Abstract
Although proton pump inhibitors (PPIs) are widely used, their relative potency and ideal dosing regimens remain unclear. We analyzed data from randomized clinical trials that performed pH testing in patients receiving solid-dose PPI formulations (omeprazole, esomeprazole, lansoprazole, pantoprazole, rabeprazole) for a minimum of 5 days. We used omeprazole equivalency and the surrogate biomarker, percentage time pH > 4 over a 24-hour period (pH4time), to compare PPI effectiveness for different PPIs given once, twice, or 3 times daily. We found that increasing strength of once-daily PPIs (9–64 mg omeprazole equivalents) increased pH4time linearly from approximately 10.0 to 15.6 hours; higher doses produced no further increase in pH4time. Increasing the frequency to twice-daily PPI increased pH4time linearly, from approximately 15.8 to 21.0 hours. Three-times daily PPIs performed similarly to twice-daily PPIs. The costs of PPIs varied greatly, but the cost variation was not directly related to potency. We conclude that PPIs can be used inter-changeably based on potency. Using twice-daily PPIs is more effective in increasing efficacy increasing once-daily PPI dosage. Omeprazole and lansoprazole (30 mg) and 20 mg of esomeprazole rabeprazole are functionally equivalent.
Keywords: Efficacy Comparison, GERD, Treatment, Reflux, Drug
Since their introduction approximately 3 decades ago, proton pump inhibitors (PPIs) have become one of the most widely used drugs worldwide. Recently, the prevalence of PPI use was estimated at 7% to 9% in ambulatory patients,1,2 and more than 20% among ambulatory patients older than 80 years.2 Increased PPI use results in high health care costs, exaggerated further by the use of proprietary rather than generic PPIs.3 It has been estimated that 5-year excess costs associated with proprietary PPIs exceeded $47 billion dollars in the United States.3
The relative potency of different histamine-2–receptor antagonists (H2RAs) was based historically on healing rates of peptic ulcers in which the duration of the intragastric pH level of 3 or higher was a reliable surrogate marker for the relative effectiveness of H2RAs.4,5 PPIs largely have replaced H2RAs because they proved to be more effective for ulcer healing and relieving symptoms and healing of erosive gastroesophageal reflux disease (GERD) than H2RAs.6–9 Analyses of the relationship between PPI effectiveness and intragastric pH level also showed the duration the pH level was maintained at a pH of 4 or greater over the 24-hour day (pH4time) could be used as a surrogate marker for symptom relief in GERD, and in the healing and prevention of relapse of erosive esophagitis.6 The pH4time biomarker subsequently has been used widely to compare antisecretory drugs, especially in marketing studies designed to prove the superiority of one PPI over another.10–14 PPIs also have proven to be useful adjuvants to antimicrobial therapy for Helicobacter pylori infection.15
Although there are numerous studies claiming that one PPI is superior to another, consensus conferences have suggested that PPIs are more similar than different. For example, the 2005 Canadian Consensus Conference concluded that 20 mg omeprazole, 40 mg esomeprazole, 30 mg lansoprazole, 40 mg pantoprazole, and 20 mg rabeprazole were equivalent for the treatment of GERD.16 The World Health Organization Collaborating Centre for Drug Statistics Methodology proposed that 20 mg omeprazole, 30 mg esomeprazole, 30 mg lansoprazole, 40 mg pantoprazole, 20 mg rabeprazole, and 30 mg of dexlansoprazole were equivalent for the treatment of GERD (http://www.whocc.no/atc_ddd_index/?code1/4A02BC&showdescription1/4yes; accessed August 27, 2016). These apparent contradictions emphasize the need for objective data and possibly for surrogate markers to reliably rank comparative PPI effectiveness and allow more cost-effective use.
Based on results from 57 clinical studies (including 3831 subjects), Kirchheiner et al17 proposed pH4time as a surrogate marker of relative PPI potency. They analyzed the dose-effect relationships for the mean (or median intragastric pH when the mean was not provided) intragastric pH as well as the pH4time for each PPI and then used pharmacodynamic modeling to define the relative potencies for the different PPIs. Relative potencies subsequently were standardized to omeprazole. Kirchheiner et al17 reported the relative potencies were 0.23, 0.90, 1.00, 1.60, and 1.82 for pantoprazole, lansoprazole, omeprazole, esomeprazole, and rabeprazole, respectively (ie, 40 mg of pantoprazole and 9 mg of omeprazole were similarly effective when assessed by pH4time) (Table 1). In their analyses, Kirchheiner et al17 pooled the daily cumulative dosages used, but did not examine the effects of the frequency or timing of administration. The conversion of other PPIs as to potency in relation to omeprazole was termed by the authors of this paper as omeprazole equivalents (OEs).
Table 1.
Potency of PPIs Based on OE
| Drug at lowest available dosage | OE |
|---|---|
| Pantoprazole 20 mg | 4.5 mg |
| Lansoprazole 15 mg | 13.5 mg |
| Omeprazole 20 mg | 20 mg |
| Esomeprazole 20 mg | 32 mg |
| Rabeprazole 20 mg | 36 mg |
NOTE. PPIs are listed in order of increasing potency.17
OE, omeprazole equivalent; PPIs, proton pump inhibitors.
Here, we used the relative potencies reported by Kirchheiner et al17 to compare available PPIs in terms of OEs in relation to dose–pH time. We further restricted our analyses to data from Western countries where CYP2C19 genotypes are skewed toward rapid metabolizers. In addition, we only included studies that tested pH4time PPIs after reaching a steady state (ie, after a minimum of 5 days of PPI therapy). We furthered the study by Kirchheiner et al17 by also investigating the potency of PPIs by frequency of administration (eg, once daily, twice daily, or 3 times/d). We also provide estimates for both dexlansoprazole and the newest PPI, the potassium-competitive acid blocker vonoprazan. PH4time, a surrogate marker, provides composite data and thus helps compensate for differences among PPIs in relation to pharmacokinetics and pharmacodynamics (eg, effect of CYP2C19), provided that the relative potencies are derived from similar populations (eg, Asian vs Western). Finally, we determined the cost in relation to OEs to provide an estimate of which PPIs provide the best value in terms of acid suppression.
