Abstract
Purpose
To report the long-term clinical outcomes for patients with neovascular age-related macular degeneration (nAMD) who received anti-vascular endothelial growth factor (anti-VEGF) therapy as part of a standardised treatment protocol in a real-world setting.
Patients and methods
This is a retrospective audit of all treatment-naïve patients with nAMD who commenced a pro re nata (PRN) treatment regimen of intravitreal Ranibizumab from January to December 2009 and completed 8 years of follow-up in one single-treatment centre. Electronic medical notes were reviewed to evaluate the outcome measures. Outcome measures included progression of visual acuity (VA), central retinal thickness (CRT) and treatment frequency.
Results
95 eyes from 86 patients had complete data for 8 years of follow-up. Baseline median CRT was 295µm [IQR 254–349] and improved to 209µm [IQR 182–254] in year 8 (p<0.001); baseline median VA was 61 ETDRS letters which increased to 70 letters post-loading however was reduced to 55 letters by year 8 (mean VA change from baseline was −9.1 letters); 47.4% had stable or improved vision, 10.5% gained ≥15 letters and 33.7% had lost ≥15 letters. The highest visual gain was achieved after the initial loading-phase, with a subsequent steady decline, 26.3% (compared to baseline 33.4%) achieved driving vision standard. Median injection frequency was 6 (range 3–10) in year 1 and 3 injections (range 0–10) in year 8. 51.6% of eyes required at least one injection each year and only 34.7% required no injections in year 8.
Conclusion
Our real-world nAMD treatment cohort using Ranibizumab PRN regimen achieved an encouraging almost 50% stable or improved VA at year 8 and total injections of 31.6 injections per patient over an 8-year period.
Keywords: age-related macular degeneration, Ranibizumab, real-world, 8 year
Plain Language Summary
Understanding long-term, real-world treatment outcomes in patients with neovascular age-related-macular degeneration is essential for patient counselling, service provision and as a “standard” for future audit. Our cohort provides a useful guidance in real-world expectation for visual prognosis and treatment frequencies over 8 years using regimen of Ranibizumab injections based on “treat-as-required for active disease only”. We found an encouraging 47.4% of eyes maintained improved or stable vision at year 8, and 26.3% achieved driving vision standard compared to baseline of 33.4%. The average number of total injections received per eye was 31.6 injections over an 8-year period.
Introduction
Age-related macular degeneration (AMD) is the most common cause of registered blindness affecting the elderly population.1 The pivotal ANCHOR trial found that treatment with monthly, intravitreal Ranibizumab (Lucentis, Genentech Inc, South San Francisco, California, USA) injections was superior to photodynamic therapy, the previous standard of care.2 Ranibizumab received The National Institute of Health and Clinical Excellence approval in the UK in 2008, making it the new standard of care.3 The introduction of this revolutionary treatment modality has modified the progression of nAMD and altered our understanding of outcomes.
While improved visual results were welcomed, the short-acting duration of anti-VEGF agents has demanded very frequent intravitreal injections and assessments in order to prevent vision loss.2,4,5 The burden of care is therefore high for patients, healthcare providers and funding authorities. As clinical trials rarely reflect the complexity of clinical practice, most healthcare providers translate clinical trials recommendation to their best practice by modifying the treatment regimen seen as the best acceptable adaptation. In the early days of Ranibizumab use in the UK, the pathway for delivering treatment was the initiation of three 1-monthly injections as a loading dose followed by pro re nata (PRN) injections as guided by disease activity.6 Here we present a large cohort of treatment-naïve nAMD patients who received intravitreal Ranibizumab therapy with a complete follow-up period of 8 years. To our knowledge, this is the first audit reporting on a large cohort with real-world visual outcomes in a single National Health Service (NHS) clinical practice in the UK.
