Abstract
Objective:
The aim of this study was to examine the local myocardial segments in hypertrophic cardiomyopathy (HCM) by MRI T1 and T2 mapping, and to investigate how tissue remodeling correlates with structural and functional remodeling in HCM.
Methods:
47 patients with HCM and 19 healthy volunteers were enrolled in this study. All subjects underwent cardiac MRI at 3.0 T. Native T1 and T2 values, end-diastolic wall thickness (EDTH), and percentage of systolic wall thickening (PSWT) were assessed in the left ventricular segments according to the American Heart Association model. Myocardial segments were categorized as normal, non-hypertrophic, mild-hypertrophic, moderate-hypertrophic, and severe-hypertrophic based on EDTH. The difference among all five groups, and the correlation between native T1 and T2 values, EDTH, and PSWT were evaluated.
Results:
Native T1 and T2 values were significantly elevated in both non-hypertrophic and hypertrophic segments of HCM patients compared to controls (both p < 0.001). PSWT was preserved in non-hypertrophic segments (p = 0.838), while significantly impaired (p < 0.001) in hypertrophic segments. Native T1 value of severe hypertrophic segments in HCM was significantly higher than segments of mild and moderate hypertrophy (p < 0.05).
Conclusion:
In HCM patients, the non-hypertrophic myocardial segments already demonstrated significantly elevated T1 and T2 values, despite normal wall thickness and preserved contraction function. The finding suggests that tissue remodeling may precede morphological and functional remodeling in HCM. MRI native T1 and T2 mapping can provide additional value for HCM diagnosis at an early stage.
Advances in knowledge:
Myocardial tissue remodeling, as detected by MRI native T1 and T2 mapping, occurs earlier than morphological and functional changes in HCM patients.
Introduction
Hypertrophic cardiomyopathy (HCM) is a common type of hereditary cardiomyopathy, and is the leading cause of sudden cardiac death in the young population.1 Different genotypes lead to different pathophysiological features, such as ventricular hypertrophy, myocardial fibrosis, and myocardial edema, resulting in different clinical symptoms and prognosis.2,3 Early identification and intervention are important for HCM patients to prevent life-threatening events, especially in the young population.
Cardiac magnetic resonance (CMR) is an advanced imaging tool for diagnosis and risk stratification in HCM. CMR can assess structural, functional, and tissue features of the myocardium by different pulse sequences. CMR was shown to be superior to echocardiography in evaluating left ventricular (LV) wall thickness,4,5 the most important structural marker of HCM. The focal myocardial fibrosis detected by late gadolinium enhanced (LGE) MRI showed to improve risk stratification of adverse cardiovascular events in HCM patients.6,7 Recent development of mapping techniques further enables quantitative assessment of myocardium tissue using the non-invasive T1 and T2 mapping techniques.8,9
Previous studies have focused on the hypertrophic myocardium in HCM, or the myocardium as a whole,4–7,10 while less attention was given to the regional variation in different myocardium segments. Depending on the HCM stage, there are often myocardial segments that fall into the normal thickness range. Such segments appear non-hypertrophic, recognized as wall thickness less than 15 mm by the European Society of Cardiology guideline on diagnosis of HCM.11–13 Non-hypertrophic segments in HCM are at risk of developing into mild or severe hypertrophy, and may have subexpressed abnormalities that fail to be recognized by structural or functional features normally measured in the clinic. Studying myocardium segments with different degree of hypertrophy may provide insight on the course of HCM, thereby facilitating early intervention.
The purpose of this study was to examine the local myocardial segments in HCM, including those non-hypertrophic ones, and to study how tissue remodeling correlates with structural and functional remodeling. We aim to quantitatively measure myocardial fibrosis and edema by MRI native T1 and T2 mapping, and to investigate their association with two other important markers also from CMR: (1) local morphological structure as characterized by the end-diastolic thickness (EDTH) and (2) local contraction function as characterized by the percentage of systolic wall thickening (PSWT).