Methods
We performed a search of articles published in PubMed from inception through August 2016 using a combination of multiple medical subject heading (MeSH) terms and keywords for PPI, clinical trials, and pH (Supplementary Appendix 1). We did not apply any restrictions to our search strategy. We manually searched bibliographies of relevant articles for references not captured by the search strategy. We included studies if they were randomized clinical trials and performed pH testing after a minimum of 5 days on PPIs. Given the variability in CYP2C19 metabolism in Asian populations, Asian studies were excluded. Studies also were excluded if they were abstracts, published in non–peer-reviewed journals, or manufacturer data. Studies or study arms were excluded if the PPI was not an oral formulation (intravenous), used a non–widely available medication (ie, tenatoprazole), or used a nonstandard formulation of available medication (ie, different from tablet or capsule). In addition, we excluded study arms if patients took histamine H2RAs concomitantly with the PPI. Both authors screened all results retrieved by the search and reviewed all articles for possible inclusion. Any disagreements were resolved through discussion. To ensure reliability, both authors separately extracted the same data from 50 study arms with excellent Cohen’s k (>0.8). In addition, several random and targeted re-evaluations of the data extraction by both authors were performed to continue to ensure reliability of the data extraction.
Finally, to compare intragastric pH data reliably, we attempted to obtain additional information regarding measurement methods and contacted investigators when necessary. Important variable information extracted included the pH measurement methods used (ie, from pH electrodes placed in the stomach or measurements of intermittently aspirated gastric contents), the type of electrode used (ie, antimony or glass), and whether an external reference electrode was used. Because intragastric data obtained by antimony electrodes with external reference electrodes often is unreliable, we only included studies using combination electrodes (Supplementary Methods).18–20 We excluded studies if they assessed only esophageal pH or used aspiration techniques to assess intragastric pH.
We abstracted data from each study arm: study population (ie, healthy, H pylori, symptomatic GERD, duodenal ulcers, and so forth), number of participants, number of women, number of patients with H pylori infection if provided, whether the study was blinded, CYP2C19 testing if provided, the percentage of time pH level was 4 or higher (mean or median), 24-hour intragastric median pH (or mean), the percentage of time the pH level was 6 or higher (mean or median) if reported, and the number of days on therapy before pH testing. The criteria used to define the specific study populations such as heartburn or GERD, were extracted. Given the wide variability in reporting strategies, we collected mean and median values separately. Means and medians were compared in studies that provided both mean and median values and also by comparing the final results. We compared values for each PPI regimen as weighted and unweighted medians and means. Studies were weighted based on study sample sizes and variance was calculated as previously described.17 We created best-fit lines to assist providers in determining drug dosage regimens and calculated linear regression models for daily, twice-daily, and 3 times daily regimens. PPIs used in study arms were plotted with OE as the independent variable based on previously reported OE data with pH4time as the dependent variable. We performed post hoc analyses limiting the results to OEs of 9 to 64 mg because most data were extracted between this range. Data analysis was performed using Stata version 13.1 (College Station, TX) and SigmaPlot 10 (San Jose, CA). P values less than .05 were considered statistically significant. We also constructed dose-response curves for the different PPIs using linear regression analysis of the combined weighted median/median time the pH was equal to or greater for each designated pH (eg, pH 4).
Results
A total of 56 randomized studies, which included 146 randomized arms, met our inclusion criteria and included at least 1 study arm evaluating the potency of omeprazole, rabeprazole, pantoprazole, esomeprazole, or lansoprazole (Table 2 and Supplementary Table 1). Seven studies (14 treatment arms) using esophageal probes (without gastric probe), 7 studies (19 treatment arms) using external reference electrodes, and 4 studies with 11 treatment arms using gastric aspiration were excluded (Supplementary Table 2). In the majority of studies, pH4times were presented as medians. We examined whether the results differed significantly if we used median or mean results for the time the pH was equal to or greater than a designated pH level (eg, pH4time using several methods). Two studies reported both median and mean values of ph4time; the reported results were similar (paired t test, P = .75).21,22 We also calculated weighted medians from median-only values and mean-only values separately. There were no significant differences between mean and median values for ph4time with a normal distribution of the differences. As a result, if median data were not provided, we substituted the mean result. Data are presented as the weighted percentage of time over a 24-hour period the pH was equal to or greater than pH 4. We used the relative potencies of Kirchheiner et al17 and expressed the dose of each PPI in OEs (Table 1).17 Supplementary Table 1 summarizes relevant data regarding the clinical studies used.
Table 2.
Studies That Reported pH Time > 4
| Studies | Study arms, na |
|---|---|
| Randomized study arms | 146 |
| Patient population | |
| Healthy | 94 |
| Heartburn/GERD/esophagitis | 38 |
| Duodenal ulcer | 7 |
| Barrett’s esophagus | 7 |
| Dosing | |
| Daily | 116 |
| Twice daily | 25 |
| Three times daily | 5 |
| Days of therapy before testing | |
| 5–6 | 100 |
| 7–9 | 32 |
| ≥10 | 14 |
GERD, gastroesophageal reflux disease.
For ease of interpretation, the table shows study arms. Some studies have multiple study arms included. A total of 56 studies, comprising 148 studies, were included.
