Abstract
Lung cancer is the most commonly diagnosed cancer and biggest cause of cancer mortality worldwide with non-small cell lung cancer (NSCLC) accounting for most cases. Radiotherapy (RT) plays a key role in its management and is used at least once in over half of patients in both curative and palliative treatments. This narrative review will demonstrate how the evolution of RT for NSCLC has been underpinned by improvements in RT technology. These improvements have facilitated geometric individualization, increasingly accurate treatment and now offer the ability to deliver truly individualized RT. In this review, we summarize and discuss recent developments in the field of advanced RT in early stage, locally advanced and metastatic NSCLC. We highlight limitations in current approaches and discuss future potential treatment strategies for patients with NSCLC.
Introduction
Lung cancer is the most commonly diagnosed malignancy and the biggest cause of cancer mortality worldwide, accounting for 1.6 million deaths per year.1 Non-small cell lung cancer (NSCLC) makes up the majority of lung cancer cases. Treatment for NSCLC depends mostly on the stage of disease and patient fitness. Radiotherapy (RT) is used in all stages of lung cancer treatment and is required at least once in over half of patients for either cure or palliation.2
Historically, RT for lung cancer was planned in a simulator using parallel opposed fields and anatomical landmarks to define the target.3 The introduction of three-dimensional (3D) conformal RT using CT planning in the 1990s allowed improved tumour coverage and reduction in dose to organs at risk (OARs). Even more conformal treatment has become possible with the advent of intensity modulated radiotherapy (IMRT) in which the RT beam fluence, weight and shape are varied for multiple beams during treatment.4
Imaging capabilities have progressed alongside RT. Four-dimensional CT (4DCT), in which the respiratory motion of the tumour is visualized, has facilitated smaller margins individualized to a patient’s breathing cycle. This motion adaptation reduces the risk of a geographical miss in lung cancer RT.5 Cone beam CT (CBCT) has replaced two-dimensional megavoltage portal imaging to provide a more accurate set-up.6 These advances in imaging and RT technology allow individualized RT plans. Although the treatment dose, fractionation and dose limits for OARs do not change between patients, the dose distribution can be tailored to each individual’s anatomy.7 Whilst approaches to systemic therapy have become increasingly personalized according to tumour histology and molecular status,8 curative RT is still prescribed mainly according to the TNM stage, performance status and comorbidities, taking no account of the tumour biology. This narrative review will demonstrate how the evolution of RT for NSCLC has been underpinned by improvements in RT technology. This has facilitated geometric individualization, increasingly accurate treatment and now offers the ability to deliver truly individualized RT. In this review, we summarize and discuss recent developments in the field of advanced RT in early stage, locally advanced (LA) and metastatic NSCLC.
Geometric individualiZation
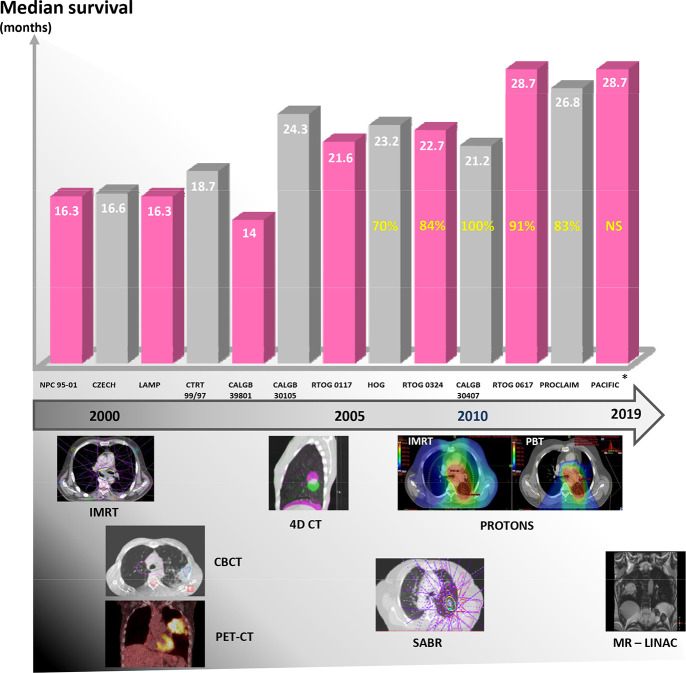
There are a number of challenges involved in the delivery of safe and accurate RT to the thorax.9 The lung has a low electron density. This can result in altered lateral dose, thus reducing the steepness of the dose fall-off and the conformality of the dose distribution. Physiological motion, tumour heterogeneity and the proximity of radiation sensitive healthy tissues, such as the lung, oesophagus and heart, increase the complexity of RT delivery. Historically, the lack of image guidance may have increased the likelihood of a geographical tumour miss, while simple beam arrangements and the uniform dose of large fields might have limited the potential of delivering a curative dose.3 Significant developments in advanced RT technologies have helped address these challenges.10–13 Figure 1 charts the development of RT technologies over time and the corresponding improvement in median survival in trials of patients with Stage 3 NSCLC.
Figure 1. .
The improving (median) survival of patients with Stage 3 NSCLC (based on trial data). This is presented with a timeline showing the introduction of technologies that have been attributed to improving the accuracy of RT (note this does not necessarily correlate with implementation into the clinical workflow). Yellow numbers: percentage of patients within each trial staged with PET-CT, this information was NS by the PACIFIC investigators. *PACIFIC survival data: This is from the control arm only. The median figure has not yet been reached in the durvalumab arm. Note randomization occurred post chemoradiation. Therefore, survival is measured from this point onwards. 4DCT,four-dimensional CT; CBCT, cone beam CT; IMRT, intensity modulated radiotherapy;MR-linac, magnetic resonance linear accelerator; NS, not supplied; NSCLC, non-smallcell lung cancer; PET-CT, positron emissiontomography-CT; RT, radiotherapy; SABR, stereotactic ablative radiotherapy.
The first of these developments to improve RT conformality was the introduction of the multileaf collimator (MLC), which shapes the RT field to the target. In addition to beam shaping, IMRT uses multiple MLC arrangements in order to provide fluence modulation. This enables sculpting of high doses around the target and away from OARs.14 Volumetric modulated arc therapy (VMAT), also called rotational IMRT, allows further modulation. This is achieved by varying the dose rate and enabling dynamic gantry rotation during beam delivery. In combination with a flattening-filter-free technique, VMAT can reduce treatment delivery time compared to fixed-beam IMRT.15 A shorter delivery time is particularly useful when delivering a high dose per fraction as in stereotactic ablative radiotherapy (SABR).16
As a result of the additional level of modulation compared to 3D conformal RT, IMRT can enhance the therapeutic ratio by optimizing dose to the tumour whilst sparing normal tissues. Consequently, IMRT will reduce toxicity and permit the radical treatment of patients with large tumours, who in the past would have been treated palliatively.4 Although there has not been a randomized trial comparing IMRT and 3D conformal RT for the treatment of lung cancer, some evidence for the use of IMRT is provided from a planned secondary analysis of data from the multi-institutional Phase III RTOG 0617 trial. This trial compared dose escalated chemoradiotherapy (CRT) with standard CRT. 47% of patients in this trial were treated with IMRT. Significantly more patients in the IMRT group had Stage 3B NSCLC and these patients had larger planning target volumes (PTVs) than those treated with 3D conformal RT.12 Despite this imbalance, which did not favour the IMRT group, there was no difference in survival between patients treated with 3D conformal RT, or IMRT. Furthermore, despite the larger PTVs, patients treated with IMRT had significantly less G3-5 pneumonitis and lower heart doses. This provides some evidence to support the use of IMRT and demonstrates how geometric individualization with IMRT can be used to treat patients with larger lung tumours.
