (22.34) | 0.05 |
| eGFR (mean ± SD) | 53.9 (20.16) | 52.2 (20.84) | 0.06 | 59.4 (18.15) | 56.9 (18.18) | 0.06 |
| Troponin >3x ULN | 1145 (89.7%) | 871 (90.0%) | 0.97 | 387 (92.4%) | 364 (96.3%) | <0.001 |
| Shock | 32 (2.5%) | 25 (2.6%) | 0.91 | 35 (8.4%) | 23 (6.1%) | 0.22 |
| NSTEMI/STEMI TIMI Score (mean ± SD) | 4.7 (1.16) | 4.5 (1.21) | <0.001 | 5.9 (1.64) | 6.4 (1.61) | <0.001 |
| Grace Score (mean ± SD) | 148.7 (23.92) | 148.0 (22.66) | 0.43 | 137.0 (17.65) | 138.7 (18.62) | 0.18 |
based on chi-squared or Fisher’s Exact test for categorical variables and the T-test or Wilcoxon rank sum test for continuous variables.
Abbreviations: CAD=Coronary Artery Disease, AMI=Acute Myocardial Infarction, COPD=Chronic Obstructive Pulmonary Disease, PCI=Percutaneous Coronary Intervention, CHF=Congestive Heart Failure, BMI=Body Mass Index, BP=Blood Pressure
FUNCTIONAL IMPAIRMENTS.
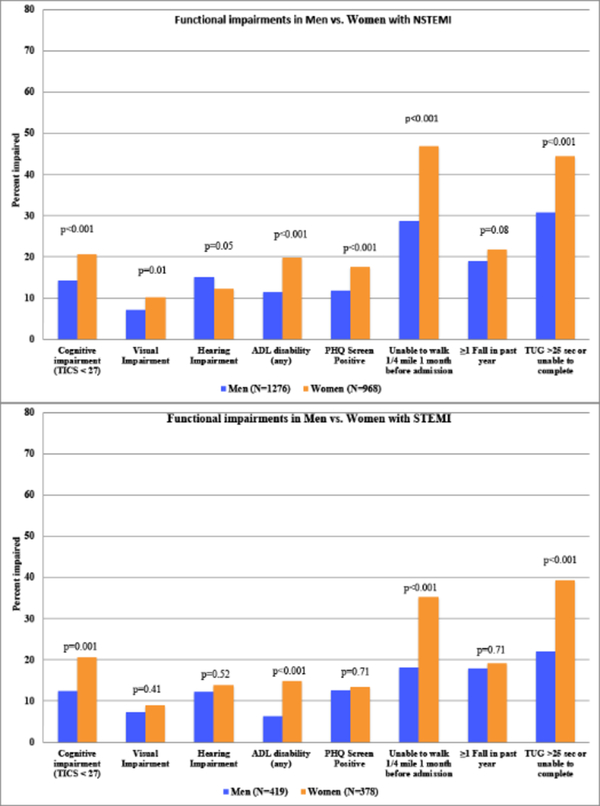
Time from admission to baseline evaluation was similar between men and women (eTable 1). Among NSTEMI and STEMI subgroups, most functional impairments were significantly more common in women compared with men (Figure 2). For example, women had a higher prevalence of cognitive impairment compared with men (NSTEMI: 20.6% vs. 14.3%, p<0.001; STEMI: 20.6% vs. 12.4%, p=0.001) as well as slower gait speed with TUG>25 seconds or unable to complete (NSTEMI: 44.5% vs. 30.7%, p<0.001, STEMI: 39.4% vs. 22.0%, p<0.001).
Figure 2. Functional impairments in Men vs. Women with NSTEMI & STEMI.
This figure displays functional impairments in Men vs. Women within NSTEMI and STEMI Subtypes of AMI. The Timed Up and Go (TUG) test was used to evaluate mobility impairment, with >25 seconds or unable to complete due to functional limitations used as a cutoff for slow gait speed. P-values were calculated using chi-squared or Fisher’s exact test.
TREATMENT.
There were selected differences among in-hospital medications between the sexes, in both the NSTEMI and STEMI subgroups (Table 2). Women with NSTEMI were more likely to receive an ACE-I or ARB (54.3% vs. 47.5%, p<0.01) or a GpIIb/IIIa inhibitor (2.7% vs. 4.3%, p=0.04), while being more likely to receive low molecular weight heparin (LMWH) (19.2% vs. 15.4%, p=0.02) and less likely to receive a statin (70.7% vs. 75.2%, p=0.02). Women with STEMI were also less likely to receive a statin (78.0% vs. 86.2%, p<0.01). There were no other significant differences in medication use among patients with STEMI.