Proton Pump Inhibitors Administered Once Daily
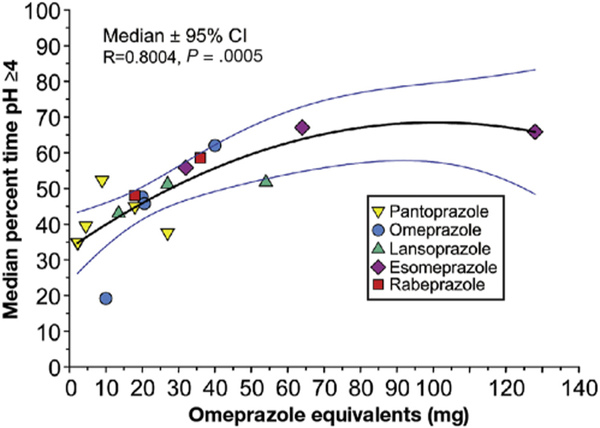
Data were available from 116 treatment arms and 3713 subjects with once-daily therapy. Figure 1 shows the results for the different OEs using a single daily dose of PPI taken for at least 5 days. Even the lowest dose of PPI (2.5 mg OE) had a marked effect on pH4time, suggesting that most of the proton pumps were inactivated at this dose. For example, pantoprazole 10 mg once daily resulted in a ph4time of 34.9% (8.3 h),23 compared with placebo arms that reported a ph4time between 1.2% and 1.7% (weighted mean, 1.5%).24–28 The curve tended to taper off after approximately 70 mg OE, suggesting that the duration of the PPI in the blood stream limits the effective dose. We obtained the best fit for the data using a quadratic polynomial equation (r = 0.769; P = .0019) (Figure 1). The increase in pH4 time was linear (between approximately 9 and 64 mg OE) (Figure 2). The linear equation for doses between 9 and 64 mg OE once daily was as follows: ph4time = (35.9 + [0.477 * OE]) with strong correlation (R = 0.738) and the standard error of estimate = 8.7. Based on this best-fit line, pH4time is approximately 40% (9.6 h over a 24-hour period) with 9 mg OE and increases to a peak of approximately 65% (15.6 h) with 64 mg OE.
Figure 1.
The effect of different PPIs given once daily as OEs on the proportion of the day the intragastric pH remained at 4 or higher. Data are presented as weighted medians and 95% CIs as omeprazole equivalents ranging from 2.5 mg OE/d to 128 mg OE/d. All data are after at least 5 days of therapy in Western populations.
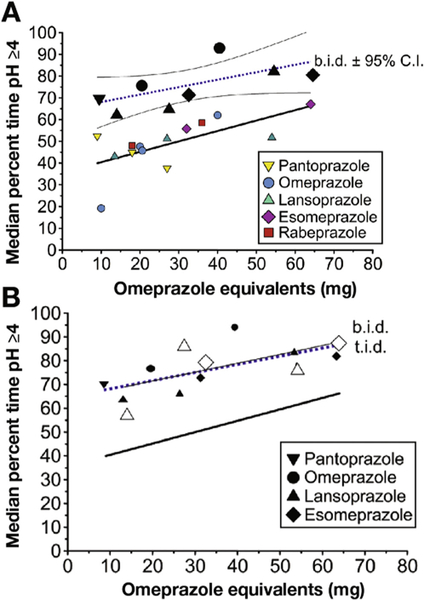
Figure 2.
(A) Comparison of the effects of once-daily and twice-daily PPI administration as OEs on the proportion of the day the intragastric pH remained at 4 or higher. Once-a-day PPI ranged from 9 to 64 mg OEs. Twice-daily administration ranged from 18 to 64 mg OEs. For both, the linear regression line is shown and for twice-daily administration the 95% CI is shown. All data are after at least 5 days of therapy in Western populations. (B) Comparison of the effects of once-daily, twice-daily (solid black symbols), and 3 times/d (open symbols) administration as OEs on the proportion of the day the intragastric pH remained at 4 or higher. The regression line for twice-daily and 3 times/d administration superimpose each other. bid, twice daily; tid, 3 times/d.
Twice-Daily and Three Times per Day Dosing Regimens
We examined the effect of twice-daily dosing (25 studies and 592 subjects) (Supplementary Table 1). The lowest dose of twice-daily dosing (9 mg OE twice daily, which corresponds to 40 mg pantoprazole twice daily) resulted in a pH4time comparable with the highest dose of PPI tested in once-daily dosing (approximately 65% or 15.6 h over a 24-hour period) (Figure 3A). Increasing the OE resulted in a further increase in pH4time, peaking at approximately 85% (20.4 h) with 64 mg OE twice daily (40 mg esomeprazole twice daily), the highest dose tested (Figure 3B). The linear equation was as follows: pH4time = 65.231 + (0.331 * OE), R = 0.629, and standard error of estimate = 8.540. Increasing the dosing interval to 3 times/d (5 studies, 106 subjects) did not provide any further improvement (the 3 times/d regression was superimposed on the twice-daily regression) (Figure 2B).
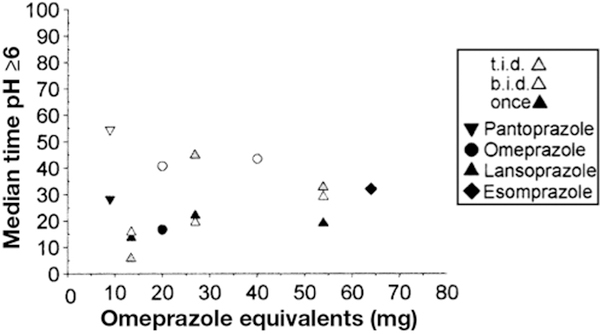
Figure 3.
Comparison of the effectiveness of various PPIs in maintaining the median pH at 6 or higher for 24 hours (pH6time) when given once, twice, or 3 times daily (Supplementary Table 4 for details). bid, twice daily; tid, 3 times/d.