Our aims are to provide an understanding of long-term effectiveness of one commonly used model of care (PRN treatment regimen) in a real-world NHS setting to report on the patient-relevant outcomes including visual changes from the time of disease diagnosis over the 8 years of treatment. Our audit would also provide useful information to help in counselling nAMD patients about long-term visual expectation and prognosis, as well as future service-provision planning and a real-life “standard” for similar future audits.
Methods
This is a retrospective clinical audit undertaken at one NHS tertiary referral eye centre (Birmingham and Midland Eye Centre, United Kingdom). Data analyses from electronic medical record were done on consecutive, treatment-naïve patients who had a confirmed diagnosis of active nAMD from January to December 2009 and commenced on anti-VEGF treatment with a full follow-up of eight years. Research ethical approval was not applicable to this observational study; however, it was registered as an audit with our local audit department (audit number 634).
All patients attended dedicated medical retina clinic or a macular fast track clinic and underwent slit-lamp bio-microscopy assessment by qualified sub-specialty clinicians at presentation. At this visit, recordings of visual acuity (VA), central retinal thickness (CRT) (using spectral domain Optical Coherence tomography (3D-OCT 2000, Topcon, Tokyo, Japan) were documented and diagnosis was confirmed with fundus fluorescein angiography FFA (Topcon TRC-50DX Retinal Camera, Topcon, Tokyo, Japan). All cases of macular nAMD were included; however, other causes of choroidal vascular membranes (CNVM) were excluded, such as myopic CNVM or peripapillary CNVM.
Primary outcome measures included VA, CRT and frequency of anti-VEGF injections. Our VA recordings in clinics were measured using Snellen charts, LogMAR charts or Early Treatment of Diabetic Retinopathy Study (ETDRS) chart with the patient’s habitual refractive correction when possible, supplemented with pin-hole correction for best-corrected vision. All VA values were converted to LogMAR figures for the purposes of statistical analysis using a published standardised conversion chart.7
Treatment Protocol
All patients newly diagnosed with active nAMD were treated in accordance with our local, standardised, protocol: a standard loading dose of three intravitreal injections of 0.5mg Ranibizumab at four-weekly intervals. All injections were performed by trained doctors and nurse practitioners using an aseptic technique under topical anaesthesia in a designated sterile procedure room. Re-treatment was decided by assessing clinicians based on the evidence of disease activity (presence of intra-retinal or sub-retinal fluid on OCT scans, macular haemorrhage or exudation). All patients were re-assessed at 4-weekly intervals for the first 6 months and extended to 8-weekly reviews if disease remained inactive. Treatment of complications such as RPE tears or decisions regarding treatment futility was entirely at the discretion of the treating clinician. It is our local policy to discharge patients who have had “stable” disease not requiring treatment for at least one year with a “patients-discharge-guidance leaflet” including Amsler grid monitoring.
Statistical Analysis
Wilcoxon signed-rank and Mann–Whitney U-tests were used to compare paired visual acuities and CRT values as appropriate. Normality was tested for using the Shapiro–Wilk Test. Parametric data are presented as mean ( standard deviation (SD)) and non-parametric averages presented as median [interquartile range (IQR)]. VA statistical analysis was performed based on the LogMAR visual acuity conversion. In tables depicting VA results, we expressed VA in ETDRS letter scores and/or Snellen scores for ease of a familiar comparison for clinical practitioners as these are the two most common VA analysis in real-world practice.
standard deviation (SD)) and non-parametric averages presented as median [interquartile range (IQR)]. VA statistical analysis was performed based on the LogMAR visual acuity conversion. In tables depicting VA results, we expressed VA in ETDRS letter scores and/or Snellen scores for ease of a familiar comparison for clinical practitioners as these are the two most common VA analysis in real-world practice.
All statistical analysis was performed using IBM SSPS Statistics software (Version 25. Armonk, NY: IBM Corp) and Microsoft Excel 2011. The level of statistical significance was set at p=0.05.