Methods and materials
Study population
The patients who were diagnosed of HCM by the CMR exam between June 2016 and June 2017 were retrospectively included in this study. The inclusion criteria were: LV maximal wall thickness ≥15 mm in adults without HCM family history, or ≥13 mm in adults with HCM family history. 68 patients met the inclusion criteria. The following exclusion criteria were applied: (1) 14 (21%) patients with inconsistent field of view on different slices of short-axis T1 mapping, (2) 7 (10%) patients with poor CMR images quality. Finally, 47 HCM patients (age 50 ± 15 years, 35 male) were enrolled in this study. In addition, 19 age and sex matched healthy volunteers (age 47 ± 14 years, 11 male) without any cardiovascular diseases or systemic diseases were enrolled as the control group.
This study was approved by the hospital institutional review board, and written informed consent was obtained from all participants.
CMR acquisition
All CMR examinations were performed on a 3.0 T scanner (Magnetom Skyra, Siemens Healthineers, Erlangen, Germany) using an 18-channel cardiac coil. The imaging protocol included LV short-axis cine, native T1 and T2 mapping at the same sectional planes with ECG gating during breath-hold. A True-FISP sequence [echo time (TE) = 1.39 ms, repetition time (TR) = 2.50 ms, field of view (FOV) = 320–360 mm, matrix = 192×146, flip angel (FA) = 47◦, slice thickness = 6 mm, slice gap = 2 mm] was acquired for cardiac cine in a series of LV short-axis planes from the apex to base. The ECG-gated single-shot modified Look-Locker inversion recovery (MOLLI) method with scan protocol of 5(3)3 was used for T1 mapping: 8 steady state free precession (SSFP) readouts in 11 heartbeats, TE = 1.07 ms, TR = 2.58 ms, FOV = 320–360 mm, matrix = 192×144, FA = 35°, slice thickness = 6 mm, 72 segments, minimum TI = 100 ms, TI increment = 80 ms, GRAPPA acceleration factor = 2, imaging window = 136 ms. T2 mapping technique involved a T2 prepared pulse sequence to produce single-shot T2 prepared SSFP images (T2p-SSFP), with different T2 preparation times (0, 30 and 55 ms). The images were generated with three heartbeat recovery durations for each T2 preparation (total acquisition time of nine heartbeats). For both T1 and T2 maps, a built-in algorithm was applied for motion correction before curve fitting. Motion correction and curve fitting were both performed by the MR scanner.
CMR images analysis
LV function, native T1, and T2 values were evaluated by two observers (L.H with 8 years’ experience and L.R with 3 years’ experience, respectively) using a commercial software (CVI42, Circle Cardiovascular Imaging, Calgary, Canada).
LV functional parameters were assessed by manually contouring endocardial and epicardial borders at end-systolic and end-diastolic phase of short-axis cine. LV volumes and mass were normalized to body-surface area (BSA, calculated with height and weight by MR scanner automatically). LV EDTH and the PSWT were calculated automatically in the 16 segments according to the American Heart Association (AHA) model.
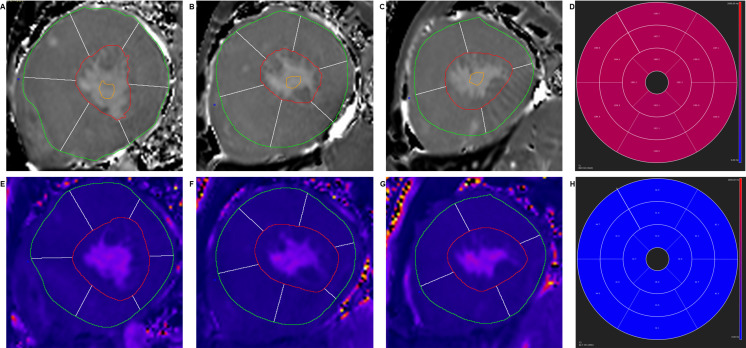
Native T1 and T2 values were evaluated by manually tracing endocardial and epicardial contours on all motion-corrected native T1 and T2 images, with the reference point at the anterior LV insertion to generate the AHA 16-segment model (Figure 1). The mean native T1 and T2 values of all 16 segments were automatically computed.
Figure 1. .
