Abstract
Personalized medicine aims at offering optimized treatment options and improved survival for cancer patients based on individual variability. The success of precision medicine depends on robust biomarkers. Recently, the requirement for improved non-biologic biomarkers that reflect tumor biology has emerged and there has been a growing interest in the automatic extraction of quantitative features from medical images, denoted as radiomics. Radiomics as a methodological approach can be applied to any image and most studies have focused on PET, CT, ultrasound, and MRI. Here, we aim to present an overview of the radiomics workflow as well as the major challenges with special emphasis on the use of multiparametric MRI datasets. We then reviewed recent studies on radiomics in the field of pelvic oncology including prostate, cervical, and colorectal cancer.
Introduction
MRI is routinely performed for staging and pretherapeutic work-up before treatment in several cancer subtypes in the pelvic area, including prostate, rectal, and cervical cancer. Standard MRI-derived features such as tumor volume and major axis length have been identified as prognostic factors1 but do not quantitatively exploit the complementary value of all the available MRI sequences (T1W pre- and post-contrast, T2W, FLAIR, and diffusion) usually acquired in routine pelvic imaging protocols. In addition, they are mostly based on visual interpretation and do not fully incorporate shape and intratumor heterogeneity characteristics.
Heterogeneity is a hallmark of malignant tumors and manifests at different scales (e.g., phenotypic, physiologic, and genomic). Tumor heterogeneity also has prognostic importance as heterogeneity is commonly associated with malignancy and aggressiveness,2–5 and may also influence response to therapy. Radiomics, which refer to quantitative features automatically extracted from medical images, have shown great potential as a source of quantitative biomarkers and have been used to build descriptive and predictive clinical models that relate imaging characteristics to intratumoral heterogeneity and biology phenotypes. In addition to overall tumor assessment, radiomics analysis can be a promising methodology in radiotherapy toward the goal of providing precision medicine and stratifying cancer patients for personalized care.6
Radiomics can be applied to any image, although in pelvic oncology, most studies have focused on PET and CT. Radiomics on MRI have not been investigated extensively7 but its potential value has been shown in some studies.8,9 The purpose of the present review is to provide an update on the rapid evolving field of radiomics for MRI application. Here, we describe the workflow of MRI radiomics, its clinical application in pelvic oncology, and the future challenges in this field.
The radiomics workflow
In radiomics, data (images or parts of images, e.g. a tumor) are fed into a feature extractor that calculates “engineered” or “handcrafted” features, which are then fed into an algorithm that maps a selected subset of the features with the classification task. More specifically, it involves the following steps: image acquisition and/or collection, images preprocessing (filtering, registration of sequences, inhomogeneity correction, interpolation, etc.), determination of the volume of interest (manual or semi-automatic), calculation of features (potentially several variants), and finally training and validation of models through statistical analysis (machine learning).2,4 Since the calculated number of features often exceeds by far the number of patients, a robust approach is needed. Relying on appropriate methods and splitting the data into training, validation and testing are therefore crucial. A more complete radiomics workflow description is available in the Image Biomarkers Standardization Initiative (IBSI) reference document.10 Lack of reproducibility and validation of radiomics analysis studies are considered to be a major challenge for the field and the IBSI aims at standardizing the radiomics workflow, as well as nomenclature, definitions, and implementation of commonly used features.
In the context of radiomics, features are usually evaluated for their robustness (versus variations of factors such as image acquisition or reconstruction, delineation method) or reproducibility/repeatability, where reproducibility informs on the stability of features when measured several times under different conditions (e.g. by changing the software), whereas repeatability informs on their stability when measured several times under the same conditions (as much as possible), for example by repeating an acquisition twice the same day for the same patient, keeping all factors the same. Preselection of only those features that contribute to clinical prediction, that are robust and reproducible/repeatable, and not correlated with one another is a potential approach to reduce the number of features that will be fed into a classifier/regression method (whether it is a basic or a more complex modeling approach).
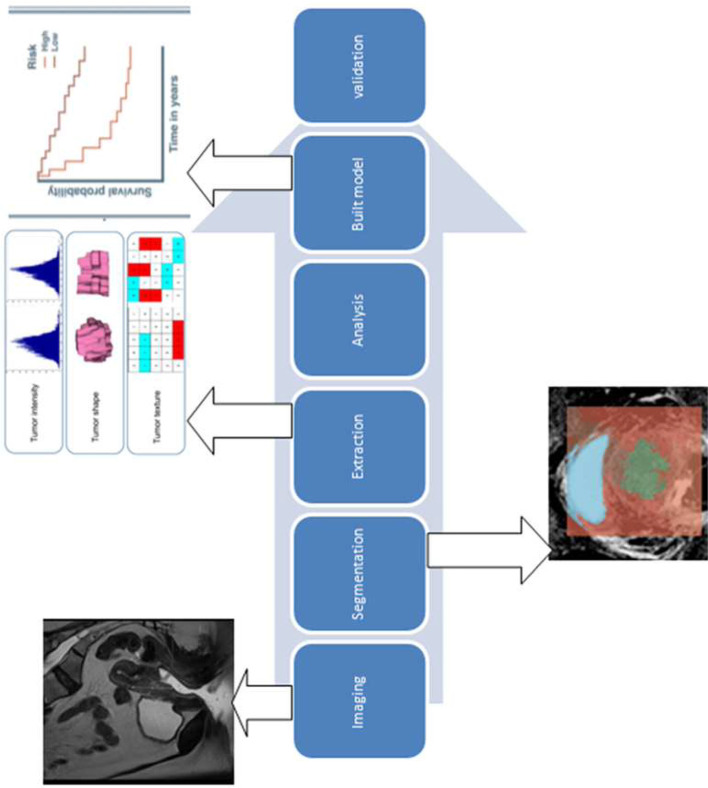
The methodology has been extensively presented in the review of Larue et al11 and therefore will be presented concisely here. The steps of the radiomics process for analysis of pelvic multiparametric MRI (mpMRI) are shown in Figure 1.
Figure 1. .
Workflow MRI radiomics as applied for images of cervical cancer
Image acquisition
MRI provides high-contrast structural and functional information to characterize soft tissue. Sequence preferences varied among studies reporting on radiomics extracted from MRI. Multiparametric approaches typically including T2W, DWI, and DCE-MRI sequences may reduce the risk of bias from features extracted from one sequence alone.12 An ADC map is then also usually calculated. MRI modalities may differ in their scanner properties, which would affect the reproducibility of images and thus the extracted radiomics features. This will be discussed in the last section of the present review.
Segmentation of region of interest
Accurate identification and segmentation of the tumor, denoted as ROI, is a critical and challenging step of the workflow, as it defines the spatial extent from which the subsequent radiomics feature data are derived. Manual delineation is a straightforward solution, but aside from being very time-consuming, it is well recognized to be susceptible to intraoperator and interoperator variability.13–15 Efforts have been made for automation or semi-automation of this process regarding MRI: 3D Slicer16 (http://www.slicer.org/) and the Growcut algorithm are available for free and require minimal inputs from the user.17 MRI segmentation using deep learning approaches, typically convolutional neural networks (CNN), is increasingly being studied to perform prostate or/and organs at risk segmentation.18 Cheng et al proposed an end-to-end prostate segmentation method by integrating holistically (image-to-image) nested edge detection. Their nested networks automatically learned a hierarchical representation that could improve prostate boundary detection and led to very good results on MRI scans from 250 patients.19 Similarly, Tian et al developed a deep learning method to automatically segment the prostate on T2W MRI with satisfactory performance: The proposed CNN model obtained a mean Dice of 85.3±3.2%, compared with the manual segmentation.20 Another group proposed a semi-supervised learning algorithm based on the multitask residual fully CNN. Best Dice values were 0.95, 0.89, and 0.88 for bladder, prostate, and rectum. Of note, this methodology focused on the whole prostatic gland segmentation, but no data using deep learning algorithms for tumors segmentation are available for now.