Results
Study Population
174 treatment-naïve eyes from 156 patients with nAMD who received Ranibizumab treatment protocol were identified. Eighty-six of these patients completed 8 years follow-up resulting in 95 eyes (55% of eyes) for final analysis. The median age of patients who completed 8 years follow-up was 72 [IQR: 67–76] years with twice as many females in the cohort.
Patients Not Completing 8 Years Follow-Up
Reasons for loss to follow-up included “intentional discharge” from service with disease stability (12%), failure to attend (10%, patient did not attend despite reminders), transfer of care to another hospital (3%) and death (18%). There were 5 patients’ notes with incomplete data, which were therefore excluded (3%). The overall median age was 81 years [IQR 77–85], higher than the age of the patients completing 8 years follow-up. The median VA for this group at baseline was 55 letters (0.60 LogMAR) and final recorded VA at “last attended visit” was 35 letters (1.00 LogMAR).
Visual Outcomes
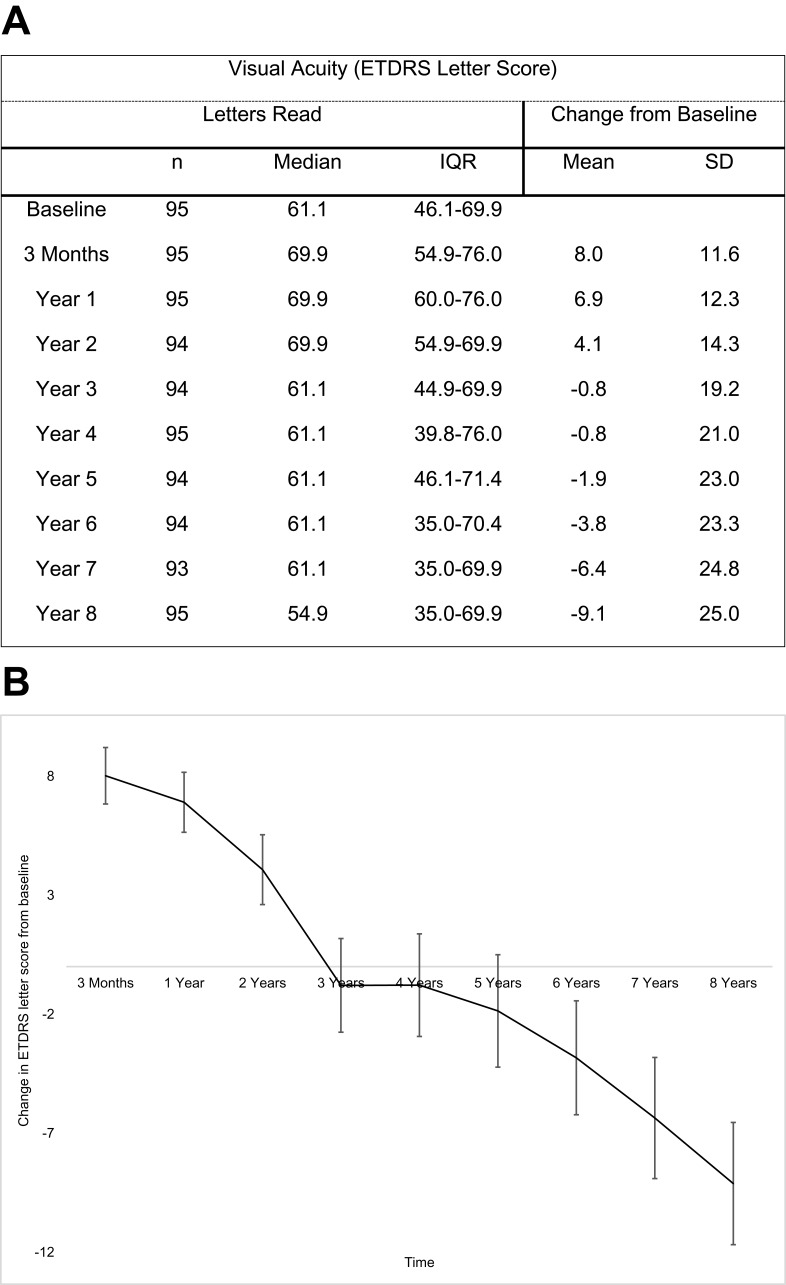
Baseline median VA was 61.1 ETDRS letters [IQR: 46.1–69.9] (Snellen VA 6/18). At year 8, median VA was 54.9 ETDRS letter [IQR 35.0–69.9] (Snellen 6/24). There was a mean loss of 9.1±25.0 letters. Figure 1A shows yearly visual acuity average measurements and changes in ETDRS scores against baseline over the study duration of 8 years. The highest visual gain was achieved after the initial loading-phase and continued up to year 2, with a subsequent steady decline (Figure 1B).
