Abstract
Objective:
Unsuspected pulmonary embolism (UPE) has been increasingly diagnosed as an incidental finding on CT scans for routine staging in cancer patients. Previous studies suggest that obesity is an independent risk factor for venous thromboembolism in patients with malignant tumors. In this study, we aimed to investigate the association between abdominal adipose tissue, especially visceral adipose tissue (VAT) and the occurrence of UPE in hospitalized patients with gastrointestinal cancer.
Methods:
Routine contrast-enhanced chest and abdominal CT scans of 1974 patients were retrospectively assessed for the presence of UPE, of which 58 patients were identified with UPE and 108 non-UPE patients were selected as the non-UPE control group based on several matching criteria. Abdominal adipose tissue was measured by volumes of VAT and subcutaneous adipose tissue (SAT) at the navel level.
Results:
VAT, SAT, indwelling venous catheters, surgery, chemotherapy, and bed rest or immobilization were associated with the occurrence of UPE. Higher VAT volumes were associated with increased risk of UPE (odds ratio: 1.96; 95% confidence interval: 1.25, 3.06; p = 0.003) adjusting body mass index (BMI), bed rest or immobilization, surgery, chemotherapy and smoking, while SAT was not associated with UPE adjusting the same confounders (p = 0.117). No statistical association was found between BMI and UPE (p = 0.102).
Conclusion:
Higher VAT rather than SAT is associated with an increased risk of unsuspected pulmonary embolism on routine CT scans in hospitalized gastrointestinal cancer patients.
Advances in knowledge:
Our findings indicate that VAT is a stronger risk factor for unsuspected pulmonary embolism than BMI and SAT in hospitalized patients with gastrointestinal cancer.
Introduction
Pulmonary embolism (PE) and deep venous thrombosis (DVT) together, known as venous thromboembolism (VTE), is one of the most common and severe complications in patients with malignant tumors.1 Thrombotic event is the second leading cause of death in cancer patients (after cancer itself) and associated with lower short- and long-term survival.2–4 A number of malignancies are associated with increased risk of VTE, including gastrointestinal cancers and particularly adenocarcinoma.5
In recent years, VTE has been increasingly diagnosed as an incidental finding on CT scans for routine staging in cancer patients. The incidence of VTE in patients with malignancy varied from 0.6% to 7.8% in previous studies.6–10 These VTE events are referred to as “incidental” or “unsuspected” VTE,11–13 and accordingly incidental finding of PE on the routine chest CT examination is defined as unsuspected PE (UPE). According to an estimation based on an epidemiological model in Europe, 59% of the deaths resulting from PE failed to be diagnosed during life.14 Previous studies suggest that UPE shares a similar impact on long-term survival and recurrent VTE compared to symptomatic PE.15 Unlike symptomatic PE, currently available evidence of the risk factors and management of UPE is still insufficient and controversial.15,16
Obesity [body mass index (BMI) ≥ 30 kg/m2] has been reported as an independent risk factor of VTE in patients with malignant tumors.17 Although BMI correlates well with total body adiposity at the population level,18 there are considerable variations in adiposity distribution in individuals with the same BMI.19 Previous studies have suggested that relying solely on BMI could lead to misclassification of cardiometabolic health risks.20,21 Abdominal adipose tissue is mainly consisted of visceral adipose tissue (VAT) around the internal organs inside the abdominal cavity, and subcutaneous adipose tissue (SAT) underneath the skin. While both excess VAT and SAT contribute to obesity, they are metabolically distinct fat compartments.22 Previous studies indicated that VAT was correlated with various diseases including cancer, cardiovascular diseases and metabolic diseases.23–28 However, whether abdominal adipose tissue is associated with an increased risk of UPE has not been addressed yet. In this case–control study, we investigated the risk factors of UPE in hospitalized patients with gastrointestinal cancer, and particularly emphasized on the potential association between UPE and different compartments of abdominal adipose tissue.
Methods
Study design and patients
This study was approved by the institutional review board and the written informed consents of the patients were waived due to its retrospective observational design.
We retrospectively analyzed the routine contrast-enhanced chest and abdominal CT examinations of 1974 consecutive hospitalized patients with gastrointestinal cancer who have been admitted to The First Affiliated Hospital of Sun Yat-sen University between January 2011 to September 2014. Two experienced radiologists (one with 20 years’ experience and the other with 2 years’ experience in clinical thoracic CT) who were blinded to clinical data separately analyzed the CT images of all the cases for the presence of PE. Diagnosis of PE was made if they were in concordance, and cases with disagreements were reviewed by a third radiologist (with more than 15 years’ experience in clinical thoracic CT). Clinical files were subsequently reviewed to determine the documented clinical suspicion of PE in each case. The clinical suspicion of PE was defined by the records documented by the referring clinician with the indication of detection/exclusion of PE.
Considering the large number of potential risk factors for UPE that needed to be adjusted during the analysis, and the relatively small anticipating sample size of the UPE group, non-UPE control cases were selected from the rest of the cohort to match the UPE cases with a ratio of 2:1 by certain rules. As age and cancer characteristics are associated with the occurrence of UPE in cancer patients,29 the matching rules for non-UPE controls were: age variation within 5 years, same tumor site and pathological type, same tumor stage or higher stage. To elaborate the matching rules, e.g. one 55-year-old UPE patient with Stage II stomach adenocarcinoma was matched with two non-UPE controls who were also suffered from stomach adenocarcinoma, in Stage II or higher stage, and aged between 50 and 60 years. When more than two non-UPE cases met all the matching conditions, the two cases whose ages were closest to the UPE case were selected.