4DCT constitutes another major technological development; it has helped to individualize RT planning by incorporating patient-specific information on respiratory motion.17 The patient’s respiratory cycle is recorded using a respiratory monitoring system, multiple axial CT images are then acquired throughout respiration and sorted into 8–10 phases ("bins").5 Each bin is then reconstructed as a 3DCT image representing the thoracic anatomy during a specific phase of the respiratory cycle. This process allows for accurate characterization of the displacement of the target volume (e.g. tumour and lymph nodes) and OARs due to breathing. 4DCT has improved treatment individualization by facilitating smaller margins and reducing the risk of a geographical tumour miss.5 The European Organization for Research and Treatment of Cancer and the European Society for Radiotherapy and Oncology Advisory Committee on Radiation Oncology Practice planning guidelines strongly recommend the use of a contrast-enhanced 4DCT for planning RT for lung cancer. These guidelines are summarized in Supplementary Material.7,18
On-treatment imaging [or image-guided radiotherapy (IGRT)] is important for correcting variations in patient set-up or anatomy between RT fractions, which can lead to a geographical tumour miss. The first forms of on-treatment imaging, i.e. kilo or megavoltage radiographs (portal imaging) could be used to match to anatomical surrogates (e.g. bone) but were often not sufficient to visualize the target. These approaches have largely been replaced by cone beam CT (CBCT) which provides better soft-tissue visualization, hence a more accurate set-up.6 If set-up errors are observed on the pre-treatment CBCT, the patient position is adjusted by shifting the couch before delivery. The importance of correcting set-up errors is underlined by recent evidence that uncorrected shifts, moving the high dose region towards the heart, were associated with worse survival.19 However, shifting the patient position is unable to account for changes in tumour shape and volume. As a result, the European Organization for Research and Treatment of Cancer guidelines recommend daily CBCT with soft tissue set-up to assess the presence of intrathoracic anatomical changes (ITACs) which may negatively impact upon the dose distribution.7 If these occur a new treatment plan, adapted to account for the impact of the ITAC should be created. This is known as reactive adaptation.7 Kwint et al have demonstrated that ITACs can occur in around 70% of patients undergoing curative radiotherapy.20 This was assessed predominantly in patients with Stage 3 disease (76%) and patients treated with SABR were excluded. They developed a decision support system to guide therapy radiographers in assessing the potential impact of ITACs upon the treatment and when to request clinician or physics support. Recent work has demonstrated that ITACs can occur in up to 22% of patients being treated with SABR.21 Most are minor but can be associated with unplanned clinician or physics review and significantly impact upon set-up time. A decision support may help identify SABR patients who may need replanning in order to avoid a geographical miss of the tumour and reduce the impact upon the workflow of clinically insignificant ITACs.
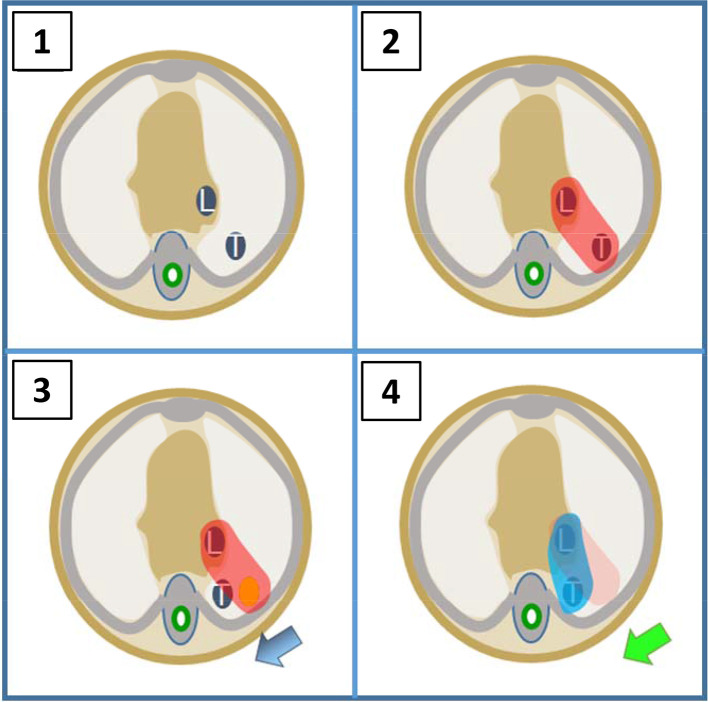
There is emerging interest in using on-treatment imaging to undertake proactive adaption in which potential ITACs are anticipated and the treatment plan adapted to account for changes in target geometry on a regular (even daily) basis (Figure 2). Such a strategy of frequent plan adaptation incorporated into fractionated RT could maximize tumour coverage, whilst minimizing the dose to OARs.22 There are however, concerns that reducing the target volumes in response to tumour regression may leave microscopic disease undertreated and increase the risk of local failure.23 This was assessed in the Phase 2 LARTIA trial where 217 patients with Stage 3 NSCLC were treated with CRT and underwent weekly chest CT. A new treatment plan was created if there was clinically significant tumour shrinkage and the treatment fields were reduced accordingly.24 In total, new plans were created in 23% of patients. While this trial showed encouragingly low rates of toxicitiy (2% acute and 4% late ≥Grade 3 lung toxicity) and acceptable numbers of marginal failures (6%), there was no consistent definition of what constituted clinically significant tumour shrinkage and therefore no objective criterion for the decision to create a new plan. Consequently, this approach should not be implemented in the routine setting until further validation is performed, ideally in the context of randomized studies. The MR-linac gives better target visualization compared to CBCT and provides the ideal platform to facilitate the investigation of daily online plan adaptation.25
Figure 2. .
Treatment adaptation. (1) RTP CT demonstrates locally advanced lung cancer, tumour (T) and lymph node (L). (2) RTP CT with PTV covering T and L. (3) On-treatment image shows baseline shift causing tumour to move outside of PTV and high risk of geographical miss. (4) Treatment adapted to produce new PTV covering new position of L and T. PTV, planning target volume; RTP, radiotherapy treatment planning.
Early stage NSCLC
Conformal RT, 4DCT and IGRT have led to greater confidence in the accuracy of RT delivery and supported the development of SABR. This is a technique in which an ablative dose of RT is delivered with high precision in a small number of fractions. SABR is accepted worldwide as a standard of care in early stage NSCLC patients not suitable for surgery.7,26,27 In the UK, 54–60 Gy is usually delivered over 3–8 alternate day fractions.28
The CHISEL trial comparing SABR with radical fractionated RT in medically inoperable patients with Stage I NSCLC found that SABR is associated with better local tumour control at 2 years—89% compared to 65% [hazard ratio (HR) 0.32, 95% CI 0.13–0.77, p = 0.008].29 Moreover, SABR was found to improve the median overall survival (OS, 5 years vs 3 years—HR 0·53; 95% CI 0·30–0·94, p = 0·027) which is consistent with the results of retrospective series of SABR.30
SABR for peripherally located tumours is typically associated with low toxicity. This is, in part, due to the small volumes treated (maximum tumour size ≤5 cm) but also the highly conformal dose distribution and steep dose gradients that minimize irradiation of healthy tissues. However, in early series of SABR, patients with tumours within 2 cm of the proximal bronchial tree (PBT) treated with a biologically equivalent dose ≥210 Gy had a higher incidence of treatment related mortality.31 As a result, the recommendation is that these central lung tumours should be treated with more fractionated regimens such as 60 Gy in 8 fractions, provided the OAR tolerances are met.32 The rate of non-cancer death in patients who receive these risk-adapted schedules for SABR to tumours from 1 to 2 cm of the PBT has been found, in a large retrospective analysis, to be equivalent to that of patients who receive SABR to more peripheral tumours. Patients in this study with tumours within 1 cm of the PBT had a higher rate of non-cancer death, although the number of patients in this group was small.33 A recent Phase I study showed that the maximum tolerated dose in centrally located tumours treated with a five fraction regime is 12 Gy per fraction.34 The organs closest to the PTV, and therefore most at risk, were the main bronchus and the large vessels. There is a need for prospective clinical trials of SABR for central tumours in order to assess efficacy and define OAR constraints; Table 1 details ongoing SABR trials in centrally located early stage NSCLC. Unfortunately the LungTech trial, a multicentre Phase II trial in early stage NSCLC patients with central tumours, has closed early due to slow patient accrual. This was in part due to by two safety-related halts in recruitment.35,36
Table 1. .
Trials of SABR in central lung tumours
| Trial | Study population | Study design | Primary outcome | Status |
|---|---|---|---|---|
| SUNSET NCT03306680a |
Stage 1 NSCLC, ultra-central tumours –i.e. where PTV touches the central bronchial tree, great vessels or oesophagus | Phase I dose escalation study using a time-to-event continual re-assessment method Starting dose: 60 Gy in eight fractions Will escalate to 60 in five fractions (or de-escalate to 60 in 15 fractions if needed) |
MTD i.e. dose associated with a < 30% rate of Grade 3–5 toxicity occurring within 2 years of treatment | Recruiting |
| LUNGTECH NCT01795521 |
Stage I-II NSCLC, centrally located in or abutting the 2 cm zone around the proximal bronchial tree and mediastinum),≤7 cm | Single arm Phase II study 60 Gy in eight fractions |
Freedom from local progression | Closed early due to poor accrual |
| HILUS trial | Stage I-II NSCLC or progressive metastasis from another solid tumour, centrally located (≤1 cm from the proximal bronchial tree),≤5 cm | Single arm Phase II 56 Gy in eight fractions |
Assessment of toxicity | Closed |
NSCLC, non-small cell lung cancer; PTV, planning target volume; MTD, maximally tolerated dose
ClinicalTrials.gov identifier
Clinical trials of SABR for ultracentral tumours, in which the PTV overlaps with central structures such as PBT, oesophagus or heart, are also required. In a Dutch retrospective study of 47 patients with ultracentral early stage NSCLC treated with SABR (60 Gy in 12 fractions), treatment-related deaths were reported in 21% of patients including fatal pulmonary haemorrhage in 15%.37 There is therefore an unmet need to define OAR constraints in such a clinical scenario.
