Abstract
Objective:
To evaluate role of multiparametric MRI (mp-MRI) in differentiation between invasive and non-invasive bladder cancer and accuracy of vesical imaging reporting and data system (VI-RADS) score.
Methods and materials:
50 patients diagnosed as cancer bladder were enrolled in this study, mp-MRI including conventional (T1 weighted imaging and high resolution T2 weighted imaging) and functional sequences (diffusion-weighted imaging and dynamic contrast enhanced-MRI) were done, all data were regrouped to evaluate the accuracy of each separate sequence and mp-MRI in distinguishing non-muscle invasive from muscle-invasive tumors, with VI-RADS score application and comparison with pathological findings, then interobserver agreement for detection of muscle invasion according to mp-MRI and VI-RADS scoring system findings was calculated.
Results:
Diagnostic accuracy of mp-MRI in differentiation between muscle invasive and non-muscle invasive bladder cancer was (84%) with highest sensitivity (78%), very good agreement between mp-MRI and histopathological data (k = 0.87), and highest area under curve (AUC) reaching 0.83, dynamic contrast enhanced-MRI sequence showed the highest accuracy in muscle invasion detection by (88%), with highest AUC 0.83. Diagnostic accuracy of VI-RADS score in detection of muscle invasion was 84%, with specificity and negative predictive value of 88% and AUC was 0.83. Interobserver agreement was strong as regard diagnostic performance of mp-MRI and VI-RADS scoring for detection of muscle invasion reaching (K = 0.82, p < 0.001) and (K = 0.87, p < 0.001) respectively.
Conclusion:
mp-MRI is considered as comprehensive and effective tool for determination of muscle invasion in cases of urinary bladder cancer. Also VI-RADS scoring system can accurately differentiate between invasive and non-invasive bladder cancer.
Advances in knowledge:
The VI-RADS system was recently suggested for the uniform evaluation of muscle invasion in cancer bladder by mp-MRI. In this paper, we applied this system to 50 cases to evaluate its ease and compared the results with the histopathological findings for evaluation of its accuracy.
Introduction
Urinary bladder cancer (BC) is considered one of the most common malignancies. Its incidence increases with age with male to female ratio about 3:1.1 Most BC (more than 90%) are urothelial in origin2 and approximately 80% of them are non-muscle invasive at diagnosis.3
Clinical staging of an individual bladder tumor is critical to the care of affected patient and is usually performed through a combination of clinical, pathologic, and radiologic means.3 Also differentiation between muscle invasive (MIBC) and non-muscle invasive bladder cancer (NMIBC) is mandatory for treatment planning, as NMIBC (stage T1) are treated by transurethral resection (TUR), but MIBC (stage T2 or higher) are treated by radical cystectomy or by radiation therapy and palliative chemotherapy.4
Various methods have been explored to improve accuracy of bladder cancer staging, however there is still need for advanced imaging tool to optimize pre-operative staging and improve treatment outcome. MRI is currently the best imaging technique for bladder cancer local regional staging because of its superior soft tissue contrast, lack of ionizing radiation, in addition, it clearly differentiates the layer of bladder wall and enables accurate assessment of tumor depth invasion in bladder wall and extra vesical extension.3
During the past few years, important improvements in MRI technology have been achieved and led to the introduction of multiparametric MRI (mp-MRI) which combines functional sequences as diffusion-weighted imaging (DWI) and dynamic contrast enhanced MRI (DCE-MR) with anatomic T1 and T2 weighted images (T1WI and T2WI), that improves the accuracy of tumor detection and staging, helps to monitor post-therapy response and identify local disease recurrence.5
Recently also standardized approach for imaging and reporting mp-MRI for bladder cancer was created by developing VI-RADS score (Vesical Imaging Reporting and Data System). This aims to standardize bladder mp-MRI for clinical and research applications to create a systematic approach for reporting bladder mp-MRI and defining the risk of muscle invasion (NMIBC vs MIBC).6 The final score is firstly based on T2WI findings for evaluation integrity of muscularis propria, then the presence of muscle invasion decided by DCE-MRI and DWI, and if there is any difference between results of DCE-MRI and T2WI, DWI can improve the accuracy.7
In the current study, we aim to evaluate the applicability of the newly developed VI-RADS which aims to create a standardized systematic approach for reporting bladder mp-MRI and defining the risk of muscle invasion with improved accuracy.