Extraction of radiomic features
From the identified ROI, various quantitative features can be derived. The most often used radiomic features can be grouped into four major categories: I, size and shape features; II, first-order histogram or global statistics; III, second-order histogram or textural features; and IV, transform-based features. Size and shape features describe the size of the ROI (such as volume, surface area…) and its shape (surface-to-volume ratio, compactness, eccentricity…).21–26 First-order histogram or global statistics describe features related to the distribution of the intensities of voxels within the ROI, ignoring the spatial interactions between them, and can be calculated for instance through histogram analysis (e.g. mean, minimum, maximum, standard deviation, skewness, or kurtosis). The features description will not be detailed in this review. Instead, we refer to the publicly available most up-to-date IBSI reference document for complete features description (including mathematical formulas and texture matrix design strategies).10
Second-order histogram or textural features, introduced by Haralick et al,27 describe the spatial distribution of voxel intensity levels. The term image texture refers to the perceived or measured spatial variation in the intensity levels, which can be visualized as a gray-level scale.8 Second-order and higher features are calculated from different matrices: the gray-level co-occurrence matrix (GLCM),22 the gray-level run-length matrix,28 the gray-level size zone matrix,29 and the neighborhood gray-tone difference matrix21,30–32 construction are presented in Supplementary Material 1. And finally, transform-based features depict repetitive or non-repetitive spatial patterns through imposing kernel functional transformation to the segmented image content. Some of the popularly used transformations are Fourier transform, Gabor transform, wavelet transform, Laplacian transforms of Gaussian bandpass filters.33–35 Matrices details are described in Supplementary Material 1 and the complete features description can be found in the reference manual of the image biomarker standardization initiative.36
Several in-house, open source (e.g. pyradiomics,37 LIFEx,38 MITK,39 or CERR40), or commercial (e.g. RADIOMICSTM, TexRADTM) software packages are available to perform radiomics analyses. One issue is the lack of standardization of the feature calculation and preprocessing steps that led to vastly different features obtained by different software packages. The IBSI has begun addressing this important issue 2 years ago and has released a reference document with guidelines and benchmark values for a large number of most commonly encountered radiomic features.36 Following these guidelines is recommended to improve and facilitate the reproducibility of radiomics studies.41
Reproducibility and stability of MRI radiomics features
A number of studies have explored the sensitivity of radiomics features in terms of variability in MRI acquisition parameters and reconstruction settings, as well as the impact of image preprocessing steps. But few studies were done in the context of pelvic cancers. One study investigated the interobserver agreement of the entropy (see10 for definition) from the co-occurrence matrix in diffusion-weighted MRI of cervical cancers.42 Results showed excellent interobserver agreement (all intraclass correlation coefficients (ICC) > 0.9). The stability of radiomic features in cervical cancer has been also very recently studied from T2W MRI in 20 females, in three ways: repeatability via test–retest, reproducibility between diagnostic MRI and simulation MRI and reproducibility in interobserver setting. The interobserver cohort had the most reproducible features (95.2% with ICC ≥0.75), whereas the diagnostic-simulation cohort had the fewest (14.1% with ICC ≥0.75). Overall, 229 features had ICC ≥0.75 in all three tests. Shape features emerged as the most stable features in all cohorts.43 In another exploratory study, Traverso et al sought to determine to what extent various commonly used radiomics features are reproducible with regard to interobserver variability, and imaging filtering applied prior to features extraction using ADC maps in 56 patients with rectal cancer. Features derived from intensity distribution histograms seem to be less sensitive to manual tumor delineation differences, noise in ADC images, pixel size resampling, and intensity discretization. Shape features however appeared to be strongly affected by delineation quality. Overall, textural features appeared to be poorly or moderately reproducible with respect to the image preprocessing perturbations.44
Schwier et al confirmed these findings in the field of prostate cancer MRI. Under different preprocessing and extraction configurations including various image normalization schemes, different image prefiltering, and different bin widths for image discretization, the radiomic features repeatability seemed to be highly sensitive to the processing parameters. Whole-image normalization led to improved ICCs for most features calculated from ADC images, while for T2W images, whole-image normalization did not result in ICC improvements. neither image normalization, using a variety of approaches, nor the use of prefiltering options resulted in consistent improvements in repeatability.45
As mentioned, the gray-level discretization method also has a direct impact on texture feature reproducibility, independent of observers, software, or method of delineation. Varying the bin number seems to lead to statistically significantly more variable results than varying the bin width.46
The impact of tumor segmentation variability using two segmentation methods has been reported on brain tumors only. Indeed, Tixier et al studied the impact of tumor segmentation variability on the robustness of MRI radiomic features using two different segmentation methods, a semi-automatic algorithm, and an interactive segmentation with two different raters. Both segmentations seem to be reliable with an intraclass correlation coefficient >0.96. Features computed from the histogram and co-occurrence matrices were found to be the most robust, whereas shape and gray-level size zone matrix features were the most impacted by the choice of segmentation method with the interactive method resulting in more robust features than the semi-automatic method.47
Yang et al also examine the dependence of image texture features extracted from brain high-grade and low-grade tumors on MR acquisition parameters and reconstruction using a digital MRI phantom. MR signal was simulated with varying levels of acquisition noise, acceleration factors, and image reconstruction algorithms. The feature variance due to reconstruction algorithm and acceleration factor were generally smaller than the clinical effect size that is, the feature difference between high-grade and low-grade tumors. They also presented a general simulation framework for assessing the robustness and accuracy of radiomic textural features under various MR acquisition/reconstruction scenarios.48 In patients with glioblastoma, it seems that histogram standardization contributes the most in reducing radiomic feature variability as it is the technique to reduce the covariate shift for three feature categories and successfully discriminate patients into groups of different survival risks.49
Harmonization
One of the main challenges in validating models in cohorts from different centers is the need to pool imaging data generated with different imaging and MRI reconstruction protocols, as it has been shown that most radiomics features are sensitive to the acquisition and reconstruction parameters, amongst other factors. As a result of this variability, a radiomics-based model trained on data from a given clinical center using a specific MRI scanner and associated acquisition/reconstruction protocol might not be directly applicable to data from another MRI scanner.
The current IBSI guidelines10 regarding MRI radiomic analyses recommend to avoid using fixed bin width discretization for images with arbitrary units (e.g. T1 or T2 sequences). It could be used on the other hand for ADC maps that are quantitative. Similarly, image interpolation and resampling to obtain isotropic voxels should be performed cautiously depending on variations in slice thickness and voxel sizes: in cases of very large slice thickness, a 2D analysis (slice by slice) may be more reliable than resampling to isotropic voxels for a 3D analysis).
Ideally, the acquisition protocols should be harmonized a priori in this context, and the scans comparable between centers. But, as the vast majority of multicenter studies are retrospective for now, MRI processing requires an a posteriori harmonization. A gradient non-linearity distortion correction can improve the voxel-based image intensity reproducibility of the data, as this has been shown on brain MRI.50 Karayumak reported on the retrospective harmonization of multisites diffusion MRI capable of removing scanner-specific effects, while accounting for minor differences in acquisition parameters such as b-value, spatial resolution and number of gradient directions. The intersubject anatomical variability such as gender-related and age-related maturation differences in different age groups was however preserved.51 Several methods have also been proposed to correct the signal artifacts represented by intensity non-uniformity, which can be induced by the choice of the radio-frequency coil or the acquisition pulse sequence.52
It is also possible to calibrate scanning settings using source-based morphometry: independent components are extracted from the data and investigated for associations with scanning parameters to assess the influence. The identified scanning-related components can be eliminated from the original data for correction.53
The feature-based harmonization ComBat is another option efficient at removing the multicenter effect for textural feature. The ComBat harmonization method initially described in 2007 and proposed for genomic studies in order to correct the so-called “batch effect” has the advantage over other methods that it provides satisfactory results even for small samples.54,55 This a posteriori harmonization statistical method was recently successfully applied to harmonize radiomics features extracted from PET images with different acquisition and reconstruction properties,56 and demonstrated an important improvement of the models performance when evaluated in external cohorts: this simple additional statistical step allowed for instance specificity of the models in the testing cohorts to increase from 62 to 88% for disease-free survival prediction and from 80 to 100% for locoregional control prediction in external cohorts of patients treated with chemoradiotherapy for cervical cancer. The ComBat method thus appears to be a valuable yet simple to use tool facilitating radiomics studies in a multicentric context, whether the data are collected retrospectively or prospectively.57
Model building
One of the most important specificities in radiomics analysis is the number of features which is often far greater than the number of available patients, potentially leading to a high risk of false-positive rate, as recently highlighted.4,58,59 There is no consensus about the optimal statistic or machine learning methodology to obtain the best results, that is, the model with the best accuracy, as results seem to depend highly on both data and methodology.60–62
Two different approaches can be considered: supervised or unsupervised. Supervised classification or regression assumes that clinical labels (e.g. dead or alive, responder or non-responder, etc.) are available, and the algorithms are trained and validated according to these labels. Unsupervised on the other hand performs clustering or other kind of analysis in unlabeled data to identify patterns and then potentially relate these patterns to a clinical outcome. Because in most radiomics studies labels are available, most of them are supervised.