While SABR has been shown as the best treatment for early stage lung cancer in people for whom surgery is not an option due to medical comorbidities, the role of SABR in those who are fit for surgery is more controversial. Retrospective analyses have shown that surgery and SABR have equivalent cancer specific survival.38–40 Patients who have surgery have improved OS, however, this is partly because these patients are younger and have fewer comorbidities. Clinical trials of SABR vs surgery for peripheral tumours are shown in Table 2. Some of these have closed early due to poor recruitment (highlighted pink) as patients and physicians often have a preference for one treatment modality. The UK SABR-TOOTH trial attempted to remove this lack of equipoise by having the diagnosing respiratory team discuss and consent patients with borderline operable early stage NSCLC to the trial.41 Following randomization, the patient was then reviewed by either the surgeon or the clinical oncologist. Despite this study design, the trial also failed to recruit patients within the predefined recruitment timelines. It is hoped that other ongoing randomized trials will provide clarity on the question of surgery or SABR for early stage lung cancer.
Table 2. .
Trials of SABR vs surgery for early stage NSCLC
| Trial | Study population | Study design | Primary outcome | Status |
|---|---|---|---|---|
| ROSEL NCT00687986a | Stage 1A NSCLC,>2 cm from PBT, fit for surgery | Phase III Arm A: SABR (60 Gy in three or five fractions) Arm B: Primary surgical resection |
Local and regional control | Closed early due to poor accrual |
| STARS NCT00840749 | Stage 1A/B NSCLC, fit for surgery | Phase III Arm A: SABR (60 Gy in four fractions if central or three fractions if peripheral using Cyberknife) Arm B: Surgery |
OS | Closed early due to poor accrual |
| ASOSOG-RTOG NCT01336894 | Stage 1 NSCLC, fit for surgery | PhaseIII Arm A: SABR (3 fractions) Arm B: Sub-lobar resection+/- intra-operative brachytherapy |
OS | Closed early due to poor accrual |
| SABR-TOOTH ISRCTN13029788b |
Stage I NSCLC, peripheral tumours, patients at higher risk from surgery | Phase II feasibility study Arm A: SABR Arm B: Surgery |
Recruitment rate | Closed early due to poor accrual |
| STABLE-MATES NCT02468024 |
Stage 1 NSCLC (≤4 cm), high risk operable patients | Phase III, patients are randomized before they consent to trial Arm A: SABR (54 Gy in three fractions) Arm B: Sub lobar resection |
OS | Recruiting |
| POSTILV NCT01753414 |
Stage 1 NSCLC (≤3 cm), fit for surgery | Randomized Phase II Arm A: SABR (55 gy in five fractions) Arm B: Complete surgical resection |
Local-regional control | Recruiting |
| VALOR NCT02984761 |
Stage 1 NSCLC (≤5 cm), includes central tumours (≥1 cm from trachea, oesophagus, brachial plexus, first bifurcation of the PBT, or spinal cord) fit for surgery | Phase III Arm A: SABR (54 Gy in three fractions, 56 Gy in four fractions or 57.5 Gy in five fractions. Central tumours will receive 50 Gy in five fractions) Arm B: Anatomic pulmonary resection |
OS | Recruiting |
NSCLC, non-small cell lung cancer; OS, overall survival; PBT, proximal bronchial tree; SABR, stereotactic ablative radiotherapy.
ClinicalTrials.gov identifier
ISRCTN.com identifier
Locally advanced NSCLC
The current standard of care for treating fit patients with inoperable LA NSCLC is concurrent CRT.42 While improved imaging and RT technology have allowed the development of ablative doses for early stage NSCLC, the RT dose fractionation in LA-NSCLC has changed very little for over 30 years.43 Nevertheless, as shown in Figure 1, survival for patients with LA-NSCLC has improved over this time. One reason for the improved survival of these patients is better staging prior to treatment with positron emission tomography CT (PET-CT) and endobronchial ultrasound transbronchial needle aspiration.18 PET-CT has been demonstrated to upstage 24% of LA-NSCLC cases to Stage 4.44
Radiobiological evidence suggests that in NSCLC the delivery of higher radiation doses results in improved tumour control probability.45 Therefore, in the late 1990s and early 2000s, multiple Phase I/II RT dose escalation trials were performed in patients with surgically inoperable lung cancer in an attempt to improve patient survival.46–51 These led to a randomized Phase III trial (RTOG 0617) of CRT comparing 60 Gy and 74 Gy RT with or without cetuximab in patients with Stage 3A/B disease. This trial found that the median survival for patients in the dose escalated arm was worse than for the 60 Gy arm (20.3 months vs 28.7 months).52 Nevertheless, as shown in Figure 1, patients in the standard dose arm of RTOG 0617 had better survival than those in previous trials. This may partly be due to stage migration as 91% of patients in RTOG 0617 had a staging PET-CT. The reasons behind the poor survival of patients in the high dose arm have been extensively discussed and are summarized in box 1.53,54
Box 1. Summary of reasons for the poor survival of patients in the 74 Gy high dose arm of RTOG 061752.

Although RTOG 0617 failed to demonstrate the benefit of dose escalation with conventional fractionation, a more individualized strategy deserves to be investigated. For example, functional imaging information can be used to increase the RT dose to particular areas. This is being used in the PET-boost trial, detailed in Table 3. Early data from the study report higher acute and late toxicities compared to standard CRT.55 Five cases (4.7%) of Grade 5 pulmonary haemorrhage have been observed in patients with central tumours encasing the vasculature; suggesting caution may be required in this scenario.
Table 3. .
