Abstract
Purpose
To analyze the effectiveness and stability of the refractive, topographic and visual outcomes of the standard cross-linking (SCXL) in keratoconus (KC) management.
Patients and methods
This study was designed as a retrospective non-comparative study that included 28 KC patients (n=49 eyes) who performed SCXL as a single procedure to treat KC and completed five-year follow-up period. The topographic, refractive and visual data were recorded preoperatively and at 12, 24, 36 and 60 months postoperatively.
Results
Forty eyes (81.6%) showed achieved postoperative spherical equivalent (SE) refraction better than the attempted refraction. Ten eyes (20.4%) improved by <1 D, 23 eyes (46.9%) improved from 1 D to <2 D and 7 eyes (14.3%) improved by ≥2 D. Both uncorrected distant visual acuity (UDVA) and corrected distant visual acuity (CDVA) showed statistically significant improvement from preoperative 1.34±0.29 (mean±SD) and 0.74±0.23 LogMAR to postoperative 0.99±0.32 and 0.50±0.22 LogMAR (P<0.0001) respectively. Both Kmax and SE refraction showed statistically significant and stable improvement from preoperative 51.95±1.90 and −7.90±3.14 D to postoperative 50.19±1.96 and −6.35±2.49 D (P<0.0001) respectively. Two eyes (4%) showed KC progression at the end of 5th follow-up year.
Conclusion
SCXL had good effectiveness and stability that halted KC progression over 5-year follow-up period. It had also unexpected improvement in the KC refractive components mainly the spherical and SE components.
Keywords: keratoconus, standard cross-linking, eye rubbing, ectasia, KC progression
Introduction
Corneal collagen structural weakness and biomechanical instability are the main causes that lead to progressive stromal thinning and cone formation in keratoconus with subsequent progressive visual impairment.1 The definite KC etiology and pathogenesis have not been strictly determined or fully understood. Many genetic, hormonal and mechanical factors have been proposed as possible causes of KC development and progression.2 A significant correlation between KC progression with hormonal changes, as in pregnancy, lactation and thyroid eye disease,3,4 was elucidated in the literature.5 Vernal keratoconjunctivitis (VKC), chronic eye rubbing, dry eye disease and limbal stem cell inefficacy have been reported as aggressive factors that both promote KC progression and treatment failure especially in pediatric patients.6,7
Standard crosslinking (SCXL) was first introduced in 2003 by Wollensak and associates, also known as Dresden Protocol, and drastically changed the future of KC management.5 SCXL mainly halts KC progression, helps corneal flattening and sometimes associated with unexpected improvement in the refractive status of the ectatic eye.8 In comparison with other CXL protocols, SCXL has greater improvements in KC indices.9 Despite the superiority of SCXL in comparison with transepithelial CXL in halting KC progression,10,11 the combination procedures; sometimes called cross-linking plus (CXL Plus);12,13 have become popular procedures that include combination of CXL with a refractive procedure such as wavefront-guided photorefractive keratectomy (WFG PRK),14 refractive lens surgery (RLS)15 and intracorneal ring segments (ICRS) to correct the refractive status of the ectatic eyes.12,16
Despite several studies have reported postoperative visual and topographic improvements following SCXL, most have addressed relatively short-term results while long-term outcomes have not been frequently outlined. In this study, we reviewed the long-term visual and topographic outcomes of SCXL in patients with progressive KC. We further evaluated the effectiveness, stability, durability and associated complications of SCXL 5 years after the first intervention.
Patients and Methods
The study was designed as a retrospective non-comparative analysis of the patients with previously documented KC progression who underwent SCXL in 2013 and completed 5-year follow-up at the Sohag University Hospital, Sohag, Egypt. Ethics Committee Faculty of Medicine, Sohag University, approved the study and all the tenets of the Declaration of Helsinki were abided. All data were obtained from the patients’ medical files including. All patients and/or their parents signed written consents before surgery.
The inclusion criteria included documented preoperative KC progression, grades 1, 2 and 3 (Amsler-Krumeich classification17) regardless of age, the thinnest corneal location >400 µm, SCXL was the only surgical intervention performed and the patients completed 5-year follow-up. The follow-up corneal topographies were performed using Pentacam HR (Pentacam® HR, OCULUS Inc., Wetzlar, Germany).
This study included only patients who had SCXL, in the year 2013, as a primary sole procedure till the last follow visit in the year 2018. Among 273 eyes performed SCXL in 2013, only 49 eyes fulfilled the inclusion criteria and included in this study. The remaining 224 eyes showed good post-SCXL stability till their last follow-up visit; however, they were excluded from this study because of 2 main reasons. The first reason was that most of these 224 eyes underwent post-CXL additional surgical interference to improve their vision based on their own requirements. These procedures included ICRS, particularly Kera ring, implantation or PRK. Addition of such refractive procedures prevented the inclusion of these eyes as our study evaluated only SCXL as a single and sole treatment procedure but not the combined procedures as CXL-Plus. The second reason was that few cases did not continue the targeted 5-year follow-up period for multiple causes mainly working, travelling, moving to other cities or they were satisfied with their surgery outcomes; so they stopped their routine follow-up visits. These drop-out cases were also excluded from our study. That is why the full data of these 224 eyes were excluded from being outside the scope of our study.
The preoperative and postoperative data (at postoperative 6, 12, 24, 36 and 60 follow-up months) of these 49 eyes were obtained from the patients’ medical files for reviewing analysis and comparison. Data collection included uncorrected distance visual acuity (UDVA), corrected visual distance acuity (CDVA), subjective refraction, pachymetry, keratometry (K readings), coma aberrations and Q value.