Illustration of segmental analysis of basal (A, E), middle (B, F) and apical (C, G) slices of native T1 and T2 mapping images and the bull’s eye plot (D, H) of LV according to AHA 16-segments’ model. LV,left ventricular.
The AHA segments in the HCM patients were classified into non-hypertrophic (EDTH <15 mm) and hypertrophic (EDTH ≥15 mm) based on the maximal LV EDTH. The hypertrophic segments were further stratified as mild (15 mm ≤ EDTH <20 mm), moderate (20 mm ≤ EDTH <25 mm), and severe (EDTH ≥25 mm) according to the criteria used in previous studies.12,14,15
Statistical analysis
All statistical analyses were performed using SPSS v. 18 (Chicago, IL). Quantitative results were expressed as mean ± standard deviation (95% confidence intervals, CI). Categorical variables are expressed as percentages. Normality of distribution was tested by the Kolmogorov–Smirnov test. Kruskal–Wallis test were performed for three-group comparison, and Mann–Whitney U-test was used to evaluate the differences between two groups without assuming normal distribution. Spearman’s rank correlation was applied to determine the relationship between CMR parameters (native T1 and T2 values, EDTH, and PSWT). p < 0.05 was considered statistically significant.
Results
Baseline characteristics and CMR parameters
The baseline clinical characteristics and LV global morphological and functional measures derived from CMR of all subjects are reported in Table 1.
Table 1. .
Baseline characteristics and LV global morphological and functional features derived from MRI in HCM patients and healthy volunteers
| Healthy volunteers (n = 19) |
Patients with HCM (n = 47) |
p-value (Mann–Whitney U-test) |
|
|---|---|---|---|
| Age (year) | 47 ± 14 (42, 56) |
50 ± 15 (46, 54) |
0.270 |
| Gender male (%) | 11 (57.9) | 35 (74.5) | 0.185 |
| BMI (kg/m2) | 23.2 ± 3.0 (21.8, 24.6) |
24.5 ± 4.3 (23.3, 25.8) |
0.217 |
| BSA (m2) | 1.7 ± 0.1 (1.7, 1.8) |
1.8 ± 0.2 (1.7, 1.9) |
0.120 |
| HR (beats/minute) | 69 ± 9 (65, 73) |
71 ± 13 (67, 75) |
0.498 |
| LVEDVI (ml/ m2) | 58.6 ± 17.2 (50.4, 66.9) |
62.2 ± 21.4 (55.9, 68.5) |
0.521 |
| LVESVI (ml/ m2) | 25.5 ± 10.7 (20.3, 30.6) |
25.6 ± 16.8 (20.7, 30.6) |
0.970 |
| LVSVI (ml/ m2) | 33.2 ± 7.2 (29.7, 36.6) |
36.6 ± 9.3 (33.9, 39.3) |
0.158 |
| LVEF (%) | 58.2 ± 6.3 (55.1, 61.2) |
60.4 ± 7.3 (58.3, 62.5) |
0.246 |
| LVmassI (g/m2) | 52.0 ± 7.8 (48.1, 55.9) |
80.0 ± 39.0 (68.2, 91.9) |
<0.001 |
Continuous variables are expressed as mean ± standard deviation(95% CI). HCM, hypertrophic cardiomyopathy; BMI, body mass index; BSA, body surface area; HR, heart rate; LVEDVI, left ventricular end-diastolic volume index; LVESVI, left ventricular end-systolic volume index; LVSVI, left ventricular stroke volume index; LVEF, left ventricular ejection fraction; LVmassI, left ventricular mass index.
CMR-derived functional parameters, including LV end-diastolic volume index, LV end-systolic volume index, LV stroke volume index, and LV ejection fraction, showed no significant difference between HCM and controls (p > 0.05), except for the LV mass index (p < 0.001). The phenotypes of the 47 HCM patients were 10 cases (21%) with obstructive HCM and 37 (79%) with non-obstructive HCM. The later included 7 (19%) apical HCM and 30 (81%) asymmetric septal HCM. 40 HCM subjects (85%) had abnormal ST-T wave changes, 32 subjects (68%) had occasional ventricular premature beat, and 1 case (2%) had paroxysmal supraventricular tachycardia on electrocardiography.