Patients and methods
This prospective study was conducted on 50 patients who were diagnosed as urinary bladder cancer either by cystoscopic examination or by any other previous radiologic investigations.
Patients were referred from the Urology clinic to the Radiology Department of Assiut University Hospital for mp-MRI examination of urinary bladder. The study was carried out after obtaining the permission of the Ethics Committee of Scientific Research, Faculty of Medicine, Assiut University, with informed consent from the patients.
Each patient was subjected to the following
mp-MRI which is the combination of T1WI, high resolution T2WI and functional imaging techniques including: DWI and DCE imaging.
All patients underwent TURBT after mp-MRI in urology department, all high risk patients underwent re-TURBT 4 weeks after the first one with resection of previous primary tumor site to eliminate any residual suspicious areas and for accurate staging of tumor and histopathological correlation as a gold-standard.
Inclusion criteria
All patients who were diagnosed as urinary bladder cancer either by cystoscopic examination or any other previous radiologic investigations with no age or sex predilection.
Exclusion criteria
Patients with general contraindication for MRI examination (as metallic prosthesis or pacemaker),
Patients with contraindication for TUR (as those unfit for anesthesia or with urethral stricture),
Patients with high renal functions, as they are contraindicated for DCE imaging.
Patient preparation
Before MRI examination, adequate bladder distention must be achieved by instructing patients to void 1–2 h before imaging, then start to drink 500–1000 ml of water half an hour before examination. Ultrasound scan prior to MRI can be useful to judge when the bladder is optimally full (300 ml). As without distension, the bladder wall appears thick leading to either a misdiagnosis of bladder cancer or over staging of tumor that was present, and over distension may cause a motion artifact due to discomfort of the patient.
Standard mp-MRI technique
MRI was performed in supine position using 1.5 T MRI scanner (Magentom Avanto, Siemens Healthcare) with four channel and phased array pelvic coil.
First spin-echo axial T1WI [repetition time/echo time (TR/TE) 550/9, slice thickness 6 mm and intersection gap 2 mm], and high resolution T2WI (TR/TE 7000–8000/90–102, slice thickness 3 mm and intersection gap 1 mm) with at least two planes of multiplanar (axial, sagittal or coronal) and without fat suppression were performed.
This was followed by axial DWI during free breathing with axial plane fat suppressed water-excited single-shot spin echo-planar sequence (TR/TE, 4000/78), slice thickness 4 mm, 0.4 mm intersection gap and b-values of 0,400,800 and 1000). Then quantitative assessment of diffusion as regard apparent diffusion coefficient (ADC) values (mm2/s) that were automatically calculated by placing the region of interest (ROI) manually within the most hypointense areas of the tumors.
Finally, DCE-MRI was done with axial T1 fat suppressed hree-dimensional-gradient echo sequence before and after i.v. injection of contrast (Magnevist, Bayer Pharma AG Germany ) using power injector with a dose of 0.1 mm/kg at a rate of 1.5–2 ml s−1. Dynamic images were obtained at 20 s (arterial phase), followed by 70 s (venous phase), then delayed phase at 3 min.
All images should include the whole urinary bladder, proximal urethra, and prostate if the patient is a male. In females, adjacent pelvic viscera (uterus, ovaries, and vagina) should also be included.
Data analysis and image interpretation
All images were transferred to work station (Syngo Siemens Medical Solutions software) for image analysis.
T1WI, T2WI images as well as DWI and DCE MRI images were interpreted and the tumors were assigned a T stage. Then MRI VI-RADS score was applied aiming to standardize mp-MRI in cancer bladder for clinical and research applications.6
Axial T1WI
is used only for the assessment of extra vesical fat infiltration, as both bladder tumor and normal detrusor muscle have intermediate signal intensity on T1WI while extra vesical fat has high signal intensity, so it is valuable in identifying luminal extension and perivesical fat infiltration, but not helpful in differentiation between non-muscle invasive and muscle-invasive tumors.