Surgical Procedure
All eyes were subjected to standard CXL 3.0mW/cm2 for 30 mins under topical anesthesia (Benox, Benoxinate hydrochloride 0.4% Sterile Ophthalmic Solution, Pharmaceutical Industries Company, E.I.P.I.CO., Egypt). Marking the cornea was performed by an 8-mm zone marker followed by epithelial removal with a blunt-tipped spatula. Iso-osmolar Riboflavin 0.1% solution with dextran (RICROLIN®, Sooft Italia S.p.A., Montegiorgio FM, Italy) was applied on a 3-min basis for complete 30 mins till full stromal saturation. Continues 30-min UVA corneal irradiation with a total dose of 5.4J/cm2 (1.50 mW power and 2.98 mW/cm2 intensity) was accomplished using the OptoXLink CXL system (Opto Global Pty Ltd, Adelaide, Australia). At the end of the procedure, bandage soft contact lens (CooperVision, The Cooper Companies, Inc. California, USA) was applied onto the cornea.
Postoperative Medication and Follow-Up
All patients received postoperative topical eye drops including prednisolone acetate 1% eye drops (Pred Forte, Allergan, Inc, Jersey city, USA), Gatifloxacin 0.3% (Zymar, Allergan, Inc, Jersey city, USA) and sodium hyaluronate 0.15% eye drops (Hyabak, THEA laboratories, Clermont-Ferrand, France) on an hourly basis in the first postoperative day. The installation frequency was five times daily in the first week and tapered gradually. Bandage contact lens was removed after completion of epithelial healing (2–5 postoperative days). All patients were followed-up on a weekly basis in the first month then every 3 months in the first year then annually.
Postoperative corneal haze score was graded from 0 to +5 using slit-lamp examination (0= clear cornea; +1= mild haze, +2= moderate haze, +3= severe haze, +4= iris details could not be visualized and +5= anterior chamber details could not be visualized).18
Statistical Analysis
Analysis of data was performed with STATA version 14.2 (Stata Statistical Software: Release 14.2 College Station, TX: StataCorp LP.). Representation of quantitative data included mean, standard deviation, median and range. Repeated-measures ANOVA (RMANOVA) test was used for comparison between preoperative and postoperative follow-up data at 12, 24, 36 and 60 months. Mauchly’s Test of Sphericity was used to examine Sphericity. The differences at each time point were examined using the Bonferroni post-hoc test. The different time points and surgeons were used as within-subject factors. Statistical significance was considered when P value <0.05.
Results
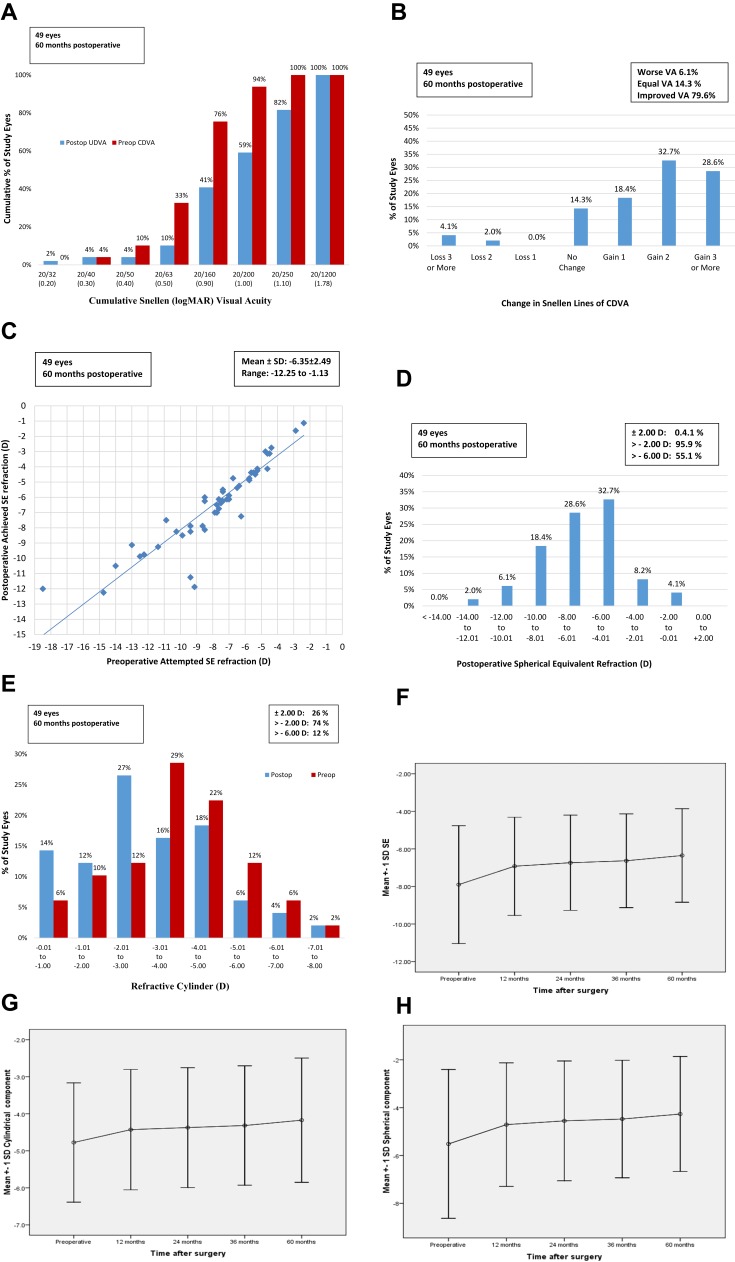
This study included 49 eyes of 28 patients with a mean age of 18.46±4.41 (range 11–26 years old). Seventeen patients were males (60.71%) and 11 patients were females (39.29%). Table 1 shows the summary of SCXL preoperative and postoperative visual, topographic and refractive data analysis using RMANOVA test. Figure 1 shows the standard graphs of the visual and refractive outcomes.
Table 1.
