Abstract
Objective:
Confirming the surgical osseous margin of a resected malignant bone tumor macroscopically before reconstruction with a prosthesis is ideal. However, making the cut-surface of the femur specimen during surgery is difficult because of the hard bone tissue. In order to resolve this problem, the possibility of intraoperative MRI was considered.
Methods:
MRI was performed at the surgical unit for five malignant femoral bone tumors that included two osteosarcomas and one undifferentiated high-grade sarcoma, and two metastatic tumors immediately after the tumor resection. The specimens were prepared in plastic containers with saline.
Results:
The osseous surgical margins were confirmed to be those planned pre-operatively in all cases without metal-induced artifacts. The T1 weighted image (WI) was useful for evaluation of the osseous surgical margin, whereas the T2WI was useful for confirmation of extraosseous soft-tissue.
Conclusion:
The MRI was performed post-operatively as a preliminary evaluation of the technique. However, a limited sequence (i.e. coronal T1WI) with short examination time could be performed during surgery for the sole purpose of assessing the osseous margin.
Advances in knowledge:
MRI examination of a resected malignant bone tumor specimen has not been reported, and can be an option for assessment of the osseous surgical margin.
Introduction
A malignant bone tumor is resected with adequate margins according to the pre-operative plan based upon MRI. Reconstruction with a tumor prosthesis is standard after resection of a malignant tumor in the long bone. After resection of the affected bone, but during surgery, pathological evaluation of the bone marrow at the surgical margin is performed on frozen specimens1 to confirm the absence of tumor cells. However, assessment of an adequate surgical margin macroscopically is best done before preparation of the prosthesis to confirm whether the resection is performed correctly according to the preoperative plan. With the modular system for current tumor prostheses, the length of the prosthesis can be adjusted during surgery. Therefore, intraoperative assessment of the osseous surgical margin macroscopically is important and can be incorporated into the surgical plan.
For a resection of malignant bone tumors, assessment of the osseous surgical margin requires a longitudinal cut in the specimen. However, making the longitudinal cut of the femur is difficult during the operation, because of the hard bone tissue. Furthermore, to make a fine cut surface, special equipment, such as a diamond coated cutter, may be necessary, but this equipment is not always available in the operating theater. In addition, when surgeons themselves make the cut surface, the operation needs to be suspended, and there might be concern about potential tumor contamination when the procedure is performed in the same room.
In a previous report, MRI for renal tumor specimens was used to assess the surgical margin and the skip metastasis.2 The possibility of intraoperative MRI of the resected bone tumor specimen to assess the osseous surgical margin was considered. In this preliminary study, MRI was performed to assess the surgical margin of five resected primary and metastatic malignant femoral bone tumors immediately after tumor resection and prosthesis replacement.
Methods and materials
Materials
Five cases of malignant femoral bone tumors (three males and two females; mean age, 71 ± 9.1 years; range, 60–80 years) were operated. Three cases were primary and the other two cases were metastatic tumors. The three primary cases were two osteosarcomas (one was osteoblastic and one was fibroblastic), and one undifferentiated high-grade sarcoma. The two metastatic cases derived from bladder and kidney cancer. The osseous surgical margin was determined based upon a preoperative MRI. The bone was cut with a single-use bone saw. Reconstruction was performed with a GMRS (Global Modular Replacement System, Stryker) tumor endoprosthesis. Proximal end replacement was used for one case, and distal end replacement in four cases. Informed consent for the MRI examination was obtained in each case.
Specimen MRI
Immediately after surgery, the resected specimen was placed in a plastic container and stabilized with non-woven fabric gauze and saline. The plastic container was used according the sample size. T1 and T2 weighted images were collected for specimen examination with a spin echo sequence using a 3 Tesla MRI (Magnetom Verio 3, Siemens). Image processing was performed using Synapse Vincent (Fujifilm Medical). After MRI examination, histological examination was performed.
Characterization of the specimen MRI was performed to assess the accuracy of defining the surgical margin of the malignant bone tumors. The proper sequence for examining the surgical margin of the bone tumor with marrow and extraosseous soft tissue was examined. For clinical evaluation, the resected specimen was compared to the planned resection based upon the preoperative MRI.