Once a subset of useful features has been identified, they are then fed to an algorithm of time-dependent outcome regression or a regression algorithm that aims at classifying the patient (e.g. responder vs non-responder to radiotherapy), or to predict time-to-event data (e.g. survival).
One of the most important guidelines regarding this step is to rely on splitting the available data into training, validation, and testing sets. The training set is used solely to train a model (i.e. select and combine relevant features), whereas the validation set is used solely to optimize parameters of the model. Finally, the testing set is used to evaluate the performance of the trained/optimized model on data that was never used for training and validation. Ideally, according to the TRIPOD guidelines,63 this testing should be performed on external datasets (i.e. training, validation, and testing occur in a multicentric context).
Representative results in the field of pelvic oncology
Using free word and control terms related to ”radiomics”, “texture”, “colorectal cancer”, “prostate adenocarcinoma”, and “cervical cancer”, we searched studies from Medical Literature Analysis via PubMed. We included studies written in English.
Table 1 provides an overview of the existing studies in the field of pelvic oncology, including their radiomics quality score, which helps to evaluate the quality of radiomics studies.64
Table 1. .
Overview of radiomics studies in the field of pelvic oncology
| Author | Pathology | N Training (Validation) |
MRI sequences | Treatment modality | Endpoints | Features overview | Radiomic quality score (%) | TRIPOD Analysis |
|---|---|---|---|---|---|---|---|---|
| Khalvati | prostate | 40.975 | T2W and DWI | Healthy vs cancerous | I-IV | 23.1 | Diagnostic | |
| Wibmer | Prostate | 147 | T2W and DWI | Healthy vs cancerous and Gleason Score | I-IV | 23.1 | Diagnostic | |
| Vignati | prostate | 93 | T2W and DWI | Biological aggressiveness | I-IV | 23.1 | Diagnostic | |
| Gnep | Prostate | 144 | T2W and ADC | RT | Gleason score, Biochemical recurrence | I-III | 23.1 | Diagnostic &prognostic models |
| Shiradkar | Prostate | 70 (50) | T2W and ADC | Surgery | Biochemical recurrence | I-III | 23.1 | Prognostic |
| Cui | rectal | 131 (55) | T1W, T2W, ADC | CRT | Pathological response | I-IV | 46.2 | Prognostic |
| Horvat | rectal | 114 | T2W | CRT | Pathological response | I-IV | 23.1 | Diagnostic |
| Nie | rectal | 48 | T1W, T2W, ADC | CRT | Pathological response | I-III | 19.2 | Prognostic |
| Liu | rectal | 152 (70) | T2W and ADC | CRT | Pathological response | I-IV | 50 | Prognostic |
| Cusumano | rectal | 173 (25) | T2W | CRT | Pathological response | I-IV | 50 | Prognostic |
| Giannini | rectal | 52 | T2W and ADC | CRT | Pathological response | I-III | 19.2 | Prognostic |
| Guan | cervical | 51 | ADC | Healthy vs cancerous | I-III | 23.1 | Diagnostic | |
| Kan | cervical | 100 (43) | T1W, T2W, ADC | Lymph node metastasis | I-IV | 53.8 | Diagnostic | |
| Wu | cervical | T1W, T2W, ADC | Surgery | LVSI, LN, Tumor grade | I-IV | 19.2 | Diagnostic | |
| Lucia | cervical | 112 (78) | T1W, T2W, ADC | CRT | recurrence | I-III | 53.8 | Prognostic |
ADC, Apparent diffusion coefficient; CRT, chemoradiotherapy; DWI, diffusion-weighted imaging; LN, lymph nodes; LVSI, lymphovascular space invasions;TRIPOD, Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis.
Radiomics application in prostate cancer
MpMRI of the prostate consists of T2W sequences, which provide anatomical information and “functional” sequences, which are DWI, dynamic contrast-enhanced sequences, and magnetic resonance spectroscopy. Prostate MRI is the most accurate imaging method for local staging of prostate cancer and also serves as a guide for targeted transrectal biopsies. Several recent studies investigated radiomics in prostate cancer for diagnosis, prognosis, and therapy.
Regarding diagnosis, most algorithms in the literature incorporate few imaging sequences into their models. Often, only three sequences (DWI, T2W, and DCE) are analyzed to build a model.65 Khalvati, et al aimed to design a radiomics-based auto-detection method for prostate cancer using mpMRI data including not only T2W and DWI but also computed high-b DWI (CHB-DWI) and a new diffusion imaging modality called correlated diffusion imaging (CDI), as well as features from individual b-value images of DWI (i.e. b-values at 0, 100, 400, and 1000 s mm-2). Indeed, CDI has shown promise in separating healthy tissues from cancerous ones and individual b-value images may also contain information to help further distinguish healthy tissues from cancerous tissues compared with ADC alone. The performance of the developed mpMRI texture feature models was evaluated via leave-one-out cross-validation using a support vector machine classifier trained on 40,975 cancerous and healthy tissue samples obtained from real clinical MP-MRI datasets. After cross-validation, the models outperformed the conventional model (i.e. T2W + DWI) with regard to cancer detection with an AUC of 0.86 (CI 95% 0.81–0.91) when using sensitivity as a performance evaluation criteria. This model was however not assessed by clinicians to investigate whether it improves over the radiologist performance or not.66 Regarding differentiation, higher values of entropy scores defined by the combined entropy derived from different sequences were also found to be correlated with a high Gleason score in another report.67
Wibmer, et al also evaluated MRI-derived radiomics for the detection of prostate cancer in 146 patients with one to three lesions on the surgical specimen.68 Four GLCM-based texture features (energy, entropy, correlation, and homogeneity) were significantly associated with the presence of cancerous tissue. Cameron et al developed a quantitative radiomics approach for prostate cancer detection combining all imaging sequences and aiming to improve MRI sensitivity and specificity.69 First, tumoral tissues were automatically delineated using computer-aided prostate cancer detection algorithm based on a mpMRI. The morphology, asymmetry, physiology, and size (MAPS) feature model was then used to score the candidate regions. This model was built using 42 features (geometrical, signal intensities or textural features, and size). In order to produce a single feature score for any region, the feature value was averaged and the mean value used as the region’s feature score. After cross-validation, the MAPS model outperformed all other feature sets with a sensitivity of 86%, a specificity of 88%, and an accuracy of 87%. Moreover, when comparing performance between the MAPS feature model and the model resulting from the T2W and ADC sequences only, the MAPS outperformed the combination.
Intratumoral heterogeneity is a possible leading confounding factor contributing to the underperformance of the current pretreatment staging assessment. Thus, efforts have been made to improve the staging accuracy and studies have shown interesting results in the setting of disease local staging, a delicate assessment in which radiologists do not always have high accuracy: For instance, a radiomics signature based on 17 features on T2WIs has been established with the potential to predict the side-specific probability of pathological extracapsular extension status, and this signature showed a good discrimination in the validation set (AUC 0.821).70,71
The Gleason score, although also highly prognostic, is frequently underestimated at the time of diagnostic biopsy. Therefore, the added value of radiomics to score the tumor biopsies is a topic of research. Interestingly, only texture radiomic features extracted from the ADC (energy, entropy) were correlated with the Gleason score in the above mentioned study.68 Fehr et al reported on the accuracy of a software-based automatic classification by Gleason score using T2W and ADC.72 An accuracy of 93% was obtained after cross-validation for discrimination of Gleason 6 (3 + 3)=6 versus Gleason ≥7, and 92% for discrimination of Gleason 7 (3 + 4)=7 versus (4 + 3)=7.
Beyond MRI, textural analysis can also be applied directly on the biopsy specimen to predict tumor differentiation: an automated system for Prostate cancer biopsies samples classification according to Gleason grading has been developed and showed that four textural features from prostatic biopsies after conversion to gray-level images (sum entropy, entropy, different average, and contrast) were strongly correlated with the Gleason score with a high accuracy. The highest levels of texture analysis (except sum Entropy) meet the highest Gleason grading scores.73
The predictive value of radiomics on the response to treatment has also been studied. Textural features may correlate with biochemical recurrence after definitive radiotherapy.8 Recently, we also found that a specific feature extracted from the ADC map was strongly predictive of biochemical recurrence after radical prostatectomy and may allow identifying in the future the patients who would mostly benefit from treatment intensification such as adjuvant radiotherapy or androgen deprivation (data currently under review).