Summary of S-CXL Preoperative and Postoperative Visual, Topographic and Refractive Data Analysis
| Parameters | Preoperative Mean ± SD Median [Range] |
Postoperative 12th Month Mean ± SD Median [Range] |
Postoperative 24th Month Mean ± SD Median [Range] |
Postoperative 36th Month Mean ± SD Median [Range] |
Postoperative 60th Month Mean ± SD Median [Range] |
Difference (Post-Pre) Mean ± SD [95% CI] |
P value Within Subject (Time) | P value Between Subject (Surgeon) |
|---|---|---|---|---|---|---|---|---|
| Visual Outcomes: | ||||||||
| Mean UDVA (logMAR) | 1.34±0.29 1.3 [0.78:1.78] | 1.10±0.26 1.12 [0.30:1.78] | 1.06±0.27 1.12 [0.30:1.78] | 1.05±0.28 1.12 [0.22:1.78] | 0.99±0.32 1.00 [0.10:1.78] | −0.34±0.32 [0.90:1.08] | <0.0001 | 0.77 |
| P1<0.0001, P2<0.0001, P3<0.0001, P4<0.0001, P5<0.0001 P6<0.0001, P7<0.0001 | ||||||||
| Mean CDVA (logMAR) | 0.74±0.23 0.78 [0.30:1.12] | 0.55±0.20 0.60 [0.18:1.22] | 0.51±0.19 0.52 [0.18:1.22] | 0.51±0.20 0.52 [0.18:1.22] | 0.50±0.22 0.52 [0.18:1.22] | −0.24±0.20 [−0.30:-0.19] | <0.0001 | 0.56 |
| P1<0.0001, P2<0.0001, P3<0.0001, P4<0.0001, P5<0.0001, P6<0.0001, P7<0.0001 | ||||||||
| Topographic Outcomes: | ||||||||
| Mean k1 reading | 46.66±1.19 46.75 [42.75:49.5] | 45.52±1.50 45.75 [40.25:49.5] | 45.49±1.55 45.5 [40.25:49.5] | 45.53±1.58 45.75 [40.25:49.5] | 45.57±1.65 45.75 [40.5:49.25] | −1.09±1.31 [−1.46:-0.71] | <0.0001 | 0.76 |
| P1<0.0001, P2<0.0001, P3<0.0001, P4<0.0001, P5 | ||||||||
| Mean k2 reading | 50.87±1.73 51 [46:55.5] | 49.39±1.73 49 [45.25:52.75] | 49.25±1.81 49 [45:52.75] | 49.20±1.83 49 [45:53] | 49.11±1.86 49 [45:5.] | −1.76±1.56 [−2.20:-1.30] | <0.0001 | 0.80 |
| P1<0.0001, P2<0.0001, P3<0.0001, P4<0.0001, P5<0.0001, P6<0.0001, P7<0.0001, P9=0.005, P10=0.009 | ||||||||
| Mean K max | 51.95±1.90 52.25 [46.5:58.82] | 51.15±2.03 51.5 [45.8:58.7] | 50.64±1.86 51 [45.5:57.5] | 50.27±1.92 50.11 [45.75:57.7] | 50.19±1.96 50 [45.8:59.93] | −1.77±1.77 [−2.26:-1.27] | <0.0001 | 0.85 |
| P1<0.0001, P2<0.0001, P3<0.0001, P4<0.0001, P5<0.0001, P6=0.001 1, P7=0.001 | ||||||||
| Mean K reading average (D) | 48.77±1.27 48.88 [44.63:51.5] | 47.46±1.47 47.5 [43:50.63] | 47.37±1.52 47.5 [42.88:50.5] | 47.36±1.55 47.5 [42.88:50.63] | 47.34±1.60 47.5 [43:50.75] | −1.42±1.39 [−1.82:-1.02] | <0.0001 | 0.21 |
| P1<0.0001, P2<0.0001, P3<0.0001, P4<0.0001, P5<0.0001 | ||||||||
| Mean corneal thickness at thinnest location, (µm) | 437.73±18.65 436 [400:485] | 430.18±20.20 428 [389:480] | 427.29±21.13 428 [365:478] | 424.31±21.99 425 [338:475] | 420.67±22.73 421 [319:470] | −17.06±11.90 [−20.75:-10.07] | <0.0001 | 0.71 |
| P1<0.0001, P2<0.0001, P3<0.0001, P4<0.0001, P5<0.0001, P6<0.0001, P7<0.0001, P8<0.0001, P9=0.03 | ||||||||
| Coma aberrations | 3.08±1.92 2.52 [0.24:10.56] | 2.79±1.66 2.02 [0.24:8.25] | 2.71±1.63 1.99 [0.13:7.90] | 2.68±1.52 1.95 [0.14:7.35] | 2.69±1.50 1.79 [0.13:7.24] | −0.39±0.48 [−0.94:-0.61] | <0.0001 | 0.61 |
| P1<0.0001, P2<0.0001, P3<0.0001, P4<0.0001, P5<0.0001, P6<0.0001, P7<0.0001, P8=0.01, P9<0.0001 | ||||||||
| Q value | −0.55±0.66 0.62 [−1.55:1.57] | −0.39±0.60 0.27 [−1.02:1.25] | −0.27±0.64 –0.10 [−1.23:1.35] | −0.24±0.71 –0.21 [−1.33:1.45] | −0.32±0.71 –0.35 [−1.5:1.50] | 0.23±0.19 [−0.61:-0.11] | <0.0001 | 0.52 |
| P1=0.001, P2<0.0001, P3<0.0001, P4<0.0001, P5<0.0001, P6<0.0001, P7<0.0001, P8=0.02, P9<0.0001, P10=0.04 | ||||||||
| Refractive Outcomes: | ||||||||
| Mean spherical component | −5.52±3.11 –4.75 [−15.5:-1.25] | −4.71±2.58 –4.25 [−10.25:-0.5] | −4.55±2.50 –4.25 [−10:-0.5] | −4.47±2.46 –4.25 [−9.75:-0.5] | −4.27±2.40 –4 [−9.5:-0.5] | 1.25±1.33 [0.87:1.63] | <0.0001 | 0.35 |
| P1<0.0001, P2<0.0001, P3<0.0001, P4<0.0001, P5=0.04, P6=0.001, P7<0.0001, P8=0.001, P9<0.0001, P10<0.0001 | ||||||||
| Mean cylindrical component | −4.78±1.61 –4.75 [−8.25:-1.5] | −4.43±1.63 –4.25 [−8:-1.25] | −4.37±1.62 –4.25 [−8:-1.25] | −4.32±1.61 –4.25 [−7.75:-1.25] | −4.17±1.67 –4 [−8.25:-1.25] | 0.60±0.57 [0.44:0.77] | <0.0001 | 0.33 |
| P1<0.0001, P2<0.0001, P3<0.0001, P4<0.0001, P5=0.06, P6<0.0001, P7<0.0001, P8=0.02, P9<0.0001, P10=0.002 | ||||||||
| Mean SE | −7.90±3.14 –7.38 [−18.5:-2.38] | −6.92±2.61 –6.63 [−13:-1.38] | −6.74±2.54 –6.5 [−12.63:-1.38] | −6.63±2.49 –6.5 [−12.63:-1.25] | −6.35±2.49 –6.13 [−12.25:-1.13] | 1.55±1.47 [1.13:1.97] | <0.0001 | 0.42 |
| P1<0.0001, P2<0.0001, P3<0.0001, P4<0.0001, P5=0.02, P6<0.0001, P7<0.0001, P8<0.0001, P9<0.0001, P10<0.0001 | ||||||||
Notes: P1 compared pre with 12 ms, P2 compared pre with 24 ms, P3 compared pre with 36 ms, P4 compared pre with 60 ms, P5 compared 12 ms with 24 ms, P6 compared 12ms with 36 ms, P7 compared 12ms with 60 ms, P8 compared 24ms with 36 ms, P9 compared 24ms with 60 ms, P10 compared 36ms with 60 ms.