Exclusion criteria were: patients complicated with non-digestive tract malignancy; tumor had been removed for more than 1 year or patient was in complete remission; outpatients; patients with no pathological results of the tumor; images with CT values lower than 100 Hounsfield unit in the contrast-enhanced pulmonary trunk30 and/or with severe artifacts (e.g. respiratory motion artifact, flow artifacts caused by pleural effusion and/or atelectasis etc.),31which were inadequate for diagnosis; patients with ongoing anticoagulant therapy.
Clinical parameters
Detailed medical history and clinical records of both UPE group and the non-UPE control group were reviewed, and the following clinical parameters were collected: (1) baseline information including age, sex, height, weight, BMI (kg/m2), body surface area (BSA, m2) and performance status (Zubrod/ECOG/WHO score).32 (2) Basic characteristics of the tumor, including tumor site (esophagus, stomach, small bowl, colon, rectum, pancreas or appendix), pathological type (adenocarcinoma, squamous cell carcinoma, etc.), tumor stage (TNM stage). (3) Clinical management, including indwelling venous catheters within 3 months, surgery within 2 months, chemotherapy within 30 days, and targeted cancer therapy within 30 days. (4) VTE-related histories, including comorbidities (Charlson comorbidity index, CCI), smoking, alcohol intake, bed rest or immobilization (entirely best rest ≥75% within 2 weeks), varicose vein of lower extremity, history of VTE. Smoking and alcohol intake were dichotomy variables depicting both former and current users regardless of the quantity of consumption.
Analysis of CT images and measurements of abdominal adipose tissue
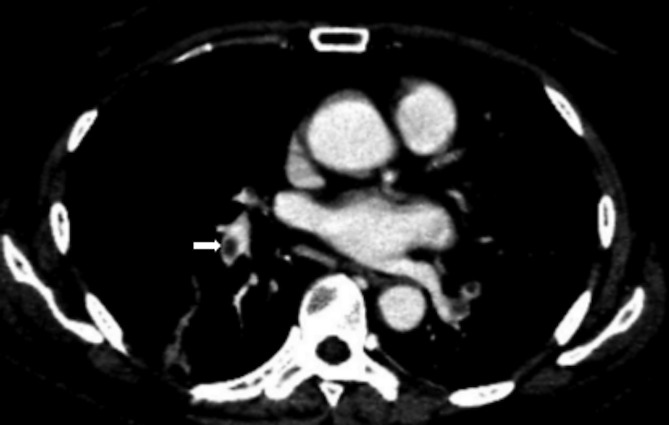
All subjects had undergone routine contrast-enhanced chest and abdominal CT scan by 64-row spiral CT with a supine position. All CT images were retrospectively reviewed using interactive axial and multiplanar reformats at individual window settings without prior knowledge of the clinical or radiological diagnosis. The diagnostic criterion for PE was the presence of filling defect within the contrast-enhanced pulmonary arterial tree in at least two continuous thin-section images (Figure 1).
Figure 1. .
The diagnostic criterion for pulmonary embolism was the presence of filling defect (white arrow) within the contrast-enhanced pulmonary arterial tree on axial contrast-enhanced CT images.
Abdominal adipose tissue was measured using post-processing workstation (Vitrea, Vital Images, Minnetonka, MN). According to previous studies,23,33–35 adipose tissue was measured on continuous axial slices at the navel level. The volumetric raw data were reconstructed with a slice thickness of 10 mm (Figure 2). Abdominal adipose tissue was defined by CT attenuation ranging from −150 to −50 Hounsfield units. All the pixels within this range of CT values were demonstrated automatically in the selected slice. VAT and SAT areas were manually labelled according to anatomy. SAT was defined by the skin and the outer margin of abdominal muscles and fascia, and VAT was delineated by the inner margin of the abdominal wall and the anterior margin of the vertebral body (Figure 3). After manually editing, VAT and SAT volumes (cm3) at the navel level were automatically obtained in the post-processing software, by multiplying the VAT and SAT areas with the 10 mm slice thickness.
Figure 2. .
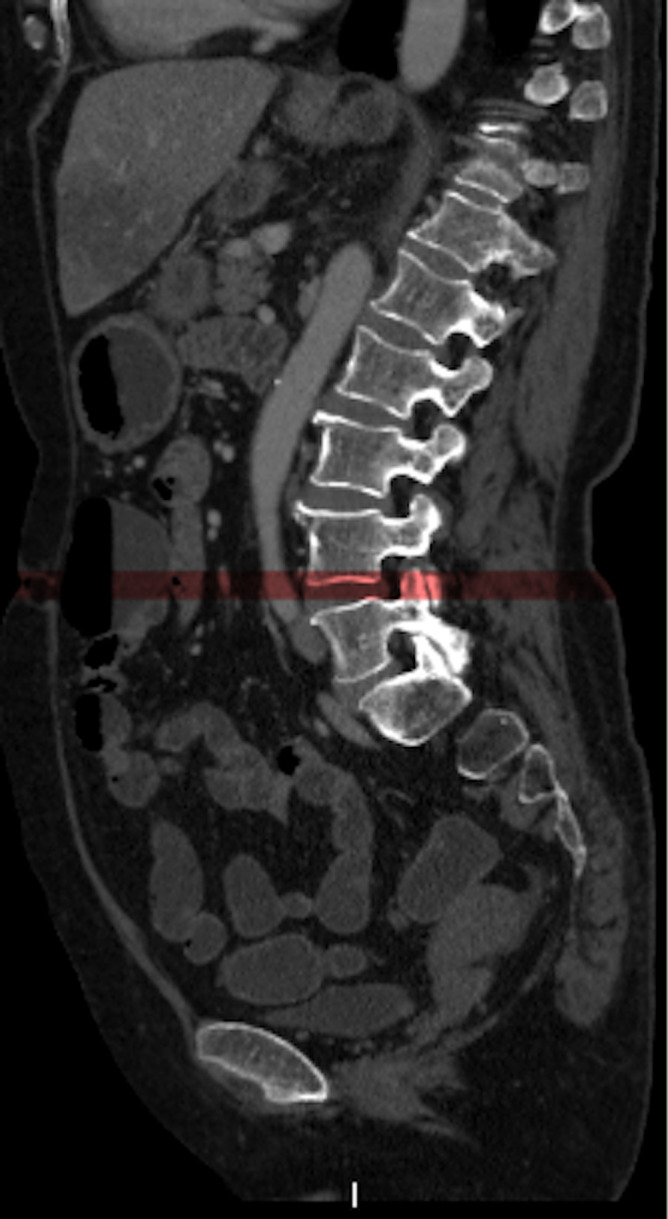
Abdominal adipose tissue was measured on continuous axial slices at the navel level, by reconstructing the volumetric raw data with a slice thickness of 10 mm.