Figure 1.
Evolution of visual acuity in cohort over 8 years. (A) Yearly visual acuity and changes in ETDRS scores in comparison to baseline. (B) Mean change per year in ETRDS letter score in comparison to baseline, including standard error bars.
Abbreviations: n, eyes number; IQR, interquartile range; SD, standard deviation.
Overall 47.4% eyes maintained or improved vision at year 8, with 10.5% who gained ≥15 letters and 33.7% that lost ≥15 letters (Table 1A). Table 1B shows achievements of different visual levels against baseline at year 8, with 41% of patients presenting with 6/12 vision at baseline (eligible for driving in the UK) maintaining this vision at 8 years. Table 2 compares our visual outcomes with those from similar trials. Visual loss in this audit was attributed to common macular changes such as foveal atrophy, fovea scarring and retinal pigment epithelial (RPE) tear.
Table 1.
Visual Outcomes At Year-8
| A) Visual acuity in ETDRS letters change at Year 8 | ||||
|---|---|---|---|---|
| n | % | |||
| Gain ≥ 15 letters | 10 | 10.5 | ||
| Gain 5–14 letters | 20 | 21.1 | ||
| Gain 0–4 letters | 15 | 15.8 | ||
| Loss 1–4 letters | 4 | 4.2 | ||
| Loss 5–14 letters | 14 | 14.7 | ||
| Loss ≥ 15 letters | 32 | 33.7 | ||
| B) Range of visual acuity levels achievements at Year-8 compared to baseline | ||||
| Snellen Visual Acuity | Baseline VA | Year-8 VA | ||
| n | % | n | % | |
| VA 6/6–6/12 | 32 | 33.4 | 25 | 26.3 |
| VA 6/6–6/18 | 49 | 51.6 | 39 | 41.1 |
| VA 6/30–PL | 24 | 25.3 | 38 | 40 |
| VA 6/60 –PL | 11 | 11.6 | 29 | 30.5 |
Abbreviations: VA, visual acuity; n, eyes number; PL, perception of light.
Table 2.
Visual Acuity Comparisons Against Other Published Studies Over The Period Of Years 6 To 8
| Jacob et al10 (Belgium) | Our Results (NHS UK) | 7-up Trial8 (USA) | Gillies et al9 (Australia) | Our Results (NHS UK) | Our Results (NHS UK) | |
|---|---|---|---|---|---|---|
| Follow-up period (years) | 6 | 6 | 7 | 7 | 7 | 8 |
| Number of patients | 88 | 95 | 65 | 131 | 95 | 95 |
| Baseline average VA in ETDRS letters score | 57.4 (12.7) | 61.1 [46–70] | 52.6, 53.2, 54.3 | 55.1 (18.8) | 61.1 [46–70] | 61.1 [46–70] |
| VA gain ≥15 letters | 23% | 17% | 12% | 16% | 14% | 11% |
| VA gain ≥ 0 letters | 53% | 60% | 43% | – | 56% | 47% |
| VA loss ≥15 letters | 21% | 27% | 34% | 28% | 30% | 34% |
Notes: For parametric data, it is presented as mean (standard deviation) and for non-parametric data, it is presented as median [interquartile range].
Abbreviation: VA, visual acuity.