Percentage Time Gastric pH ≥6
We also evaluated the pH6time because this has been suggested as possibly important for the success of H pylori triple therapy and in control of upper gastrointestinal bleeding.29 We found 28 studies with 687 subjects using once-daily (17 studies and 528 patients), twice-daily, and 3 times/d dosing. The pH6time increased to greater than 50% irrespective of the OE in only 1 study (Figure 4). The maximum OE tested was 54 mg (lansoprazole 60 mg 3 times/d), which achieved a mean pH6 time of 33% (7.8 h over a 24-hour period) (Figure 3). With pH6time, the mean and median data differed significantly, reflecting the need for standardization of reporting; it also suggested that data for pH of 6 or higher does not follow normal distribution and is right-skewed (Figure 3, Supplementary Table 4). This is not surprising considering that pH 6 = 0.001 mmol/L H+ and secretion of even a minute amount of acid can result in a marked decrease in pH.
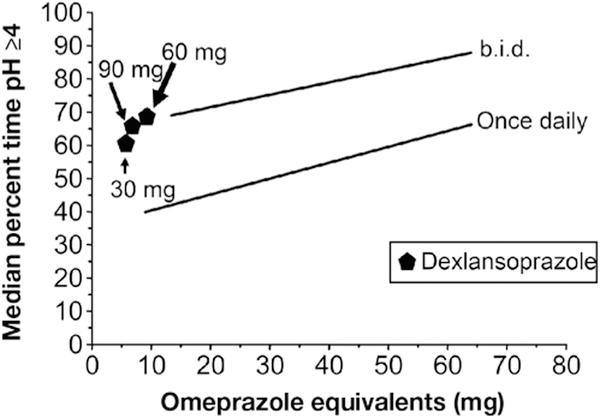
Figure 4.
Effect of dexlansoprazole, a quasi–twice-daily therapy, on maintaining the intragastric pH level at 4 or higher.
Costs
Table 3 provides the US costs of different PPIs in standard tablet or capsule formulations by strength. The corresponding OE and the resulting cost per OE also is provided. Brands are significantly more expensive than generic formulations. Low-dose (20 mg) pantoprazole had the highest cost per OE. Generic formulation had the lowest cost per OE. A formulation that cost less than $0.10 per OE was available for all PPIs reported earlier.
Table 3.
PPI Cost: Brand vs Generic PPIs
| Drug | OE | Cost | Cost per OE | ||
|---|---|---|---|---|---|
| Brand price | Generic median price (minimum-maximum price) |
Brand | Generic (based on median price) |
||
| Pantoprazole | |||||
| 20 mg | 4.5 | $14.38 | $4.09 ($0.13-$10.79) | $3.19 | $0.91 |
| 40 mg | 9 | $14.37 | $0.33 ($0.13-$10.79) | $1.60 | $0.04 |
| Omeprazole | |||||
| 10 mg | 10 | - | $3.77 ($0.22-$3.99) | - | $0.38 |
| 20 mg | 20 | - | $0.64 ($0.07-$8.79) | - | $0.03 |
| 40 mg | 40 | - | $7.40 ($0.15-$7.40) | - | $0.18 |
| Lansoprazole | |||||
| 15 mg | 13.5 | $16.60 | $0.60 ($0.16-$6.01) | $1.23 | $0.04 |
| 30 mg | 27 | $16.60 | $4.25 ($2.05-$13.24) | $0.61 | $0.16 |
| Esomeprazole | |||||
| 20 mg | 32 | $10.04 | $8.52 ($0.65-$9.02) | $0.31 | $0.27 |
| 22.3 mg | 35.7 | $0.70 ($0.65-$0.77) | - | $0.02 | |
| 40 mg | 64 | $10.04 | $8.64 ($4.50-$9.02) | $0.16 | $0.13 |
| Rabeprazole | |||||
| 20 mg | 36 | $20.79 | $2.75 ($1.20-$11.46) | $0.58 | $0.08 |
| Dexlansoprazole | |||||
| 30 mg | N/A | $9.76 | N/A | - | - |
| 60 mg | N/A | $9.76 | N/A | - | - |
NOTE. Drugs are presented in order of increasing potency and only forsolid dosage formulations (ie, excluded sprinkle and liquid formulations). Drugs are ordered in terms of potency based on OEs.
OE, omeprazole equivalent; PPIs, proton pump inhibitors.
Dexlansoprazole and Vonoprazan
Finally, we assessed the effects of newer acidsuppressing formulations, dexlansoprazole, the (R)-(+)-enantiomer of lansoprazole, and vonoprazan, a potassium-competitive acid blocker. Data for both medications were limited in Western populations.
Dexlansoprazole releases drug at 2 points in time as a result of a dual delayed-release formulation with 25% of the drug being released at pH 5.5 and 75% being released at pH 6.8.30 After 5 days of therapy, weighted median pH4time with dexlansoprazole once daily at 30 mg, 60 mg, and 90 mg were 59% (83 patients), 70% (232 patients), and 67% (83 patients), respectively.31–33 By using the pH4time, 30 mg dexlansoprazole was equivalent to approximately 50 to 60 mg OE once daily, 60 mg was equivalent to 15.5 OE twice daily, and 90 mg was equivalent to 9.5 mg OE twice daily (Figure 4). Considering their high cost (Supplementary Table 3) and low relative effectiveness, dexlansoprazole cannot generally be recommended.