Table 2:
Treatment Characteristics of Older Men and Women Hospitalized with AMI: Medical Therapies, Interventions, and In-hospital Outcomes (N=3041)
| NSTEMI(n=2244) | STEMI (n=797) | |||||
|---|---|---|---|---|---|---|
| Treatment Characteristics | Men (n=1276) | Women (n = 968) | p-value* | Men (n=419) | Women (n = 378) | p-value* |
| Medical Therapies on Admission (%) | N (%) | N (%) | ||||
| Aspirin | 979 (76.7%) | 740 (76.4%) | 0.77 | 279 (66.6%) | 234 (61.9%) | 0.16 |
| P2Y12 Inhibitor | 688 (53.9%) | 503 (52.0%) | 0.82 | 369 (88.1%) | 329 (87.0%) | 0.94 |
| Clopidogrel | 593 (46.5%) | 431 (44.5%) | 233 (55.6%) | 208 (55.0%) | ||
| Ticlopidine | 0 | 0 | 1 (0.2%) | 2 (0.5%) | ||
| Prasugrel | 10 (0.8%) | 7 (0.7%) | 13 (3.1%) | 13 (3.4%) | ||
| Ticagrelor | 85 (6.7%) | 65 (6.7%) | 122 (29.1%) | 106 (28.0%) | ||
| Anticoagulant use (intravenous or subcutaneous) | 1013 (79.4%) | 777 (80.3%) | 0.61 | 373 (89.0%) | 340 (90.0%) | 0.67 |
| GpIIb/IIIa inhibitor | 55 (4.3%) | 26 (2.7%) | 0.04 | 111 (26.5%) | 91 (24.1%) | 0.43 |
| UFH | 869 (68.1%) | 627 (64.8%) | 0.10 | 331 (79.0%) | 299 (79.1%) | 0.97 |
| LMWH | 197 (15.4%) | 186 (19.2%) | 0.02 | 39 (9.3%) | 44 (11.6%) | 0.28 |
| Bivalirudin | 78 (6.1%) | 63 (6.5%) | 0.70 | 97 (23.2%) | 80 (21.2%) | 0.50 |
| Statin | 959 (75.2%) | 684 (70.7%) | 0.02 | 361 (86.2%) | 295 (78.0%) | <0.01 |
| Beta blocker | 1007 (78.92%) | 0764 (78.93%) | 0.96 | 337 (80.4%) | 282 (74.6%) | 0.05 |
| ACE-I or ARB | 606 (47.5%) | 526 (54.3%) | <0.01 | 172 (41.1%) | 169 (44.7%) | 0.34 |
| Interventions (%) | ||||||
| Coronary angiography | 1042 (81.7%) | 755 (78.0%) | 0.03 | 411 (98.1%) | 362 (95.8%) | 0.06 |
| PCI | 605 (47.4%) | 437 (45.1%) | 0.29 | 375 (89.5%) | 321 (84.9%) | 0.05 |
| CABG | 214 (16.8%) | 105 (10.9%) | <0.001 | 28 (6.7%) | 15 (4.0%) | 0.09 |
| Revascularization (PCI or CABG) | 811 (63.6%) | 538 (55.6%) | <0.001 | 391 (93.3%) | 330 (87.3%) | 0.01 |
| Coronary angiography (%) | (N=1042) | (N=755) | (N = 411) | (N = 362) | ||
| Obstructive coronary disease (%) | <0.001 | 0.02 | ||||
| None | 28 (2.7%) | 71 (9.4%) | 0 | 5 (1.4%) | ||
| 1 vessel | 171 (16.4%) | 212 (28.1%) | 138 (33.6%) | 145 (40.1%) | ||
| 2 vessels | 231 (22.2%) | 179 (23.7%) | 141 (34.3%) | 124 (34.3%) | ||
| 3 vessels or left main | 612 (58.7%) | 293 (38.8%) | 132 (32.1%) | 88 (24.3%) | ||
| LVEF % from echo | 0.001 | <0.01 | ||||
| >=50% | 609 (47.7%) | 525 (54.2%) | 200 (47.7%) | 203 (53.7%) | ||
| 40–50% | 230 (18.0%) | 161 (16.6%) | 128 (30.5%) | 81 (21.4%) | ||
| 30–40% | 179 (14.0%) | 102 (10.5%) | 55 (13.1%) | 66 (17.5%) | ||
| <30% | 112 (8.8%) | 58 (6.0%) | 33 (7.9%) | 20 (5.3%) | ||
| In-hospital Outcomes | ||||||
| Hospital Length of Stay | 6.6 (6.14) | 6.2 (5.50) | 0.09 | 4.6 (4.06) | 4.9 (3.80) | 0.26 |
| Discharge to home (%) | 1023 (80.2%) | 719 (74.3%) | <0.001 | 363 (86.6%) | 312 (82.5%) | 0.11 |
based on chi-squared or Fisher’s Exact test for categorical variables and the T-test or Wilcoxon rank sum test for continuous variables.
Abbreviations: IV=intravenous, SQ=subcutaneous, UFH=unfractionated heparin, LMWH=low molecular weight heparin, ACE-I=angiotensin converting enzyme inhibitor, ARB=angiotensin receptor blocker, LVEF=Left ventricular ejection fraction, PCI=Percutaneous Coronary Intervention, CABG=coronary artery bypass grafting
Women with NSTEMI had lower rates of obstructive coronary disease than their male counterparts (p<0.001), with less 3 vessel and/or left main disease (38.8% vs. 58.7%) (Table 2). Overall, revascularization was less common among women in both the NSTEMI (55.6% vs. 63.6%, p<0.001) and STEMI (87.3% vs. 93.3%, p=0.01) subgroups. This difference was driven by a significantly lower rate of CABG among women in the NSTEMI group (10.9% vs. 16.8%, p<0.001), while there was no significant difference in the rate of PCI (45.1% vs. 47.4%, p=0.29). In the STEMI subgroup, lower rates of revascularization overall were observed due to trends toward lower rates (albeit statistically non-significant) of both PCI and CABG (PCI: 84.9% vs. 89.5%, p=0.05; CABG: 4.0% vs. 6.7%, p=0.09). When stratified by mobility impairment (as a proxy for frailty), similar treatment patterns were seen in both NSTEMI & STEMI subgroups, albeit with loss of statistical significance in all but CABG for NSTEMI (Table 3).