Figure 3. .
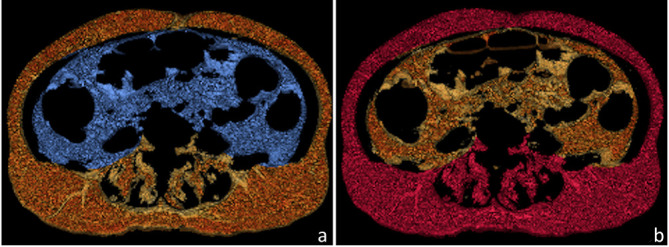
VAT and SAT volumes at the navel level were obtained by the post-processing software. (a) VAT was defined by the inner margin of abdominal wall and the anterior margin of the vertebral body (blue). (b) SAT was defined by the skin and the outer margin of abdominal muscles and fascia (red). SAT,subcutaneous adipose tissue; VAT, visceral adipose tissue.
Statistical analysis
Data were expressed as mean and standard deviation, median and interquartile range, or percent frequency (proportion), as appropriate. χ2 test was used for comparisons of qualitative data. One-way analysis of covariance was utilized for comparisons of VAT and SAT between UPE and non-UPE groups adjusting age and BMI. Conditional univariable logistic regression analysis was performed to evaluate the associations between UPE and the potential risk factors. The associations of VAT and SAT with the occurrence of UPE were evaluated with adjustment of possible confounders using conditional multivariable logistic regression.
p value < 0.05 was considered statistically significant. All analyses were performed using a standard statistical package (SPSS for Windows, v. 20.0, SPSS Inc., Chicago, IL).
Results
Characteristics of the UPE group and non-UPE controls
None of the 1974 patients was under clinical suspicion of PE when they underwent routine CT scans. 32 cases were excluded due to poor image quality. Eight cases were excluded because they were taking anticoagulant medications, and no sign of PE was found in the CT scans of these patients. Among the rest 1934 patients, 58 patients (age from 35 to 88 years, 60.3% males) were found to have UPE. During the enrollment of the non-UPE matched subjects, four cases in the UPE group could not be completely matched due to their rare tumor site and pathological type. Therefore, 108 cases (age 36–86 years, 72.2% males) were enrolled in the non-UPE control group. The demographic, tumor-related and clinical parameters are presented in Table 1. There was no statistical difference in sex distribution between the two groups (p = 0.118). Stomach (25 cases, 43.10%) and colon (20 cases, 34.48%) were the most frequent tumor sites. Adenocarcinoma was the main pathological type (86.21%). Most subjects were in an advanced stage and a number of them had metastasis (75.86%).
Table 1. .
Characteristics of the UPE group and non-UPE controls
| Indexes | Total (n = 166) |
UPE group (n = 58) |
Non-UPE control group (n = 108) |
|---|---|---|---|
| Age (y/o) | 62.0 (58.0, 69.0) | 62.00 (58.0, 68.0) | 63.00 (58.0, 69.0) |
| Sex, n (%) | |||
| Male | 113 (68.07) | 35 (60.34) | 78 (72.22) |
| Female | 53 (31.93) | 23 (39.66) | 30 (27.78) |
| BMI, kg/m2 | 21.81 ± 3.13 | 22.51 ± 3.71 | 21.44 ± 2.72 |
| Performance status | 1.0 (1.0,2.0) | 1.0 (1.0,1.0) | 1.0 (1.0,2.0) |
| Tumor site, n (%) | |||
| Esophagus | 10 (6.02%) | 4 (6.90%) | 6 (5.56%) |
| Stomach | 75 (45.18%) | 25 (43.10%) | 50 (46.30%) |
| Small bowl | 4 (2.41%) | 2 (3.45%) | 2 (1.85%) |
| Colon | 60 (36.14%) | 20 (34.48%) | 40 (37.04%) |
| Rectum | 1 (0.61%) | 1 (1.72%) | 0 (0.00%) |
| Pancreas | 16 (9.64%) | 6 (10.34%) | 10 (9.26%) |
| Pathological type, n (%) | |||
| Adenocarcinoma | 150 (90.36%) | 50 (86.21%) | 100 (92.59%) |
| Other type | 16 (9.64%) | 8 (13.79%) | 8 (7.41%) |
| Tumor stage, n (%) | |||
| Ⅰ | 3 (1.81%) | 2 (1.85%) | 1 (1.72%) |
| Ⅱ | 35 (21.08%) | 14 (24.14%) | 21 (19.44%) |
| Ⅲ | 62 (37.35%) | 20 (34.48%) | 42 (38.89%) |
| Ⅳ | 66 (39.76%) | 23 (39.66%) | 43 (39.81%) |
| Metastasis, n (%) | |||
| Yes | 134 (80.72%) | 44 (75.86%) | 90 (83.33%) |
| No | 32 (19.28%) | 14 (24.14%) | 18 (16.67%) |
| Indwelling venous catheters<3 months | 51 (30.72%) | 34 (58.62%) | 17 (15.74%) |
| Surgery<2 months | 41 (24.70%) | 27 (46.55%) | 14 (12.96%) |
| Chemotherapy<30 days | 17 (10.24%) | 11 (18.97%) | 6 (5.56%) |
| Targeted cancer therapy<30 days | 3 (1.81%) | 2 (3.45%) | 1 (0.93%) |
| Charlson comorbidity index | 5.0 (4.0, 8.0) | 5.0 (4.0, 7.0) | 5.5 (4.0, 8.0) |
| Smoking | 45 (27.11%) | 11 (18.97%) | 34 (31.48%) |
| Alcohol intake | 18 (10.84%) | 6 (10.34%) | 12 (11.11%) |
| Bed rest or immobilization | 30 (18.07%) | 16 (27.59%) | 14 (12.96%) |
| VTE history | 2 (1.20%) | 2 (3.45%) | 0 (0.00%) |
UPE, unsuspected pulmonary embolism; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; BMI, body mass index; VTE, venous thromboembolism.