Vonoprazan, a new class of PPI (potassium competitive-acid blocker), is both long acting and does not require acid activation.34 Although most pH data come from Asian populations, 2 studies examined the effects of vonoprazan in a Western (UK) population.35,36 Of these, only 1 study met our criteria examining effects of vonoprazan in a Western population.37 The weighted median pH4time was 60.2%, 85.2%, 90.1%, and 93.2% for 10, 20, 30, and 40 mg of vonoprazan, respectively, when given once daily after 7 days of therapy.37 Based on the PPI pH4time plots (Figures 1 and 2A), when given once daily, 10 mg of vonoprazan was equivalent to 60 mg OE once daily, and 20 mg was equal to 60 mg OE twice daily (Supplementary Table 3).
Discussion
Overall, we confirmed that PPIs differ in potency in terms of acid suppression as assessed by pH4time. In addition, we found the following: (1) the slope of the increase in effectiveness was low and peaked at approximately 70 mg OE; (2) PPI administration twice a day was more effective in increasing the pH4time than a once-daily dose; (3) increasing the dosing frequency from twice to 3 times daily did not increase the effectiveness of acid suppression; (4) combining data regarding relative potencies with PPI costs allowed easy determination of the relative cost effectiveness of PPIs (Table 3); (5) dexlansoprazole, a quasi–twice-a-day formulation produced similar acid suppression to the lowest twice-daily PPI regimen; and (6) 20 mg vonoprazan once daily provided similar efficacy as high-dose twice-daily PPI.
We used relative potencies of different PPIs proposed by Kirchheiner et al17 to compare pH4time data from a large sample of patients receiving PPIs once, twice, or 3 times daily. This approach allows one to predict the outcome and effectiveness of different strategies such as decreasing or increasing the OE in once-daily therapy or increasing the dose to twice daily or 3 times/d dosing intervals.
Our study differed from that of Kirchheiner et al,17 who calculated the relative PPI potency based on total daily PPI dosage and included nonrandomized studies and conference abstracts. More importantly, our review focused on providing data that would allow PPI regimens to be tailored at the level of individual patients. There were several strengths of our study including our rigorous criteria to reduce bias, construction of multiple models to evaluate efficacy, and analysis of the effects of dosage frequency.38–44 Many comparative studies of PPIs assessing specific indications (such as relief of heart burn, healing of esophageal erosions, prevention of relapse of esophageal erosions, or rapidity of ulcer healing) have used the surrogate marker pH4time. The use of pH4time allows comparison of relative PPI potency across different studies. This is especially important for studies that are not double-blinded, which can be subject to higher degrees of bias in qualitative assessments such as symptom improvement.
Although many head-to-head comparisons of different PPIs have been performed, we found that few attempted to relate the outcome to differences in relative potency of the different PPIs used. For example, Rohss et al14 reported that 40 mg of esomeprazole was superior to 20 mg of omeprazole, 30 mg of lansoprazole, 40 mg of pantoprazole, or 20 mg of rabeprazole for intragastric pH control as assessed by pH4time greater than 12 hours for patients with symptomatic GERD. In this and other studies, PPIs with similar or greater OEs have not been compared directly (eg, 40 mg of rabeprazole compared with 72 mg OE). When considered in terms of relative potency, that study actually compared a high acid-suppression dose of esomeprazole with markedly lower acid-suppression doses of other PPIs (ie, 64 mg OE [esomeprazole] vs 20 mg OE [omeprazole], 27 mg OE [lansoprazole], 9 mg OE [pantoprazole], and 36 mg OE [rabeprazole]). Studies claiming superiority of one PPI over another almost invariably have been based on comparisons of drugs and doses with markedly different OEs. Although direct comparisons are lacking, it appears reasonable to believe that when given at equivalent OEs, all PPIs are equivalent, which is consistent with the Canadian Consensus Conference and the World Health Organization Collaborating Centre’s conclusions.16
To further confirm and extend the usefulness of OEs and pH4time, head-to-head PPI comparisons, based on similar OEs, both in terms of acid suppression and in relation to clinical end points are necessary. The available clinical data suggest there are ph4time thresholds for achieving different end points (eg, to achieve healing of esophageal erosions). Studies comparing different antisecretory therapies for erosive GERD have suggested a maximum rate with a pH4time of longer than approximately 16 hours (66%).6 How-ever, that conclusion was based on only 2 data points for data on more than 8 to 10 hours (33 and 50%) pH4time and none between approximately 10 and 16 hours.6 Another small comparison of PPIs for the prevention of relapse of erosive GERD showed a marked benefit with 4.5 and 13.5 mg OE (approximately 8–10 hours pH4time), with the best results obtained with pH4times of longer than 12 hours (50% pH4time).45 Such data also are consistent with the notion that single doses of PPI between 27 mg OE (30 mg of lansoprazole) through 36 mg OE (rabeprazole 20 mg) would produce similar outcomes.
The results of our review suggest that increasing dosing frequency to twice daily may be a more effective strategy than escalating once-a-day dosing (Figure 3). Our results suggest that it is possible to reasonably predict the relative outcome of commonly used doses of PPIs given once or twice daily in relation to the percentage of time the intragastric pH remains at 4 or higher over a 24-hour period.17 Interestingly, when administered twice a day, even the lowest OE tested (pantoprazole 20 mg or 4.5 mg OE) equaled or exceeded the effectiveness of the highest dose of the most potent PPI (rabeprazole 40 mg or 72 mg OE) given once a day. Twice-daily dosing resulted in pH4 times ranging between approximately 65% and 85% (15.6–20.4 h). Increasing dosing to 3 times daily did not appear to provide an additional benefit. The caveat to that statement is that there were only a few studies with small sample sizes using 3 times/d dosing.