Treatment Exposure
The median number of injections per year is represented in Table 3. There was a decrease in the average number of injections from 6 during year 1 to 3 in year 8. The mean number of total injections per eye over the 8 years was 31.6 (range 3–60). However, looking more closely at individual treatment patterns, 49 eyes (51.6%) required injections every year, while 23 eyes (24.2%) had a break from injections for at least 1 year due to inactive disease before reactivation. A total of 33 eyes (34.7%) did not require treatment in year 8. Reasons for stopping injections included stability of disease (either stable and improved VA with no reactivation of choroidal neovascularisation) and non-response to treatment (VA stabilised to an unaltered level despite signs of active disease). Analysis of fovea atrophy progression was not possible from the available data.
Table 3.
Comparison In Yearly Anti-VEGF Injections Frequency Against Other Published Studies
Anatomical Outcomes
Baseline median CRT was 295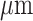 [IQR 254–349], with statistically significant improvement to 209µm [IQR 182–254] at year 8 (p<0.001).
[IQR 254–349], with statistically significant improvement to 209µm [IQR 182–254] at year 8 (p<0.001).
Discussion
In this study, we present the visual outcomes of a large group of treatment-naïve patients managed with Ranibizumab for nAMD in a real-world NHS setting over a follow-up period of 8 years. We also found a lower rate of attrition; 45% compared to 80–89% reported by other longitudinal studies.8,9
Our analysis of yearly visual changes depicted an improvement in average VA up to year 2, with subsequent steady decline (Figure 1B). The highest mean VA gain of 8 letters was noted after the initial loading-phase (3 months), comparable with other published studies (Table 1).2,5,9 Comparing our results to those of the largest published UK electronic medical-record (EMR) study on nAMD patients treated with Ranibizumab, despite sharing a similar baseline mean VA, their reported trend of VA change was less favourable throughout.6 In this study, the reported mean VA gain was only 2 and 1 letters in years 1 and 2, respectively, compared to our improvement of 8 and 7 letters. There was little difference with our mean injection frequency that would account for the difference in VA over the 3 years, recording 5, 4, 4 injections in each year, respectively, in the UK EMR/AMD study, compared with 6, 4, 4 injections in our study (Table 3).
Although the UK EMR/AMD report may serve as a useful benchmark by providing the largest real-world visual outcomes on treated nAMD patients, the complexity of the retrospective data collection from 14 UK AMD treatment centres undoubtedly posed many challenges, especially in interpreting results from incomplete data, a large diversity on re-treatment decisions and varied review plans. Our data suggest that implementation of a standardised local treatment and review pathway, with a robust auditing process, allows us to have the potential to achieve better results, providing valid and more relevant information for future local service provision planning.
There are limited published papers on the long-term VA outcomes of treated nAMD patients using anti-VEGF injections at non-fixed intervals and the average follow-up duration of those available was 6 to 7 years.8–10 Although all three studies are a reflection of real-world results, direct comparisons are less than ideal as they were conducted in different countries (Belgium, USA and Australia) with varied health service systems. Specifically, the vast majority of patients were treated in private settings with differing incentives for treatment, local government’s restriction over the number of treatments per year,10 variation in drug availability and choices, local preference to a different treatment posology and lastly patients’ choice had also played a significant role. On the other hand, the comparison of results from such varied health service settings would add value to the understanding of the intricacies of nAMD treatment.
Interestingly, despite the local variations, all studies shared our trend of initial VA gain in early years and VA declining after year 5.9,10 Our baseline average VA is similar to the Belgium teams 6-year follow-up study, but slightly higher than Australia’s and Seven-up papers (Table 2).8–10 All studies reported on treatment-naive patients except Belgium, which was inclusive of patients switched from other therapies such as photodynamic therapy.
Comparing the VA gain and stability results at year 6, we reported 60% stability as opposed to 53% in Belgium paper (Table 2).10 This may be related to the comparatively fewer injections per year in Belgium’s cohort, as we recorded a mean of 5 injections in year 5 whilst Belgium had only injected 3 (Table 3). However, overall our visual outcome results are similar at 6 years (Table 2).