A total of 752 myocardial segments were evaluated in the HCM patient group and 304 segments in the control group. Within the HCM patient group, 588 (78%) non-hypertrophic and 164 (22%) hypertrophic myocardial segments were identified. Of the 164 hypertrophic segments, 105 (64%) were mild, 35 (21%) moderate, and 24 (15%) severe.
Segmental analysis: non-hypertrophic myocardium in HCM vs healthy myocardium in controls
Native T1 and T2 values, PSWT, and EDTH in healthy volunteers and HCM patients are reported in Table 2. In HCM patients, the native T1 and T2 values in the non-hypertrophic segments were significantly elevated as compared to controls (p < 0.001, Figure 2A, B). However, there was no significant difference in PSWT between the non-hypertrophic segments in HCM patients and normal segments in healthy volunteers (p = 0.838, Figure 2C). Within the HCM group, native T1, and T2 in the hypertrophic segments were significantly higher than those in the non-hypertrophic segments, and PSWT were significantly reduced (p < 0.001; Figure 2).
Table 2. .
Native T1 and T2 values, PSWT, and EDTH in normal myocardial segments of healthy controls, non-hypertrophic, and hypertrophic myocardial segments of HCM patients
| Parameters | Healthy volunteers | HCM patients | ||
|---|---|---|---|---|
| Normal segments (n = 304) |
Non-hypertrophic segments (n = 588) |
Hypertrophic segments (n = 164) |
p-value (Kruskal–Wallis test) |
|
| EDTH (mm) | 6.3 ± 1.9 (6.1, 6.5) |
8.3 ± 2.8* (8.1,8.6) | 19.8 ± 5.8* (18.9, 20.7) |
<0.001 |
| Native T1 (ms) | 1237.1 ± 55.8 (1230.8, 1243.4) |
1283.6 ± 86.0* (1266.6, 1278.0) | 1336.6 ± 65.1* (1324.1, 1343.4) |
<0.001 |
| T2 (ms) | 40.4 ± 3.3 (40.0, 40.8) |
42.3 ± 3.9* (41.9, 42.5) |
43.8 ± 3.4* (43.2,44.2) |
<0.001 |
| PSWT (%) | 97.3 ± 51.8 (91.4, 103.1) |
100.7 ± 59.8 (94.7, 104.2) |
29.0 ± 20.0* (26.0, 32.2) |
<0.001 |
Continuous variables are expressed as mean ± standard deviation(95% CI). HCM, hypertrophic cardiomyopathy; EDTH, end-diastolic thickness; PSWT, percentage of systolic wall thickening. Column 3 and 4: * statistical significance compared to normal segments (column 2) using Mann–Whitney U-test.
Figure 2. .
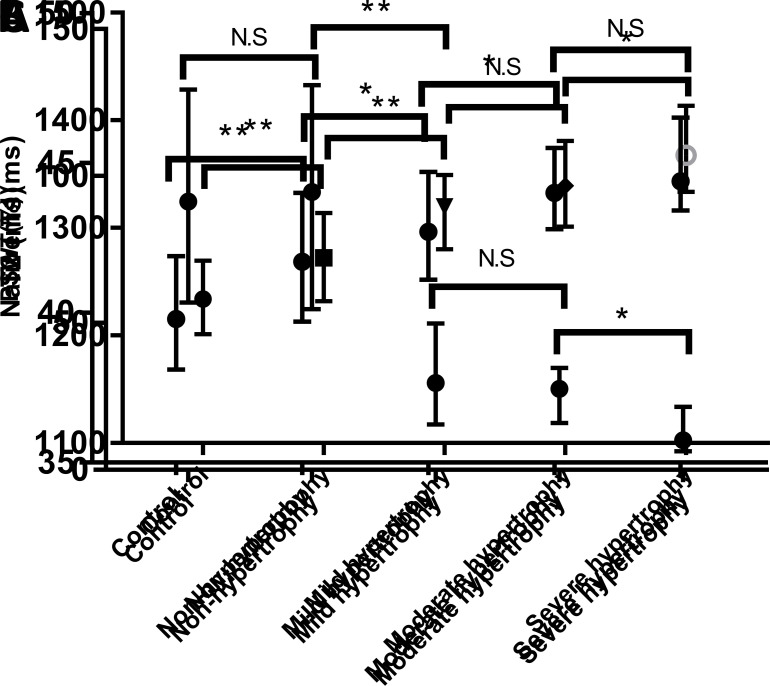
Native T1 values (A), T2 values (B) and PSWT (C) in the normal segments in healthy controls, non-hypertrophic, mild-hypertrophic, moderate-hypertrophic, and severe hypertrophic segments. The error bars represents the median with interquartile range. (**: p < 0.001;*: p < 0.05; N.S : p > 0.05). PSWT,percentage of systolic wall thickening.