Omeprazole Equivalents in Gastroesophageal Reflux Disease and Ulcer Healing
Our data help explain apparent discrepancies between comparative studies using clinical outcomes that actually were comparing PPIs with markedly different relative potencies. For example, one large comparative meta-analysis compared clinical differences between different PPIs in terms of relative risk in healing of GERD, healing of peptic ulcers, or H pylori therapy. The meta-analysis compared relative risks, using omeprazole 20 mg as the control group, for pantoprazole 40 mg, lansoprazole 30 mg, rabeprazole 20 mg, and esomeprazole 40 mg.15 The only significant difference when comparing relative risk for endoscopic healing at 4 weeks for GERD (based on 40 study arms) was that 40 mg of esomeprazole (64 mg OE) was found to be superior to 20 mg of omeprazole (20 mg OE) (relative risk, 1.18; 95% CI, 1.14–1.23).15 In the assessment of peptic ulcer healing at 4 weeks (based 18 study arms, none of which used esomeprazole), the only significant difference reported was that 40 mg of pantoprazole (9 mg OE) was found to be superior to omeprazole 20 mg (20 mg OE) (relative risk, 1.07; 95% CI, 1.02–1.13). There was no significant difference in H pylori eradication (42 study arms). There were several potential limitations of this meta-analysis including, but not limited to, potential heterogeneity between study groups, publication bias, small sample sizes, short follow-up period, and potential for selection bias. Another large meta-analysis of 10 studies (15,316 patients) examined healing of erosive GERD at 8 weeks. Daily single doses of esomeprazole at 40 mg (64 mg OE) were compared with omeprazole 20 mg, lansoprazole 30 mg (27 mg OE), pantoprazole 40 mg (9 mg OE), or omeprazole 40 mg (40 mg OE). Overall, at 8 weeks there was only a 5% (95% CI, 2%–8%) increase in the probability of healing with the highest PPI dose. The effectiveness of high-dose PPI was related inversely to the severity of esophagitis, with the numbers needed to treat ranging from 50 for Los Angeles grade A (based on 4138 patients) to 8 at grade D (based on 920 patients).46
How These Results May Affect Practice
PPIs often are prescribed for long periods. There has been increasing interest in the consequences of long-term use including effects on bone metabolism, the microbiota, vitamin B12 availability, susceptibility of different infections, cardiovascular disease, and so forth. In most instances, long-term PPI use is for the treatment and prevention of symptoms.47 It is prudent to use the lowest effective dose as assessed by OE and many patients can be managed by on-demand therapy. Cost is also an important consideration. In the United States, the price varies markedly without any clear relation to efficacy. Physicians should be provided with cost information and OE data to be able to choose the most cost-effective combination of an individual patient in relation to their method of paying for care.
Table 3 provides information regarding PPI potency in relation to cost. Potential applications of this table may be to help patients and providers decide what may best meet their needs. Patients with erosive GERD or Barrett’s likely require long-term therapy. As shown here, twice-daily therapy likely is preferred for its improved ability to achieve a prolonged increase in pH4time compared with high-dose once-daily therapy. For patients with erosive esophagitis, it may be reasonable to initiate therapy with one of the least expensive PPIs given twice daily. If successful, after approximately 8 weeks, when healing is likely to have been achieved, one might consider reducing dosage to once a day. Pantoprazole is likely best avoided unless one wishes to focus on low-dose PPI therapy and in certain populations that find it effective at symptom control. The cost-effectiveness table may help guide clinicians with escalation of therapy and de-escalation of therapy. For instance, patients requiring proton pump inhibitors but who have risk factors or concerns for Clostridium difficile infection (in which the biological plausibility is related to acid suppression) may wish to use a less-potent regimen that will continue to control symptoms.
Conclusions
This article attempts to clarify the obfuscation surrounding PPI dosing for tailored acid suppression. These data allow one to address common PPI-related questions such as potency of different PPIs, dosages, and regimens. Clearly, PPIs differ in relative potency and this should be taken into account both in terms of degree of acid suppression desired and in relation to cost effectiveness, while also being tailored for the individual patient and the indication for PPI. Although these data highlight the effectiveness of higher OE dosages and twice-daily regimens in acid suppression, the choice of PPI regimen and dosing should be based on indication for use and the lowest effective dose should be used to limit adverse effects related to PPIs. There are also important implications for PPI research. Studies that have measured effects of PPIs by pooling all PPIs fail to account for differences in the amount of acid suppression seen with different dosages, drugs, and regimens, and as a result reported associations (or lack thereof) may be inaccurate. Future well-designed clinical trials are needed that examine different PPIs based on similar omeprazole equivalents and the resulting effects on both clinical and patient-reported outcomes.
Supplementary Material
Acknowledgments
Funding
Supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grant DK56338, which funds the Texas Medical Center Digestive Diseases Center (D.Y.G.); and by Public Health Service grant 5T32DK083266–07 (A.T.). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the VA or the National Institutes of Health.
Abbreviations used in this paper:
- GERD
gastroesophageal reflux disease
- H2RA
histamine-2–receptor antagonists
- OE
omeprazole equivalent
- pH4time
percentage time pH > 4 over a 24-hour period
- PPI
proton pump inhibitor
Footnotes
Conflicts of interest
This author discloses the following: David Graham is a paid consultant for RedHill Biopharma regarding novel Helicobacter pylori therapies and for Bio-Gaia regarding the use of probiotics for H pylori infections. The remaining author discloses no conflicts.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2017.09.033.