the inTrials of individualiszed RT
| Trial | Study population | Study design | Primary outcome | Status |
|---|---|---|---|---|
| Adaption based on functional imaging | ||||
| RTOG 1106 NCT01507428a |
PET-CT avid (SUV ≥4) inoperable stage 3 NSCLC | Randomized Phase II Arm A: SOC CRT (60 Gy in 30 fractions) Arm B: Daily CRT over 30 fractions. Individualized adaptive RT; 2.4–3.5 Gy daily to MLD 20 Gy (≤86 Gy), based on PET-CT between fractions 18/19 Both arms will receive 3 cycles of consolidation chemotherapy (carboplatin/paclitaxel) |
|
Closed |
| PET boost NCT01024829 |
Stage IB-III NSCLC, SUVmax on pre-treatment PET-CT ≥5 |
Randomized Phase II Stage IB-II patients receive RT alone, stage 3 patients receive sequential or concurrent CRT Arm A: 66 Gy in 24 daily fractions with integrated boost (≤72 Gy) to primary tumour Arm B: 66 Gy in 24 daily fractions with integrated boost (≤72 Gy) to the 50% SUVmax area of the primary (of pre-treatment PET-CT) |
Local PFS | Closed |
| IFCT14-02/RTEP7 | Inoperable locally advanced NSCLC | Randomized Phase II Arm A: Individualized RT ≤74 Gy in 33 fractions if a PET-CT at 42 Gy is positive Initial dose of 50 Gy will be given in 5 weeks (2 Gy daily), then ≤24 Gy in 1.6 weeks (bd fractions) Arm B: 66 Gy in 33 daily fractions with no adaptation Both arms will undergo 2 cycles of induction and subsequent concurrent platinum based chemotherapy |
Local regional control rate | Recruiting |
| High Intensity Functional Image Guided VMAT Lung Evasion (HI-FIVE) NCT03569072 |
Stage 3 NSCLC | Single arm interventional pilot study (20pts) 60 Gy in 30 fractions with simultaneous integrated boost to primary tumour to 69 Gy RT adapted using ventilation/perfusion PET-CT to avoid regions of functional lung and boost tumour |
To assess the technical feasibility of the delivery of personalized functional lung radiotherapy (Treatment will be considered feasible if all of the following criteria are met: Reduction in mean functional lung dose of ≥2%, functional lung volume receiving 20 Gy of ≥4%, Mean heart dose is ≤30 Gy and relative heart volume receiving 50 Gy is <25%) |
Recruiting |
| FLARE RT NCT02773238 |
Inoperable stage IIB-IIIB NSCLC, PS 0–1 | Single arm Phase II CRT with functional lung avoidance based on SPECT-CT. RT dose escalation if no response on PET-CT after 3 weeks |
OS (in the functional lung avoidance and response-adaptive dose escalation RT cohort will be compared to 60 Gy cohort from RTOG 0617) | Recruiting |
| Individualized RT | ||||
| N12HYB NCT01933568 |
Inoperable stage II or III NSCLC Peripheral tumour <5 cm |
Phase I Combined SABR to primary tumour and CRT to lymph nodes |
Mean lung dose associated with a 15% probability of DLT;≥Grade three radiation pneumonitis and radiation induced dyspnoea | Closed |
| Hypofractionated IGRT NCT01459497 |
Stage II-III NSCLC, PS ≥ 2, PS0-1 and >10% wt loss or unfit for combined modality RT | Phase III Arm A: 60 Gy in 15 daily fractions with daily IGRT Arm B: 60–66 Gy in 30–33 daily fractions |
OS | Recruiting |
| ADSCAN ISRCTN47674500b |
Stage 3 NSCLC patients to be treated with sequential CRT | Randomized Phase II Arm A Standard RT arm 55 Gy in 20 daily fractions (2.75 Gy per fraction) Arm B CHART-ED, 54 Gy in 36 fractions (3 × 1.5 Gy fractions per day)+10.8 Gy in six fractions (days 15–17) Arm C IDEAL, 30 daily fractions of isotoxic RT, 63–71 Gy Arm D I-START, 20 daily fractions of isotoxic RT, 55–65 Gy Arm E Isotoxic IMRT, bi-daily fractions of isotoxic RT over 4 weeks, max dose 79.2 Gy |
PFS | Recruiting |
CRT, chemoradiotherapy; DLT, dose-limiting toxicity; MLD, mean lung dose; NSCLC, non-small cell lung cancer;PET-CT, positron emission tomography-CT; PFS, progression-free survival; RT, radiotherapy; SABR, stereotactic ablative radiotherapy; SOC, standard of care; SPECT, single-photon emission computed tomography; SUV, standard uptake volume; SUVmax, maximum standard uptake volume; VMAT, volumetric-modulated arc therapy.
ClinicalTrials.gov identifier
ISRCTN.com identifier
Another example of individualized dose-escalation is the delivery of RT based on the maximum dose that can be tolerated by the OARs (so called "isotoxic RT"). Studies have investigated this approach both in the sequential and the concurrent CRT setting.56,57 This led to a feasibility study in the UK (Isotoxic IMRT) showing a median tumour dose of 77.4 Gy (61.2–79.2 Gy; two fractions per day) can be delivered in LA-NSCLC treated with sequential CRT and IMRT.58,59 Isotoxic IMRT has been incorporated into the ongoing UK randomized Phase 2 ADSCAN trial comparing standard of care sequential CRT with three other dose escalated regimes in patients not suitable for concurrent CRT.60 The most efficacious RT regimen will then be compared to the standard of care in a Phase 3 trial. Other studies have looked at individualizing treatment by using ablative doses to boost residual or recurrent disease in patients with LA-NSCLC. However, this approach requires caution; data from four studies evaluating this technique show 5/80 (6.3%) patients experienced fatal toxicities such as haemorrhage.61–64 This was most notable when boosting centrally located disease and is similar to the outcomes observed when using primary SABR to treat central tumours.65 This and other ongoing trials examining ways to individualize dose escalated RT in NSCLC are shown in Table 3.
In many of these studies of isotoxic RT, the dose escalation is constrained by normal tissue tolerances of surrounding organs such as the oesophagus, lungs, airways and heart. These tissue tolerances are based on preclinical and clinical studies and have been synthesized in the Qualitative Analyses of Normal Tissue Effects in the Clinic.66 At present, the same dose volume constraints are used in all patients with little regard to patient factors, such as pre-existing comorbidities, smoking status, baseline lung or cardiac function, which could affect tissue tolerances.
Between 66 and 76% of patients with LA-NSCLC have at least one concomitant medical condition.67,68 Patients with comorbidities are often not included in clinical trials. Only 41% of patients on the Maastrict cancer registry would have met the inclusion criteria for RTOG 0617 due to comorbidities and no patient over the age of 75 would have been eligible.69 Trials which investigate RT in unfit or older patients (Table 3), are therefore important in attempting to answer questions about individualizing RT based on patient factors.
Despite the improvements in RT over the last 20 years, the largest improvement in survival of patients with inoperable LA-NSCLC has come, not through RT, but immunotherapy (IO). The addition of 1 year of durvalumab following CRT has been shown to improve 2 year OS (66.3% with durvalumab vs 55.6 with placebo) and progression-free survival (PFS) (median, 28.3 months vs 16.2 months).70 Such consolidation treatment is now considered standard of care in fit patients with LA-NSCLC who have responded to definitive concurrent CRT.27 In the UK, durvalumab is approved by the National Institute for Health and Care Excellence only in patients treated with concurrent CRT and with PDL1 >1%.71 Questions remain about the best way of integrating targeted therapies and RT in LA patients with driver mutations.
Metastatic NSCLC
Traditionally, patients with Stage 4 disease received chemotherapy with a platinum doublet. Over the last decade, the treatment of this group of patients has become increasingly tailored to tumour histology, and outcomes have improved. Targeted agents are used in patients with tumours with molecular drivers such as EGFR, ALK and ROS-1 mutations. More recently, IO has transformed the treatment of metastatic NSCLC.8 Palliative RT for Stage 4 disease is used to improve symptoms such as pain, cough or haemoptysis.72 In recent years the concept of oligometastatic disease (OMD) has emerged and there is evidence that RT can improve survival in these patients.73 OMD is defined differently depending on the point in the disease process the distant disease is diagnosed (Table 4). The first evidence for the benefit of RT in OMD came from a randomized trial of whole brain radiotherapy with or without stereotactic brain RT in patients with 1–3 brain metastases. Over half of the patients in this trial had brain metastases from NSCLC. OS was significantly improved with the addition of stereotactic brain RT in patients with a single brain metastasis (6.5 months vs 4.9 months).74
Table 4. .