Results
For the specimen MRI, the pulse sequence parameters of repetition time (TR) and echo time (TE) were 670 ± 52.6 ms (650–778) and 13.8 ± 0.5 ms (13–14), respectively, for T1 weighted images. For T2 weighted images, the TR was 4665 ± 1199.2.2 msec (3500–6240), and TE was 95.3 ± 3.6 ms (91–102). The signal intensity of the specimen MRI was different than the pre-operative MRI. The most prominent difference was that the signal intensity of fat tissue including bone marrow on T2 weighted images gave an intermediate signal intensity, whereas the signal intensity in the preoperative MRI should be high (Figure 1).
Figure 1.
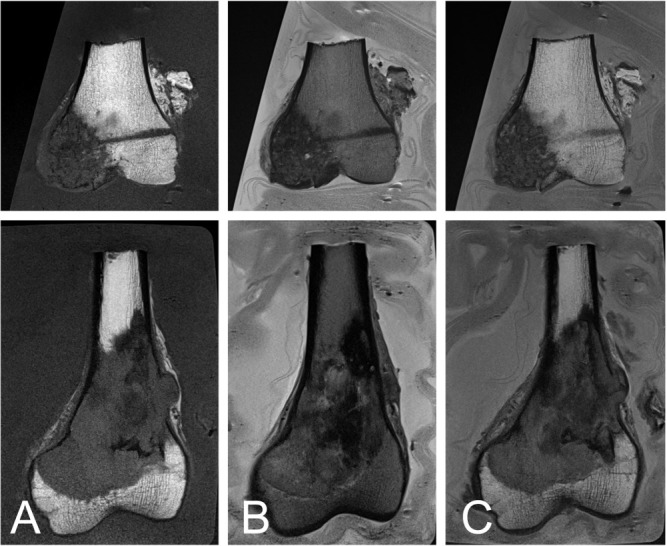
Renal cell carcinoma, metastatic to the bone, of a 74-year-old female (top), and a fibroblastic osteosarcoma of a 60-year-old male (bottom). The lesions with low signal intensity on the T1 weighted image have a clear surgical margin with the bone marrow that has high signal intensity (A). The extraosseous soft tissue is clearly contrasted to saline that has high signal intensity (B). The fusion images of the T1 and T2 weighted images depict the osseous margin and existence of extraosseous soft tissue (C).
For assessment of the surgical margin of the bone marrow, T1 weighed images were useful because the signal intensity of fat tissue within the bone marrow is high, allowing contrast with the tumor, which has low signal intensity (Figure 1A). On the other hand, for evaluation of extraosseous soft tissue including fat, muscle and tendon, T2 weighted images were useful to distinguish the tumor because the saline provides high signal intensity (Figure 1B). For simultaneous assessment of the surgical margin of the bone marrow and existence of extraosseous tissue, fusion images of T1 and T2 weighted images each at 50% intensity were useful (Figure 1C). The reconstructed MRI including the fusion images were consistent with the same cut surface of the pathology sections as long as the plane was the same (Figure 2).
Figure 2.
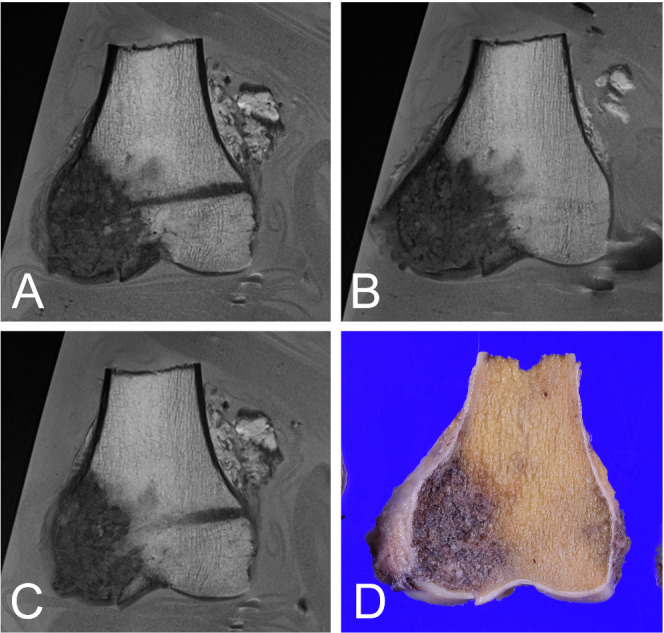
Renal cell carcinoma, metastatic to the bone in a 74-year-old female. Various sections in the same plane (A-C) and the pathological section (D). The MRI of the fusion image is reconstructed to adjust to the pathology section.