Table 3:
Interventions and Complications Among Older Men and Women Hospitalized with AMI in Strata defined By Mobility Impairment (N=2557*)
| NSTEMI (N=1,880) | ||||||
|---|---|---|---|---|---|---|
| Mobility Impaired | Normal or Near Normal Mobility | |||||
| Treatment Characteristics | Men (n=392) | Women (n = 431) | p-value † | Men (n=681) | Women (n = 376) | p-value † |
| Coronary angiography | 284 (72.4%) | 297 (68.9%) | 0.27 | 590 (86.6%) | 329 (87.5%) | 0.69 |
| PCI | 135 (34.4%) | 154 (35.7%) | 0.70 | 373 (54.8%) | 208 (55.3%) | 0.86 |
| CABG | 80 (20.4%) | 53 (12.3%) | <0.01 | 93 (13.7%) | 40 (10.6%) | 0.16 |
| Revascularization (PCI or CABG) | 212 (54.1%) | 204 (47.3%) | 0.05 | 462 (67.8%) | 247 (65.7%) | 0.48 |
| In-hospital Outcomes | ||||||
| Bleeding event (any) ‡ | 76 (24.4%) | 85 (22.5%) | 0.56 | 64 (10.9%) | 45 (13.4%) | 0.26 |
| Major bleeding event | 14 (4.5%) | 16 (4.2%) | 0.87 | 10 (1.7%) | 6 (1.8%) | 0.92 |
| Non-major bleeding event | 61 (19.6%) | 69 (18.3%) | 0.66 | 54 (9.2%) | 39 (11.6%) | 0.24 |
| Bleeding Event following PCI | 31 (9.9%) | 46 (12.2%) | 0.35 | 36 (6.1%) | 35 (10.4%) | 0.02 |
| Acute Kidney Injury | 126 (32.1%) | 143 (33.2%) | 0.75 | 129 (18.9%) | 64 (17.0%) | 0.43 |
| STEMI (N=677) | ||||||
| Mobility Impaired | Normal or Near Normal Mobility | |||||
| Treatment Characteristics | Men (N=92) | Women (N=149) | p-value† | Men (N=262) | Women (N=174) | p-value† |
| Coronary angiography | 89 (96.7%) | 137 (91.9%) | 0.13 | 258 (98.5%) | 171 (98.3%) | 0.87 |
| PCI | 76 (82.6%) | 118 (79.2%) | 0.52 | 241 (92.0%) | 157 (90.2%) | 0.52 |
| CABG | 10 (10.9%) | 10 (6.7%) | 0.26 | 12 (4.6%) | 2 (1.1%) | <0.05 |
| Revascularization (PCI or CABG) | 83 (90.2%) | 126 (84.6%) | 0.21 | 247 (94.3%) | 157 (90.2%) | 0.11 |
| In-hospital Outcomes | ||||||
| Bleeding event (any) ‡ | 19 (23.2%) | 44 (31.7%) | 0.18 | 35 (14.0%) | 37 (21.5%) | 0.04 |
| Major bleeding event | 8 (9.8%) | 11 (7.9%) | 0.64 | 3 (1.2%) | 6 (3.5%) | 0.11 |
| Non-major bleeding event | 9 (11.0%) | 32 (23.0%) | 0.03 | 31 (12.4%) | 31 (18.0%) | 0.11 |
| Bleeding Event following PCI | 18 (22.0%) | 39 (28.1%) | 0.32 | 33 (13.2%) | 32 (18.6%) | 0.13 |
| Acute Kidney Injury | 33 (35.9%) | 38 (25.5%) | 0.09 | 33 (12.6%) | 21 (12.1%) | 0.87 |
Patients excluded where TUG was missing
based on chi-squared or Fisher’s Exact test for categorical variables and the T-test or Wilcoxon rank sum test for continuous variables.
In-hospital bleeding excludes patients who underwent CABG
Abbreviations: IV=intravenous, SQ=subcutaneous, UFH=unfractionated heparin, LMWH=low molecular weight heparin, ACE-I=angiotensin converting enzyme inhibitor, ARB=angiotensin receptor blocker, LVEF=Left ventricular ejection fraction, PCI=Percutaneous Coronary Intervention, CABG=coronary artery bypass grafting
IN-HOSPITAL COMPLICATIONS.
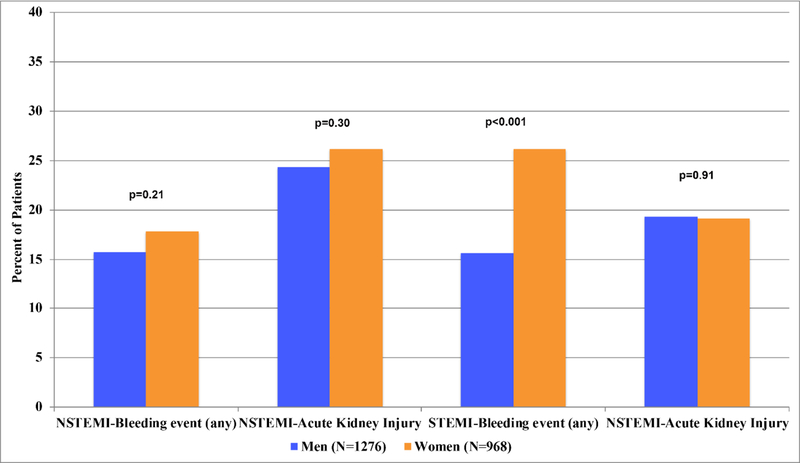
Among patients with NSTEMI, overall bleeding events were statistically similar between women and men (15.7% vs. 17.8%, p=0.21) (Figure 3). We did observe a higher frequency of bleeding following PCI in women among those with NSTEMI (11.0% vs. 7.8%, p=0.04). In patients with STEMI, bleeding events were more common in women than men both overall (26.2% vs. 15.6%, p<0.001) and following PCI (22.6% vs. 14.8%, p=0.02) (Figure 3). The increased overall bleeding rate observed in women with STEMI was largely driven by a higher rate of non-major bleeding (20.4% vs. 11.5%, p=0.006). Major bleeding events constituted 18% of all bleeding events overall (N= 87/477); the remainder were classified as non-major bleeding. The rate of major bleeding in women was similar to men in those with STEMI (major bleeding in women: 5.5% vs. 3.3% in men, p=0.17) and NSTEMI (major bleeding in women: 2.7% vs. 2.9% in men, p=0.74).