Comparing the VA results at year 7, our findings and the Australian-based real-world study show a higher percentage of patients with visual gain in comparison to the Seven-up study.8,9 On the other hand, the Seven-up study reported a mean loss of 8.2 letters at year 7, similar to our loss of 6.4 letters, which is still much higher than the 2.6 letters loss in the Australian report. However, as discussed further below, the difference could be attributed to attrition bias.
There may be several factors influencing such differences, one of which may again be the frequency of injections. Patients included in the Seven-up study were trial patients from the MARINA, ANCHOR AND HORIZON trials, who received monthly injections for the first 24 months and their subsequent reported injection-rate was only 6.8 over 3.4 years, averaging 2 injections per year compared to our average of 4 per year. In contrast, the Australian report, which was based on predominantly a treat-and-extend posology and perhaps also with greater incentive to treat in the Australian private healthcare system, their mean frequency of injections was persistently 5 to 6 in each year including the final year 7 when we see our reduction to 3 injections in year 7 and 8 (Table 3).
The Australian cohort started with 1212 eyes, with only 11% followed up at year 7. This inevitably introduces bias, with longer term follow-up linked to better vision as patients with poorer vision tend to drop out of their study either by the decision of treating clinicians or patient or both.9 Our report shared a similar pattern of “unintentional selection bias” with a much lower baseline median VA for “excluded group” compared to patients completed 8-year follow-up: 55 letters vs 61 letters at baseline; and 35 letters vs 54 letters at final recorded VA, respectively.
It is worth mentioning at this point of Peden’s 7-year real-world study, which adopted a continuous fixed-interval dosing (4–8 weekly) of anti-VEGF for nAMD patients whereby patients received an average of 10.5 injections per year.5 They have reported a staggering mean VA gain of 12.1 letters even in 7 years. Such high demand for service provision is shown plausible in the United States where private health service predominates, but it is faced as a difficult hurdle by many other countries with different healthcare provisions. For this very reason, we are eager to know and understand the efficacy of other treatment pathways that may offer to achieve reasonable goals for both patients and healthcare providers.
Extending to our year 8 analyses, we see an average loss of 9.1 letters, alongside a reduction in injection frequency to 3 injections (range 0−10) in year 8. However, nearly half (47.4%) of the cohort still achieved stable or improved VA in comparison to baseline and 10.5% continued to maintain a ≥15 letter gain (Table 1A).
Eligibility to continue driving remains a common concern for patients when first diagnosed with nAMD. In our cohort at presentation, 33.4% reached the UK driving standard vision of 6/12; this was reduced overall to 26.3% at year 8, a reduction of 7.1% (Table 1B). Specifically, out of those 33.4% eligible drivers at presentation, 41% of these patients maintained their licence at year 8. This result could serve as a useful guide in part of the patients’ counselling regarding long-term expectation of independence.
Our study shows that even in year 8, only 34.7% of those patients with ongoing follow-up received 0 injections, and 51.6% required at least one injection each year. This reflects the ongoing need for re-treatment, likely in the subsequent years and beyond. Interestingly, 24.2% had a least one-year break from treatment before disease reactivation and prompted re-treatment. Given the expectation of a natural disease progression, most nAMD would eventually resolve to macular scarring to some degree, continuation of visual reduction might, therefore, be inevitable despite ongoing treatment in the longer study duration.