Note: Parametric variables are mean ± standard deviation; non-parametric variables are median (interquartile range).
Compared with non-UPE control group, patients in the UPE group had higher levels of SAT (202.38 ± 91.39 cm3 vs 163.70 ± 63.10 cm3) and VAT (192.14 ± 65.36 cm3 vs 157.02 ± 69.69 cm3) than the non-UPE group after adjustment for age and BMI (Table 2).
Table 2. .
Comparisons of abdominal adipose tissue between UPE group and non-UPE controls adjusting age and BMI
| Indexes | Total(n = 166) | UPE group(n = 58) | Non-UPE control group (n = 108) | F value | p-value |
|---|---|---|---|---|---|
| VAT, cm3 | 169.29 ± 70.05 | 192.14 ± 65.36 | 157.02 ± 69.69 | 6.789 | 0.01 |
| SAT, cm3 | 176.75 ± 75.83 | 202.38 ± 91.39 | 163.70 ± 63.10 | 5.202 | 0.024 |
UPE, unsuspected pulmonary embolism; BMI, body mass index; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue.
Risk factors for the occurrence of UPE
Factors found to be associated with the occurrence of UPE in univariable conditional logistic regression analysis were VAT, SAT, indwelling venous catheters within 3 months, surgery within 2 months, chemotherapy within 30 days, bed rest or immobilization (Table 3). BMI was not associated with the occurrence of UPE (p = 0.102). CCI, alcohol intake, smoking, targeted cancer therapy and VTE history was not associated with the occurrence of UPE.
Table 3. .
The associations between the occurrence of UPE and potential risk factors in conditional univariable logistic regression analysis
| Variables | χ2 | p-value | OR (95% CI) |
|---|---|---|---|
| VAT, cm3 | 7.839 | 0.005 | 1.442 (1.117, 1.861)a |
| SAT, cm3 | 6.918 | 0.023 | 1.289 (1.035, 1.605)a |
| Indwelling venous catheters < 3 months | 22.042 | <0.0001 | 8.349 (3.443, 20.247) |
| Surgery <2 months | 16.993 | <0.0001 | 6.736 (2.720, 16.682) |
| Chemotherapy <30 days | 5.436 | 0.0198 | 3.333 (1.211, 9.171) |
| Smoking | 3.064 | 0.080 | 0.483 (0.214, 1.091) |
| Bed rest or immobilization | 6.420 | 0.011 | 3.103 (1.292,7.452) |
| BMI, kg/m2 | 2.679 | 0.102 | 1.089 (0.983, 1.205) |
BMI, body mass index;CI, confidential interval; OR, odds ratio; SAT, subcutaneous adipose tissue;UPE, unsuspected pulmonary embolism;VAT, visceral adipose tissue.
With increment of 50 cm3.
Associations between the occurrence of UPE and VAT, SAT
As mentioned above, larger VAT volume was positively associated with higher odds of UPE [odds ratio (OR): 1.44; 95% confidence interval (CI) (1.12, 1.86); p = 0.005] in univariable logistic regression analysis (Table 3). After the adjustment of surgery within 2 months, chemotherapy within 30 days, bed rest and immobilization, smoking and BMI, the OR of UPE increased to 1.96 [95% CI (1.26, 3.06); p = 0.003] (Table 4). Each 50 cm3 increase of the VAT volume at the navel level was associated with almost twofold risk of the occurrence of UPE in hospitalized patients with gastrointestinal malignancy. As for SAT, despite a positive association existed between larger SAT and higher odds of UPE in univariable logistic regression [OR: 1.29; 95% CI (1.04, 1.61); p = 0.023] (Table 3), the statistical association was lost after adjusting the same confounders of VAT [OR: 1.34; 95% CI (0.93, 1.94); p = 0.117] (Table 4).
Table 4. .