Trials in OMD
| Type of OMD | Trial | Study population | Study design | Primary outcome |
|---|---|---|---|---|
|
SYNCHRONOUS OMD is detected at diagnosis of the primary tumour 
|
SARON NCT02417662a |
NSCLC with ≤3 synchronous oligo-metastasesa | Phase III Arm A: Standard treatment, chemotherapy alone Arm B: Chemotherapy followed by radical RT (SABR or conventional RT) to the primary and SABR to metastases |
OS |
|
NRG-LU002 NCT03137771 |
NSCLC with ≤3 metastases with no progression after four cycles chemotherapy | Randomized Phase II/III Arm A: Maintenance SACT only ARM B: Local consolidative therapy (LCT- SABR, IMRT, 3DCRT or possibly surgery) followed by maintenance SACT |
Phase II: PFS Phase III: OS |
|
|
LONESTAR NCT 03391869 |
Metastatic NSCLC | Phase III Arm A: Nivolumab and ipilimumab alone Arm B: Nivolumab +ipilimumab and LCT (radiotherapy ± surgical resection, radiofrequency ablation or cryoablation) |
OS in overall population OS in OMD (subgroup ≤3 lesions) |
|
|
METACHRONOUS Oligo-recurrent OMD is detected after treatment of the primary tumour 
|
CORE ISRCTN45961438˜ |
Patients with NSCLC, breast or prostate cancer (previously completed radical treatment) with ≤3 extra cranial metastases suitable for SABR | Randomized Phase II/III Arm A: SABR to metastases and standard of care treatment (e.g., SACT, palliative RT or observation) Arm B: Standard of care |
PFS |
|
SABR-COMET 10 NCT03721341 |
Patients with 4–10 new metastases from any primary tumour (who completed radical treatment for any >3 months previously) and have stable disease at primary site | Phase III Arm A: SABR plus standard of care treatment (SACT or observation) Arm B: Standard of care |
OS | |
|
OLIGO-PROGRESSIVE OMD that occurs after response to systemic therapy. Progression of a limited number of areas of disease occurs while all other tumours remain controlled 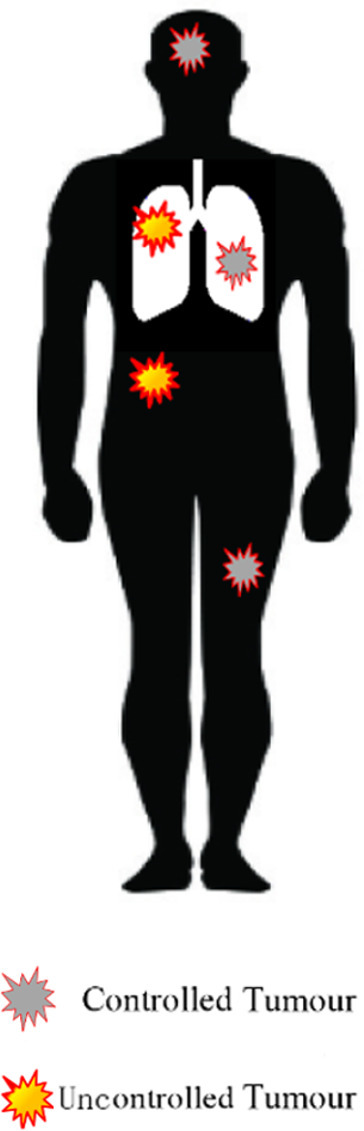
|
HALT ISRCTN53398136 |
Metastatic NSCLC with actionable mutation receiving TKI therapy, ≤3 extracranial sites of progressive disease |
Randomized Phase II Arm A: SABR to progressive lesions and continuation of TKI Arm B: Continuation on TKI |
PFS |
|
STOP-NSCLC NCT02756793 |
Metastatic NSCLC with actionable mutation receiving TKI therapy, ≤5 extracranial sites of progressive disease |
Randomized Phase II Arm A: SABR to progressive lesions + continuation of TKI Arm B: Continuation on TKI |
PFS | |
|
OLIGO-PERSISTENT This is a scenario where there are a limited number of controlled persistent lesions following a response to systemic therapy 
|
NORTHSTAR NCT03410043 |
EGFR mutant metastatic NSCLC, treated with 6–12 weeks of osimertinib, and oligo or poly persistent disease | Randomized Phase II Arm A: LCT (RT or surgery ± RT) and continue osimertinib Arm B: Osimertinib only |
PFS |
3DCRT, three-dimensional conformal radiation therapy; EGFR, epidermal growth factor receptor; IMRT, intensity modulated radiotherapy; NSCLC, non-small cell lung cancer;OMD, oligometastatic disease; OS, overall survival; PFS, progression-free survival; RT, radiotherapy; SABR, stereotactic ablative radiotherapy; SACT, systemic anticancer therapy; TKI, tyrosine kinase inhibitor.
ClinicalTrials.gov identifier, ˜ISRCTN.com identifier
More recently, evidence is emerging for the use of SABR to treat extracranial metastatic disease in NSCLC from a number of randomized Phase II studies. Iyengar et al compared maintenance chemotherapy alone to SABR and maintenance chemotherapy in 29 patients with OMD from NSCLC. This was stopped early due to a significant improvement in PFS in the SABR arm (9.7 months vs 3.5 months).75 Another similar study of local therapy (including SABR, intermediate hypofractionated-RT, CRT or surgery) versus standard of care following systemic anticancer therapy was also stopped early for the same reason.76 Subsequently, an updated analysis of this trial reported an OS benefit with local therapy of 24 months.77 A larger randomized trial of 99 patients with any primary tumour (18% of whom had NSCLC) found improved OS with SABR to between 1 and 5 metastases (28 vs 41 months).73 Although the results of these trials are encouraging, the numbers of patients with OMD from NSCLC included are small and there are important ongoing questions regarding patient selection. Consequently, randomized Phase III evidence is required. There are many ongoing studies examining radiotherapy including both metastatic NSCLC and other cancer sites, shown in Table 4.
Future developments
Over the past two decades radiotherapy has evolved into a highly precise treatment. This has led to an improvement in patient outcomes such as that seen with SABR for early stage disease, however in LA disease there is still an unmet need to improve results. Undoubtedly, future progress in lung cancer RT will come from the individualization of our advanced treatment strategies and the better integration with systemic therapies, including IO.
The MR-guided linear accelerator (MRL) and proton beam therapy both represent exciting developments in RT technology which may further advance targeting and individualization of treatment.
The MRL combines a linear accelerator with on-board diagnostic quality MRI. The superior soft tissue discrimination of MR gives better target visualization compared to CBCT. The improved target visualization may facilitate the investigation of daily online plan adaptation and isotoxic dose intensification without the concerns of excessive ionizing radiation that exist with CBCT.22,25,59 The MRL will also enable real-time gating and permit assessment of delivered dose compared to planned dose.78 Ultimately, the anatomical and dosimetric information obtained in real time may facilitate intrafractional plan adaptation, to ensure the intended daily dose is delivered. This should permit smaller PTV margins and further reduce the dose to healthy tissues. Therefore, MR-guided RT potentially represents truly individualized RT with a move away from an image-guided to a "dose-guided" technique.22 The MRL also offers the potential to incorporate biological information via functional imaging. This may enable the exciting possibility of individualized adaptation and intensification based on tumour metabolic activity and hypoxia.79
Proton beam therapy (PrBT) is another technology that has the potential to improve the outcome of NSCLC patients. The physical characteristics of protons mean their energy deposition increases with increasing depth in tissue. As a result, the maximum energy deposition occurs toward the end of the proton range (the Bragg peak). Therefore, the dose to the normal tissues beyond this range and the integral dose are reduced. A trial of IMRT vs passive scattering PrBT in patients with LA-NSCLC found that PrBT reduced low dose bath to the lungs although more lung tissue was exposed to doses ≥ 20 Gy. Moreover, PrBT spared more heart volume at all dose levels compared to IMRT.80 In spite of this dosimetric advantage, this randomized Phase III trial did not show a clinical benefit for patients treated with protons. There are many unsolved technological challenges in delivering PrBT to mobile targets such as lung tumours, as the sharp dose gradient of proton plans makes them particularly sensitive to geometric uncertainties. There are a number of studies ongoing in early and LA lung cancer patients comparing IMRT with PrBT (ClinicalTrials.gov:NCT 01993810, NCT 02731001 and NCT 03818776).
The addition of consolidation IO after concurrent CRT has recently become standard of care in LA NSCLC.70 There are currently many ongoing trials investigating a similar strategy in patients with early stage NSCLC and in LA patients not suitable for concurrent CRT. Clinical trials are also investigating the addition of IO to thoracic RT concurrently in combination with consolidation treatment. Many questions remain about the timing of the administration of IO and thoracic RT and the impact of the volume of irradiation on the immune system. Further research is also required to identify the patients most likely to benefit from consolidation IO. One other promising area of investigation is the concept of "minimal residual disease" with circulating tumour deoxyribonucleic acid (ctDNA).81 ctDNA represents an attractive biomarker and may have benefits in screening, assessing response to treatment and surveillance. Blood collection for circulating tumour cells and ctDNA is included in some of the trials of RT in OMD (Table 4).
Conclusion
In this review, we have described the advances in imaging, tumour localization and RT technology that have facilitated the development of SABR for early stage NSCLC and allowed the treatment of large volume LA NSCLC. We have also highlighted ongoing research in areas such as dose escalation in LA disease and the use of SABR in the treatment of oligometastatic disease.
Advances in RT technologies have improved the precision of RT but in order to make further progress, there is an unmet need to tailor treatment to individual patients and tumour characteristics.
Footnotes
Acknowledgment: Sean Brown acknowledges speaker’s fees and travel support from Elekta. Corinne Faivre-Finn acknowledges research funding and travel support from Astra Zeneca, Merck and Elekta.
Funding: Corinne Faivre-Finn is supported by the NIHR Biomedical Research Centre. Marianne Aznar is supported by Cancer Research UK – United Kingdom (CRUK, grant C8225/A21133). This work is also supported by a CRUK Centres Network Accelerator Award Grant (A21993) to the ART-NET consortium and via CRUK funding to Cancer Research UK Manchester Centre: [C147/A18083] and [C147/A25254].
Sean Brown and Kathryn Banfill have contributed equally to this study and should be considered as joint first authors.
Contributor Information
Sean Brown, Email: sean.brown@christie.nhs.uk.
Kathryn Banfill, Email: kathryn.banfill@manchester.ac.uk.
Marianne C. Aznar, Email: marianne.aznar@manchester.ac.uk.