Figure 1.
The standard graphs of the visual and refractive outcomes: (A) the cumulative logMAR visual acuity; (B) the change in CDVA; (C) the attempted versus achieved postoperative SE refraction in a scatterplot; (D) the refractive accuracy of SE; (E) the refractive cylinder; (F) the refractive stability of SE; (G) the refractive stability of cylindrical component and (H) the refractive stability of spherical component.
Visual Outcomes (Figure 1A and B)
Both UDVA and CDVA showed statistically significant improvement at postoperative 12 follow-up month compared with the preoperative and postoperative 24, 36 and 60 follow-up months (all P <0.0001). On the other hand, both UDVA and CDVA showed statistically insignificant improvement after the postoperative 24 follow-up month (all P >0.05). However, there was a good stability of the achieved postoperative improvement from postoperative 24 to 60 follow-up months. Moreover, 39 eyes (79.6% of study eyes) showed improvement in postoperative CDVA one or more logMAR lines, 7 eyes (14.3%) showed the same preoperative CDVA while 3 eyes (6.1%) lost 2 or more logMAR lines and finally achieved postoperative CDVA less than the preoperative values. Figure 1A shows cumulative logMAR visual acuity and Figure 1B shows the change in the logMAR of CDVA.
UDVA showed good improvement from preoperative 1.34±0.29 (mean±SD) to 1.10±0.26, 1.06±0.27, 1.05±0.28 and 0.99±0.32 at postoperative 12, 24, 36 and 60 follow-up months. CDVA showed good improvement from preoperative 0.74±0.23 (mean±SD) to 0.55±0.20, 0.51±0.19, 0.51±0.20 and 0.50±0.22 at postoperative 12, 24, 36 and 60 follow-up months.
Refractive Outcomes (Figures 1C–H and 2B)
Figure 2.
The relationship graphs of topographic and refractive outcomes: (A) K readings relationships; (B) spherical, cylindrical and SE components relationships.
Our substantial purpose was to stabilize the already present preoperative refractive status of all eyes by SCXL and to prevent further KC progression. Surprisingly, 40 eyes (81.6%) showed achieved better postoperative SE refraction than the attempted refraction (Figure 1C). Ten eyes (20.4%) improved by <1 D, 23 eyes (46.9%) improved from 1 D to <2 D, 7eyes (14.3%) improved by ≥2 D. However, 6 eyes (12.3%) had the same preoperative SE at the postoperative 60-month follow-up while 3 eyes (6.1%) had lower achieved SE refraction due to postoperative complications.
SE refraction showed good improvement from preoperative −7.90±3.14 D (mean±SD) to −6.92±2.61, −6.74±2.54, −6.63±2.49 and −6.35±2.49 D at postoperative 12, 24, 36 and 60 follow-up months (P < 0.001). The preoperative values of the mean spherical component (Figure 1H), mean cylindrical component (Figure 1E and G) and SE showed significant improvement at postoperative 12, 24, 36 and 60 months (all P < 0.001) (Table 1). As well as, there was constantly improving in the three components throughout all follow-up periods. Figure 1D and F shows the postoperative refractive accuracy and stability of SE along the 60 months follow-up period. Figure 1E shows the comparison between the preoperative and postoperative 60-month refractive cylinder. Figure 2B shows the relationship between the pathways of the 3 refractive components.
Topographic Outcomes (Figure 2A)
There was a statistically significant flattening in the baseline K max, K1 and K2 readings. The mean preoperative mean K max reduced from preoperative 51.95±1.90 D (mean±SD) to 51.15±2.03, 50.64±1.86, 50.27±1.92 and 50.19±1.96 D at postoperative 12, 24, 36 and 60 follow-up months (P < 0.001). Likewise, the mean K max, K1, K2, and average K readings improved constantly at each time point compared to the baseline readings (Table 1).
The corneal thickness at the thinnest location (CT) showed progressive significant thinning at each time point compared to the preoperative values (P < 0.001). It revealed thinning by 17.06µm at the postoperative 60 follow-up month. On the other hand, there was an insignificant change in pachymetry between postoperative 24 to 60 months (Table 1).
Regarding the corneal asphericity (Q value), the post-CXL corneas became less prolate through overall time points of follow-up. Q value was reduced by 0.23 at the postoperative 60 follow-up month (P < 0.001) and achieved better aspheric corneal values (Table 1). At the same time, there was a statistically significant improvement in total coma aberration coefficient, its mean was changed from 3.08±1.92 preoperatively to 2.69±1.50 at the postoperative 60 follow-up month (P < 0.001).