Conditional multivariable logistic regression analysis for the associations of VAT and SAT with the occurrence of UPE
| Adjusting covariates | χ2 | p-value | OR (95% CI) | |
|---|---|---|---|---|
| VAT, cm3 | Surgery <2 months Chemotherapy <30 days Bed rest or immobilization Smoking BMI |
8.783 | 0.003 | 1.960 (1.256, 3.057)a |
| SAT, cm3 | 2.461 | 0.117 | 1.343 (0.929, 1.940)a |
BMI, body mass index; BSA, body surface area; OR, odds ratio;SAT, subcutaneous adipose tissue;UPE, unsuspected pulmonary embolism; VAT, visceral adipose tissue.
With increment of 50 cm3.
Considering that excessive covariates could hamper the statistical power of multiple regression models, not all potential confounders were involved in the above analysis. Furthermore, if other confounders including indwelling venous catheters within 2 months, CCI and alcohol intake were also involved the regression models, the correlations of VAT and SAT with UPE remained essentially unaltered.
Discussion
In this case–control study, we identified that large abdominal adipose tissue, indwelling venous catheters, bed rest or immobilization, surgery and chemotherapy were risk factors for the occurrence of UPE in hospitalized patients with gastrointestinal cancer. Particularly, larger volumes of VAT rather than SAT was associated with higher risk of UPE when adjusting previously identified confounders.
Associations between obesity, VAT and UPE
Obesity, conventionally evaluated by BMI, is a known independent risk factor of VTE in cancer patients.36 Nevertheless, BMI has not been found to be a risk factor for UPE in cancer patients based on the current evidence.15 Similarly, no statistical association was found between BMI and the occurrence of UPE in our study. Recent studies have demonstrated the distribution of adipose tissue in obese patients may play a role in determining the untoward effects other than obesity itself.37 Especially, VAT has been increasingly shown to be a more important and correlated obese index in metabolic disorders, cardiovascular disease, carcinoma, brain tissue damage and inflammatory diseases.23,28,37–43 Our study revealed that larger VAT, rather than SAT and BMI, was associated with UPE event, indicating that VAT might have stronger impact than BMI or SAT in the development of UPE in cancer patients.
The possible mechanisms for the relationship between VAT and UPE
The pathophysiological pathways linking VAT and UPE are not completely understood. However, several mechanisms could be proposed based on previously published literature. Firstly, an increased amount of abdominal visceral fat has been suggested to play a role in disturbances in hemostasis and fibrinolysis. Fibrinogen and plasminogen activator inhibitor 1 (PAI-1), as the most important inhibitor of the fibrinolytic system, have been reported to be associated with visceral obesity, with the most convincing evidence found for the involvement of PAI-1.44 VAT produces more PAI-1 compared with SAT, and PAI-1 inhibits the degradation of fibrin clots, contributing to an increased atherothrombotic risk.45 Secondly, inflammation may be a potential mechanism of the relationship between VAT and UPE. VAT functions not only as endocrine organ secreting hormones, but also as inflammatory tissue secreting cytokines and chemokines.34 It is biologically active in producing proinflammatory cytokines (adipokines) such as TNF-α, interleukin 6, interleukin 8, and leptin.46,47 Elevation of these cytokines was reported to be associated with increased VTE risk.48 It has been found that obese people have more inflammatory cells and mediums than people with normal weight.49 These cytokines and accumulated inflammatory cells may influence the vascular endothelium, resulting in endothelial damage and dysfunction,50 thus higher thrombosis risk. Thirdly, a recent study suggested that immune activation and inflammatory adherence existed throughout the formation of acute VTE. It was hypothesized that VTE was the result of a biological filamentous mesh-like structure formed when circulated allotype antigen cells were not cleared effectively and timely in the presence of immune dysfunction.51 Relative studies provide new visual angle in the theoretical knowledge of the impact of VAT on the genesis of VTE. However, verification of the true mechanism requires further investigations.
Other risk factors for UPE in hospitalized patients with gastrointestinal cancer
Besides VAT related variables, our study found that indwelling venous catheters within 3 months, bed rest or immobilization, surgery within 2 months and chemotherapy within 30 days were risk factors for UPE in hospitalized patients with gastrointestinal cancer, which were consistent with Donadini et al’s review data15 and den Exter et al’s52 results. However, in a similar prior study,53 immobilization, the presence of a central venous line and chemotherapy within 30 days were not found as risk factors for UPE. This different result might be attributed to the more specific patient group in our study. Meanwhile, other risk factors such as physical performance declination and VTE history which were identified in several studies15,33 were of no statistical significance in our study. In addition, some uncertain factors such as comorbidity, smoking and alcohol intake were not correlated with UPE in our study either. These might be attributed to an inadequate sample size.
Clinical implications for UPE prevention
Gastrointestinal cancer is a group of malignant tumors afflicting a major portion of all the cancer patients worldwide. UPE were increasingly detected in routine tumor staging CT scans due to both the high sensitivity of multidetector CT and high incidence of PE among these patients.15,53 Even so, clinicians may fail in correctly diagnosing fatal PE in up to 70% of hospitalized patients.54 Therefore, the identification of the risk factors for UPE in cancer patients is crucial for clinicians and radiologists to get better knowledge of the prevention and diagnosis of PE. The most significant finding in our study is that VAT is associated with UPE in cancer patients. Since VAT volume can be managed by diet and exercises, this finding may have clinical value for UPE prevention. Furthermore, VAT measurements during tumor staging for high risk populations (i.e. obese patients) could be recommended in clinical scenarios.