Segmental analysis
The CMR-derived parameters of myocardial segments with different degrees of hypertrophy are reported in Table 3 and displayed in Figure 3. The native T1 values of segments with severe hypertrophy were significantly higher than those with mild and moderate hypertrophy (p < 0.001 and p = 0.009; Figure 2A). No significant difference in native T1 values was observed between mild and moderate hypertrophic segments (p = 0.075; Figure 2A). Mean T2 values of segments with severe hypertrophy and moderate hypertrophy were significantly higher than those with mild hypertrophy (p = 0.001 and p = 0.036; Figure 2B). T2 values of segments with moderate hypertrophy had no significant difference as compared to those with severe hypertrophy (p = 0.238, Figure 2B). PSWT was significantly higher in segments with mild and moderate hypertrophy than in severe hypertrophic segments (p < 0.001 and p = 0.001; Figure 2C). As compared to mild hypertrophic segments, PSWT in moderate hypertrophic segments showed no significant difference (p = 0.142, Figure 2C).
Table 3. .
Native T1 and T2 values, PSWT, and EDTH in mild, moderate, and severe hypertrophic myocardial segments in the HCM patient group
| Parameters | Mild hypertrophic segments (n = 105) |
Moderate hypertrophic segments (n = 35) |
Severe hypertrophic segments (n = 24) |
p-value (Kruskal–Wallis test) |
|---|---|---|---|---|
| EDTH (mm) | 16.5 ± 1.4 (16.2, 16.8) |
21.7 ± 1.3* (21.3, 22.2) |
31.5 ± 5.3* (29.3, 33.8) |
<0.001 |
| Native T1 (ms) | 1323.2 ± 60.5 (1308.2, 1329.5) |
1340.8 ± 58.0 (1320.8, 1360.1) |
1389.0 ± 69.2* (1359.8, 1418.2) |
<0.001 |
| T2 (ms) | 43.3 ± 3.5 (42.6, 43.9) |
44.3 ± 3.4* (43.1, 45.4) |
45.1 ± 2.6* (44.0, 46.2) |
0.002 |
| PSWT (%) | 33.3 ± 21.0 (29.3, 37.4) |
25.4 ± 14.1 (21.2, 26.1) |
15.0 ± 15.6* (8.4, 21.5) |
<0.001 |
Continuous variables are expressed as mean ± standard deviation(95% CI). EDTH, end-diastolic thickness; PSWT, percentage of systolic wall thickening. Column 3 and 4: * statistical significance compared to mild hypertrophic segments (column 2) using Mann-Whitney U-test.
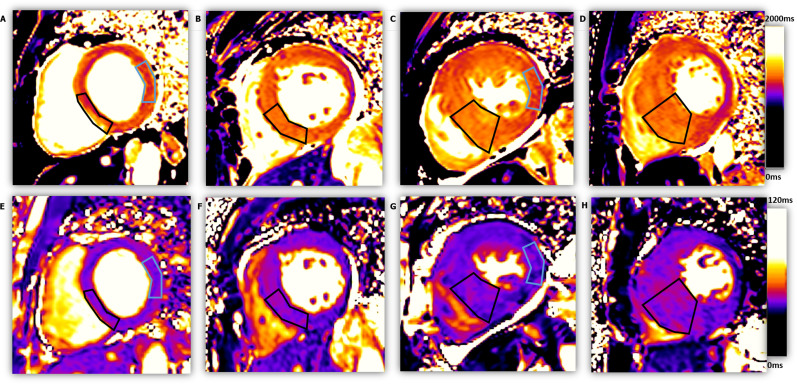
Figure 3. .