Radiomics application in colorectal cancer
Colorectal carcinoma (CRC) is characterized by a marked intratumor genetic heterogeneity.74 To date, data on the added value of radiomics or radiogenomics in patients with CRC are limited.
Preliminary data showed that radiomic features based on intensity histograms analysis (entropy, uniformity, kurtosis, skewness, and standard deviation) extracted from CT could be useful for CRC staging, as they correlate with 5 year overall survival.75 Higher values of the entropy on hepatic perfusion CT may predict poorer survival.76,77 Some of these features (skewness and kurtosis) on CT and on PET/CT could also help to differentiate primary CRC from liver metastases.78
Radiomics on MRI have also been studied in the attempt to predict the response to neoadjuvant chemoradiotherapy in rectal cancer. On a prospective study, Nie et al aimed to investigate the value of MR radiomics after chemoradiotherapy compared with conventional MR metrics (such as volume from anatomical MRI, mean-maximum signal difference from DCE-MRI, and mean-ADC from DWI) for diagnosis of clinical complete response in 48 patients with rectal cancer. The conventional volume-averaged analysis could provide an area under ROC curve ranging from 0.54 to 0.73 in predicting pathological complete response. While if the models were replaced by heterogeneity analysis, the prediction accuracy measured by AUC could be improved to 0.71–0.79.79 A similar analysis was performed in 222 patients who all underwent T2W and DWI before and after chemoradiotherapy; 2252 radiomic features were extracted from each patient before and after treatment imaging. The two-sample t-test and the least absolute shrinkage and selection operator regression were used for feature selection, whereupon a radiomics signature was built with support vector machines. The proposed radiomics model showed excellent performance for individualized, non-invasive prediction of pathological complete response with an area under the receiver operating characteristic curve of 0.9756 (95% CI, 0.9185–0.9711) in the validation cohort.80
Finally, Horvat et al aimed to retrospectively investigate the value of T2W-based radiomics compared with qualitative assessment at T2W imaging and DWI carried out after chemoradiotherapy for diagnosis of clinical complete response in 114 patients with rectal cancer.81 A random forest classifier was trained to separate the patients by their outcomes after balancing the number of patients in each response category using the synthetic minority oversampling technique. 18% of patients achieved pathological complete response. After cross-validation, the radiomic classifier demonstrated an area under the curve of 0.93, sensitivity of 100%, specificity of 91%, positive predictive value of 72%, and negative predictive value of 100%. The diagnostic performance of radiomics was significantly higher than qualitative assessment was at T2WI or DWI alone (p < .02), and the specificity and positive predictive values obtained with the radiomics model were significantly higher than the model combining T2W and DWI (p < .0001) . Fractal analysis, used to examine underlying tumor structural geometry, could play an important role in radiomics in rectal cancer, providing valuable information not only about the tumor volume structure but also about its inner subpopulations.82
Imaging analysis of tumor characteristics also offers a powerful tool for assessment of tumor biology to assist with therapeutic decision-making. Kirsten rat sarcoma viral oncogene homolog (KRAS) and epidermal growth factor receptor gene mutations are independent prognostic factors for survival, and a predictive marker of tumor response to antiepidermal growth factor receptor targeted antibodies in patients with colorectal cancer. Alongside with microsatellite instability, they have been linked to stratification of prognosis in patients with CRC.83 Recent data showed that CT-based radiomics signature can predict KRAS mutations in primary and metastatic colorectal cancer.84 Recently, Xu et al reported that rectal carcinoma with different KRAS mutation statuses demonstrated distinctive diffusion and perfusion characteristics on the basis of diffusion MRI techniques. No significant correlations were found between semi-quantitative DCE MRI parameters and KRAS mutation or microsatellite instability.85
Radiomics application in cervical cancer
FDG-PET/CT and MRI play an essential role in the initial staging, therapeutic strategy,86 and treatment response assessment87 in cervical cancer. Radiomics extracted from PET/CT have been shown to provide prognostic information in cervical cancer,42,88–91 especially regarding the response to therapy and the risk of pelvic recurrence.88,90,91 Texture analysis from MRI has first been used to characterize cervical cancer lesions using diffusion sequences.42 The added values of radiomics extracted from MRI have also been studied to predict lymph-vascular space invasion92 and lymph nodes metastases. Using support vector machine algorithms, a multiple-sequence radiomics signature could be identified that allowed good discrimination between patients with lymph nodes metastases and those without metastases, with an AUC of 0.75 [95% CI (0.66–0.85)] in the validation cohort.93 These findings were confirmed by others94 who found that functional maps exhibit better discriminative values than anatomical images for discriminating the pathological features of cervical cancer, with ADC maps showing the best discrimination performance for lymph node metastasis and Ve (fractional volume of extravascular extracellular space) maps showing the best discriminative value for lymph-vascular space invasion and tumor grade, although on a relatively small population (n = 56).
We recently showed that a radiomics signature from both FDG-PET and MRI is associated with the outcome of patients with histologically proven staged IB1-IVA cervical cancer and treated with definitive curative chemoradiotherapy and subsequent brachytherapy. Our results suggest that gray-level non-uniformity GLRLM in FDG-PET and/or entropyGLCM in ADC maps from DWI MRI are powerful predictors of the efficacy of treatment in the treatment of cervical cancer. Higher values of these parameters were associated with worse outcome, confirming that more heterogeneous tumor have a poor prognosis. In our study, we could obtain near-perfect accuracy by using only one or two parameters that remained significant after correction for multiple testing.9 These findings were successfully validated in two external cohorts thanks to the ComBat harmonization technique.57 The disease-free survival model reached an accuracy of 90% [95% CI (79–98%)] in both external cohorts, and the locoregional control model an accuracy of 98% [95% CI (90–99%)]. The best prediction using standard clinical variables was 56–60% only. These findings can be acted upon to tailor treatment.
The promise and challenge of radiomics
Although MRI-based radiomics show great promise in cancer management, their application is challenged by significant issues.
There is a wide range of image acquisition and reconstruction algorithms among institutions and scanners,95 and the analysis of signal in MR images is difficult to generalize because of the problem of normalization and regularization of images.96 The image acquisition parameters,97 alongside with other factors such as tumor volume,98 gray-level discretization, and other preprocessing steps4,59 can influence radiomics findings. Indeed, unlike CT, MRI intensities do not necessarily reflect physical parameters like electron density and exhibit a larger magnitude in variability with voxel size, magnetic field strength, pulse sequence, machine vendor, and reconstruction algorithm.99 The dependence of radiomic features on MRI scanner manufacturer, field strength, and acquisition parameters has been shown in many studies.100–104 Thus, testing of MRI-based radiomic features for robustness and reproducibility is a critical initial step in the radiomic workflow. Very few studies on the repeatability and stability of radiomic features in MRI have been published yet. Given the fact that geometrical distortions are quite common in MRI,27,105 further research investigating its effect on radiomics feature extraction is needed. Some texture features seem to maintain discriminatory significance—particularly those derived from short tau inversion recovery and T2W sequences.106 Our previous work focusing on the prognostic and predictive value of radiomics using MRI and PET in cervical cancer also showed a stability of our proposed model based on the entropyGLCM from the ADC map of the DWI MRI in the external validation cohorts, although the harmonization method ComBat was necessary.57
Moreover, great efforts have to be made to assess the external validation, applicability, and re-usability of a given radiomics model. Standardization of MRI acquisition across institutions should be encouraged. The Quantitative Imaging Biomarkers Alliance® (QIBA) continues to develop protocols for optimizing acquisition parameters, but a technically confirmed profile for MRI radiomics still does not exist. Functional MRI, DWI MRI, DCE MRI, and magnetic resonance elastography imaging biomarker profiles are currently in progress.107 For instance, given the lack of uniformity in prostate MR acquisition and interpretation standards, the Prostate Imaging Reporting and Data System (PIRADS) guidelines established in 2012 and updated in 2019 has achieved important goals for standardization of image acquisition and interpretation of prostate MRI.108 QIBA also designed DWI MRI phantoms to streamline calculations of ADC parametric maps and bias estimates, signal-to-noise ratios, as well as ADC spatial and b-value dependences. Extension of this protocol to other tumor sites is warranted.109
Hopefully in the coming years, a more reproducible MRI workflow that will facilitate the development and validation of multicentric radiomics models in the classification and categorization of cancer patients will be developed, although a posteriori harmonization techniques such as ComBat may be necessary to obtain the best results. Many public datasets including MRI in prostate cancer are available from the Cancer Imaging Archive Tutorial and can be used for models’ building and validation, whereas data on cervical cancer and rectal cancer are less common.