Complications
Pain and photophobia were reported in all patients. All cases improved once epithelial healing was complete. Delayed Epithelial Healing occurred in 5 eyes (10.2%). All cases were treated with removal of contact lenses, cessation of topical steroid and adding preservative-free eye lubrications as Hyabak and Refresh Plus (carboxy methylcellulose sodium solution 0.5%, Allergan, Inc, Jersey city, USA). Four eyes (8.2%) improved with complete re-epithelialization within the first two postoperative weeks. Only one eye (2%) progressed to persistent epithelial defect (PED).
Corneal haze was reported in 37eyes (75.5%). Grading of haze was +1 in 9 eyes (18.4%), +2 in 19 eyes (38.8%), +3 in 8 eyes (16.3%) and +4 in one eye (2%). Haze was treated with topical Pred Forte, Hyabak, Refresh Plus and Thilotears gel (Alcon Laboratories, Inc, USA). All haze resolved in all eyes within the first 6 postoperative months. All 5 eyes of delayed epithelial healing had also haze that improved in 4 eyes once re-epithelization was complete but unfortunately the 5th eye with +4 corneal haze had progressed to PED that ended in permanent corneal scarring.
KC Progression was documented at the 60 months in 2 eyes (4.1%) of a pediatric patient with a history of VKC. Both eyes were stable and showed a noticeable improvement in K max and SE refraction throughout the first 36 months. KC progression in both eyes was documented (Kmax˃ 1D) at the 60 months follow-up visit. The patient underwent SCXL retreatment after the end of the study.
Discussion
Since Wollensak et al8 published the clinical results of the Dresden protocol in 2003, various clinical trials have been conducted around the safety and efficacy of SCXL in treating and halting KC progression.19–21 Our long-term 5-year outcomes revealed the effectiveness and good stability of SCXL in the treatment of progressive KC.
Currently, we confirmed a stationary improvement of the mean visual acuity over 5-year follow-up period. Both UDVA and CDVA showed statistically significant improvement from preoperative 1.34±0.29 and 0.74±0.23 LogMAR to postoperative 0.99±0.32 and 0.50±0.22 LogMAR (P<0.0001) respectively. In addition, Kmax showed statistically significant and stable improvement from preoperative 51.95±1.90 D to postoperative 50.19±1.96 D (P<0.0001).
In agreement with our findings, many previously published studies achieved visual improvement after CXL.35 Raiskup-Wolf et al22 established a statistically significant improvement in CDVA, K readings, and astigmatic refraction over 2 years. Comparable results were reported by Caporossi et al20 and Hashemi et al,21 they found an improvement in BCVA up to 4-year post-CXL. On the other hand, others found different results with almost no change in CDVA after 1 year of SCXL23 or minimal improvement by one line (0.1 logMAR).24
Visual improvement after SCXL could be attributed to improvement in the corneal surface regularity, symmetry between the corneal hemispheres and better stability of the precorneal tear film.25 Furthermore, visual improvement could be due to the neural compensatory adaptation as a long-term outcome in ectatic eyes in which the visual system with time compensates in a trial to abolish the adverse visual effect of higher-order aberrations, thus improving the visual performance in ectatic eyes.26 However, CDVA is believed as a non-sensitive index of KC progression or improvement.27 Moreover, the five-year outcomes showed a significant improvement in the mean spherical, cylindrical and SE components. These findings were similar to other published results25 and against others.21
Furthermore, Iqbal et al28 compared SCXL versus CXL Plus (combined ACXL and photorefractive keratectomy). They finally concluded that SXCL had close outcomes to CXL Plus at 24-month follow-up. In line with their results, our study had similar findings regarding SCXL late postoperative improvements in myopic and SE components with almost no significant differences in the astigmatic component. However, in another interesting study, Hafez29 tested a procedure to induce refractive improvement in the cylinder component by performing meridional CXL on the steepest meridians (RMCXL). He concluded that if we focused SCXL only on the steep meridians without cross-linking other meridians, we could have up to 1 D postoperative astigmatic correction. Our study showed that SCXL for all meridians symmetrically did improve the astigmatic correction.
We recorded statistically significant improvement in all k readings which proved the long-term effectiveness and good stability of the SCXL. K max improved by 0.8, 1.31, 1.68 and 1.76 D at 1, 2, 3 and 5 years, respectively. Some studies have reported fewer values in improvement K max than our findings. Hashemi et al21 recorded a mean K max improvement by 0.90 and 0.24 D at 4 and 5 years after SCXL. In addition, Caporossi et al30 reported a 0.57 D improved in K max over 4 years. On the other hand, other studies have described more reduction in K max than we had. Kim et al31 observed improvement in K max by 2.6 and 2.5 D at 3 and 5 years, respectively. Correspondingly, Grewal et al19 notified K max improvement by 2.57 D at 3 years. These remarkable differences in results between different studies could be attributed to various study designs, diverse sample size and different baseline K max.
Regarding the corneal thickness changes at the thinnest location, our outcomes are close to other published results.11,19,31–33 We recorded a reduction in pachymetry by 17.06 µm at the end of 5 years with an insignificant difference from the last 3 follow-up years values. The mechanism of the corneal thinning following SCXL is not yet fully explained. The mechanism of postoperative thinning might be multifactorial including anatomical and structural changes.32 Other factors may include changes in the corneal hydration, remodeling of the stromal collagen bundles and even as keratocyte apoptosis.33
The mean coma aberrations coefficient showed statistically significant reduction along the 5 years follow-up period. This may signify that the corneal surface becomes more regular following SCXL. Many authors documented similar findings.11,20 Furthermore, our results showed statistically significant improvement in the corneal asphericity (Q value) which is a good quantified indicator of asphericity of the corneal surface and could be considered as a clue of the optical and visual improvement.
One of our goals in this study was to outline the long-term drawbacks of CXL. Despite the high success rate of CXL as a durable and efficient visual-preserving and improving procedure, we documented cases of progression. Our results showed that 40 eyes (81.6%) improved and maintained this improvement with good stability following CXL. However, 2 eyes (4%1) showed KC progression and required further surgical interventions at the end of 5 years. Both cases had a history of VKC and eye rubbing. In line with our results, Shetty et al7 reported 2 pediatric patients with VKC and eye rubbing presented with keratoconus. They also reported high levels of serum immunoglobulin E (IgE) in both children.