For clinical evaluation, clear images were obtained in all cases without any metal-induced artifact. The surgical margin between the bone and marrow was confirmed to be the same as the planned surgical margin. Histological examination confirmed that the surgical margin was free of tumor cells in all cases.
Discussion
Previous studies show that assessments of resected specimens in breast cancers can be made by plain radiograph.3 The surgical margin and skip lesions in the resected kidney can be examined using 7 Tesla MRI with the scanner oriented for animal usage and interfaced to the clinical console.2 For stabilization, the kidney specimen in that study was positioned in a glass container immersed in perfluropolyether oil.2 In the current protocol, the specimens were examined with a clinical 3 Tesla MRI, and the resected specimen was placed in a plastic container stabilized with non-woven fabric gauze and saline. The MRI can generate heating of the tissue, but the 3 Tesla MRI has been reported to be safe clinically in terms of temperature changes.4
The signal intensity of the specimen MRI was somewhat different from the preoperative MRI. The specimen MRI provides an intermediate signal for fat tissue including bone marrow on T1 weighted images whereas T1 weighted images using the preoperative MRI give a high signal for fat tissue. The reason for the difference of intensity is not known. Spin echo images defined by TR and TE are semi-automatically defined on our MRI, and these parameters may not be suitable for detecting fat tissue on T2 weighted images with the specimen MRI. There was a concern that the minute metal particles in cutting the bone might interfere with the MRI examination and create artifacts on the MRI. However, no artifacts were observed at the cutting site. Furthermore, the preoperative MRI is known to create several artifacts when applied in vivo. One is the flow-void phenomenon in which the signal for blood flow is absent.5 The specimen MRI is supposed to prevent any artifacts when used in patients. In order to know the specificity of the specimen MRI, subtraction analysis between the specimen MRI and the preoperative MRI would be necessary.
In the current report, the surgical margin of bone tumor specimens was evaluated successfully in five cases. Most bone tumors have low signal intensity on T1 weighted images and variable signals on T2 weighted images. The coronal T1 weighted image is reported to be excellent for evaluating bone marrow spread in the long-axis of osteosarcoma and Ewing sarcoma tumors.6,7 For the current specimen MRI, the T1 weighted image was useful to evaluate tumor extension in the bone marrow. The specimen MRI can be used to confirm the planned surgical margin. However, in order to confirm the absence of tumor cells at the surgical margin, an intraoperative pathology diagnosis would be necessary.
However, it was difficult to analyze the existence of the soft tissue around the bone on T1 weighted images because saline gives a low signal intensity and provides less contrast. On the other hand, T2 weighted images provide clear contrast between extraosseous soft-tissue and saline with a high signal intensity. In a previous report using specimen MRI for the kidney, both T1 and T2 weighted images were useful for assessment of the surgical margin and satellite lesions.2 Using spin echo MRI, a combination of T1 and T2 weighted images may be useful for assessment. In the current report, fusion images of T1 and T2 weighted images each at 50% intensity were made. With the fusion image of the resected femur specimen, the osseous surgical margin and existence of extraosseous soft tissue could be analyzed simultaneously. Reconstruction of the T1 weighted image or the fusion image provides images in the same plane as those of the cut sections viewed macroscopically for pathology examination. It would be difficult without the reconstruction to match the MRI and histopathology of the cut section.