References
- 1.Rotman SR, Bishop TF. Proton pump inhibitor use in the U.S. ambulatory setting, 2002–2009. PLoS One 2013;8:e56060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pottegard A, Broe A, Hallas J, et al. Use of proton-pump inhibitors among adults: a Danish nationwide drug utilization study. Therap Adv Gastroenterol 2016;9:671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johansen ME, Huerta TR, Richardson CR. National use of proton pump inhibitors from 2007 to 2011. JAMA Intern Med 2014;174:1856–1858. [DOI] [PubMed] [Google Scholar]
- 4.Jones DB, Howden CW, Burget DW, et al. Acid suppression in duodenal ulcer: a meta-analysis to define optimal dosing with antisecretory drugs. Gut 1987;28:1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt RH, Cederberg C, Dent J, et al. Optimizing acid suppression for treatment of acid-related diseases. Dig Dis Sci 1995; 40:24S–49S. [DOI] [PubMed] [Google Scholar]
- 6.Bell NJ, Burget D, Howden CW, et al. Appropriate acid suppression for the management of gastro-oesophageal reflux disease. Digestion 1992;51(Suppl 1):59–67. [DOI] [PubMed] [Google Scholar]
- 7.Howden CW, Burget DW, Hunt RH. Appropriate acid suppression for optimal healing of duodenal ulcer and gastro-oesophageal reflux disease. Scand J Gastroenterol Suppl 1994;201:79–82. [DOI] [PubMed] [Google Scholar]
- 8.Huang JQ, Hunt RH. pH, healing rate and symptom relief in acid-related diseases. Yale J Biol Med 1996;69:159–174. [PMC free article] [PubMed] [Google Scholar]
- 9.Hunt RH. Importance of pH control in the management of GERD. Arch Intern Med 1999;159:649–657. [DOI] [PubMed] [Google Scholar]
- 10.Dent J Review article: pharmacology of esomeprazole and comparisons with omeprazole. Aliment Pharmacol Ther 2003; 17(Suppl 1):5–9. [DOI] [PubMed] [Google Scholar]
- 11.Hatlebakk JG. Review article: gastric acidity–comparison of esomeprazole with other proton pump inhibitors. Aliment Pharmacol Ther 2003;17(Suppl 1):10–15. [DOI] [PubMed] [Google Scholar]
- 12.Dent J Review article: initial therapy of reflux disease with esomeprazole. Aliment Pharmacol Ther 2003;17(Suppl 1):18–20. [DOI] [PubMed] [Google Scholar]
- 13.Rohss K, Wilder-Smith C, Naucler E, et al. Esomeprazole 20mg provides more effective intragastric acid control than maintenance-dose rabeprazole, lansoprazole or pantoprazole in healthy volunteers. Clin Drug Investig 2004;24:1–7. [DOI] [PubMed] [Google Scholar]
- 14.Rohss K, Lind T, Wilder-Smith C. Esomeprazole 40 mg provides more effective intragastric acid control than lansoprazole 30 mg, omeprazole 20 mg, pantoprazole 40 mg and rabeprazole 20 mg in patients with gastro-oesophageal reflux symptoms. Eur J Clin Pharmacol 2004;60:531–539. [DOI] [PubMed] [Google Scholar]
- 15.Klok RM, Postma MJ, van Hout BA, et al. Meta-analysis: comparing the efficacy of proton pump inhibitors in short-term use. Aliment Pharmacol Ther 2003;17:1237–1245. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong D, Marshall JK, Chiba N, et al. Canadian Consensus Conference on the management of gastroesophageal reflux disease in adults - update 2004. Can J Gastroenterol 2005;19:15–35. [DOI] [PubMed] [Google Scholar]
- 17.Kirchheiner J, Glatt S, Fuhr U, et al. Relative potency of proton-pump inhibitors-comparison of effects on intragastric pH. Eur J Clin Pharmacol 2009;65:19–31. [DOI] [PubMed] [Google Scholar]
- 18.Opekun AR, Smith JL, Graham DY. Antimony electrodes.Mucosal potential differences and buffer composition adversely affect pH measurements in the stomach. Dig Dis Sci 1990; 35:950–955. [DOI] [PubMed] [Google Scholar]
- 19.Rovelstad RA, Owen CA Jr, Magath TB. Factors influencing the continuous recording of in situ pH of gastric and duodenal contents. Gastroenterology 1952;20:609–624. [PubMed] [Google Scholar]
- 20.McLauchlan G, Rawlings JM, Lucas ML, et al. Electrodes for 24 hours pH monitoring–a comparative study. Gut 1987; 28:935–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Florent C, Forestier S. Twenty-four-hour monitoring of intragastric acidity: comparison between lansoprazole 30mg and pantoprazole 40mg. Eur J Gastroenterol Hepatol 1997; 9:195–200. [DOI] [PubMed] [Google Scholar]
- 22.Warrington S, Baisley K, Boyce M, et al. Effects of rabeprazole, 20 mg, or esomeprazole, 20 mg, on 24-h intragastric pH and serum gastrin in healthy subjects. Aliment Pharmacol Ther 2002; 16:1301–1307. [DOI] [PubMed] [Google Scholar]
- 23.Tutuian R, Katz PO, Bochenek W, et al. Dose-dependent control of intragastric pH by pantoprazole, 10, 20 or 40 mg, in healthy volunteers. Aliment Pharmacol Ther 2002;16:829–836. [DOI] [PubMed] [Google Scholar]
- 24.Gan KH, Geus WP, Lamers CB, et al. Effect of omeprazole 40 mg once daily on intraduodenal and intragastric pH in H. pylori-negative healthy subjects. Dig Dis Sci 1997;42:2304–2309. [DOI] [PubMed] [Google Scholar]
- 25.Koop H, Kuly S, Flug M, et al. Intragastric pH and serum gastrin during administration of different doses of pantoprazole in healthy subjects. Eur J Gastroenterol Hepatol 1996;8:915–918. [PubMed] [Google Scholar]
- 26.Sanders SW, Tolman KG, Greski PA, et al. The effects of lansoprazole, a new H+,K(+)-ATPase inhibitor, on gastric pH and serum gastrin. Aliment Pharmacol Ther 1992;6:359–372. [DOI] [PubMed] [Google Scholar]