Limitations and conclusions
Limitations of our study include the retrospective design and the single-center data. Another limitation is that the true risk of the prevalence of UPE in this cohort may be underestimated due to the routine contrast-enhanced CT protocol, which was not a dedicated pulmonary angiogram. As abdominal fat distributions on male and female are quite different,55,56 another limitation is that we did not match the non-UPE cases by sex. However, since there was no statistical difference in sex distribution between the UPE group (60.3% male) and non-UPE control group (72.2% male), we expected that the influence of sex on the associations of VAT and SAT with the occurrence of UPE would be limited. Additional adjustment of sex in our multiple regression models did not markedly change the results (data not shown). Finally, our results were based on a specific sample of hospitalized patients with gastrointestinal cancer. Thus, the generalization of our findings requires further validation by large-scale prospective investigations regarding patients with various malignancies. However, considering the high prevalence of underdiagnose of UPE, its impact on the survival of cancer patients, and the lack of understanding of the prognostic value of VAT in these patients, this study may inspire further investigations in the mechanism of VAT's contribution in UPE.
In summary, our study indicated that abdominal adipose tissue, indwelling venous catheters, bed rest or immobilization, surgery and chemotherapy were risk factors for UPE in hospitalized patients with gastrointestinal cancer. Our findings revealed an association between larger VAT and increased risk of UPE with adjustment of previously identified confounders, suggesting that VAT is a stronger risk factor for UPE than BMI and SAT in hospitalized patients with gastrointestinal cancer. Further investigation is required for the underlying mechanisms of the association between VAT and UPE.
Footnotes
Acknowledgments: The authors thank Xiangxiang Chen from Canon Medical System (China) for her technical support in abdominal adipose tissue measurements on CT, but she did not have any role in the design or conducting of the study, the collection, management, analysis or interpretation of the data, the preparation of the manuscript, or the decision to publish.
Xiaojuan Xiao and Yao Wang have contributed equally to this study and should be considered as co-first authors.
Contributor Information
Xiaojuan Xiao, Email: xiaoxj90@126.com.
Yao Wang, Email: bigfaceyao@163.com.
Ying Gao, Email: gyinger423@163.com.
Qiuxia Xie, Email: www.xqx@qq.com.
Xuhui Zhou, Email: zhouxuh@mail.sysu.edu.cn.
Ling Lin, Email: l.lin@lumc.nl.
Ilona A. Dekkers, Email: I.A.Dekkers@lumc.nl.
Hildo J. Lamb, Email: h.j.lamb@lumc.nl.
REFERENCES
- 1.Prandoni P, Falanga A, Piccioli A. Cancer and venous thromboembolism. Lancet Oncol 2005; 6: 401–10. doi: 10.1016/S1470-2045(05)70207-2 [DOI] [PubMed] [Google Scholar]
- 2.Sørensen HT, Mellemkjær L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000; 343: 1846–50. doi: 10.1056/NEJM200012213432504 [DOI] [PubMed] [Google Scholar]
- 3.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. Journal of Thrombosis and Haemostasis 2007; 5: 632–4. doi: 10.1111/j.1538-7836.2007.02374.x [DOI] [PubMed] [Google Scholar]
- 4.Khorana AA. Venous thromboembolism and prognosis in cancer. Thromb Res 2010; 125: 490–3. doi: 10.1016/j.thromres.2009.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rickles FR, Levine MN. Epidemiology of thrombosis in cancer. Acta Haematol 2001; 106(1-2): 6–12. doi: 10.1159/000046583 [DOI] [PubMed] [Google Scholar]
- 6.Sallah S, Wan JY, Nguyen NP. Venous thrombosis in patients with solid tumors: determination of frequency and characteristics. Thrombosis and haemostasis 2002; 87: 575–9. [PubMed] [Google Scholar]
- 7.Levitan N, Dowlati A, Remick SC, Tahsildar HI, Sivinski LD, Beyth R, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. risk analysis using Medicare claims data. Medicine 1999; 78: 285–91. doi: 10.1097/00005792-199909000-00001 [DOI] [PubMed] [Google Scholar]
- 8.Khorana AA, Francis CW, Culakova E, Fisher RI, Kuderer NM, Lyman GH. Thromboembolism in hospitalized neutropenic cancer patients. J Clin Oncol 2006; 24: 484–90. doi: 10.1200/JCO.2005.03.8877 [DOI] [PubMed] [Google Scholar]
- 9.Stein PD, Beemath A, Meyers FA, Skaf E, Sanchez J, Olson RE. Incidence of venous thromboembolism in patients hospitalized with cancer. Am J Med 2006; 119: 60–8. doi: 10.1016/j.amjmed.2005.06.058 [DOI] [PubMed] [Google Scholar]
- 10.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH, Frequency LGH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer 2007; 110: 2339–46. doi: 10.1002/cncr.23062 [DOI] [PubMed] [Google Scholar]
- 11.Dentali F, Ageno W, Becattini C, Galli L, Gianni M, Riva N, et al. Prevalence and clinical history of incidental, asymptomatic pulmonary embolism: a meta-analysis. Thromb Res 2010; 125: 518–22. doi: 10.1016/j.thromres.2010.03.016 [DOI] [PubMed] [Google Scholar]