T2WI
Provides information on tumor depth whether it is muscle-invasive or non-muscle invasive, extra vesical extension and surrounding organs infiltration. As on T2WI bladder tumors have intermediate signal intensity and normal detrusor muscle appears as hypointense line, so in case of non-muscle invasive cancer the low signal intensity of the muscle is seen preserved denoting stage (T1), but in muscle invasive cancer an interrupted irregular detrusor lining is observed denoting stage (T2), while stage (T3) shows interrupted detrusor lining with extension to perivesical fat, and stage (T4) shows extension of tumor to adjacent organs.
In DWI
the signal intensity of tumor is identified at b-value 1000 and it will be hyperintense signal. In case of non-muscle invasion, the hyperintense signal of tumor is seen within bladder lumen denoting stage (T1), while in muscle invasive tumors the hyperintense signal is partially seen within wall of urinary bladder denoting stage (T2), in stage (T3) the hyperintense tumor is seen disrupting bladder wall, and in stage (T4) the hyperintense tumor is seen extending into adjacent organs.
In DCE imaging
Bladder tumors, mucosa and submucosa enhance early, but muscle layer remain hypointense and enhance late. Linear submucosal enhancement with intact low signal intensity of muscle layer denoting stage (T1) (non-muscle invasive), while stage (T2) (muscle-invasive) shows interrupted hypointense muscle layer with early enhancement without extension to perivesical fat, in stage (T3) the disrupted muscle layer and enhancing areas in perivesical fat are seen, and in stage (T4) the lesion is seen extending into adjacent organs. Then, all data were regrouped to evaluate the accuracy of each separate sequence and MP-MRI in distinguishing non-muscle invasive from muscle-invasive tumors compared to pathological findings.
Regarding MRI VI-RADS classification
Tumors were scored according to T2WI, DWI and DCE-MRI to create final invasion score (Table 1).
Table 1. .
MRI VI-RADS classification according to T2WI, DWI and DCE-MRI
| VI-RADS category |
MRI sequence | ||
|---|---|---|---|
|
T2WI (SC) |
DWI | DCE-MRI | |
| 1 |
|
|
|
| 2 |
|
|
|
| 3 |
|
|
|
| 4 |
|
|
|
| 5 |
|
|
|
ADC, apparent diffusion coefficient; DCE, dynamic contrast enhanced; DWI, diffusion-weighted imaging; SC, structural category; SI, signal intensity; T2WI, T2 weighted imaging.
Final VI-RADS scoring was done using all categories and suggest probability of muscle invasion as the following7 .
VI-RADS 1 (muscle invasion is highly unlikely): SC, CE, and DW category 1
-
VI-RADS 2 (muscle invasion is unlikely)
SC, CE, and DW category 2; both CE and DW category two with SC category 3.
-
VI-RADS 3 (muscle invasion is equivocal):
SC, CE, and DW category 3; SC category 3, CE or DW category 3, the remaining sequence category 2.
-
VI-RADS 4 (muscle invasion is likely):
at least SC and/or DW and CE category 4: the remaining category three or 4; SC category three plus DW and/or CE category 4; SC category five plus DW and/or CE category 4.
VI-RADS 5 (invasion of muscle and beyond the bladder is very likely): at least SC plus DW and/or CE category 5; the remaining category four or 5.
Then VI-RADS scoring system results were compared to pathological data to evaluate its accuracy.
Finally, all mp MRI were reviewed by two radiologists and then interobserval agreement for detection of muscle invasion according to mp-MRI and VI-RADS scoring system findings was calculated.
Statistical analysis
Data were collected and analyzed using SPSS (Statistical Package for the Social Science, v. 20, IBM, and Armonk, NY). Continuous data were expressed in form of mean ± standard deviation or median (range), while nominal data was expressed in form of frequency (percentage). Receiver operating characteristic curve (ROC) analysis was used to calculate the area under the curve (AUC) for evaluation the MIBC diagnostic accuracy of mp-MRI and VI-RADS scoring system.