As a limitation, the specimen MRI was applied only after surgery, though intraoperative usage was considered for the assessment of the osseous surgical margin. To apply this MRI approach intraoperatively, a definite surgical plan and preparation for MRI examination would be necessary. However, the exact time of completion of the resection cannot be anticipated in surgery. Furthermore, a time-consuming MRI examination might interrupt surgery. However, limiting the MRI sequence only to confirm the osseous surgical margin on a coronal T1 weighted image is sufficient. In our study, the specimen was placed in a plastic container with gauze and saline. Other conditions for the specimen MRI, such as different container materials or liquids other than saline, were not examined. For use during surgery, materials used for the preparation should be available within the surgical unit and should not interfere with the pathology analysis or genetic examination. Finally, the current MRI examination was performed within the surgical unit without taking the specimen outside the unit. It is possible to examine the specimen MRI outside the surgical unit, although such facilities are rare.
Conclusion
In summary, the specimen MRI was applied to resected malignant tumors of the femur. T1 weighted images were useful for assessment of the osseous surgical margin and T2 weighted images were useful for assessment of extraosseous soft tissue. There is the possibility that T1, T2 weighted fusion images can be used for both purposes simultaneously. Although the specimen MRI was performed post-operatively this time, a short intraoperative examination with a limited sequence, such as a coronal T1 weighted image, could be applicable for the assessment of the surgical margin.
Footnotes
Patient consent: All patients represented in this study were informed that the data from their case would be de-identified and used in a journal publication.
Contributors: AS arranged the study design and drafted the manuscript. TO, RI, and SM participated in planning the study. All authors confirmed the final version of the manuscript.
Ethics approval: The MRI examination was performed after the operation, and there was no negative effect on these patients. Therefore, the working group determined that ethics approval was not required.
Contributor Information
Akio Sakamoto, Email: akiosaka@kuhp.kyoto-u.ac.jp.
Takeshi Okamoto, Email: okaken@kuhp.kyoto-u.ac.jp.
Ryosuke Ikeguchi, Email: ikeguchi@kuhp.kyoto-u.ac.jp.
Shuichi Matsuda, Email: smat522@kuhp.kyoto-u.ac.jp.
REFERENCES
- 1.Bhaker P, Mohan H, Handa U, Kumar S. Role of intraoperative pathology consultation in skeletal tumors and tumor-like lesions. Sarcoma 2014; 2014: 6: 902104. doi: 10.1155/2014/902104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Oostenbrugge TJ, Runneboom W, Bekers E, Heidkamp J, Langenhuijsen JF, Veltien A, et al. Mri as a tool to assess surgical margins and pseudocapsule features directly following partial nephrectomy for small renal masses. Eur Radiol 2019; 29: 509–16. doi: 10.1007/s00330-018-5630-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SHH, Cornacchi SD, Heller B, Farrokhyar F, Babra M, Lovrics PJ. An evaluation of intraoperative digital specimen mammography versus conventional specimen radiography for the excision of nonpalpable breast lesions. Am J Surg 2013; 205: 703–10. doi: 10.1016/j.amjsurg.2012.08.010 [DOI] [PubMed] [Google Scholar]
- 4.Angelone LM, Ahveninen J, Belliveau JW, Bonmassar G. Analysis of the role of lead resistivity in specific absorption rate for deep brain stimulator leads at 3T MRI. IEEE Trans Med Imaging 2010; 29: 1029–38. doi: 10.1109/TMI.2010.2040624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wedeen VJ, Weisskoff RM, Poncelet BP. Mri signal void due to in-plane motion is all-or-none. Magn. Reson. Med. 1994; 32: 116–20. doi: 10.1002/mrm.1910320116 [DOI] [PubMed] [Google Scholar]
- 6.Putta T, Gibikote S, Madhuri V, Walter N. Accuracy of various MRI sequences in determining the tumour margin in musculoskeletal tumours. Pol J Radiol 2016; 81: 540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmad S, Stevenson J, Mangham C, Cribb G, Cool P. Accuracy of magnetic resonance imaging in planning the osseous resection margins of bony tumours in the proximal femur: based on coronal T1-weighted versus stir images. Skeletal Radiol 2014; 43: 1679–86. doi: 10.1007/s00256-014-1979-2 [DOI] [PubMed] [Google Scholar]