Philip Whitehurst, Email: Philip.Whitehurst@christie.nhs.uk.
Corinne Faivre Finn, Email: Corinne.Finn@christie.nhs.uk.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–E386. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Kong F-MS, Zhao J, Wang J, Faivre-Finn C. Radiation dose effect in locally advanced non-small cell lung cancer. J Thorac Dis 2014; 6: 336–47. doi: 10.3978/j.issn.2072-1439.2014.01.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ragazzi G, Cattaneo GM, Fiorino C, Ceresoli G, Verusio C, Villa E. Use of dose^volume histograms and biophysical models to compare 2D and 3D irradiation techniques for non-small cell lung cancer. Available from: http://www.birpublications.org/doi/pdf/10.1259/bjr.72.855.10396219 [cited 2018 Jan 9]. [DOI] [PubMed]
- 4.Chan C, Lang S, Rowbottom C, Guckenberger M, Faivre-Finn C. IASLC Advanced Radiation Technology Committee Intensity-Modulated radiotherapy for lung cancer: current status and future developments. J Thorac Oncol 2014; 9: 1598–608. doi: 10.1097/JTO.0000000000000346 [DOI] [PubMed] [Google Scholar]
- 5.Liu HH, Balter P, Tutt T, Choi B, Zhang J, Wang C, et al. Assessing respiration-induced tumor motion and internal target volume using four-dimensional computed tomography for radiotherapy of lung cancer. Int J Radiat Oncol Biol Phys 2007; 68: 531–40. doi: 10.1016/j.ijrobp.2006.12.066 [DOI] [PubMed] [Google Scholar]
- 6.Borst GR, Sonke J-J, Betgen A, Remeijer P, van Herk M, Lebesque JV. Kilo-voltage cone-beam computed tomography setup measurements for lung cancer patients; first clinical results and comparison with electronic portal-imaging device. Int J Radiat Oncol Biol Phys 2007; 68: 555–61. doi: 10.1016/j.ijrobp.2007.01.014 [DOI] [PubMed] [Google Scholar]
- 7.De Ruysscher D, Faivre-Finn C, Moeller D, Nestle U, Hurkmans CW, Le Péchoux C, et al. European organization for research and treatment of cancer (EORTC) recommendations for planning and delivery of high-dose, high precision radiotherapy for lung cancer. Radiother Oncol 2017; 124: 1–10. doi: 10.1016/j.radonc.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 8.Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018; 553: 446–454. doi: 10.1038/nature25183 [DOI] [PubMed] [Google Scholar]
- 9.Christodoulou M, Bayman N, McCloskey P, Rowbottom C, Faivre-Finn C. New radiotherapy approaches in locally advanced non-small cell lung cancer. Eur J Cancer 2014; 50: 525–34. doi: 10.1016/j.ejca.2013.11.027 [DOI] [PubMed] [Google Scholar]
- 10.Chetty IJ, Devpura S, Liu D, Chen D, Li H, Wen NW, et al. Correlation of dose computed using different algorithms with local control following stereotactic ablative radiotherapy (SABR)-based treatment of non-small-cell lung cancer. Radiother Oncol 2013; 109: 498–504. doi: 10.1016/j.radonc.2013.10.012 [DOI] [PubMed] [Google Scholar]
- 11.Keall PJ, Mageras GS, Balter JM, Emery RS, Forster KM, Jiang SB, et al. The management of respiratory motion in radiation oncology report of AAPM task group 76. Med Phys 2006; 33: 3874–900. doi: 10.1118/1.2349696 [DOI] [PubMed] [Google Scholar]
- 12.Chun SG, Hu C, Choy H, Komaki RU, Timmerman RD, Schild SE, et al. Impact of intensity-modulated radiation therapy technique for locally advanced Non–Small-Cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. JCO 2017; 35: 56–62. doi: 10.1200/JCO.2016.69.1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen AB, Neville BA, Sher DJ, Chen K, Schrag D. Survival outcomes after radiation therapy for stage III Non–Small-Cell lung cancer after adoption of computed Tomography–Based simulation. JCO 2011; 29: 2305–11. doi: 10.1200/JCO.2010.33.4466 [DOI] [PubMed] [Google Scholar]
- 14.Bree Ide, van Hinsberg MGE, van Veelen LR. High-dose radiotherapy in inoperable nonsmall cell lung cancer: comparison of volumetric modulated arc therapy, dynamic IMRT and 3D conformal radiotherapy. Med Dosim 2012; 37: 353–7. doi: 10.1016/j.meddos.2011.12.002 [DOI] [PubMed] [Google Scholar]
- 15.Boda-Heggemann J, Mai S, Fleckenstein J, Siebenlist K, Simeonova A, Ehmann M, et al. Flattening-filter-free intensity modulated breath-hold image-guided SABR (stereotactic ablative radiotherapy) can be applied in a 15-min treatment slot. Radiother Oncol 2013; 109: 505–9. doi: 10.1016/j.radonc.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 16.Verbakel W, Senan S, Cuijpers JP, Slotman BJ, Lagerwaard FJ. Rapid delivery of stereotactic radiotherapy for peripheral lung tumors using volumetric intensity-modulated arcs. Radiother Oncol 2009; 93: 122–4. doi: 10.1016/j.radonc.2009.05.020 [DOI] [PubMed] [Google Scholar]
- 17.Slotman BJ, Lagerwaard FJ, Senan S. 4D imaging for target definition in stereotactic radiotherapy for lung cancer. Acta Oncol 2006; 45: 966–72. doi: 10.1080/02841860600902817 [DOI] [PubMed] [Google Scholar]
- 18.Nestle U, De Ruysscher D, Ricardi U, Geets X, Belderbos J, Pöttgen C, et al. ESTRO ACROP guidelines for target volume definition in the treatment of locally advanced non-small cell lung cancer. Radiother Oncol 2018; 127: 1–5. doi: 10.1016/j.radonc.2018.02.023 [DOI] [PubMed] [Google Scholar]
- 19.Johnson-Hart CN, Price GJ, Faivre-Finn C, Aznar MC, van Herk M. Residual setup errors towards the heart after image guidance linked with poorer survival in lung cancer patients: do we need stricter IGRT protocols? Int J Radiat Oncol Biol Phys 2018; 102: 434–42. doi: 10.1016/j.ijrobp.2018.05.052 [DOI] [PubMed] [Google Scholar]
- 20.Kwint M, Conijn S, Schaake E, Knegjens J, Rossi M, Remeijer P, et al. Intra thoracic anatomical changes in lung cancer patients during the course of radiotherapy. Radiother Oncol 2014; 113: 392–7. doi: 10.1016/j.radonc.2014.10.009 [DOI] [PubMed] [Google Scholar]
- 21.Beasley M, Brown S, Chuter R, Faivre-Finn C, Franks K, Murray L, et al. PV-0579 the impact of intra-thoracic anatomical changes on the delivery of lung SABR. Radiotherapy and Oncology 2019; 133(Supplement 1): S303–S304. doi: 10.1016/S0167-8140(19)30999-5 [DOI] [PubMed] [Google Scholar]
- 22.Bainbridge H, Salem A, Tijssen RHN, Dubec M, Wetscherek A, Van Es C, et al. Magnetic resonance imaging in precision radiation therapy for lung cancer. Transl Lung Cancer Res 2017; 6: 689–707. doi: 10.21037/tlcr.2017.09.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonke J-J, Belderbos J. Adaptive radiotherapy for lung cancer. Semin Radiat Oncol 2010; 20: 94–106. doi: 10.1016/j.semradonc.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 24.Ramella S, Fiore M, Silipigni S, Zappa MC, Jaus M, Alberti AM, et al. Local control and toxicity of adaptive radiotherapy using Weekly CT imaging: results from the LARTIA trial in stage III NSCLC. J Thorac Oncol 2017; 12: 1122–30. doi: 10.1016/j.jtho.2017.03.025 [DOI] [PubMed] [Google Scholar]
- 25.Choudhury A, Budgell G, MacKay R, Falk S, Faivre-Finn C, Dubec M, et al. The future of image-guided radiotherapy. Clin Oncol 2017; 29: 662–6. doi: 10.1016/j.clon.2017.04.036 [DOI] [PubMed] [Google Scholar]
- 26.Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017; 28(September): iv1–21. doi: 10.1093/annonc/mdx222 [DOI] [PubMed] [Google Scholar]
- 27.Wood DE, Chair V, Aggarwal C, Aisner DL, Akerley W, Bauman JR. NCCN Guidelines Version 4.2019 - Non-Small Cell Lung Cancer. 2019. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf [cited 2019 May 2].