Degree of agreement was determined by Cohen’s κ statistic (K) for comparison of data. The K value can be interpreted as follows (<0.20 considered poor, 0.21–0.40 considered fair, 0.41–0.60 considered moderate, 0.61–0.80 considered good, and 0.81–1.00 considered excellent). The level of confidence was kept at 95% hence a p-value < 0.05 indicated a significant association.8
Results
50 patients (46 males and 4 females with ages ranging from 44 to 70 years and mean of 57.16 ± 7.32 years) were enrolled in this study. Regarding to pathological data, 32 patients out of 50 (64%) presented with stage T1, 12 patients (24%) presented with stage T2, and only 6 patients (12%) were stage T4.
mp-MRI correctly diagnosed local T stage of urinary BC in 42 patients (84%). DCE-MRI was the most accurate sequence as it correctly diagnosed 46 patients (92%), with only 4 cases (8%) understaged and none of studied cases was over staged by such technique, followed by T2W-MRI which correctly diagnosed 38 patients (76%) then DW-MRI that correctly diagnosed 36 patients (72%) and lastly, the least one was T1W-MRI which correctly diagnosed only 32 patients (64%). The frequency of over and understaging by different MRI sequences was presented in (Table 2).
Table 2. .
Distribution of cases based on different MRI sequences
| MRI sequences | Correct | Understaging | Overstaging |
|---|---|---|---|
| T1W-MRI | 32 (64%) | 8 (16%) | 10 (20%) |
| T2W-MRI | 38 (76%) | 4 (4%) | 8 (16%) |
| DW-MRI | 36 (72%) | 10 (20%) | 4 (8%) |
| DCE-MRI | 46 (92%) | 4 (8%) | 0 |
| Multiparametric MRI | 42 (84%) | 4 (8%) | 4 (8%) |
DCE, dynamic contrast enhanced; DW, diffusion-weighted.
Data were expressed in form of frequency (percentage).
Extent of agreement between local T staging by mp-MRI compared with histopathological data was very good (k = 0.87). DCE-MRI sequence showed the greatest degree of agreement by 0.80, followed by T2W-MRI which showed good agreement (k = 0.75) then T1W-MRI and DW-MRI that showed moderate agreement by 0.41 and 0.40 respectively (Table 3).
Table 3. .
Degree of agreement between hispathological and radiological staging
| MRIs’ techniques | Histopathological stages | p-value | ||
|---|---|---|---|---|
| Stage I | Stage II | Stage IV | ||
|
T1W-MRI Stage I Stage II Stage IV |
20 (62.5%) 12 (37.5%) 0 |
4 (33.3%) 8 (66.7%) 0 |
2 (33.3%) 0 4 (66.7%) |
<0.001 |
| Degree of agreement | 0.41 (moderate agreement) | |||
|
T2W-MRI Stage I Stage II Stage III Stage IV |
28 (87.5%) 4 (12.5%) 0 0 |
4 (33.3%) 4 (33.3%) 4 (33.3%) 0 |
0 0 0 6 (100%) |
<0.001 |
| Degree of agreement | 0.75 (good agreement) | |||
|
DW-MRI Stage I Stage II Stage IV |
32 (100%) 0 0 |
4 (33.3%) 8 (66.7%) 0 |
0 0 6 (100%) |
<0.001 |
| Degree of agreement | 0.40 (moderate agreement) | |||
|
DCE-MRI Stage I Stage II Stage III |
28 (87.5%) 4 (12.5%) 0 |
4 (33.3%) 8 (66.7%) 0 |
2 (33.3%) 0 4 (66.7%) |
<0.001 |
| Degree of agreement | 0.80 (very good agreement) | |||
|
Multiparametric MRI Stage I Stage II Stage IV |
28 (87.5%) 4 (12.5%) 0 |
4 (33.3%) 8 (66.7%) 0 |
0 0 6 (100%) |
<0.001 |
| Degree of agreement | 0.87 (very good agreement) | |||
DCE, dynamic contrast enhanced.
Data were expressed in form of frequency (percentage). p-value was significant if <0.05.