In conclusion, ongoing efforts are made to correlate radiomics features acquired from pelvic cancer MRI prior to radiation treatment to patient outcome. Clinical trials are warranted to assess the robustness of radiomics-based predictive models and to maximize the efficacy of these models. Integrating radiomics into clinical decision-making is an important innovation that has the potential to provide a significant step toward the goal of personalized patient care for pelvic cancer. Our understanding of how genetic, histologic, and physiologic tumor features correlate spatial and temporal changes in imaging parameters must be developed further.
Contributor Information
Ulrike Schick, Email: ulrike.schick@chu-brest.fr.
François Lucia, Email: francois.lucia@chu-brest.fr.
Gurvan Dissaux, Email: gurvan.dissaux@chu-brest.fr.
Dimitris Visvikis, Email: visvikis@univ-brest.fr.
Bogdan Badic, Email: bodgan.badic@chu-brest.fr.
Ingrid Masson, Email: ingrid-masson@hotmail.fr.
Olivier Pradier, Email: olivier.pradier@chu-brest.fr.
Vincent Bourbonne, Email: vicnent.bourbonne@chu-brest.fr.
Mathieu Hatt, Email: hatt@univ-brest.fr.
REFERENCES
- 1.Lee JH, Lee S-W, Kim JR, Kim YS, Yoon MS, Jeong S, et al. Tumour size, volume, and marker expression during radiation therapy can predict survival of cervical cancer patients: a multi-institutional retrospective analysis of KROG 16-01. Gynecol Oncol 2017; 147: 577–84. doi: 10.1016/j.ygyno.2017.09.036 [DOI] [PubMed] [Google Scholar]
- 2.Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology 2016; 278: 563–77. doi: 10.1148/radiol.2015151169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avanzo M, Stancanello J. El Naqa I: Beyond imaging: The promise of radiomics. Physica medica : PM : an international journal devoted to the applications of physics to medicine and biology : official journal of the Italian Association of Biomedical Physics 2017; 38: 122–39. [DOI] [PubMed] [Google Scholar]
- 4.Yip SSF, Aerts HJWL. Applications and limitations of radiomics. Phys Med Biol 2016; 61: R150–R166. doi: 10.1088/0031-9155/61/13/R150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang F, Young L, Grigsby P. Predictive value of standardized intratumoral metabolic heterogeneity in locally advanced cervical cancer treated with chemoradiation. International Journal of Gynecologic Cancer 2016; 26: 777–84. doi: 10.1097/IGC.0000000000000616 [DOI] [PubMed] [Google Scholar]
- 6.Rutman AM, Kuo MD. Radiogenomics: creating a link between molecular diagnostics and diagnostic imaging. Eur J Radiol 2009; 70: 232–41. doi: 10.1016/j.ejrad.2009.01.050 [DOI] [PubMed] [Google Scholar]
- 7.Rios Velazquez E, Parmar C, Liu Y, Coroller TP, Cruz G, Stringfield O, et al. Somatic mutations drive distinct imaging phenotypes in lung cancer. Cancer Res 2017; 77: 3922–30. doi: 10.1158/0008-5472.CAN-17-0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gnep K, Fargeas A, Gutiérrez-Carvajal RE, Commandeur F, Mathieu R, Ospina JD, et al. Haralick textural features on T 2 -weighted MRI are associated with biochemical recurrence following radiotherapy for peripheral zone prostate cancer. Journal of Magnetic Resonance Imaging 2017; 45: 103–17. doi: 10.1002/jmri.25335 [DOI] [PubMed] [Google Scholar]
- 9.Lucia F, Visvikis D, Desseroit M-C, Miranda O, Malhaire J-P, Robin P, et al. Prediction of outcome using pretreatment 18F-FDG PET/CT and MRI radiomics in locally advanced cervical cancer treated with chemoradiotherapy. Eur J Nucl Med Mol Imaging 2018; 45: 768–86. doi: 10.1007/s00259-017-3898-7 [DOI] [PubMed] [Google Scholar]
- 10.Zwanenburg ALS, Vallières M. Löck S: The Image Biomarkers Standardization Initiative. see. 2019;.
- 11.Larue RTHM, Defraene G, De Ruysscher D, Lambin P, van Elmpt W. Quantitative radiomics studies for tissue characterization: a review of technology and methodological procedures. Br J Radiol 2017; 90: 20160665. doi: 10.1259/bjr.20160665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang B, Tian J, Dong D, Gu D, Dong Y, Zhang L, et al. Radiomics features of multiparametric MRI as novel prognostic factors in advanced nasopharyngeal carcinoma. Clinical Cancer Research 2017; 23: 4259–69. doi: 10.1158/1078-0432.CCR-16-2910 [DOI] [PubMed] [Google Scholar]
- 13.Isebaert S, Van den Bergh L, Haustermans K, Joniau S, Lerut E, De Wever L, et al. Multiparametric MRI for prostate cancer localization in correlation to whole-mount histopathology. Journal of Magnetic Resonance Imaging 2013; 37: 1392–401. doi: 10.1002/jmri.23938 [DOI] [PubMed] [Google Scholar]
- 14.Hoeks CMA, Hambrock T, Yakar D, Hulsbergen–van de Kaa CA, Feuth T, Witjes JA, et al. Transition zone prostate cancer: detection and localization with 3-T multiparametric MR imaging. Radiology 2013; 266: 207–17. doi: 10.1148/radiol.12120281 [DOI] [PubMed] [Google Scholar]
- 15.Eminowicz G, McCormack M. Variability of clinical target volume delineation for definitive radiotherapy in cervix cancer. Radiotherapy and Oncology 2015; 117: 542–7. doi: 10.1016/j.radonc.2015.10.007 [DOI] [PubMed] [Google Scholar]
- 16.Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin J-C, Pujol S, et al. 3D slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging 2012; 30: 1323–41. doi: 10.1016/j.mri.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egger J, Kapur T, Fedorov A, Pieper S, Miller JV, Veeraraghavan H, et al. Gbm volumetry using the 3D slicer medical image computing platform. Sci Rep 2013; 3: 1364. doi: 10.1038/srep01364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundervold AS, Lundervold A. An overview of deep learning in medical imaging focusing on MRI. Zeitschrift für Medizinische Physik 2019; 29: 102–27. doi: 10.1016/j.zemedi.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 19.Cheng R, Roth HR, Lay N, Lu L, Turkbey B, Gandler W, et al. Automatic magnetic resonance prostate segmentation by deep learning with holistically nested networks. Journal of Medical Imaging 2017; 4: 1. doi: 10.1117/1.JMI.4.4.041302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian ZLL. Fei B: Deep convolutional neural network for prostate MR segmentation. Int J Comput Assist Radiol Surg 2018; 13: 1687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vallières M, Freeman CR, Skamene SR, El Naqa I. A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys Med Biol 2015; 60: 5471–96. doi: 10.1088/0031-9155/60/14/5471 [DOI] [PubMed] [Google Scholar]
- 22.Aerts HJWL, Velazquez ER, Leijenaar RTH, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 2014; 5: 4006. doi: 10.1038/ncomms5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coroller TP, Agrawal V, Narayan V, Hou Y, Grossmann P, Lee SW, et al. Radiomic phenotype features predict pathological response in non-small cell lung cancer. Radiotherapy and Oncology 2016; 119: 480–6. doi: 10.1016/j.radonc.2016.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Zhu Y, Burnside ES, Drukker K, Hoadley KA, Fan C, et al. Mr imaging Radiomics signatures for predicting the risk of breast cancer recurrence as given by research versions of MammaPrint, Oncotype DX, and PAM50 gene assays. Radiology 2016; 281: 382–91. doi: 10.1148/radiol.2016152110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie H, Hu J, Zhang X, Ma S, Liu Y, Wang X. Preliminary utilization of radiomics in differentiating uterine sarcoma from atypical leiomyoma: comparison on diagnostic efficacy of MRI features and radiomic features. Eur J Radiol 2019; 115: 39–45. doi: 10.1016/j.ejrad.2019.04.004 [DOI] [PubMed] [Google Scholar]