Figure 3: Complications in Men vs. Women with AMI.
This figure displays complications in Men vs. Women with NSTEMI and STEMI. Complications included any bleeding event and acute kidney injury. P-values were calculated using chi-squared or Fisher’s exact test.
In-hospital AKI was common among both men and women, occurring in 23.6% of the study sample. Men and women had similar rates of AKI in both the NSTEMI (26.2% of women vs. 24.3% of men, p= 0.30) and STEMI (19.1% vs. 19.3%, p=0.91) subgroups. In-hospital complications stratified by mobility impairment revealed similar trends in bleeding complications regardless of mobility impairment (Table 3).
DISCUSSION
To our knowledge, this is the first large investigation of sex differences among older adults with AMI that incorporates a description of aging-related functional impairments. There are several key findings: first, in our study, women had a higher prevalence of functional impairments than men on presentation, including slow gait speed and cognitive impairment. Second, women differed from men in their presentation characteristics by less frequently reporting chest pain (in the NSTEMI group) while more often reporting other types of discomfort in both groups. Third, we observed treatment differences in both medical therapies and invasive strategies, with older women being less likely than men to receive statins and undergo revascularization for both NSTEMI and STEMI. Fourth, whereas rates of AKI were similar between women and men, women with STEMI had significantly higher bleeding rates than men, which was largely driven by differences in non-major bleeding and bleeding following PCI.
While sex differences in clinical presentation, patient characteristics, and treatment patterns among younger patients presenting with AMI have been well-described in the literature, with women generally having lower rates of revascularization and worse outcomes,1–3, 6, 33 studies specifically investigating sex differences in older populations that capture functional impairments have yielded mixed results; both women and older adults (age ≥75 years) have historically been underrepresented in many large AMI studies,33–36 and even when enrolled, functional assessments are typically not performed. However, small studies have shown these impairments may influence management and outcomes. For example, one single-center prospective study of 471 patients in Japan hospitalized with STEMI identified an association between slow gait speed (a known marker of frailty) and an increased risk of subsequent cardiovascular events.20, 37 Forman et al. have proposed incorporating functional assessment in the management for older adults with cardiovascular disease,16, 20 although to date this has not been implemented in standard practice. Functional impairments may contribute to patients’ ability to adhere to and tolerate medication regimens, as well as participate in cardiac rehabilitation, which has potential implications for management. Identifying these impairments during the index hospitalization may allow for targeted interventions in these areas that may improve outcomes. Examples might include a home safety check in patients at high-risk for falls due to vision impairment, a pill-box for patients with cognitive impairment that might limit medication adherence, and intensive physical rehabilitation for patients with functional mobility impairment.
A distinctly geriatric phenotype of older women presenting with AMI emerged from our data, with more frequent rates of functional impairment, cognitive impairment, and ADL disability compared with men, although we did not find consistent differences in treatments received attributable to this finding. These findings contrast with the results of a prior study using the New Zealand Mental Health Survey, a general population survey of mental health and health-related disability including functional limitations. The New Zealand Mental Health Survey included 618 patients with cardiovascular disease (median age 63 years old in women, 61 years old in men) and found similar degrees of functional limitations in women compared with men38. While the New Zealand Mental Health Survey relied solely on survey data in a smaller cohort of younger patients, SILVER-AMI captured more objective data on functional impairment by including a TUG assessment data in a large population of older adults most likely to be impacted by geriatric impairments. We hypothesized that greater recognition of functional impairment in older women may influence prescribing patterns. Although older women in SILVER-AMI received similar rates of several medications (aspirin, P2Y12 inhibitors, overall anticoagulants), they were more likely to receive treatment with ACE-I/ARBs and LMWH in the NSTEMI group and less likely to receive statin therapy in both the NSTEMI & STEMI sub-groups. Lower use of statins may represent greater hesitancy on the part of clinicians to prescribe medications which may exacerbate weakness and falls39, 40 in the setting of a more geriatric phenotype, though this explanation should be interpreted with caution since we did not see this pattern with other medications.
Regarding invasive management, we observed women with NSTEMI in SILVER-AMI underwent less frequent coronary angiography than men (78.0% vs. 81.7%, p=0.03) with a trend toward lower rates of angiography in women with STEMI (95.8% vs. 98.1%, p=0.06). This is in line with findings from a recent retrospective cohort study of 1,414 older adult AMI patients in Sweden (mean age 84 years old), which demonstrated a trend toward more coronary angiography among men compared with women (OR 1.34, CI 1.00–1.79, p=0.05).41 While slightly lower rates of coronary angiography among female patients with NSTEMI may be attributable to clinician recognition of functional impairments among older women and the higher risk that these impairments may confer, our stratified analysis using frailty (operationalized as normal versus impaired functional mobility), as a proxy for geriatric phenotype, demonstrated no differential propensity to perform coronary angiography. Lower rates of chest pain reported among women with NSTEMI may also influence provider decision-making to pursue less aggressive care.42 Lower subsequent revascularization rates among women with NSTEMI may be plausibly explained by the less frequent presence of 3-vessel and/or left main disease necessitating CABG and the greater incidence of myocardial infarction with non-obstructive coronary arteries (MINOCA) seen among women.43 Similar to NSTEMI patients in SILVER-AMI, a study from De Carlo et al. investigated outcomes in NSTEMI patients age ≥75 from the Italian Elderly ACS study and found that women were less likely to undergo coronary revascularization.44 Women with STEMI also had lower revascularization rates compared with men but these differences were not entirely explained by their coronary anatomy alone. Analyses stratified by mobility impairment were underpowered for comparisons but did not seem to effect the observed sex differences. The degree to which additional factors contribute to the lower observed revascularization rates among older women presenting with STEMI, such as the higher frequency of cognitive impairment among women with STEMI, individual patient beliefs and preferences, and provider biases, merit future investigation.