Many studies reported KC progression following initial SCXL treatment.34,35 The failure rates ranged from 0%12 to 16.5%.36 While the recorded postoperative period for documentation of KC progression following SCXL varied from early postoperative months34 to several postoperative years32,35 and even reached 10 years in Raiskup et al.37 Our study recorded 4.1% failure rate at 5 years which is similar to some studies38 and lower than others.34,36
The actual risk factors of KC progression after CXL are still not fully clear. The relationship between KC and VKC with eye rubbing has been previously postulated.31,39 Chronic eye rubbing leads to epithelial trauma with the release of metalloproteinase and cytokine enzymes. The subsequent corneal biochemical and biomechanical changes may end in progressive stromal thinning and KC progression.40 Raiskup-Wolf et al22 stated the similar findings of the impact of eye rubbing on CXL failure.
Many studies reported insignificant differences between SCXL and other CXL protocols at a short-term follow-up period concerning keratometric, visual and refractive parameters.9,41 Furthermore, Bouheraoua et al42 reported similar outcomes between SCXL and ACXL regarding postoperative findings with confocal microscopy and also optical coherence tomography. On the other hand, Woo et al43 reported that ACXL has advantages over SCXL regarding biomechanical stability. Iqbal et al44 showed the superiority of SCXL protocol over accelerated and transepithelial protocols.
Our study showed that the associated postoperative corneal haze was universal in 75.5% of eyes which were also associated with improvements in SE by 0.50 D or more at postoperative 60 months thus proving that the postoperative corneal haze is an early postoperative indicator of successful SCXL. We certainly cannot consider these improvements in the postoperative refractive indices resulting from post-SCXL corneal flattening as a definitive landmark of refractive CXL. This is because these recorded refractive improvements are lawless, timeless, unguaranteed, unpredictable and unexpectable with the possibility of regression in the future. Our study documented the steady positive relationship between the postoperative corneal haze, flattening and thinning. The more the postoperative corneal haze is, the more the corneal flattening and thinning. This relationship might be responsible for the recorded postoperative improvements in the refractive components and might be based on the actual amount and depth of the cross-linked corneal tissues as demonstrated in the early postoperative demarcation line (DL). In other words, if this relationship breaks down, then the refractive improvements regress to baseline and KC may even start to progress again.
Most of our study patients were adults; however, we only documented one child with postoperative KC progression (Kmax>1D), at postoperative 60 months. This case was associated with VKC and the patient underwent a repeat SCXL bilaterally after the end of the study. The authors are not sure whether or not the collagen turnover rate is faster in pediatrics than in adults and if this rate is partially responsible for this progression. In other words, the etiology of KC progression in our case might be multifactorial particularly associated eye rubbing with VKC, rapid collagen turnover rate or even hormonal changes at puberty. This, of course, proved the unstable aggressive progressive nature of the pediatric KC in comparison to adult KC and explains the importance to routinely discuss with the parents, at the time of 1st intervention, the possibility of needing a repeat CXL in the future.
Our study had some limitations, mainly the small number of eyes included in our study. This is because most of our patients; who underwent SCXL 5 or more years ago, had actually undergone additional types of surgeries to improve their vision particularly WFG PRK or ICRS implantation while only a few patients continued to wear spectacles or hard contact lenses after SCXL. This why we included bilateral cases in this study; however, we do acknowledge that this could be a potential source of bias.
The problem of including both eyes of each patient in the study can, of course, result in a classical dilemma and creates the inter-eye dependence obstacle with possible bias in statistical interpretation. Definitely including only one eye from each patient in the study is ideal and undoubtedly more conclusive for accurate statistical outcomes. However, many published studies included both eyes in the same patient and some opinions; including ours, think that they are still two separate eyes through they are from one patient as, for example, Keratoconus stage may be quite different in the two eyes of the same patient. Finally, we do acknowledge that we included both eyes in most patients in our study, which could be a potential source of bias. However, our main excuse was the rarity of the disease so we aimed to boost the number of the study eyes. Being a retrospective study was also another limitation towards increasing the number of the study eyes that fulfilled the inclusion criteria.
In conclusion, SCXL had good effectiveness and stability that halted KC progression along the 5-year follow-up. SCXL had also unexpected improvement in the KC refractive states mainly the spherical and SE components. Chronic eye rubbing and VKC were identified to be important risk factors for KC progression following SCXL. Therefore, such patients need a close follow-up even after a long-term disease stability. However, further prospective clinical studies are needed to validate our results.
Acknowledgment
The authors are grateful for the help and support of Dr. Mona Abo Ali, Mr. Hamza Mohammed, Mr. Seif Mohammed and Ms. Lina Mohammed as well as the EPK Group, they also appreciate the help and support of Prof. Foad Metry Yousef.
Data Sharing Statement
The Excel sheet and patient data used to support the findings of this study are available from the corresponding author upon request.
Disclosure
The authors report no conflicts of interest.