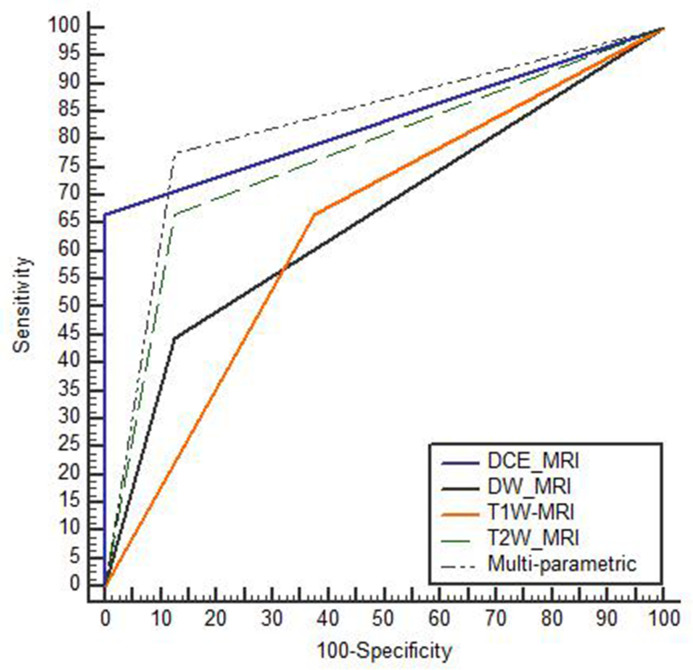
Diagnostic accuracy of mp-MRI in differentiation between MIBC and NMIBC was 84% with highly statistically significance as p-value < 0.001. DCE-MRI sequence showed the highest accuracy by 88%, followed by T2W-MRI (80%) then DW-MRI (72%) and the least accurate sequence was T1W-MRI by (64%), with AUC was highest in DCE-MRI and mp-MRI reaching to 0.83. Table (4) and Figure (1).
Table 4. .
Diagnostic accuracy of MRI sequences in muscle invasion detection
| T1W-MRI | T2W-MRI | DW-MRI | DCE-MRI | mp-MRI | |
|---|---|---|---|---|---|
| Sensitivity | 67% | 67% | 44.4% | 67% | 78% |
| Specificity | 63% | 88% | 87.5% | 100% | 88% |
| PPV | 50% | 75% | 66.7% | 100% | 78% |
| NPV | 77% | 82% | 73.7% | 84.3% | 88% |
| Accuracy | 64% | 80% | 72% | 88% | 84% |
| AUC | 0.64 | 0.77 | 0.66 | 0.83 | 0.83 |
| p-value | 0.03 | 0.01 | 0.04 | <0.001 | <0.001 |
AUC, area under the curve; DCE, dynamic contrast enhanced; DW, diffusion-weighted; NPV, negative predictive value; PPV, positive predictive value.
p-value was significant if <0.05
Figure 1.
Diagnostic accuracy of MRI sequences in muscle invasion
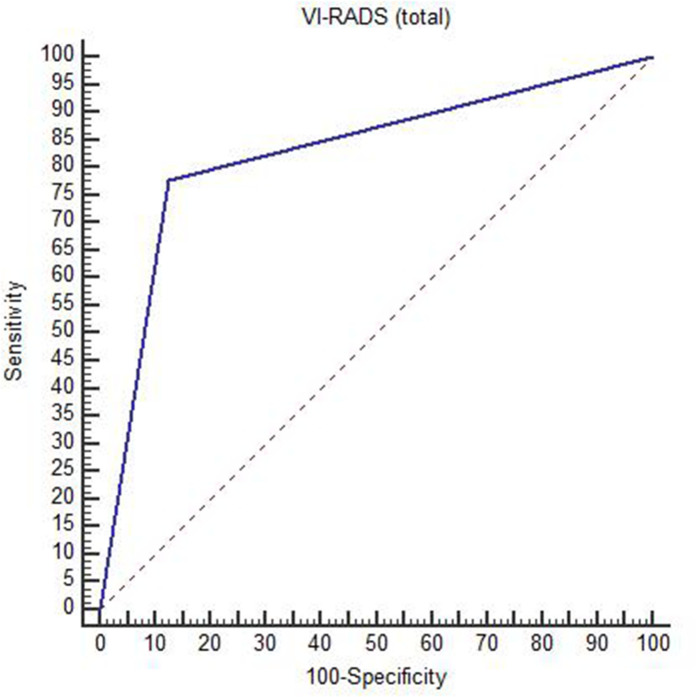
Diagnostic accuracy of VI-RADS score in prediction of muscle invasion reached 84% in this current study with sensitivity and specificity of 78 and 88% respectively and area under curve was 0.83, with highly statistically significance as p value < 0.001. Table (5) and Figures (2), (3) , (4).
Table 5. .