Overall bleeding risk was higher for women versus men with STEMI in our study, but similar overall between men and women with NSTEMI. The difference in bleeding risk in the STEMI subgroup was largely driven by a higher incidence of non-major bleeding events among women and higher rates of bleeding following PCI. This difference occurred despite lower rates of revascularization among women and similar utilization of antiplatelet agents and anticoagulants. While NSTEMI presentation and management can be quite heterogeneous, the standardization of care among patients presenting with STEMI may unmask true biological differences between the sexes. There are several potential explanations for a higher incidence of bleeding among older women with STEMI. The more geriatric phenotype observed among women in SILVER-AMI may have contributed to higher bleeding risk; frailty alone has been identified as a risk factor for bleeding in several prior studies.45–47 However, our stratified analysis (normal versus impaired mobility as a proxy for frailty) demonstrated no significant difference in bleeding complications by mobility sub-groups. Loss of statistical significance on TUG stratified analysis in categories where statistically significant sex differences were seen in the overall analysis may have been partially due to a decrease in statistical power within these sub-groups. Second, standardized anticoagulation protocols used in emergency settings for STEMI patients may not account for weight-adjusted dosing and lead to overdosing in smaller patients. Third, our results may be influenced by underlying biological differences that have yet to be differentiated. For example, some have suggested a sex-based response to antiplatelet agents based on differences in platelet biology.6 These findings mirror prior studies conducted in younger populations that have generally, although not uniformly, found a higher bleeding risk for women versus men presenting with AMI.2, 6–9, 48 Observational data from the TRANSLATE-ACS study examining patients after PCI for either NSTEMI or STEMI revealed a higher adjusted risk of severe bleeding for women versus men,6 a finding that is consistent with other studies.7–9 Future investigation into the potential driving factors behind the observed increased bleeding risk among women presenting with STEMI will be critical for designing strategies to mitigate bleeding risk.
In contrast with bleeding complications, AKI rates were similar between women and men (in both NSTEMI and STEMI subgroups), although notably the overall incidence of AKI was high (23.6%). Risk of AKI increases with age in AMI patients and has been associated with worse procedural outcomes and higher mortality.49, 50 Attention to preventive measures such as pre-hydration and minimizing contrast dye remains critical in this population.
We recognize several key limitations in this study. AMI patients with the highest level of acuity were likely to have been underrepresented in our cohort, including those who died in-hospital prior to enrollment. However, we have no indication that there would be dissimilar representation among men and women among AMI patients with the highest acuity, with similar rates of enrollment and inclusion in the final analysis cohort. Furthermore, the time from admission to baseline interview and assessment of functional impairments was similar between male and female participants. Second, we did not have access to anticoagulant dosages administered on admission or in the cardiac catheterization laboratory, which may have contributed to differential bleeding risk. Prior studies have shown that inappropriate dosing of antithrombotic agents including glycoprotein IIb/IIIa inhibitors influence bleeding risk, and that this phenomenon is more common in women.6, 14, 51–53 Third, coronary anatomy was based on angiogram reports in SILVER-AMI and were not adjudicated with cardiac catheterization core laboratory, which therefore subjects the results to inter-operator variability. Fourth, our analyses were descriptive in nature, as our purpose was to provide a broad overview of presentation characteristics and treatment patterns between men and women; we therefore did not attempt to investigate the independent association between sex and outcomes (bleeding and AKI) after adjusting for potential confounders. Fifth, while patients with decisional impairment without legally authorized representatives and those deemed unable to complete the interview were excluded from SILVER-AMI, patients with subtle delirium may have been missed, which could have influenced performance on functional assessments. Furthermore, the assessment of mobility at the time of hospitalization may underestimate baseline functional status due to rapid deconditioning that can occur during an inpatient admission; patients may have had better baseline functional statuses prior to their AMI admission. In addition, the deconditioning that occurs during an inpatient admission can persist long after discharge or even represent a new functional baseline without proper rehabilitation. Finally, we did not have access to the rationale behind clinical decisions to withhold certain therapies, including contraindications to certain medications, and thus cannot make definitive conclusions about reasons for their non-use.
In conclusion, older adult women hospitalized with AMI differ significantly from men in their presentation characteristics, management, burden of coronary artery disease and bleeding complications. Recognition of higher rates of functional impairment among older women with AMI and higher incidence of overall bleeding among older women with STEMI, driven by higher rates of non-major bleeding and bleeding following PCI, represents a critical step towards optimizing in-hospital and post-discharge care for this vulnerable population.
Supplementary Material
ACKNOWLEDGMENTS
SOURCES OF FUNDING
This research was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health (R01HL115295). This work was conducted at the Yale Program on Aging/Claude D. Pepper Older Americans Independence Center (P30AG021342). The project described used REDCap which is supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH), through grant UL1 TR00000. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Nanna is supported by NIH training grant 5T32-HL069749-15. Dr. Dodson receives support from a Patient Oriented Career Development Award (K23 AG052463) from the National Institutes of Health/National Institute of Aging. Dr. Murphy is supported by the Yale Claude D. Pepper Older Americans Independence Center (P30 AG021342).