References
- 1.Davis LJ, Schechtman KB, Wilson BS, et al.; Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study Group. Longitudinal changes in visual acuity in keratoconus. Invest Ophthalmol Vis Sci. 2006;47(2):489–500. doi: 10.1167/iovs.05-0381 [DOI] [PubMed] [Google Scholar]
- 2.Mas Tur V, MacGregor C, Jayaswal R, O’Brart D, Maycock N. A review of keratoconus: diagnosis, pathophysiology, and genetics. Surv Ophthalmol. 2017;62(6):770–783. doi: 10.1016/j.survophthal.2017.06.009 [DOI] [PubMed] [Google Scholar]
- 3.Thanos S, Oellers P, Meyer M, et al. Role of thyroxine in the development of keratoconus. Cornea. 2016;35(10):1338–1346. doi: 10.1097/ICO.0000000000000988 [DOI] [PubMed] [Google Scholar]
- 4.Lee R, El-Massry A, El-Massry Y, Randleman J. Bilateral, asymmetric keratoconus induced by thyrotoxicosis with long-term stability after corneal cross-linking. J Refract Surg. 2018;34:354–356. doi: 10.3928/1081597X-20180301-02 [DOI] [PubMed] [Google Scholar]
- 5.Bilgihan K, Hondur A, Sul S, Ozturk S. Pregnancy-induced progression of keratoconus. Cornea. 2011;30(9):991–994. doi: 10.1097/ICO.0b013e3182068adc [DOI] [PubMed] [Google Scholar]
- 6.Solomon A. Corneal complications of vernal keratoconjunctivitis. Curr Opin Allergy Clin Immunol. 2015;15(5):489–494. doi: 10.1097/ACI.0000000000000202 [DOI] [PubMed] [Google Scholar]
- 7.Shetty R, Sureka S, Kusumgar P, Sethu S, Sainani K. Allergen-specific exposure associated with high immunoglobulin E and eye rubbing predisposes to progression of keratoconus [published correction appears in Indian J Ophthalmol. 2017 Jul;65(7):642–643]. Indian J Ophthalmol. 2017;65(5):399–402. doi: 10.4103/ijo.IJO_217_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–627. doi: 10.1016/S0002-9394(02)02220-1 [DOI] [PubMed] [Google Scholar]
- 9.Lang P, Hafezi N, Khandelwal S, Torres-Netto E, Hafezi F, Randleman J. Comparative functional outcomes after corneal crosslinking using standard, accelerated, and accelerated with higher total fluence protocols. Cornea. 2019;38:433–441. doi: 10.1097/ICO.0000000000001878 [DOI] [PubMed] [Google Scholar]
- 10.Hafez MI. Comparison of epithelium-off and transepithelial corneal collagen cross-linking for treatment of keratoconus. J Egypt Ophthalmol Soc. 2014;107:181–186. doi: 10.4103/2090-0686.148163 [DOI] [Google Scholar]
- 11.Wen D, Li Q, Song B, et al. Comparison of standard versus accelerated corneal collagen cross-linking for keratoconus: a meta-analysis. Invest Ophthalmol Vis Sci. 2018;59:3920–3931. doi: 10.1167/iovs.18-24656 [DOI] [PubMed] [Google Scholar]
- 12.Saleem MIH, Ibrahim Elzembely HA, AboZaid MA, et al. Three-year outcomes of cross-linking PLUS (Combined cross-linking with femtosecond laser intracorneal ring segments implantation) for management of keratoconus. J Ophthalmol. 2018;2018:8. doi: 10.1155/2018/6907573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Randleman J, Santhiago M, Kymionis G, Hafezi F. Corneal cross-linking (CXL): standardizing terminology and protocol nomenclature. J Refract Surg. 2017;33:727–729. doi: 10.3928/1081597X-20170925-01 [DOI] [PubMed] [Google Scholar]
- 14.Iqbal M, Elmassry A, Tawfik A, et al. Evaluation of the effectiveness of cross-linking combined with photorefractive keratectomy for treatment of keratoconus. Cornea. 2018;37(9):1143–1150. doi: 10.1097/ICO.0000000000001663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abou SW, Mokbel T, Elwan M, et al. Two-stage procedure in the management of selected cases of keratoconus: clear lens extraction with aspherical IOL implantation followed by WFG-PRK. Int J Ophthalmol. 2018;11(11):1761–1767. doi: 10.18240/ijo.2018.11.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed Saleem MH. Combined cross-linking with femtosecond laser myoring implantation versus combined cross-linking with femtosecond laser keraring implantation in the treatment of keratoconus. J Egypt Ophthalmol Soc. 2015;108:140–147. doi: 10.4103/2090-0686.168716 [DOI] [Google Scholar]
- 17.Kamiya K, Ishii R, Shimizu K, Igarashi A. Evaluation of corneal elevation, pachymetry and keratometry in keratoconic eyes with respect to the stage of Amsler-Krumeich classification. Br J Ophthalmol. 2014;98(4):459–463. doi: 10.1136/bjophthalmol-2013-304132 [DOI] [PubMed] [Google Scholar]
- 18.Andrade HA, McDonald MB, Liu JC, Abdelmegeed M, Varnell R, Sunderland G. Evaluation of an opacity lensometer for determining corneal clarity following excimer laser photoablation. Refract Corneal Surg. 1990;6(5):346–351. [PubMed] [Google Scholar]
- 19.Grewal DS, Brar GS, Jain R, Sood V, Singla M, Grewal SP. Corneal collagen crosslinking using riboflavin and ultraviolet-A light for keratoconus: one-year analysis using Scheimpflug imaging. J Cataract Refract Surg. 2009;35(3):425–432. doi: 10.1016/j.jcrs.2008.11.046 [DOI] [PubMed] [Google Scholar]
- 20.Caporossi A, Mazzotta C, Baiocchi S, Caporossi T. Long-term results of riboflavin ultraviolet a corneal collagen cross-linking for keratoconus in Italy: the Siena eye cross study. Am J Ophthalmol. 2010;149:585–593. doi: 10.1016/j.ajo.2009.10.021 [DOI] [PubMed] [Google Scholar]
- 21.Hashemi H, Seyedian MA, Miraftab M, Fotouhi A, Asgari S. Corneal collagen cross-linking with riboflavin and ultraviolet a irradiation for keratoconus: long-term results. Ophthalmology. 2013;120(8):1515–1520. doi: 10.1016/j.ophtha.2013.01.012 [DOI] [PubMed] [Google Scholar]
- 22.Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet-A light inkeratoconus: long-term results. J Cataract Refract Surg. 2008;34:796–801. doi: 10.1016/j.jcrs.2007.12.039 [DOI] [PubMed] [Google Scholar]