Diagnostic accuracy of total VI-RADS in muscle invasion detection
| Indices | Value |
|---|---|
| Sensitivity | 78% |
| Specificity | 88% |
| Positive predictive value | 78% |
| Negative predictive value | 88% |
| Accuracy | 84% |
| Area under curve | 0.83 |
| p-value | <0.001 |
p-value was significant if <0.05
Figure 2. .
Diagnostic accuracy of VI-RADS score. VI-RADS, vesical imagingreporting and data system
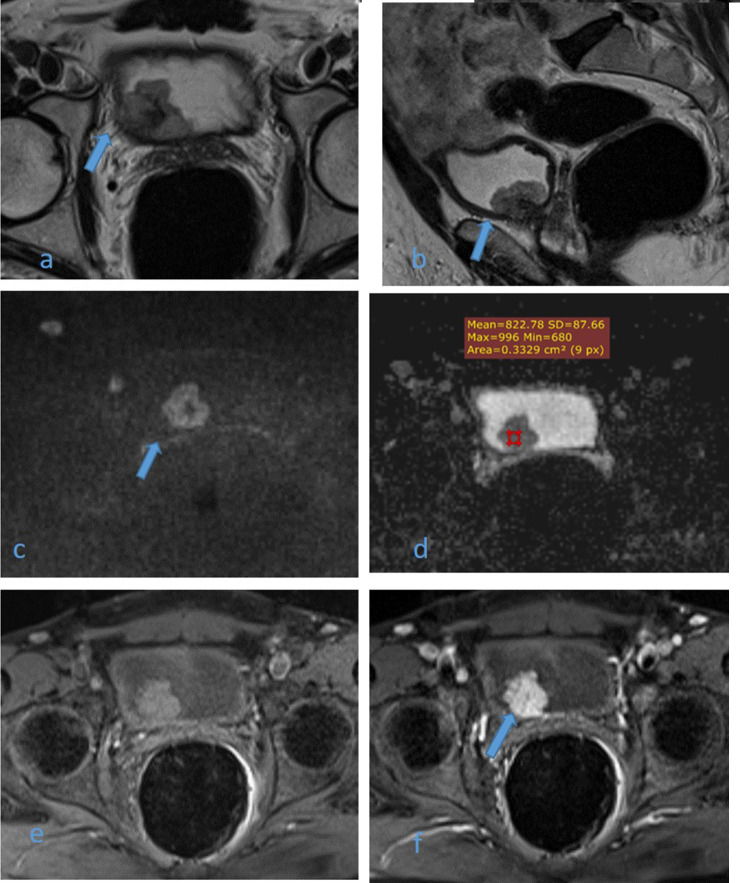
Figure 3. .
61-year-old male patient with history of terminal hematuria. High resolution axial (a) and sagittal (b) T2WI images shows non-muscle invasive right posterolateral U.B. polypoidal lesion not disrupting the hypointense detrusor lining (arrows). DW image (c) and ADC map image (d) show highly restricted diffusion mass with facilitated diffusion of muscle layer exclude muscle invasion (arrow) and low ADC value about 0.8 × 10–3 mm2/s denoting malignant nature. DCE-MRI (e) non-contrast subtracted image and (f) early arterial phase image are displaying early intense enhancement of the mass with no enhancement of muscle layer exclude muscle invasion (arrow). Multiparameteric MRI are consistent with non-muscle invasive bladder cancer (Stage T1) and VI-RADS II, which confirmed histopathologically. ADC, apparent diffusioncoefficient; T2WI,T2 weightedimaging; VI-RADS, vesical imaging reporting and data system.