ABBREVIATIONS
- AKI
Acute kidney injury
- AMI
Acute myocardial infarction
- ASCVD
atherosclerotic cardiovascular disease
- CI
confidence interval
- KDIGO
Kidney Disease: Improving Global Outcomes criteria
- NSTEMI
non-ST-segment elevation AMI
- SILVER-AMI
Comprehensive evaluation of risk factors in older adults with AMI study
- STEMI
ST segment elevation AMI
- TIMI
Thrombolysis in Myocardial Infarction
- TUG
Timed Up and Go test
Footnotes
CONFLICT OF INTEREST DISCLOSURES
MG Nanna: Dr. Nanna is supported by NIH training grant 5T32-HL069749–15.
A Hajduk: No relationships(s) to disclose.
HM Krumholz: Dr. Krumholz is a recipient of research agreements from Medtronic and from Johnson & Johnson (Janssen), through Yale University, to develop methods of clinical trial data sharing; is the recipient of a grant from the Food and Drug Administration and Medtronic to develop methods for post-market surveillance of medical devices; works under contract with the Centers for Medicare & Medicaid Services to develop and maintain performance measures; chairs a cardiac scientific advisory board for UnitedHealth; is a participant/participant representative of the IBM Watson Health Life Sciences Board; is a member of the Advisory Board for Element Science; and is the founder of Hugo, a personal health information platform.
TE Murphy: Dr. Murphy is supported by the Yale Claude D. Pepper Older Americans Independence Center (P30 AG021342).
RP Dreyer: No relationship(s) to disclose.
KP Alexander: No relationship(s) to disclose.
M Geda: No relationship(s) to disclose.
S Tsang: No relationship(s) to disclose.
F Welty: No relationship(s) to disclose.
B Safdar: No relationship(s) to disclose.
DK Lakshminarayan: No relationship(s) to disclose.
SI Chaudhry: No relationship(s) to disclose.
JA Dodson: No relationship(s) to disclose.
REFERENCES:
- 1.Vaccarino V, Parsons L, Every NR, Barron HV and Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. The New England journal of medicine. 1999;341:217–25. [DOI] [PubMed] [Google Scholar]
- 2.Lichtman JH, Wang Y, Jones SB, Leifheit-Limson EC, Shaw LJ, Vaccarino V, Rumsfeld JS, Krumholz HM and Curtis JP. Age and sex differences in inhospital complication rates and mortality after percutaneous coronary intervention procedures: evidence from the NCDR((R)). American heart journal. 2014;167:376–83. [DOI] [PubMed] [Google Scholar]
- 3.Pancholy SB, Shantha GP, Patel T and Cheskin LJ. Sex differences in short-term and long-term all-cause mortality among patients with ST-segment elevation myocardial infarction treated by primary percutaneous intervention: a meta-analysis. JAMA internal medicine. 2014;174:1822–30. [DOI] [PubMed] [Google Scholar]
- 4.Canto JG, Rogers WJ, Goldberg RJ, Peterson ED, Wenger NK, Vaccarino V, Kiefe CI, Frederick PD, Sopko G, Zheng ZJ and Investigators N. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. Jama. 2012;307:813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dey S, Flather MD, Devlin G, Brieger D, Gurfinkel EP, Steg PG, Fitzgerald G, Jackson EA, Eagle KA and Global Registry of Acute Coronary Events i. Sex-related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: the Global Registry of Acute Coronary Events. Heart. 2009;95:20–6. [DOI] [PubMed] [Google Scholar]
- 6.Hess CN, McCoy LA, Duggirala HJ, Tavris DR, O’Callaghan K, Douglas PS, Peterson ED and Wang TY. Sex-based differences in outcomes after percutaneous coronary intervention for acute myocardial infarction: a report from TRANSLATE-ACS. Journal of the American Heart Association. 2014;3:e000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moscucci M, Fox KA, Cannon CP, Klein W, Lopez-Sendon J, Montalescot G, White K and Goldberg RJ. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur Heart J. 2003;24:1815–23. [DOI] [PubMed] [Google Scholar]
- 8.Subherwal S, Bach RG, Chen AY, Gage BF, Rao SV, Newby LK, Wang TY, Gibler WB, Ohman EM, Roe MT, Pollack CV, Jr., Peterson ED and Alexander KP. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation. 2009;119:1873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehran R, Pocock SJ, Nikolsky E, Clayton T, Dangas GD, Kirtane AJ, Parise H, Fahy M, Manoukian SV, Feit F, Ohman ME, Witzenbichler B, Guagliumi G, Lansky AJ and Stone GW. A risk score to predict bleeding in patients with acute coronary syndromes. Journal of the American College of Cardiology. 2010;55:2556–66. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen JT, Berger AK, Duval S and Luepker RV. Gender disparity in cardiac procedures and medication use for acute myocardial infarction. American heart journal. 155:862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger JS, Elliott L, Gallup D and et al. SEx differences in mortality following acute coronary syndromes. Jama. 2009;302:874–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hochman JS, Tamis JE, Thompson TD, Weaver WD, White HD, Van de Werf F, Aylward P, Topol EJ and Califf RM. Sex, Clinical Presentation, and Outcome in Patients with Acute Coronary Syndromes. New England Journal of Medicine. 1999;341:226–232. [DOI] [PubMed] [Google Scholar]
- 13.Bucholz EM, Butala NM, Rathore SS, Dreyer RP, Lansky AJ and Krumholz HM. Sex Differences in Long-Term Mortality after Myocardial Infarction: A Systematic Review. Circulation. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterson ED, Lansky AJ, Kramer J, Anstrom K, Lanzilotta MJ and National Cardiovascular Network Clinical I. Effect of gender on the outcomes of contemporary percutaneous coronary intervention. Am J Cardiol. 2001;88:359–64. [DOI] [PubMed] [Google Scholar]