- 12.Khorana AA, O'Connell C, Agnelli G, Liebman HA, Lee AYY, , et al.Subcommittee on Hemostasis and Malignancy of the SSC of the ISTH . Incidental venous thromboembolism in oncology patients. J Thromb Haemost 2012; 10: 2602–4. doi: 10.1111/jth.12023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khorana AA. Cancer-Associated thrombosis: updates and controversies. ASH Education Program Book 2012; 2012: 626–30. [DOI] [PubMed] [Google Scholar]
- 14.Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, et al. Venous thromboembolism (VTe) in Europe. Thrombosis and haemostasis 2007; 98: 756–64. [DOI] [PubMed] [Google Scholar]
- 15.Donadini MP, Dentali F, Squizzato A, Guasti L, Ageno W. Unsuspected pulmonary embolism in cancer patients: a narrative review with pooled data. Intern Emerg Med 2014; 9: 375–84. doi: 10.1007/s11739-014-1066-7 [DOI] [PubMed] [Google Scholar]
- 16.Trujillo-Santos J, Monreal M. Management of unsuspected pulmonary embolism in cancer patients. Expert Rev Hematol 2013; 6: 83–9. doi: 10.1586/ehm.12.72 [DOI] [PubMed] [Google Scholar]
- 17.Stein PD, Beemath A, Olson RE. Obesity as a risk factor in venous thromboembolism. Am J Med 2005; 118: 978–80. doi: 10.1016/j.amjmed.2005.03.012 [DOI] [PubMed] [Google Scholar]
- 18.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr 2000; 72: 694–701. doi: 10.1093/ajcn/72.3.694 [DOI] [PubMed] [Google Scholar]
- 19.Després J-P. Excess visceral adipose tissue/ectopic fat the missing link in the obesity paradox? J Am Coll Cardiol 2011; 57: 1887–9. doi: 10.1016/j.jacc.2010.10.063 [DOI] [PubMed] [Google Scholar]
- 20.Tomiyama AJ, Hunger JM, Nguyen-Cuu J, Wells C. Misclassification of cardiometabolic health when using body mass index categories in NHANES 2005–2012. Int J Obes 2016; 40: 883–6. doi: 10.1038/ijo.2016.17 [DOI] [PubMed] [Google Scholar]
- 21.Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes 2008; 32(S3): S56–9. doi: 10.1038/ijo.2008.87 [DOI] [PubMed] [Google Scholar]
- 22.Neeland IJ, Ayers CR, Rohatgi AK, Turer AT, Berry JD, Das SR, et al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity (Silver 2013; 21: E439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katsuyama H, Kawaguchi A, Yanai H. Not visceral fat area but the ratio of visceral to subcutaneous fat area is significantly correlated with the marker for atherosclerosis in obese subjects. Int J Cardiol 2015; 179: 112–3. doi: 10.1016/j.ijcard.2014.10.112 [DOI] [PubMed] [Google Scholar]
- 24.Bergström A, Hsieh CC, Lindblad P, Lu CM, Cook NR, Wolk A. Obesity and renal cell cancer--a quantitative review. Br J Cancer 2001; 85: 984–90. doi: 10.1054/bjoc.2001.2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bianchini F, Kaaks R, Overweight VH. Obesity, and cancer risk. The lancet oncology 2002; 3: 565–74. [DOI] [PubMed] [Google Scholar]
- 26.Guiu B, Petit JM, Bonnetain F, Ladoire S, Guiu S, Cercueil J-P, et al. Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut 2010; 59: 341–7. doi: 10.1136/gut.2009.188946 [DOI] [PubMed] [Google Scholar]
- 27.Ladoire S, Bonnetain F, Gauthier M, Zanetta S, Petit JM, Guiu S, et al. Visceral fat area as a new independent predictive factor of survival in patients with metastatic renal cell carcinoma treated with antiangiogenic agents. Oncologist 2011; 16: 71–81. doi: 10.1634/theoncologist.2010-0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohashi N, Yamamoto H, Horiguchi J, Kitagawa T, Hirai N, Ito K, et al. Visceral fat accumulation as a predictor of coronary artery calcium as assessed by multislice computed tomography in Japanese patients. Atherosclerosis 2009; 202: 192–9. doi: 10.1016/j.atherosclerosis.2008.04.019 [DOI] [PubMed] [Google Scholar]
- 29.SAHUT D’IZARN M, Caumont Prim A, Planquette B, Revel MP, Avillach P, Chatellier G, et al. Risk factors and clinical outcome of unsuspected pulmonary embolism in cancer patients: a case-control study. J Thromb Haemost 2012; 10: 2032–8. doi: 10.1111/j.1538-7836.2012.04868.x [DOI] [PubMed] [Google Scholar]
- 30.Storto ML, Di Credico A, Guido F, Larici AR, Bonomo L. Incidental detection of pulmonary emboli on routine MDCT of the chest. American Journal of Roentgenology 2005; 184: 264–7. doi: 10.2214/ajr.184.1.01840264 [DOI] [PubMed] [Google Scholar]
- 31.Wittram C, Maher MM, Yoo AJ, Kalra MK, Shepard J-AO, McLoud TC. Ct angiography of pulmonary embolism: diagnostic criteria and causes of misdiagnosis. RadioGraphics 2004; 24: 1219–38. doi: 10.1148/rg.245045008 [DOI] [PubMed] [Google Scholar]
- 32.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the eastern cooperative Oncology Group. Am J Clin Oncol 1982; 5: 649–56. doi: 10.1097/00000421-198212000-00014 [DOI] [PubMed] [Google Scholar]
- 33.Dutia M, White RH, Wun T. Risk assessment models for cancer-associated venous thromboembolism. Cancer 2012; 118: 3468–76. doi: 10.1002/cncr.26597 [DOI] [PubMed] [Google Scholar]
- 34.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J 2007; 153: 907–17. doi: 10.1016/j.ahj.2007.03.019 [DOI] [PubMed] [Google Scholar]
- 35.Seidell JC, Bakker CJ, van der Kooy K. Imaging techniques for measuring adipose-tissue distribution--a comparison between computed tomography and 1.5-T magnetic resonance. Am J Clin Nutr 1990; 51: 953–7. doi: 10.1093/ajcn/51.6.953 [DOI] [PubMed] [Google Scholar]