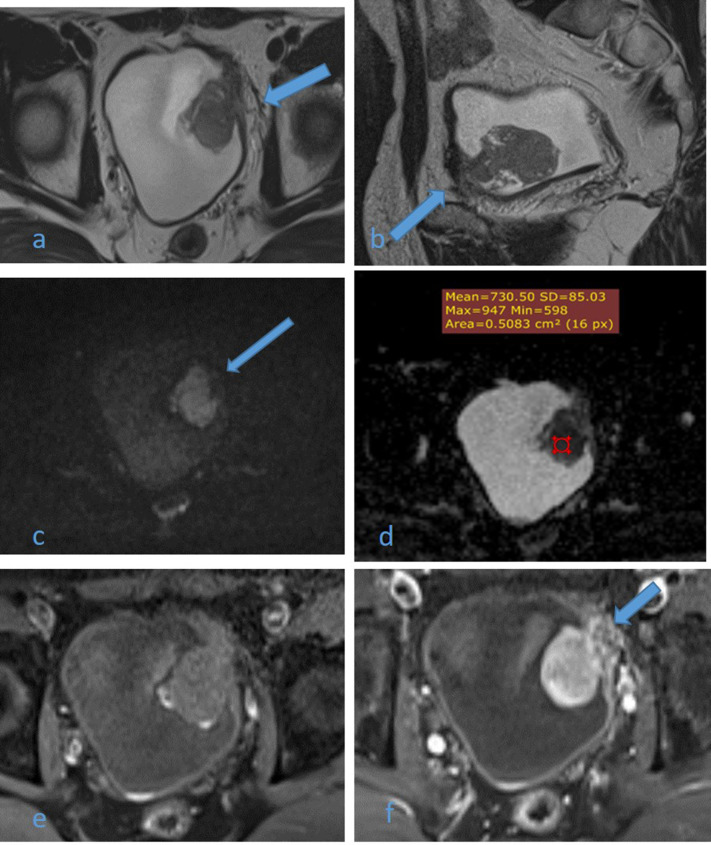
Figure 4.
A 54-year-old male patient with history of recurrent hematuria. High resolution axial (a) and sagittal (b) T2WI images shows muscle-invasive left anterolateral U.B. polypoidal lesion disrupting the underneath hypointense detrusor lining, with no extension to perivesical fat (arrows). DW image (c) and ADC map image (d) show highly restricted diffusion of mass with facilitated diffusion of muscle layer exclude muscle invasion (arrow) and low ADC value about 0.7 × 10–3 s/mm2 denoting malignant nature, DCE-MRI (e) non-contrast subtracted image and (f) early arterial phase image are displaying early intense enhancement of the mass with interrupted enhancement of muscle layer denoting muscle invasion (arrow). Multiparameteric MRI are consistent with muscle-invasive bladder cancer (Stage T2) and VI-RADS IV which confirmed histopathologically. ADC, apparent diffusioncoefficient; T2WI,T2 weightedimaging; VI-RADS, vesical imaging reporting and data system.
The interobserver agreement for detection of muscle invasion by mp-MRI and VI-RADS scoring system was strong reaching (K = 0.82, p < 0.001), and (K = 0.87, p < 0.001) respectively Tables 6 and 7.
Table 6. .
Interobserver agreement for muscle invasion detection by mp-MRI
| Muscle invasion by mp-MRI | Observer B | p-value | K degree | ||
|---|---|---|---|---|---|
| No | Yes | ||||
| Observer A | No | 31 | 2 | <0.001 | 0.82 |
| Yes | 2 | 15 | |||
mp-MRI, multiparametric MRI.
Data were expressed in form of frequency. p-value was significant if <0.05.
Table 7. .
Interobserver agreement for muscle invasion detection by VI-RADS
| Muscle invasion by VI-RADS | Observer B | p-value | K degree | ||
|---|---|---|---|---|---|
| No | Yes | ||||
| Observer A | No | 30 | 2 | <0.001 | 0.87 |
| Yes | 1 | 17 | |||
VI-RADS, vesical imaging reporting and data system.
Data were expressed in form of frequency. p-value was significant if <0.05.
Discussion
Transitional cell carcinoma of urinary bladder is considered as one of the most common malignancies affecting genitourinary tract and is characterized by multifocality and high rate of recurrence, so accurate assessment of T stage of bladder cancer is important for treatment decision direction.9
Improvement has been achieved in MRI technology with introduction of mp-MRI combining anatomic and functional sequences (including high resolution T2WI, DWI and DCE-MRI), several reports have shown superiority of it,also it seems promising in detection, staging and follow up cases of urinary bladder cancer with effective treatment decision guidance.5
In this current study, staging accuracy as regard muscle invasion by T2WI-MRI was 80%, extent of agreement with pathological data was good (k = 0.7), and overstaging detected only in eight patients (16%), this was better than Takeuchi et al10 and Abou El-Ghar ME et al11 studies that reported that T2WI diagnostic accuracy was 67 and 39.6% resepectively.