The native T1 mapping and T2 mapping images of four subjects, from left to right: one healthy control and three patients with different HCM severity. ROIs with black outline shows the mid-inferoseptal wall of each subjects for segmental analysis (from left to right: EDTH = 8.0 mm, 15.1 mm, 21.8 mm, 25.5 mm; T1 = 1241.8 ms, 1302.8 ms, 1365.3 ms, 1400.2 ms; T2 = 39.1 ms, 43.5 ms, 44.6 ms, 45.6 ms). The ROI with blue outline in C and G highlight a non-hypertrophic myocardial segment (EDTH = 7.3 mm, T1 = 1283.3 ms, T2 = 42.0 ms), as compared to a normal myocardial segment in A and E (EDTH = 7.1 mm, T1 = 1230.1 ms, T2 = 37.6 ms). EDTH, end-diastolic wall thickness; ROI, region ofinterest.
Correlation between segmental parameters
Native T1 and T2 values were positively correlated with EDTH, but only weakly (Spearman’s ρ = 0.367, p < 0.001 and Spearman’s ρ = 0.183, p < 0.001, respectively). PSWT was negatively correlated with EDTH (Spearman’s ρ = −0.692, p < 0.001). Native T1 and T2 values showed no significant correlation with PSWT (Spearman’s ρ = −0.083, p = 0.288 and Spearman’s ρ = −0.059, p = 0.454, respectively).
Discussion
In this study, we focused on regional variation in HCM myocardium, specifically on the normal-appearing myocardium in HCM, i.e. the non-hypertrophic myocardial segments with EDTH less than 15 mm, and showed that these segments already had significantly elevated native T1 and T2 values, despite their normal-range thickness and preserved contraction function.
Our major finding suggests that myocardial tissue remodeling already occurs in seemingly normal segments of HCM. This is in line with a previous study of MR perfusion that showed myocardial perfusion of non-hypertrophic segments in HCM patients is lower than normal segments in healthy volunteers.13 In another study, elevated biomarkers of myocardial collagen synthesis were found in HCM gene mutation carriers without LV hypertrophy,14 and native T1 mapping can detect early diffuse myocardial fibrosis in these subjects.15 However, T2 mapping was not performed in previous studies to assess the possible edema. The elevated edema may be attributed to ischemia or microvascular dysfunction induced by myocardial hypertrophy and capillary endothelial dysfunction.16 The accumulation of regional collagen may also account for the elevated T2 values.17
In this study, we differentiated myocardium segments in our analysis, instead of as a whole in most previous studies.8,15,16 In the hypertrophic segments of HCM, both native T1 and T2 values gradually increase with the severity of hypertrophy. In particular, the T2 values are significantly different between segments of mild and moderate hypertrophy, at a relatively early stage in remodeling. The T1 values, although elevated, were not statistically different at this stage. This suggests that myocardial edema may be a major pathological feature in early HCM. In a later transition stage, i.e. from moderate to severe hypertrophy, the behavior of T1 and T2 values was the opposite: T1 was significantly elevated, while T2 changes did not show statistical significance. Our results were in accordance to the findings of Noureldin et al,18 who showed that increased T2 signal intensity is linked to acute myocardial injuries and appears at early phase of HCM, while LGE showed chronic injuries developed at a later stage.
Another major finding is that although tissue remodeling occurs in terms of elevated fibrosis and edema, the contraction function is still preserved in non-hypertrophic segments of HCM. PSWT is a sensitive marker reflecting the mechanical properties (i.e. stiffness) of myocardial tissue,19,20 potentially critical for prognosis as it is linked to the mechanical properties. The preserved PSWT showed that the contraction function has not been significantly impaired in non-hypertrophic segments. It is only after the hypertrophy being manifested that the PSWT drops significantly. In comparison, the native T1 and T2 values could already demonstrate statistical difference between such non-hypertrophic segments and the healthy myocardium.