- 15.Robinson TN, Wu DS, Sauaia A, Dunn CL, Stevens-Lapsley JE, Moss M, Stiegmann GV, Gajdos C, Cleveland JC, Jr. and Inouye SK Slower walking speed forecasts increased postoperative morbidity and 1-year mortality across surgical specialties. Ann Surg. 2013;258:582–8; discussion 588–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forman DE, Rich MW, Alexander KP, Zieman S, Maurer MS, Najjar SS, Cleveland JC, Jr., Krumholz HM and Wenger NK. Cardiac care for older adults. Time for a new paradigm. J Am Coll Cardiol. 2011;57:1801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dodson JA, Geda M, Krumholz HM, Lorenze N, Murphy TE, Allore HG, Charpentier P, Tsang SW, Acampora D, Tinetti ME, Gill TM and Chaudhry SI. Design and rationale of the comprehensive evaluation of risk factors in older patients with AMI (SILVER-AMI) study. BMC Health Serv Res. 2014;14:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Joint ESCAAHAWHFTFftUDoMI, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJand Mendis S. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–35. [DOI] [PubMed] [Google Scholar]
- 19.Donoghue OA, Savva GM, Cronin H, Kenny RA and Horgan NF. Using timed up and go and usual gait speed to predict incident disability in daily activities among community-dwelling adults aged 65 and older. Arch Phys Med Rehabil. 2014;95:1954–61. [DOI] [PubMed] [Google Scholar]
- 20.Savva GM, Donoghue OA, Horgan F, O’Regan C, Cronin H and Kenny RA. Using timed up-and-go to identify frail members of the older population. J Gerontol A Biol Sci Med Sci. 2013;68:441–6. [DOI] [PubMed] [Google Scholar]
- 21.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD and National Eye Institute Visual Function Questionnaire Field Test I. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–8. [DOI] [PubMed] [Google Scholar]
- 22.Owen CG, Rudnicka AR, Smeeth L, Evans JR, Wormald RP and Fletcher AE. Is the NEI-VFQ-25 a useful tool in identifying visual impairment in an elderly population? BMC Ophthalmol. 2006;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magruderhabib K, Breitner JCS and Welsh K. Performance-Characteristics of the Telephone Interview for Cognitive Status. Am J Epidemiol. 1990;132:788–788. [Google Scholar]
- 24.Folstein MF, Folstein SE and McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- 25.Fong TG, Fearing MA, Jones RN, Shi P, Marcantonio ER, Rudolph JL, Yang FM, Kiely DK and Inouye SK. Telephone interview for cognitive status: Creating a crosswalk with the Mini-Mental State Examination. Alzheimers Dement. 2009;5:492–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG and White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–47. [DOI] [PubMed] [Google Scholar]
- 27.Khwaja A KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–84. [DOI] [PubMed] [Google Scholar]
- 28.White IR, Royston P and Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30:377–99. [DOI] [PubMed] [Google Scholar]
- 29.Dodson JA, Hochman JS, Roe MT, Chen AY, Chaudhry SI, Katz S, Zhong H, Radford MJ, Udell JA, Bagai A, Fonarow GC, Gulati M, Enriquez JR, Garratt KN and Alexander KP. The Association of Frailty With In-Hospital Bleeding Among Older Adults With Acute Myocardial Infarction: Insights From the ACTION Registry. JACC Cardiovasc Interv. 2018;11:2287–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kramer DB, Tsai T, Natarajan P, Tewksbury E, Mitchell SL and Travison TG. Frailty, Physical Activity, and Mobility in Patients With Cardiac Implantable Electrical Devices. Journal of the American Heart Association. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ansai JH, Farche ACS, Rossi PG, de Andrade LP, Nakagawa TH and Takahashi ACM. Performance of Different Timed Up and Go Subtasks in Frailty Syndrome. Journal of geriatric physical therapy (2001). 2017;00:1–7. [DOI] [PubMed] [Google Scholar]
- 32.Benjamini Y and Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 33.Bucholz EM, Butala NM, Rathore SS, Dreyer RP, Lansky AJ and Krumholz HM. Sex differences in long-term mortality after myocardial infarction: a systematic review. Circulation. 2014;130:757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dodd KS, Saczynski JS, Zhao Y, Goldberg RJ and Gurwitz JH. Exclusion of older adults and women from recent trials of acute coronary syndromes. Journal of the American Geriatrics Society. 2011;59:506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sardar M, Badri M, Prince CT, Seltzer J and Kowey PR. UNderrepresentation of women, elderly patients, and racial minorities in the randomized trials used for cardiovascular guidelines. JAMA internal medicine. 2014;174:1868–1870. [DOI] [PubMed] [Google Scholar]
- 36.Dodson JA and Hochman JS. Improving outcomes in older women? JACC Cardiovasc Interv. 2015;8:797–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuzawa Y, Konishi M, Akiyama E, Suzuki H, Nakayama N, Kiyokuni M, Sumita S, Ebina T, Kosuge M, Hibi K, Tsukahara K, Iwahashi N, Endo M, Maejima N, Saka K, Hashiba K, Okada K, Taguri M, Morita S, Sugiyama S, Ogawa H, Sashika H, Umemura S and Kimura K. Association between gait speed as a measure of frailty and risk of cardiovascular events after myocardial infarction. Journal of the American College of Cardiology. 2013;61:1964–72. [DOI] [PubMed] [Google Scholar]