- 36.Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism. Circulation 2008; 117: 93–102. doi: 10.1161/CIRCULATIONAHA.107.709204 [DOI] [PubMed] [Google Scholar]
- 37.Uko V, Vortia E, Achkar J-P, Karakas P, Fiocchi C, Worley S, et al. Impact of abdominal visceral adipose tissue on disease outcome in pediatric Crohnʼs disease. Inflamm Bowel Dis 2014; 20: 2286–91. doi: 10.1097/MIB.0000000000000200 [DOI] [PubMed] [Google Scholar]
- 38.Thanassoulis G, Massaro JM, O'donnell CJ, Hoffmann U, Levy D, Ellinor PT, et al. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham heart study. Circulation: Arrhythmia and Electrophysiology 2010; 3: 345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roever LS, Resende ES, Diniz AL, Penha-Silva N, Veloso FC, Casella-Filho A, et al. Abdominal obesity and association with atherosclerosis risk factors: the Uberlandia heart study. Medicine 2016; 95: e1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diaz AA, Young TP, Kurugol S, Eckbo E, Muralidhar N, Chapman JK, et al. Abdominal visceral adipose tissue is associated with myocardial infarction in patients with COPD. J Copd F 2015; 2: 8–16. doi: 10.15326/jcopdf.2.1.2015.0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Widya RL, Kroft LJM, Altmann-Schneider I, van den Berg-Huysmans AA, van der Bijl N, de Roos A, et al. Visceral adipose tissue is associated with microstructural brain tissue damage. Obesity 2015; 23: 1092–6. doi: 10.1002/oby.21048 [DOI] [PubMed] [Google Scholar]
- 42.Oikawa M, Owada T, Yamauchi H, Misaka T, Machii H, Yamaki T, et al. Predominance of abdominal visceral adipose tissue reflects the presence of aortic valve calcification. Biomed Res Int 2016; 2016: 2174657–5. doi: 10.1155/2016/2174657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JJ, Pedley A, Hoffmann U, Massaro JM, Levy D, Long MT. Visceral and intrahepatic fat are associated with cardiometabolic risk factors above other ectopic fat depots: the Framingham heart study. Am J Med 2018; 131: 684–92e12.. doi: 10.1016/j.amjmed.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimomura I, Funahasm T, Takahashi M, Maeda K, Kotani K, Nakamura T, et al. Enhanced expression of PAI–1 in visceral fat: possible contributor to vascular disease in obeisty. Nat Med 1996; 2: 800–3. doi: 10.1038/nm0796-800 [DOI] [PubMed] [Google Scholar]
- 45.Mertens I, Van Gaal LF. Visceral fat as a determinant of fibrinolysis and hemostasis. Semin Vasc Med 2005; 5: 48–55. doi: 10.1055/s-2005-871741 [DOI] [PubMed] [Google Scholar]
- 46.Schäffler A, Fürst A, Büchler C, Paul G, Rogler G, Schölmerich J, et al. Secretion of RANTES (CCL5) and interleukin‐10 from mesenteric adipose tissue and from creeping fat in Crohn’s disease: Regulation by steroid treatment. Journal of gastroenterology and hepatology 2006; 21: 1412–8. [DOI] [PubMed] [Google Scholar]
- 47.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. Journal of Clinical Investigation 2003; 112: 1821–30. doi: 10.1172/JCI200319451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Branchford BR, Carpenter SL. The role of inflammation in venous thromboembolism. Front. Pediatr. 2018; 6: 142. doi: 10.3389/fped.2018.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitamins & Hormones 2006; 74: 443–77. [DOI] [PubMed] [Google Scholar]
- 50.Lau DCW, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol 2005; 288: H2031–41. doi: 10.1152/ajpheart.01058.2004 [DOI] [PubMed] [Google Scholar]
- 51.Wang L-M, Duan Q-L, Yang F, X-H Y, Zeng Y, Tian H-Y, et al. Activation of circulated immune cells and inflammatory immune adherence are involved in the whole process of acute venous thrombosis. International journal of clinical and experimental medicine 2014; 7: 566–72. [PMC free article] [PubMed] [Google Scholar]
- 52.den Exter PL, Kroft LJM, van der Hulle T, Klok FA, Jiménez D, Huisman MV. Embolic burden of incidental pulmonary embolism diagnosed on routinely performed contrast-enhanced computed tomography imaging in cancer patients. Journal of Thrombosis and Haemostasis 2013; 11: 1620–2. doi: 10.1111/jth.12325 [DOI] [PubMed] [Google Scholar]
- 53.O'Connell CL, Boswell WD, Duddalwar V, Caton A, Mark LS, Vigen C, et al. Unsuspected pulmonary emboli in cancer patients: clinical correlates and relevance. Journal of Clinical Oncology 2006; 24: 4928–32. doi: 10.1200/JCO.2006.06.5870 [DOI] [PubMed] [Google Scholar]
- 54.Stein PD, Henry JW. Prevalence of acute pulmonary embolism among patients in a general Hospital and at autopsy. Chest 1995; 108: 978–81. doi: 10.1378/chest.108.4.978 [DOI] [PubMed] [Google Scholar]
- 55.Lemieux S, Prud'homme D, Bouchard C, Tremblay A, Després JP. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. Am J Clin Nutr 1993; 58: 463–7. doi: 10.1093/ajcn/58.4.463 [DOI] [PubMed] [Google Scholar]
- 56.Camhi SM, Bray GA, Bouchard C, Greenway FL, Johnson WD, Newton RL, et al. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity 2011; 19: 402–8. doi: 10.1038/oby.2010.248 [DOI] [PMC free article] [PubMed] [Google Scholar]