There were several limitations in present study. The cohort is relatively small, and the number of segments in each category was not well-balanced for evaluating the remodeling at different hypertrophic stages. Furthermore, no LGE or ECV mapping were available in our study, the latter able to provide a useful range of values which can be compared across MRI vendors and mapping protocols. We have shown the trend of increasing T1 and T2 when the degree of hypertrophy increases in HCM, however, significant overlap exists between the values and no threshold could be reliably established. Future studies may specifically focus on HCM patients of different stage, and establish native T1 and T2 models aiming for diagnosis at an early stage.
Conclusion
We showed that in HCM patients, T1 and T2 tissue remodeling occurs in the normal-appearing myocardial segments with preserved contraction function. The finding suggests that tissue remodeling may precede morphological and functional remodeling in HCM. MRI native T1 and T2 mapping can provide additional value for HCM diagnosis at an early stage. Further studies are warranted to establish multiparametric CMR models involving T1 and T2 measurements for early identification of HCM.
Footnotes
Acknowledgment: This work was supported by the National Natural Science Foundation of China [grant numbers 81471637, 81401388, 81873889].
The authors Liming Xia and Qian Tao contributed equally to the work.
Contributor Information
Lu Huang, Email: jxmuhl@126.com.
Lingping Ran, Email: ranlingping15@163.com.
Peijun Zhao, Email: 827213469@qq.com.
Dazhong Tang, Email: tangdazhong2004@163.com.
Rui Han, Email: hanrui31@163.com.
Tao Ai, Email: aitao007@hotmail.com.
Liming Xia, Email: xialiming2017@outlook.com, lmxia@tjh.tjmu.edu.cn.
Qian Tao, Email: q.tao@lumc.nl.
REFERENCES
- 1.Maron BJ, Maron MS. Hypertrophic cardiomyopathy. The Lancet 2013; 381: 242–55. doi: 10.1016/S0140-6736(12)60397-3 [DOI] [PubMed] [Google Scholar]
- 2.Marian AJ, Braunwald E. Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res 2017; 121: 749–70. doi: 10.1161/CIRCRESAHA.117.311059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxi AJ, Restrepo CS, Vargas D, Marmol-Velez A, Ocazionez D, Murillo H. Hypertrophic cardiomyopathy from a to Z: genetics, pathophysiology, imaging, and management. RadioGraphics 2016; 36: 335–54. doi: 10.1148/rg.2016150137 [DOI] [PubMed] [Google Scholar]
- 4.Hindieh W, Weissler-Snir A, Hammer H, Adler A, Rakowski H, Chan RH. Discrepant measurements of maximal left ventricular wall thickness between cardiac magnetic resonance imaging and echocardiography in patients with hypertrophic cardiomyopathy. Circ Cardiovasc Imaging 2017; 10: e006309. doi: 10.1161/CIRCIMAGING.117.006309 [DOI] [PubMed] [Google Scholar]
- 5.Webb J, Villa A, Bekri I, Shome J, Teall T, Claridge S, et al. Usefulness of Cardiac Magnetic Resonance Imaging to Measure Left Ventricular Wall Thickness for Determining Risk Scores for Sudden Cardiac Death in Patients With Hypertrophic Cardiomyopathy. Am J Cardiol 2017; 119: 1450–5. doi: 10.1016/j.amjcard.2017.01.021 [DOI] [PubMed] [Google Scholar]
- 6.Steriotis AK, Sharma S. Risk stratification in hypertrophic cardiomyopathy. Eur Cardiol 2015; 10: 31–6. doi: 10.15420/ecr.2015.10.01.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green JJ, Berger JS, Kramer CM, Salerno M. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging 2012; 5: 370–7. doi: 10.1016/j.jcmg.2011.11.021 [DOI] [PubMed] [Google Scholar]