- 38.Scott KM and Collings SC. Gender differences in the disability (functional limitations) associated with cardiovascular disease: a general population study. Psychosomatics. 2012;53:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zullo AR, Olean M, Berry SD, Lee Y, Tjia J and Steinman MA. Patient-Important Adverse Events of beta-blockers in Frail Older Adults after Acute Myocardial Infarction. J Gerontol A Biol Sci Med Sci. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinman MA, Zullo AR, Lee Y, Daiello LA, Boscardin WJ, Dore DD, Gan S, Fung K, Lee SJ, Komaiko KD and Mor V. Association of beta-Blockers With Functional Outcomes, Death, and Rehospitalization in Older Nursing Home Residents After Acute Myocardial Infarction. JAMA internal medicine. 2017;177:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thang ND, Karlson BW, Karlsson T and Herlitz J. Characteristics of and outcomes for elderly patients with acute myocardial infarction: differences between females and males. Clin Interv Aging. 2016;11:1309–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McSweeney JC, Cody M, O’Sullivan P, Elberson K, Moser DK and Garvin BJ. Women’s early warning symptoms of acute myocardial infarction. Circulation. 2003;108:2619–23. [DOI] [PubMed] [Google Scholar]
- 43.Safdar B, Spatz ES, Dreyer RP, Beltrame JF, Lichtman JH, Spertus JA, Reynolds HR, Geda M, Bueno H, Dziura JD, Krumholz HM and D’Onofrio G. Presentation, Clinical Profile, and Prognosis of Young Patients With Myocardial Infarction With Nonobstructive Coronary Arteries (MINOCA): Results From the VIRGO Study. Journal of the American Heart Association. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Carlo M, Morici N, Savonitto S, Grassia V, Sbarzaglia P, Tamburrini P, Cavallini C, Galvani M, Ortolani P, De Servi S and Petronio AS. Sex-Related Outcomes in Elderly Patients Presenting With Non-ST-Segment Elevation Acute Coronary Syndrome: Insights From the Italian Elderly ACS Study. JACC Cardiovasc Interv. 2015;8:791–6. [DOI] [PubMed] [Google Scholar]
- 45.Alonso Salinas GL, Sanmartin Fernandez M, Pascual Izco M, Marco Del Castillo A, Rincon Diaz LM, Lozano Granero C, Valverde Gomez M, Pastor Pueyo P, Del Val Martin D, Pardo Sanz A, Monteagudo Ruiz JM, Recio-Mayoral A, Salvador Ramos L, Marzal Martin D, Camino Lopez A, Jimenez Mena M and Zamorano Gomez JL. Frailty predicts major bleeding within 30days in elderly patients with Acute Coronary Syndrome. International journal of cardiology. 2016;222:590–593. [DOI] [PubMed] [Google Scholar]
- 46.Alonso Salinas GL, Sanmartin M, Pascual Izco M, Rincon LM, Pastor Pueyo P, Marco Del Castillo A, Garcia Guerrero A, Caravaca Perez P, Recio-Mayoral A, Camino A, Jimenez-Mena M and Zamorano JL. Frailty is an independent prognostic marker in elderly patients with myocardial infarction. Clinical cardiology. 2017;40:925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alonso Salinas GL, Sanmartin Fernandez M, Pascual Izco M, Martin Asenjo R, Recio-Mayoral A, Salvador Ramos L, Marzal Martin D, Camino Lopez A, Jimenez Mena M and Zamorano Gomez JL. Frailty is a short-term prognostic marker in acute coronary syndrome of elderly patients. European heart journal Acute cardiovascular care. 2016;5:434–40. [DOI] [PubMed] [Google Scholar]
- 48.Clemmensen P, Roe MT, Hochman JS, Cyr DD, Neely ML, McGuire DK, Cornel JH, Huber K, Zamoryakhin D, White HD, Armstrong PW, Fox KA, Prabhakaran D, Ohman EM and Investigators TA. Long-term outcomes for women versus men with unstable angina/non-ST-segment elevation myocardial infarction managed medically without revascularization: insights from the TaRgeted platelet Inhibition to cLarify the Optimal strateGy to medicallY manage Acute Coronary Syndromes trial. American heart journal. 2015;170:695–705 e5. [DOI] [PubMed] [Google Scholar]
- 49.Vavalle JP, van Diepen S, Clare RM, Hochman JS, Weaver WD, Mehta RH, Pieper KS, Patel MR, Patel UD, Armstrong PW, Granger CB and Lopes RD. Renal failure in patients with ST-segment elevation acute myocardial infarction treated with primary percutaneous coronary intervention: Predictors, clinical and angiographic features, and outcomes. American heart journal. 2016;173:57–66. [DOI] [PubMed] [Google Scholar]
- 50.Sun YB, Liu BC, Zou Y, Pan JR, Tao Y and Yang M. Risk factors of acute kidney injury after acute myocardial infarction. Ren Fail. 2016;38:1353–1358. [DOI] [PubMed] [Google Scholar]
- 51.Alexander KP, Chen AY, Newby LK, Schwartz JB, Redberg RF, Hochman JS, Roe MT, Gibler WB, Ohman EM, Peterson ED and Investigators C. Sex differences in major bleeding with glycoprotein IIb/IIIa inhibitors: results from the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines) initiative. Circulation. 2006;114:1380–7. [DOI] [PubMed] [Google Scholar]
- 52.Alexander KP, Chen AY, Roe MT, Newby LK, Gibson CM, Allen-LaPointe NM, Pollack C, Gibler WB, Ohman EM, Peterson ED and Investigators C. Excess dosing of antiplatelet and antithrombin agents in the treatment of non-ST-segment elevation acute coronary syndromes. Jama. 2005;294:3108–16. [DOI] [PubMed] [Google Scholar]
- 53.LaPointe NM, Chen AY, Alexander KP, Roe MT, Pollack CV, Jr., Lytle BL, Ohman ME, Gibler BW and Peterson ED. Enoxaparin dosing and associated risk of in-hospital bleeding and death in patients with non ST-segment elevation acute coronary syndromes. Arch Intern Med. 2007;167:1539–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.