- 27.Wilder-Smith C, Lind T, Lundin C, et al. Acid control with esomeprazole and lansoprazole: a comparative dose-response study. Scand J Gastroenterol 2007;42:157–164. [DOI] [PubMed] [Google Scholar]
- 28.Williams MP, Sercombe J, Hamilton MI, et al. A placebo-controlled trial to assess the effects of 8 days of dosing with rabeprazole versus omeprazole on 24-h intragastric acidity and plasma gastrin concentrations in young healthy male subjects. Aliment Pharmacol Ther 1998;12:1079–1089. [DOI] [PubMed] [Google Scholar]
- 29.Green FW Jr, Kaplan MM, Curtis LE, et al. Effect of acid and pepsin on blood coagulation and platelet aggregation. A possible contributor prolonged gastroduodenal mucosal hemorrhage. Gastroenterology 1978;74:38–43. [PubMed] [Google Scholar]
- 30.Oldfield ECIV, Parekh PJ, Johnson DA. Dexlansoprazole: delayed-release orally disintegrating tablets for the treatment of heartburn associated with non-erosive gastroesophageal reflux disease and the maintenance of erosive esophagitis. Expert Rev Gastroenterol Hepatol 2016;10:1083–1089. [DOI] [PubMed] [Google Scholar]
- 31.Vakily M, Zhang W, Wu J, et al. Pharmacokinetics and pharmacodynamics of a known active PPI with a novel dual delayed release technology, dexlansoprazole MR: a combined analysis of randomized controlled clinical trials. Curr Med Res Opin 2009; 25:627–638. [DOI] [PubMed] [Google Scholar]
- 32.Kukulka M, Nudurupati S, Perez MC. Pharmacokinetics and pharmacodynamics of an orally disintegrating tablet formulation of dexlansoprazole. Therap Adv Gastroenterol 2016;9:759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee RD, Mulford D, Wu J, et al. The effect of time-of-day dosing on the pharmacokinetics and pharmacodynamics of dexlansoprazole MR: evidence for dosing flexibility with a dual delayed release proton pump inhibitor. Aliment Pharmacol Ther 2010; 31:1001–1011. [DOI] [PubMed] [Google Scholar]
- 34.Otake K, Sakurai Y, Nishida H, et al. Characteristics of the novel potassium-competitive acid blocker vonoprazan fumarate (TAK-438). Adv Ther 2016;33:1140–1157. [DOI] [PubMed] [Google Scholar]
- 35.Sakurai Y, Mori Y, Okamoto H, et al. Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects–a randomised open-label cross-over study. Aliment Pharmacol Ther 2015; 42:719–730. [DOI] [PubMed] [Google Scholar]
- 36.Ashida K, Sakurai Y, Nishimura A, et al. Randomised clinical trial: a dose-ranging study of vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the treatment of erosive oesophagitis. Aliment Pharmacol Ther 2015; 42:685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenkins H, Sakurai Y, Nishimura A, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther 2015;41:636–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lundell L, Hatlebakk J, Galmiche JP, et al. Long-term effect on symptoms and quality of life of maintenance therapy with esomeprazole 20 mg daily: a post hoc analysis of the LOTUS trial. Curr Med Res Opin 2015;31:65–73. [DOI] [PubMed] [Google Scholar]
- 39.Dehn TC, Shepherd HA, Colin-Jones D, et al. Double blind comparison of omeprazole (40 mg od) versus cimetidine (400 mg qd) in the treatment of symptomatic erosive reflux oesophagitis, assessed endoscopically, histologically and by 24 h pH monitoring. Gut 1990;31:509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vasiliadis KV, Viazis N, Vlachogiannakos J, et al. Efficacy of three different dosages of esomeprazole in the long-term management of reflux disease: a prospective, randomized study, using the wireless Bravo pH system. Am J Gastroenterol 2010;105:308–313. [DOI] [PubMed] [Google Scholar]
- 41.Galmiche JP, Zerbib F, Ducrotte P, et al. Decreasing oesophageal acid exposure in patients with GERD: a comparison of rabeprazole and omeprazole. Aliment Pharmacol Ther 2001;15:1343–1350. [DOI] [PubMed] [Google Scholar]
- 42.Simon B, Muller P, Pascu O, et al. Intra-oesophageal pH profiles and pharmacokinetics of pantoprazole and esomeprazole: a crossover study in patients with gastro-oesophageal reflux disease. Eur J Gastroenterol Hepatol 2003;15:791–799. [DOI] [PubMed] [Google Scholar]
- 43.Janczewska I, Sagar M, Sjostedt S, et al. Comparison of the effect of lansoprazole and omeprazole on intragastric acidity and gastroesophageal reflux in patients with gastroesophageal reflux disease. Scand J Gastroenterol 1998;33:1239–1243. [DOI] [PubMed] [Google Scholar]
- 44.Moawad FJ, Betteridge JD, Boger JA, et al. Reflux episodes detected by impedance in patients on and off esomeprazole: a randomised double-blinded placebo-controlled crossover study. Aliment Pharmacol Ther 2013;37:1011–1018. [DOI] [PubMed] [Google Scholar]
- 45.Wilder-Smith C, Rohss K, Bokelund SS, et al. The effects of dose and timing of esomeprazole administration on 24-h, day-time and night-time acid inhibition in healthy volunteers. Aliment Pharmacol Ther 2010;32:1249–1256. [DOI] [PubMed] [Google Scholar]
- 46.Gralnek IM, Dulai GS, Fennerty MB, et al. Esomeprazole versus other proton pump inhibitors in erosive esophagitis: a meta-analysis of randomized clinical trials. Clin Gastroenterol Hepatol 2006;4:1452–1458. [DOI] [PubMed] [Google Scholar]
- 47.Scarpignato C, Gatta L, Zullo A, et al. Effective and safe proton pump inhibitor therapy in acid-related diseases - a position paper addressing benefits and potential harms of acid suppression. BMC Med 2016;14:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.