- 23.Asri D, Touboul D, Fournié P, et al. Corneal collagen crosslinking in progressive keratoconus: multicenter results from the French National Reference Center for Keratoconus. J Cataract Refract Surg. 2011;37(12):2137–2143. doi: 10.1016/j.jcrs.2011.08.026 [DOI] [PubMed] [Google Scholar]
- 24.Henriquez MA, Izquierdo L Jr, Bernilla C, Zakrzewski PA, Mannis M. Riboflavin/Ultraviolet A corneal collagen cross-linking for the treatment of keratoconus: visual outcomes and Scheimpflug analysis. Cornea. 2011;30(3):281–286. doi: 10.1097/ICO.0b013e3181eeaea1 [DOI] [PubMed] [Google Scholar]
- 25.Greenstein SA, Fry KL, Hersh PS. Corneal topography indices after corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. 2011;37(7):1282–1290. doi: 10.1016/j.jcrs.2011.01.029 [DOI] [PubMed] [Google Scholar]
- 26.Sabesan R, Yoon G. Neural compensation for long-term asymmetric optical blur to improve visual performance in keratoconic eyes. Invest Ophthalmol Vis Sci. 2010;51:3835–3839. doi: 10.1167/iovs.09-4558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanellopoulos AJ, Asimellis G. Revisiting keratoconus diagnosis and progression classification based on evaluation of corneal asymmetry indices, derived from Scheimpflug imaging in keratoconic and suspect cases. Clin Ophthalmol. 2013;7:1539–1548. doi: 10.2147/OPTH.S44741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iqbal M, Elmassry A, Tawfik A, et al. Standard cross‐linking versus photorefractive keratectomy combined with accelerated cross‐linking for keratoconus management: a comparative study. Acta Ophthalmol. 2019;97(4):e623–e631. doi: 10.1111/aos.13986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hafez MI. Refractive meridional corneal collagen cross-linking: a new modified technique for treatment of astigmatism. Delta J Ophthalmol. 2015;16:5–9. doi: 10.4103/1110-9173.157776 [DOI] [Google Scholar]
- 30.Caporossi A, Mazzotta C, Baiocchi S, Caporossi T, Denaro R. Age-related long-term functional results after riboflavin UV A corneal cross-linking. J Ophthalmol. 2011;2011:608041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim TG, Kim KY, Han JB, Jin KH. The long-term clinical outcome after corneal collagen cross-linking in Korean patients with progressive keratoconus. Korean J Ophthalmol. 2016;30(5):326–334. doi: 10.3341/kjo.2016.30.5.326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hafez MI. Analysis of 2-year corneal cross-linking results in keratoconus patients. J Egypt Ophthalmol Soc. 2014;107:226–231. doi: 10.4103/2090-0686.150659 [DOI] [Google Scholar]
- 33.Greenstein SA, Shah VP, Fry KL, Hersh PS. Corneal thickness changes after corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. 2011;37:691–700. doi: 10.1016/j.jcrs.2010.10.052 [DOI] [PubMed] [Google Scholar]
- 34.Koller T, Mrochen M, Seiler T. Complication and failure rates after corneal crosslinking. J Cataract Refract Surg. 2009;35(8):1358–1362. doi: 10.1016/j.jcrs.2009.03.035 [DOI] [PubMed] [Google Scholar]
- 35.Kymionis GD, Karavitaki AE, Grentzelos MA, Liakopoulos DA, Tsoulnaras KI, Kontadakis GA. Topography-based keratoconus progression after corneal collagen crosslinking. Cornea. 2014;33(4):419–421. doi: 10.1097/ICO.0000000000000064 [DOI] [PubMed] [Google Scholar]
- 36.Baenninger PB, Bachmann LM, Wienecke L, Kaufmann C, Thiel MA. Effects and adverse events after CXL for keratoconus are independent of age: a 1-year follow-up study. Eye (Lond). 2014;28(6):691–695. doi: 10.1038/eye.2014.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raiskup F, Theuring A, Pillunat LE, Spoerl E. Corneal collagen crosslinking with riboflavin and ultraviolet-A light in progressive keratoconus: ten-year results. J Cataract Refract Surg. 2015;41:41–46. doi: 10.1016/j.jcrs.2014.09.033 [DOI] [PubMed] [Google Scholar]
- 38.Antoun J, Slim E, El Hachem R, et al. Rate of corneal collagen crosslinking redo in private practice: risk factors and safety. J Ophthalmol. 2015;2015:690961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iqbal M, Elmassry A, Tawfik A, et al. Analysis of the outcomes of combined cross-linking with intracorneal ring segment implantation for the treatment of pediatric keratoconus. Curr Eye Res. 2019;44(2):125–134. doi: 10.1080/02713683.2018.1540706 [DOI] [PubMed] [Google Scholar]
- 40.Wisse RP, Kuiper JJ, Gans R, Imhof S, Radstake TR, Van der Lelij A. Cytokine expression in keratoconus and its corneal microenvironment: a systematic review. Ocul Surf. 2015;13(4):272–283. doi: 10.1016/j.jtos.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 41.Choi M, Kim J, Kim EK, Seo KY. Comparison of the conventional Dresden protocol and accelerated protocol with higher ultraviolet intensity in corneal collagen cross-linking for keratoconus. Cornea. 2017;36:523–529. doi: 10.1097/ICO.0000000000001165 [DOI] [PubMed] [Google Scholar]
- 42.Bouheraoua N, Jouve L, El Sanharawi M, et al. Optical coherence tomography and confocal microscopy following three different protocols of corneal collagen-crosslinking in keratoconus. Invest Ophthalmol Vis Sci. 2014;55:7601–7609. doi: 10.1167/iovs.14-15662 [DOI] [PubMed] [Google Scholar]
- 43.Woo JH, Iyer J, Lim L, et al. Conventional versus accelerated collagen cross-linking for keratoconus: a comparison of visual, refractive, topographic and biomechanical outcomes. Open Ophthalmol J. 2017;11:262–272. doi: 10.2174/1874364101711010262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iqbal M, Elmassry A, Saad H, et al. Standard cross‐linking protocol versus accelerated and transepithelial cross‐linking protocols for treatment of paediatric keratoconus: a 2‐year comparative study. Acta Ophthalmol. 2019. doi: 10.1111/aos.14275 [DOI] [PMC free article] [PubMed] [Google Scholar]