- 26.Cuocolo R, Stanzione A, Ponsiglione A, Romeo V, Verde F, Creta M, et al. Clinically significant prostate cancer detection on MRI: a radiomic shape features study. Eur J Radiol 2019; 116: 144–9. doi: 10.1016/j.ejrad.2019.05.006 [DOI] [PubMed] [Google Scholar]
- 27.Haralick RM, Shanmugam K, Dinstein Its'Hak. Textural features for image classification. IEEE Trans Syst Man Cybern 1973; SMC-3: 610–21. doi: 10.1109/TSMC.1973.4309314 [DOI] [Google Scholar]
- 28.Galloway MM. Texture analysis using gray level run lengths. Computer Graphics and Image Processing 1975; 4: 172–9. doi: 10.1016/S0146-664X(75)80008-6 [DOI] [Google Scholar]
- 29.Tixier F, Le Rest CC, Hatt M, Albarghach N, Pradier O, Metges J-P, et al. Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. Journal of Nuclear Medicine 2011; 52: 369–78. doi: 10.2967/jnumed.110.082404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ypsilantis P-P, Siddique M, Sohn H-M, Davies A, Cook G, Goh V, et al. Predicting response to neoadjuvant chemotherapy with PET imaging using Convolutional neural networks. PLoS One 2015; 10: e0137036. doi: 10.1371/journal.pone.0137036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amadasun M, King R. Textural features corresponding to textural properties. IEEE Trans Syst Man Cybern 1989; 19: 1264–74. doi: 10.1109/21.44046 [DOI] [Google Scholar]
- 32.Bashir U, Siddique MM, Mclean E, Goh V, Cook GJ. Imaging heterogeneity in lung cancer: techniques, applications, and challenges. American Journal of Roentgenology 2016; 207: 534–43. doi: 10.2214/AJR.15.15864 [DOI] [PubMed] [Google Scholar]
- 33.van Stiphout RGPM, Lammering G, Buijsen J, Janssen MHM, Gambacorta MA, Slagmolen P, et al. Development and external validation of a predictive model for pathological complete response of rectal cancer patients including sequential PET-CT imaging. Radiotherapy and Oncology 2011; 98: 126–33. doi: 10.1016/j.radonc.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 34.Ohri N, Duan F, Snyder BS, Wei B, Machtay M, Alavi A, et al. Pretreatment 18F-FDG PET textural features in locally advanced non-small cell lung cancer: secondary analysis of ACRIN 6668/RTOG 0235. Journal of Nuclear Medicine 2016; 57: 842–8. doi: 10.2967/jnumed.115.166934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ft E, Kelly PJ, Scheithauer BW, Kall BA, Cascino TL, Ehman RL, et al. Axley PL: Cerebral astrocytomas: histopathologic correlation of MR and CT contrast enhancement with stereotactic biopsy. Radiology 1988; 166: 823–7. [DOI] [PubMed] [Google Scholar]
- 36.Zwanenburg AVM, Löck S. Image biomarker standardisation initiative - feature definitions. 2017;.
- 37.van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, et al. Computational Radiomics system to decode the radiographic phenotype. Cancer Res 2017; 77: e104–7. doi: 10.1158/0008-5472.CAN-17-0339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nioche C, Orlhac F, Boughdad S, Reuzé S, Goya-Outi J, Robert C, et al. LIFEx: a freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res 2018; 78: 4786–9. doi: 10.1158/0008-5472.CAN-18-0125 [DOI] [PubMed] [Google Scholar]
- 39.Götz M, Nolden M, Maier-Hein K. MITK phenotyping: an open-source toolchain for image-based personalized medicine with radiomics. Radiotherapy and Oncology 2019; 131: 108–11. doi: 10.1016/j.radonc.2018.11.021 [DOI] [PubMed] [Google Scholar]
- 40.Apte AP, Iyer A, Crispin-Ortuzar M, Pandya R, van Dijk LV, Spezi E, et al. Technical note: extension of CERR for computational radiomics: a comprehensive Matlab platform for reproducible radiomics research. Med Phys 2018; 45: 3713–20. doi: 10.1002/mp.13046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vallières M, Zwanenburg A, Badic B, Cheze Le Rest C, Visvikis D, Hatt M. Responsible Radiomics research for faster clinical translation. Journal of Nuclear Medicine 2018; 59: 189–93. doi: 10.2967/jnumed.117.200501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guan Y, Li W, Jiang Z, Chen Y, Liu S, He J, et al. Whole-Lesion apparent diffusion Coefficient-Based Entropy-Related parameters for characterizing cervical cancers. Acad Radiol 2016; 23: 1559–67. doi: 10.1016/j.acra.2016.08.010 [DOI] [PubMed] [Google Scholar]
- 43.Fiset S, Welch ML, Weiss J, Pintilie M, Conway JL, Milosevic M, et al. Repeatability and reproducibility of MRI-based radiomic features in cervical cancer. Radiotherapy and Oncology 2019; 135: 107–14. doi: 10.1016/j.radonc.2019.03.001 [DOI] [PubMed] [Google Scholar]
- 44.Traverso A, Kazmierski M, Shi Z, Kalendralis P, Welch M, Nissen HD, et al. Wee L: Stability of radiomic features of apparent diffusion coefficient (ADC) maps for locally advanced rectal cancer in response to image pre-processing. Physica medica : PM : an international journal devoted to the applications of physics to medicine and biology : official journal of the Italian Association of Biomedical Physics 2019; 61: 44–51. [DOI] [PubMed] [Google Scholar]
- 45.Schwier M, van Griethuysen J, Vangel MG, Pieper S, Peled S, Tempany C, et al. Repeatability of multiparametric prostate MRI Radiomics features. Sci Rep 2019; 9: 9441. doi: 10.1038/s41598-019-45766-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duron L, Balvay D, Vande Perre S, Bouchouicha A, Savatovsky J, Sadik J-C, et al. Gray-level discretization impacts reproducible MRI radiomics texture features. PLoS One 2019; 14: e0213459. doi: 10.1371/journal.pone.0213459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tixier F, Um H, Young RJ, Veeraraghavan H. Reliability of tumor segmentation in glioblastoma: impact on the robustness of MRI‐radiomic features. Med Phys 2019; 10. doi: 10.1002/mp.13624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang F, Dogan N, Stoyanova R. Ford JC: Evaluation of radiomic texture feature error due to MRI acquisition and reconstruction: A simulation study utilizing ground truth. Physica medica : PM : an international journal devoted to the applications of physics to medicine and biology : official journal of the Italian Association of Biomedical Physics 2018; 50: 26–36. [DOI] [PubMed] [Google Scholar]
- 49.Um H, Tixier F, Bermudez D, Deasy JO, Young RJ, Veeraraghavan H. Impact of image preprocessing on the scanner dependence of multi-parametric MRI radiomic features and covariate shift in multi-institutional glioblastoma datasets. Phys. Med. Biol. 2019; 64: 165011. doi: 10.1088/1361-6560/ab2f44 [DOI] [PubMed] [Google Scholar]
- 50.Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, et al. Reliability in multi-site structural MRI studies: effects of gradient Non-linearity correction on phantom and human data. Neuroimage 2006; 30: 436–43. doi: 10.1016/j.neuroimage.2005.09.046 [DOI] [PubMed] [Google Scholar]
- 51.Cetin Karayumak S, Bouix S, Ning L, James A, Crow T, Shenton M, et al. Retrospective harmonization of multi-site diffusion MRI data acquired with different acquisition parameters. Neuroimage 2019; 184: 180–200. doi: 10.1016/j.neuroimage.2018.08.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Belaroussi B, Milles J, Carme S, Zhu YM, Benoit-Cattin H. Intensity non-uniformity correction in MRI: existing methods and their validation. Med Image Anal 2006; 10: 234–46. doi: 10.1016/j.media.2005.09.004 [DOI] [PubMed] [Google Scholar]