- 28.SABR UK Consortium Stereotactic Ablative Body Radiation Therapy (SABR): A Resource. Version 6.1. 2019. Available from: https://www.sabr.org.uk/wp-content/uploads/2019/04/SABRconsortium-guidelines-2019-v6.1.0.pdf [cited 2019 May 1].
- 29.Ball D, Mai GT, Vinod S, Babington S, Ruben J, Kron T, et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol 2019; 20: 494–503. doi: 10.1016/S1470-2045(18)30896-9 [DOI] [PubMed] [Google Scholar]
- 30.Simone CB, Wildt B, Haas AR, Pope G, Rengan R, Hahn SM. Stereotactic body radiation therapy for lung cancer. Chest 2013; 143: 1784–90. doi: 10.1378/chest.12-2580 [DOI] [PubMed] [Google Scholar]
- 31.Senthi S, Haasbeek CJA, Slotman BJ, Senan S. Outcomes of stereotactic ablative radiotherapy for central lung tumours: a systematic review. Radiother Oncol 2013; 106: 276–82. doi: 10.1016/j.radonc.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 32.Hanna GG, Murray L, Patel R, Jain S, Aitken KL, Franks KN, et al. UK consensus on normal tissue dose constraints for stereotactic radiotherapy. Clin Oncol 2018; 30: 5–14. doi: 10.1016/j.clon.2017.09.007 [DOI] [PubMed] [Google Scholar]
- 33.Stam B, Kwint M, Guckenberger M, Mantel F, Hope A, Giuliani M, et al. Subgroup survival analysis in stage I-II NSCLC patients with a central tumor partly treated with Risk-Adapted SBRT. Int J Radiat Oncol Biol Phys 2019; 103: 132–41. doi: 10.1016/j.ijrobp.2018.08.040 [DOI] [PubMed] [Google Scholar]
- 34.Bezjak A, Paulus R, Gaspar LE, Timmerman RD, Straube WL, Ryan WF, et al. Safety and efficacy of a Five-Fraction stereotactic body radiotherapy schedule for centrally located non-small-cell lung cancer: NRG Oncology/RTOG 0813 trial. J Clin Oncol 2019; JCO1800622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adebahr S, Liu Y, Colette S, Faivre-Finn C, Ahmad S, Ahmed M, et al. OC-0061 EORTC 22113-8113 Lungtech trial on SBRT of central lung tumors. Radiotherapy and Oncology 2019; 133(Supplement 1): S24–S25. doi: 10.1016/S0167-8140(19)30481-5 [DOI] [Google Scholar]
- 36.Adebahr S, Collette S, Shash E, Lambrecht M, Le Pechoux C, Faivre-Finn C, et al. LungTech, an EORTC phase II trial of stereotactic body radiotherapy for centrally located lung tumours: a clinical perspective. Br J Radiol 2015; 88: 20150036. doi: 10.1259/bjr.20150036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tekatli H, Haasbeek N, Dahele M, De Haan P, Verbakel W, Bongers E. Outcomes of Hypofractionated High-Dose Radiotherapy in Poor-Risk Patients with ``Ultracentral’’ Non-Small Cell Lung Cancer. 2016. Available from: 10.1016/j.jtho.2016.03.008 [cited 2019 May 1]. [DOI] [PubMed]
- 38.Chang JY, Senan S, Paul MA, Mehran RJ, Louie AV, Balter P, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015; 16: 630–7. doi: 10.1016/S1470-2045(15)70168-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paul S, Lee PC, Mao J, Isaacs AJ, Sedrakyan A. Long term survival with stereotactic ablative radiotherapy (SABR) versus thoracoscopic sublobar lung resection in elderly people: national population based study with propensity matched comparative analysis. BMJ 2016; 354: i3570. doi: 10.1136/bmj.i3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spencer KL, Kennedy MPT, Lummis KL, Ellames DAB, Snee M, Brunelli A, et al. Surgery or radiotherapy for stage I lung cancer? an intention to treat analysis. Eur Respir J 2019; 568. [DOI] [PubMed] [Google Scholar]
- 41.Snee MP, Mcparland L, Collinson F, Lowe CM, Striha A, Baldwin DR. The SABRTooth feasibility trial protocol: a study to determine the feasibility and acceptability of conducting a phase III randomised controlled trial comparing stereotactic ablative radiotherapy (SABR) with surgery in patients with peripheral stage I non-small cell lung cancer (NSCLC) considered to be at higher risk of complications from surgical resection. 2016. Available from: https://pilotfeasibilitystudies-biomedcentral-com.manchester.idm.oclc.org/track/pdf/10.1186/s40814-016-0046-2 [cited 2019 May 1]. [DOI] [PMC free article] [PubMed]
- 42.Aupérin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010; 28: 2181–90. doi: 10.1200/JCO.2009.26.2543 [DOI] [PubMed] [Google Scholar]
- 43.Perez CA, Stanley K, Rubin P, Kramer S, Brady L, Perez-Tamayo R, et al. A prospective randomized study of various irradiation doses and fractionation schedules in the treatment of inoperable non-oat-cell carcinoma of the lung. preliminary report by the radiation therapy Oncology Group. Cancer 1980; 45: 2744–53. doi: [DOI] [PubMed] [Google Scholar]
- 44.MacManus MP, Hicks RJ, Matthews JP, Hogg A, McKenzie AF, Wirth A, et al. High rate of detection of unsuspected distant metastases by PET in apparent stage III non-small-cell lung cancer: implications for radical radiation therapy. Int J Radiat Oncol Biol Phys 2001; 50: 287–93. doi: 10.1016/S0360-3016(01)01477-8 [DOI] [PubMed] [Google Scholar]
- 45.Martel MK, Ten Haken RK, Hazuka MB, Kessler ML, Strawderman M, Turrisi AT, et al. Estimation of tumor control probability model parameters from 3-D dose distributions of non-small cell lung cancer patients. Lung Cancer 1999; 24: 31–7. doi: 10.1016/S0169-5002(99)00019-7 [DOI] [PubMed] [Google Scholar]
- 46.Bradley JD, Moughan J, Graham MV, Byhardt R, Govindan R, Fowler J, et al. A phase I/II radiation dose escalation study with concurrent chemotherapy for patients with inoperable stages I to III non-small-cell lung cancer: phase I results of RTOG 0117. Int J Radiat Oncol Biol Phys 2010; 77: 367–72. doi: 10.1016/j.ijrobp.2009.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bradley JD, Bae K, Graham MV, Byhardt R, Govindan R, Fowler J, et al. Primary analysis of the phase II component of a phase I/II dose intensification study using three-dimensional conformal radiation therapy and concurrent chemotherapy for patients with inoperable non-small-cell lung cancer: RTOG 0117. J Clin Oncol 2010; 28: 2475–80. doi: 10.1200/JCO.2009.27.1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Socinski MA, Blackstock AW, Bogart JA, Wang X, Munley M, Rosenman J, et al. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol 2008; 26: 2457–63. doi: 10.1200/JCO.2007.14.7371 [DOI] [PubMed] [Google Scholar]
- 49.Schild SE, McGinnis WL, Graham D, Hillman S, Fitch TR, Northfelt D, et al. Results of a phase I trial of concurrent chemotherapy and escalating doses of radiation for unresectable non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006; 65: 1106–11. doi: 10.1016/j.ijrobp.2006.02.046 [DOI] [PubMed] [Google Scholar]
- 50.Lee CB, Stinchcombe TE, Moore DT, Morris DE, Hayes DN, Halle J, et al. Late complications of high-dose (≥66 Gy) thoracic conformal radiation therapy in combined modality trials in unresectable stage III non-small cell lung cancer. J Thorac Oncol 2009; 4: 74–9. doi: 10.1097/JTO.0b013e3181915028 [DOI] [PubMed] [Google Scholar]
- 51.Ramroth J, Cutter DJ, Darby SC, Higgins GS, McGale P, Partridge M, et al. Dose and fractionation in radiation therapy of curative intent for non-small cell lung cancer: meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys 2016; 96: 736–47. doi: 10.1016/j.ijrobp.2016.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015; 16: 187–99. doi: 10.1016/S1470-2045(14)71207-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cox JD. Are the results of RTOG 0617 mysterious? Int J Radiat Oncol Biol Phys 2012; 82: 1042–4. doi: 10.1016/j.ijrobp.2011.12.032 [DOI] [PubMed] [Google Scholar]
- 54.Faivre-Finn C. Dose escalation in lung cancer: have we gone full circle? Lancet Oncol 2015; 16: 125–7. doi: 10.1016/S1470-2045(15)70001-X [DOI] [PubMed] [Google Scholar]