- 8.Iles LM, Ellims AH, Llewellyn H, Hare JL, Kaye DM, McLean CA, et al. Histological validation of cardiac magnetic resonance analysis of regional and diffuse interstitial myocardial fibrosis. Eur Heart J Cardiovasc Imaging 2015; 16: 14–22. doi: 10.1093/ehjci/jeu182 [DOI] [PubMed] [Google Scholar]
- 9.Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Perry M, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of cardiology (ESC. Eur Heart J 2014; 35: 2733–79. doi: 10.1093/eurheartj/ehu284 [DOI] [PubMed] [Google Scholar]
- 10.Spirito P, Bellone P, Harris KM, Bernabò P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med Overseas Ed 2000; 342: 1778–85. doi: 10.1056/NEJM200006153422403 [DOI] [PubMed] [Google Scholar]
- 11.Elliott PM, Gimeno Blanes JR, Mahon NG, Poloniecki JD, McKenna WJ. Relation between severity of left-ventricular hypertrophy and prognosis in patients with hypertrophic cardiomyopathy. The Lancet 2001; 357: 420–4. doi: 10.1016/S0140-6736(00)04005-8 [DOI] [PubMed] [Google Scholar]
- 12.Maron MS, Zenovich AG, Casey SA, Link MS, Udelson JE, Aeppli DM, et al. Significance and relation between magnitude of left ventricular hypertrophy and heart failure symptoms in hypertrophic cardiomyopathy. Am J Cardiol 2005; 95: 1329–33. doi: 10.1016/j.amjcard.2005.01.077 [DOI] [PubMed] [Google Scholar]
- 13.Huang L, Han R, Ai T, Sun Z, Bai Y, Cao Z, et al. Assessment of coronary microvascular dysfunction in hypertrophic cardiomyopathy: first-pass myocardial perfusion cardiovascular magnetic resonance imaging at 1.5 T. Clin Radiol 2013; 68: 676–82. doi: 10.1016/j.crad.2013.01.003 [DOI] [PubMed] [Google Scholar]
- 14.Ho CY, López B, Coelho-Filho OR, Lakdawala NK, Cirino AL, Jarolim P, Kwong R, González A, Ph D, et al. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med 2010; 363: 552–63. doi: 10.1056/NEJMoa1002659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinojar R, Goodman BP, Villa A, Ucar EA, Dabir D, Schaeffter T, et al. T1 mapping in discrimination between hypertrophic and hypertensive cardiomyopathy. J Cardiovasc Magn Reson 2014; 16(S1): O61. doi: 10.1186/1532-429X-16-S1-O61 [DOI] [Google Scholar]
- 16.Gommans DF, Cramer GE, Bakker J, Michels M, Dieker H-J, Timmermans J, et al. High T2-weighted signal intensity is associated with elevated troponin T in hypertrophic cardiomyopathy. Heart 2017; 103: 293–9. doi: 10.1136/heartjnl-2016-309900 [DOI] [PubMed] [Google Scholar]
- 17.Abdel-Aty H, Cocker M, Strohm O, Filipchuk N, Friedrich MG. Abnormalities in T2-weighted cardiovascular magnetic resonance images of hypertrophic cardiomyopathy: regional distribution and relation to late gadolinium enhancement and severity of hypertrophy. J Magn Reson Imaging 2008; 28: 242–5. doi: 10.1002/jmri.21381 [DOI] [PubMed] [Google Scholar]
- 18.Noureldin RA, Liu S, Nacif MS, Judge DP, Halushka MK, Abraham TP, et al. The diagnosis of hypertrophic cardiomyopathy by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2012; 14: 17. doi: 10.1186/1532-429X-14-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pennell DJ, Sechtem UP, Higgins CB, Manning WJ, Pohost GM, Rademakers FE, et al. Clinical indications for cardiovascular magnetic resonance (CMR): consensus panel report. Eur Heart J 2004; 25: 1940–65. doi: 10.1016/j.ehj.2004.06.040 [DOI] [PubMed] [Google Scholar]
- 20.Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for cardiovascular magnetic resonance (SCMR) board of trustees Task force on standardized post processing. J Cardiovasc Magn Reson 2013; 15: 35. doi: 10.1186/1532-429X-15-35 [DOI] [PMC free article] [PubMed] [Google Scholar]