- 53.Chen J, Liu J, Calhoun VD, Arias-Vasquez A, Zwiers MP, Gupta CN, et al. Exploration of scanning effects in multi-site structural MRI studies. J Neurosci Methods 2014; 230: 37–50. doi: 10.1016/j.jneumeth.2014.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.WWB G, Wang W. Wong L: Why Batch Effects Matter in Omics Data, and How to Avoid Them. Trends Biotechnol 2017; 35: 498–507. [DOI] [PubMed] [Google Scholar]
- 55.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007; 8: 118–27. doi: 10.1093/biostatistics/kxj037 [DOI] [PubMed] [Google Scholar]
- 56.Orlhac F, Boughdad S, Philippe C, Stalla-Bourdillon H, Nioche C, Champion L, et al. A Postreconstruction harmonization method for multicenter radiomic studies in PET. Journal of Nuclear Medicine 2018; 59: 1321–8. doi: 10.2967/jnumed.117.199935 [DOI] [PubMed] [Google Scholar]
- 57.Lucia F, Visvikis D, Vallieres M, Desseroit MC, Miranda O, Robin P, et al. External validation of a combined PET and MRI radiomics model for prediction of recurrence in cervical cancer patients treated with chemoradiotherapy. European journal of nuclear medicine and molecular imaging 2018;. [DOI] [PubMed] [Google Scholar]
- 58.Chalkidou A, O’Doherty MJ, Marsden PK. False discovery rates in PET and CT studies with texture features: a systematic review. PLoS One 2015; 10: e0124165. doi: 10.1371/journal.pone.0124165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hatt M, Tixier F, Pierce L, Kinahan PE, Le Rest CC, Visvikis D. Characterization of PET/CT images using texture analysis: the past, the present… any future? Eur J Nucl Med Mol Imaging 2017; 44: 151–65. doi: 10.1007/s00259-016-3427-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deist TM, Dankers FJWM, Valdes G, Wijsman R, Hsu I‐Chow, Oberije C, et al. Machine learning algorithms for outcome prediction in (chemo)radiotherapy: An empirical comparison of classifiers. Med Phys 2018; 45: 3449–59. doi: 10.1002/mp.12967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parmar C, Grossmann P, Bussink J, Lambin P, Aerts HJWL. Machine learning methods for quantitative radiomic biomarkers. Sci Rep 2015; 5: 13087. doi: 10.1038/srep13087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leger S, Zwanenburg A, Pilz K, Lohaus F, Linge A, Zöphel K, et al. A comparative study of machine learning methods for time-to-event survival data for radiomics risk modelling. Sci Rep 2017; 7: 13206. doi: 10.1038/s41598-017-13448-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ 2014; 350(jan07 4): g7594. doi: 10.1136/bmj.g7594 [DOI] [PubMed] [Google Scholar]
- 64.Sanduleanu S, Woodruff HC, de Jong EEC, van Timmeren JE, Jochems A, Dubois L, et al. Tracking tumor biology with radiomics: a systematic review utilizing a radiomics quality score. Radiotherapy and Oncology 2018; 127: 349–60. doi: 10.1016/j.radonc.2018.03.033 [DOI] [PubMed] [Google Scholar]
- 65.Duda DKM, Mathieu R, Crevoisier RD, Bezy-Wendling J, M-ITAiCoPT from, Preliminary Results MRI. Information technologies in biomedicine. Volume 3 AiISaC 2014;. [Google Scholar]
- 66.Khalvati F, Wong A, Haider MA. Automated prostate cancer detection via comprehensive multi-parametric magnetic resonance imaging texture feature models. BMC Med Imaging 2015; 15: 27. doi: 10.1186/s12880-015-0069-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Orczyk C, Villers A, Rusinek H, Lepennec V, Bazille C, Giganti F, et al. Prostate cancer heterogeneity: texture analysis score based on multiple MRI sequences for detection, stratification and selection of lesions at time of biopsy. BJU international 2018;. [DOI] [PubMed] [Google Scholar]
- 68.Wibmer AG, Vargas HA, Hricak H. Role of MRI in the diagnosis and management of prostate cancer. Future Oncology 2015; 11: 2757–66. doi: 10.2217/fon.15.206 [DOI] [PubMed] [Google Scholar]
- 69.Cameron A, Khalvati F, Haider MA, Wong A. Maps: a quantitative Radiomics approach for prostate cancer detection. IEEE Trans Biomed Eng 2016; 63: 1145–56. doi: 10.1109/TBME.2015.2485779 [DOI] [PubMed] [Google Scholar]
- 70.Ma S, Xie H, Wang H, Yang J, Han C, Wang X, et al. Preoperative prediction of extracapsular extension: Radiomics signature based on magnetic resonance imaging to stage prostate cancer. Molecular Imaging and Biology 2019; 79. doi: 10.1007/s11307-019-01405-7 [DOI] [PubMed] [Google Scholar]
- 71.Stanzione A, Cuocolo R, Cocozza S, Romeo V, Persico F, Fusco F, et al. Detection of Extraprostatic extension of cancer on Biparametric MRI combining texture analysis and machine learning: preliminary results. Acad Radiol 2019; 26: 1338–44. doi: 10.1016/j.acra.2018.12.025 [DOI] [PubMed] [Google Scholar]
- 72.Fehr D, Veeraraghavan H, Wibmer A, Gondo T, Matsumoto K, Vargas HA, et al. Automatic classification of prostate cancer Gleason scores from multiparametric magnetic resonance images. Proc Natl Acad Sci U S A 2015; 112: E6265–E6273. doi: 10.1073/pnas.1505935112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Alexandratou E: Texture analysis of tissues in Gleason grading of prostate cancer. Imaging, Manipulation and Analysis of Biomolecules, Celles and Tissues VI 2008;: 6859. [Google Scholar]
- 74.Losi L, Baisse B, Bouzourene H, Benhattar J. Evolution of intratumoral genetic heterogeneity during colorectal cancer progression. Carcinogenesis 2005; 26: 916–22. doi: 10.1093/carcin/bgi044 [DOI] [PubMed] [Google Scholar]
- 75.Ng F, Ganeshan B, Kozarski R, Miles KA, Goh V. Assessment of primary colorectal cancer heterogeneity by using Whole-Tumor texture analysis: contrast-enhanced CT texture as a biomarker of 5-year survival. Radiology 2013; 266: 177–84. doi: 10.1148/radiol.12120254 [DOI] [PubMed] [Google Scholar]
- 76.Ganeshan B, Miles KA, Young RC. Chatwin CR: Hepatic enhancement in colorectal cancer: texture analysis correlates with hepatic hemodynamics and patient survival. Academic radiology 2007; 14: 1520–30. [DOI] [PubMed] [Google Scholar]
- 77.Miles KA, Ganeshan B, Griffiths MR, Young RCD, Chatwin CR. Colorectal cancer: texture analysis of portal phase hepatic CT images as a potential marker of survival. Radiology 2009; 250: 444–52. doi: 10.1148/radiol.2502071879 [DOI] [PubMed] [Google Scholar]
- 78.Wagner F, Hakami YA, Warnock G, Fischer G, Huellner MW, Veit-Haibach P. Comparison of Contrast-Enhanced CT and [18F]FDG PET/CT Analysis Using Kurtosis and Skewness in Patients with Primary Colorectal Cancer. Molecular Imaging and Biology 2017; 19: 795–803. doi: 10.1007/s11307-017-1066-x [DOI] [PubMed] [Google Scholar]
- 79.Nie K, Shi L, Chen Q, Hu X, Jabbour SK, Yue N, et al. Rectal cancer: assessment of neoadjuvant chemoradiation outcome based on Radiomics of multiparametric MRI. Clinical Cancer Research 2016; 22: 5256–64. doi: 10.1158/1078-0432.CCR-15-2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu Z, Zhang X-Y, Shi Y-J, Wang L, Zhu H-T, Tang Z, et al. Radiomics analysis for evaluation of pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Clinical Cancer Research 2017; 23: 7253–62. doi: 10.1158/1078-0432.CCR-17-1038 [DOI] [PubMed] [Google Scholar]
- 81.Horvat N, Veeraraghavan H, Khan M, Blazic I, Zheng J, Capanu M, et al. Mr imaging of rectal cancer: Radiomics analysis to assess treatment response after neoadjuvant therapy. Radiology 2018; 287: 833–43. doi: 10.1148/radiol.2018172300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cusumano D, Dinapoli N, Boldrini L, Chiloiro G, Gatta R, Masciocchi C, et al. Fractal-based radiomic approach to predict complete pathological response after chemo-radiotherapy in rectal cancer. Radiol Med 2018; 123: 286–95. doi: 10.1007/s11547-017-0838-3 [DOI] [PubMed] [Google Scholar]