- 55.van Diessen J, De Ruysscher D, Sonke J-J, Damen E, Sikorska K, Reymen B, et al. The acute and late toxicity results of a randomized phase II dose-escalation trial in non-small cell lung cancer (PET-boost trial. Radiotherapy and Oncology 2019; 131: 166–73. doi: 10.1016/j.radonc.2018.09.019 [DOI] [PubMed] [Google Scholar]
- 56.van Baardwijk A, Wanders S, Boersma L, Borger J, Öllers M, Dingemans A-MC, et al. Mature results of an individualized radiation dose prescription study based on normal tissue constraints in stages I to III non-small-cell lung cancer. J Clin Oncol 2010; 28: 1380–6. doi: 10.1200/JCO.2009.24.7221 [DOI] [PubMed] [Google Scholar]
- 57.De Ruysscher D, van Baardwijk A, Wanders R, Hendriks LE, Reymen B, van Empt W, et al. Individualized accelerated isotoxic concurrent chemo-radiotherapy for stage III non-small cell lung cancer: 5-year results of a prospective study. Radiother Oncol 2019; 135: 141–6. doi: 10.1016/j.radonc.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 58.Haslett K, Bayman N, Franks K, Groom N, Hanna G, Harden S, et al. MA 09.11 isotoxic intensity modulated radiotherapy (IMRT) in stage III non-small cell lung cancer (NSCLC) – a feasibility study. Journal of Thoracic Oncology 2017; 12: S1838–S1839. doi: 10.1016/j.jtho.2017.09.532 [DOI] [Google Scholar]
- 59.Haslett K, Franks K, Hanna GG, Harden S, Hatton M, Harrow S, et al. Protocol for the isotoxic intensity modulated radiotherapy (IMRT) in stage III non-small cell lung cancer (NSCLC): a feasibility study. BMJ Open 2016; 6: e010457. doi: 10.1136/bmjopen-2015-010457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hatton MQF, Lawless CA, Faivre-Finn C, Landau D, Lester JF, Fenwick J, et al. Accelerated, dose escalated, sequential chemoradiotherapy in non-small-cell lung cancer (ADSCaN): a protocol for a randomised phase II study. BMJ Open 2019; 9: e019903. doi: 10.1136/bmjopen-2017-019903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feddock J, Arnold SM, Shelton BJ, Sinha P, Conrad G, Chen L, et al. Stereotactic body radiation therapy can be used safely to boost residual disease in locally advanced non-small cell lung cancer: a prospective study. Int J Radiat Oncol Biol Phys 2013; 85: 1325–31. doi: 10.1016/j.ijrobp.2012.11.011 [DOI] [PubMed] [Google Scholar]
- 62.Karam SD, Horne ZD, Hong RL, McRae D, Duhamel D, Nasr NM. Dose escalation with stereotactic body radiation therapy boost for locally advanced non small cell lung cancer. Radiat Oncol 2013; 8: 179. doi: 10.1186/1748-717X-8-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hepel JT, Leonard KL, Safran H, Ng T, Taber A, Khurshid H, et al. Stereotactic body radiation therapy boost after concurrent chemoradiation for locally advanced non-small cell lung cancer: a phase 1 dose escalation study. Int J Radiat Oncol Biol Phys 2016; 96: 1021–7. doi: 10.1016/j.ijrobp.2016.08.032 [DOI] [PubMed] [Google Scholar]
- 64.Trovo M, Minatel E, Durofil E, Polesel J, Avanzo M, Baresic T, et al. Stereotactic body radiation therapy for re-irradiation of persistent or recurrent non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2014; 88: 1114–9. doi: 10.1016/j.ijrobp.2014.01.012 [DOI] [PubMed] [Google Scholar]
- 65.Roach MC, Bradley JD, Robinson CG. Optimizing radiation dose and fractionation for the definitive treatment of locally advanced non-small cell lung cancer. J Thorac Dis 2018; 10(S21): S2465–S2473. doi: 10.21037/jtd.2018.01.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gagliardi G, Constine LS, Moiseenko V, Correa C, Pierce LJ, Allen AM, et al. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys 2010; 76(3 Suppl): S77–S85. doi: 10.1016/j.ijrobp.2009.04.093 [DOI] [PubMed] [Google Scholar]
- 67.Islam KMM, Jiang X, Anggondowati T, Lin G, Ganti AK. Comorbidity and survival in lung cancer patients. Cancer Epidemiology Biomarkers & Prevention 2015; 24: 1079–85. doi: 10.1158/1055-9965.EPI-15-0036 [DOI] [PubMed] [Google Scholar]
- 68.Janssen-Heijnen ML, Schipper RM, Razenberg PP, Crommelin MA, Coebergh JW. Prevalence of co-morbidity in lung cancer patients and its relationship with treatment: a population-based study. Lung Cancer 1998; 21: 105–13. doi: 10.1016/S0169-5002(98)00039-7 [DOI] [PubMed] [Google Scholar]
- 69.De Ruysscher D, Botterweck A, Dirx M, Pijls-Johannesma M, Wanders R, Hochstenbag M, et al. Eligibility for concurrent chemotherapy and radiotherapy of locally advanced lung cancer patients: a prospective, population-based study. Ann Oncol 2009; 20: 98–102. doi: 10.1093/annonc/mdn559 [DOI] [PubMed] [Google Scholar]
- 70.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with Durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018; 379: 2342–50. doi: 10.1056/NEJMoa1809697 [DOI] [PubMed] [Google Scholar]
- 71.Durvalumab for treating locally advanced unresectable non-small-cell lung cancer after platinum-based chemoradiation | Guidance | NICE. Available from: https://www.nice.org.uk/guidance/ta578/chapter/1-Recommendations [cited 2019 May 3].
- 72.Stevens R, Macbeth F, Toy E, Coles B, Lester JF. Palliative radiotherapy regimens for patients with thoracic symptoms from non-small cell lung cancer : Stevens R, Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; 2015. CD002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. The Lancet 2019; 393: 2051–8. doi: 10.1016/S0140-6736(18)32487-5 [DOI] [PubMed] [Google Scholar]
- 74.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. The Lancet 2004; 363: 1665–72. doi: 10.1016/S0140-6736(04)16250-8 [DOI] [PubMed] [Google Scholar]
- 75.Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS, et al. Consolidative radiotherapy for limited metastatic Non–Small-Cell lung cancer. JAMA Oncol 2018; 4: e173501. doi: 10.1001/jamaoncol.2017.3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gomez DR, Blumenschein GR, Lee JJ, Hernandez M, Ye R, Camidge DR, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol 2016; 17: 1672–82. doi: 10.1016/S1470-2045(16)30532-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gomez DR, Tang C, Zhang J, Blumenschein GR, Hernandez M, Lee JJ, et al. Local consolidative therapy (lct) improves overall survival (OS) compared to maintenance Therapy/Observation in oligometastatic non-small cell lung cancer (NSCLC): final results of a multicenter, randomized, controlled phase 2 trial. Int J Radiat Oncol Biol Phys 2018; 102: 1604. doi: 10.1016/j.ijrobp.2018.08.050 [DOI] [Google Scholar]
- 78.van Herk M, McWilliam A, Dubec M, Faivre-Finn C, Choudhury A. MRI Guided Radiotherapy: A Short SWOT Analysis. SoR Imaging Oncol. 2017. Available from: https://www.sor.org/system/files/article/201706/io_2017_lr.pdf [cited 2017 Dec 11].
- 79.Kumar S, Liney G, Rai R, Holloway L, Moses D, Vinod SK. Magnetic resonance imaging in lung: a review of its potential for radiotherapy. Br J Radiol 2016; 89: 20150431. doi: 10.1259/bjr.20150431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liao Z, Lee JJ, Komaki R, Gomez DR, O’Reilly MS, Fossella FV, et al. Bayesian adaptive randomization trial of passive scattering proton therapy and intensity-modulated photon radiotherapy for locally advanced Non–Small-Cell lung cancer. J Clin Oncol 2018; 36: 1813–22. doi: 10.1200/JCO.2017.74.0720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chaudhuri AA, Chabon JJ, Lovejoy AF, Newman AM, Stehr H, Azad TD, et al. Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discov 2017; 7: 1394–403. doi: 10.1158/2159-8290.CD-17-0716 [DOI] [PMC free article] [PubMed] [Google Scholar]