The strengths of this study are the long follow-up duration and the comparatively lower loss to follow-up rate (45% in comparison to 89% in the Gillies paper and 91% in the SEVEN-UP study), which reduces the risk of attrition bias in comparison to the current literature. We also included consecutive patients in a real-world environment, therefore minimising selection bias. However, it is still important to consider the impact of attrition, or survival bias. With such a long follow-up period in an elderly, multi-morbid population, it is inevitable that a proportion of patients will pass away. We found 18% of patients from our original cohort had died by 8 years. Unsurprisingly, sub-analysis of this group shows their average age was higher (84 vs 72) and their baseline VA was lower (46 letters vs 61 letters), as was their final recorded visual acuity reading (35 letters vs 54 letters), in comparison to the group that completed 8-year follow-up. Due to increased age and morbidity, this group of patients is more likely to have presented to secondary care with more advanced disease (hence lower baseline vision) and may not have been able to comply with the frequent injection and clinic schedule in comparison to the 8-year follow-up group.
Ultimately, attrition due to death and discharge is an inevitable part of a real-world study. However, our excellent 55% follow-up rate is significantly higher than other published literature; therefore, we minimise this impact as much as possible.
We feel this study allows us to better consent and guides our patient’s expectations of visual improvement over the first 8 years of treatment. We acknowledge this study is limited by its retrospective nature and the use of variable visual acuity scores at each clinic assessment.
Conclusion
Our real-world cohort reported the long-term effectiveness of one specific treatment protocol of Ranibizumab PRN dosing with the highest efficacy on visual outcomes from 3 months up to year 5, and with nearly 50% of patients maintaining their presenting vision at year 8. The report also reflects the potential achievement of desirable long-term VA outcomes in a single-treatment centre if there is a robust standardised local treatment protocol and review plan in place. Our cohort results could also serve as useful, relevant information in counselling nAMD patients in terms of expectation in visual prognosis and driving eligibility in 8 years of anti-VEGF therapy and for practitioners to plan for future local healthcare provision.
Disclosure
BM is on the advisory board for Alimera, Novartis, Bayer and Alcon and reports grants and personal fees from Alimera, Novartis and Bayer outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA. 2004;291(15):1900–1901. doi: 10.1001/jama.291.15.1900 [DOI] [PubMed] [Google Scholar]
- 2.Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009;116(1):57–65. e55. doi: 10.1016/j.ophtha.2008.10.018 [DOI] [PubMed] [Google Scholar]
- 3.NICE. Ranibizumab and pegaptanib for the treatment of age-related macular degeneration. 2008. Accessed October22, 2018.
- 4.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481 [DOI] [PubMed] [Google Scholar]
- 5.Peden MC, Suner IJ, Hammer ME, Grizzard WS. Long-term outcomes in eyes receiving fixed-interval dosing of anti-vascular endothelial growth factor agents for wet age-related macular degeneration. Ophthalmology. 2015;122(4):803–808. doi: 10.1016/j.ophtha.2014.11.018 [DOI] [PubMed] [Google Scholar]
- 6.Writing Committee for the UKA-RMDEMRUG. The neovascular age-related macular degeneration database: multicenter study of 92 976 ranibizumab injections: report 1: visual acuity. Ophthalmology. 2014;121(5):1092–1101. doi: 10.1016/j.ophtha.2013.11.031 [DOI] [PubMed] [Google Scholar]
- 7.Gregori NZ, Feuer W, Rosenfeld PJ. Novel method for analyzing snellen visual acuity measurements. Retina. 2010;30(7):1046–1050. doi: 10.1097/IAE.0b013e3181d87e04 [DOI] [PubMed] [Google Scholar]
- 8.Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, Group S-US. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013;120(11):2292–2299. [DOI] [PubMed] [Google Scholar]
- 9.Gillies MC, Campain A, Barthelmes D, et al. Long-term outcomes of treatment of neovascular age-related macular degeneration: data from an observational study. Ophthalmology. 2015;122(9):1837–1845. doi: 10.1016/j.ophtha.2015.05.010 [DOI] [PubMed] [Google Scholar]
- 10.Jacob J, Brie H, Leys A, et al. Six-year outcomes in neovascular age-related macular degeneration with ranibizumab. Int J Ophthalmol. 2017;10(1):81–90. doi: 10.18240/ijo.2017.01.14 [DOI] [PMC free article] [PubMed] [Google Scholar]