- 83.Conlin A, Smith G, Carey FA, Wolf CR. The prognostic significance of K-Ras, p53, and APC mutations in colorectal carcinoma. Gut 2005; 54: 1283–6. doi: 10.1136/gut.2005.066514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang L, Dong D, Fang M, Zhu Y, Zang Y, Liu Z, et al. Can CT-based radiomics signature predict KRAS/NRAS/BRAF mutations in colorectal cancer? Eur Radiol 2018; 28: 2058–67. doi: 10.1007/s00330-017-5146-8 [DOI] [PubMed] [Google Scholar]
- 85.Xu Y, Xu Q, Sun H, Liu T, Shi K, Wang W. Could IVIM and ADC help in predicting the KRAS status in patients with rectal cancer? Eur Radiol 2018; 28: 3059–65. doi: 10.1007/s00330-018-5329-y [DOI] [PubMed] [Google Scholar]
- 86.Herrera FG, Prior JO. The role of PET/CT in cervical cancer. Front Oncol 2013; 3: 34. doi: 10.3389/fonc.2013.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Choi J, Kim HJ, Jeong YH, Lee J-H, Cho A, Yun M, et al. The Role of 18 F-FDG PET/CT in Assessing Therapy Response in Cervix Cancer after Concurrent Chemoradiation Therapy. Nucl Med Mol Imaging 2014; 48: 130–6. doi: 10.1007/s13139-013-0248-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.KC H, Fang YH, Chung HW, Yen TC, TY H, Chou HH, et al. Lai CH: A preliminary investigation into textural features of intratumoral metabolic heterogeneity in (18)F-FDG PET for overall survival prognosis in patients with bulky cervical cancer treated with definitive concurrent chemoradiotherapy. American journal of nuclear medicine and molecular imaging 2016; 6: 166–75. [PMC free article] [PubMed] [Google Scholar]
- 89.Torheim T, Groendahl AR, Andersen EKF, Lyng H, Malinen E, Kvaal K, et al. Cluster analysis of dynamic contrast enhanced MRI reveals tumor subregions related to locoregional relapse for cervical cancer patients. Acta Oncol 2016; 55: 1294–8. doi: 10.1080/0284186X.2016.1189091 [DOI] [PubMed] [Google Scholar]
- 90.Chung HH, Kang SY, Ha S, Kim J-W, Park N-H, Song YS, et al. Prognostic value of preoperative intratumoral FDG uptake heterogeneity in early stage uterine cervical cancer. J Gynecol Oncol 2016; 27: e15. doi: 10.3802/jgo.2016.27.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reuzé S, Orlhac F, Chargari C, Nioche C, Limkin E, Riet F, et al. Prediction of cervical cancer recurrence using textural features extracted from <sup>18</sup>F-FDG PET images acquired with different scanners. Oncotarget 2017; 8: 43169–79. doi: 10.18632/oncotarget.17856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li Z, Li H, Wang S, Dong D, Yin F, Chen A, et al. MR-Based Radiomics nomogram of cervical cancer in prediction of the Lymph-Vascular space invasion preoperatively. Journal of magnetic resonance imaging : JMRI 2018;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kan Y, Dong D, Zhang Y, Jiang W, Zhao N, Han L, et al. Radiomic signature as a predictive factor for lymph node metastasis in early-stage cervical cancer. Journal of Magnetic Resonance Imaging 2019; 49: 304–10. doi: 10.1002/jmri.26209 [DOI] [PubMed] [Google Scholar]
- 94.Wu Q, Shi D, Dou S, Shi L, Liu M, Dong L, et al. Wang M: Radiomics Analysis of Multiparametric MRI Evaluates the Pathological Features of Cervical Squamous Cell Carcinoma. Journal of magnetic resonance imaging : JMRI 2018;. [DOI] [PubMed] [Google Scholar]
- 95.Summers RM. Texture analysis in radiology: does the emperor have no clothes? Abdominal Radiology 2017; 42: 342–5. doi: 10.1007/s00261-016-0950-1 [DOI] [PubMed] [Google Scholar]
- 96.Collewet G, Strzelecki M, Mariette F. Influence of MRI acquisition protocols and image intensity normalization methods on texture classification. Magn Reson Imaging 2004; 22: 81–91. doi: 10.1016/j.mri.2003.09.001 [DOI] [PubMed] [Google Scholar]
- 97.Galavis PE, Hollensen C, Jallow N, Paliwal B, Jeraj R. Variability of textural features in FDG PET images due to different acquisition modes and reconstruction parameters. Acta Oncol 2010; 49: 1012–6. doi: 10.3109/0284186X.2010.498437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hatt M, Majdoub M, Vallieres M, Tixier F, Le Rest CC, Groheux D, et al. 18F-FDG PET uptake characterization through texture analysis: investigating the complementary nature of heterogeneity and functional tumor volume in a multi-cancer site patient cohort. Journal of Nuclear Medicine 2015; 56: 38–44. doi: 10.2967/jnumed.114.144055 [DOI] [PubMed] [Google Scholar]
- 99.Kumar V, Gu Y, Basu S, Berglund A, Eschrich SA, Schabath MB, et al. Radiomics: the process and the challenges. Magn Reson Imaging 2012; 30: 1234–48. doi: 10.1016/j.mri.2012.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Herlidou-Même S, Constans JM, Carsin B, Olivie D, Eliat PA, Nadal-Desbarats L, et al. Mri texture analysis on texture test objects, normal brain and intracranial tumors. Magn Reson Imaging 2003; 21: 989–93. doi: 10.1016/S0730-725X(03)00212-1 [DOI] [PubMed] [Google Scholar]
- 101.Jirák D, Dezortová M, Hájek M. Phantoms for texture analysis of Mr images. long-term and multi-center study. Med Phys 2004; 31: 616–22. doi: 10.1118/1.1646231 [DOI] [PubMed] [Google Scholar]
- 102.Mayerhoefer ME, Szomolanyi P, Jirak D, Materka A, Trattnig S. Effects of MRI acquisition parameter variations and protocol heterogeneity on the results of texture analysis and pattern discrimination: an application-oriented study. Med Phys 2009; 36: 1236–43. doi: 10.1118/1.3081408 [DOI] [PubMed] [Google Scholar]
- 103.Ford J, Dogan N, Young L, Yang F. Quantitative Radiomics: impact of pulse sequence parameter selection on MRI-based textural features of the brain. Contrast Media Mol Imaging 2018; 2018: 1–9. doi: 10.1155/2018/1729071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Savio SJ, Harrison LCV, Luukkaala T, Heinonen T, Dastidar P, Soimakallio S, et al. Effect of slice thickness on brain magnetic resonance image texture analysis. Biomed Eng Online 2010; 9: 60. doi: 10.1186/1475-925X-9-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weygand J, Fuller CD, Ibbott GS, Mohamed AS, Ding Y, Yang J, et al. Wang J: Spatial Precision in Magnetic Resonance Imaging-Guided Radiation Therapy: The Role of Geometric Distortion. International journal of radiation oncology, biology. Physics 2016; 95: 1304–16. [DOI] [PubMed] [Google Scholar]
- 106.Fruehwald-Pallamar J, Hesselink JR, Mafee MF, Holzer-Fruehwald L, Czerny C, Mayerhoefer ME. Texture-Based Analysis of 100 MR Examinations of Head and Neck Tumors - Is It Possible to Discriminate Between Benign and Malignant Masses in a Multicenter Trial? Rofo 2016; 188: 195–202. doi: 10.1055/s-0041-106066 [DOI] [PubMed] [Google Scholar]
- 107.Shukla-Dave A, Obuchowski NA, Chenevert TL, Jambawalikar S, Schwartz LH, Malyarenko D, et al. Quantitative imaging biomarkers alliance (QIBA) recommendations for improved precision of DWI and DCE-MRI derived biomarkers in multicenter oncology trials. J Magn Reson Imaging 2019; 49: e101–21. doi: 10.1002/jmri.26518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol 2019; 76: 340–51. doi: 10.1016/j.eururo.2019.02.033 [DOI] [PubMed] [Google Scholar]
- 109.Jethanandani A, Lin TA, Volpe S, Elhalawani H, Mohamed ASR, Yang P, et al. Exploring applications of Radiomics in magnetic resonance imaging of head and neck cancer: a systematic review. Front Oncol 2018; 8: 131. doi: 10.3389/fonc.2018.00131 [DOI] [PMC free article] [PubMed] [Google Scholar]