In this study, we also found that the acurracy in differentiation between NMIBC and MIBC by DWI-MRI was 72% with moderate degree of agreement (k = 0.4) , and understaging in 10 cases (20%), these results were near matching the results of Kobayashi et al study12 which showed acurracy about 78.8% for DWI. However, overall staging acurracy of DCE-MRI in muscle invasion detection was the highest compared to other sequences reaching about 88% ,with very good agreement with pathological data (k = 0.8) and no over staging detected, this agree with Tekes et al study13 which reported that accuracy of DCE-MRI in differentiation between superficial and deep cancer was 85%.
Staging accuracy of mp-MRI for muscle invasion detection in this study was 84% with very good degree of agreement (k = 0.87), and overstaging detected only in four cases (8%), these resuts were matching with Afifi et al study14 who reported that accuracy of mp-MRI was 88%.
The results of our study showed that the diagnostic efficacy in differentiation between NMIBC and MIBC was increased when we combined anatomic and functional MRI sequences, as we achieved high acurracy about 84%, high sensitivity and specificity reaching about 78 and 88% respectively,strong interobserver agreement (K = 0.82, p < 0.001), and highest AUC which reached 0.83. Also, the positive and negative predictive values were significantly higher in mp-MRI compared to each single conventional, so mp-MRI allowed the elimination of the false-negative and false-positive results.
These results were in agreement with Afifi et al study14 which proved the additional value of mp-MRI, and also in agreement with Watanabe et al15 and Gupta et al16 studies’ results that reported that extent of agreement between radiological and histopathological data was greater with mp-MRI and recommended the combined approach.
As mp-MRI results seem promising, VI-RADS was created to standardize mp-MRI in BC staging for clinical and research application and to create systematic approach for reporting BC cases with defining risk of muscle invasion or not (NMIBC vs MIBC) .6
In this study, the comparison between VI-RADS scoring system results and pathological data was reported that its accuracy in prediction of muscle invasion reached about 84% with sensitivity and specificity was 78 and 88% respectively, and high AUC reaching 0.83 with highly statistically significance as p-value < 0.001 as well as strong interobserver agreement (K = 0.87, p < 0.001).
The limitations of this study include the small sample size that was due to the exclusion of unco-operative patients with motion artifacts and patients with poor renal function which represent high percentage of BC patients. Another limitation is that all cases were done on 1.5 T MR machine, as 3 T MR machine was not available. Future studies on larger sample size using 3 T MRI machine may achieve better imaging quality, as its ability to increase signal to noise ratio and acquire section of 3 mm thickness within less time.
In conclusion, mp-MRI is considered as comprehensive and effective tool for determination of muscle invasion in cases of urinary bladder cancer. Also VI-RADS scoring system can accurately differentiate between MIBCand NMIBC.
Contributor Information
Marwa Makboul, Email: makboul@aun.edu.eg.
Shimaa Farghaly, Email: Sh.f@aun.edu.eg.
Islam F. Abdelkawi, Email: Islamfaa@yahoo.com.
REFERENCES
- 1.Burger M, Catto JWF, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol 2013; 63: 234–41. doi: 10.1016/j.eururo.2012.07.033 [DOI] [PubMed] [Google Scholar]
- 2.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F, et al. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol 2017; 71: 96–108. doi: 10.1016/j.eururo.2016.06.010 [DOI] [PubMed] [Google Scholar]
- 3.Panebianco V, Barchetti F, de Haas RJ, Pearson RA, Kennish SJ, Giannarini G, et al. Improving staging in bladder cancer: the increasing role of multiparametric magnetic resonance imaging. Eur Urol Focus 2016; 2: 113–21. doi: 10.1016/j.euf.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 4.Verma S, Rajesh A, Prasad SR, Gaitonde K, Lall CG, Mouraviev V, et al. Urinary bladder cancer: role of Mr imaging. Radiographics 2012; 32: 371–87. doi: 10.1148/rg.322115125 [DOI] [PubMed] [Google Scholar]
- 5.de Haas RJ, Steyvers MJ, Fütterer JJ. Multiparametric MRI of the bladder: ready for clinical routine? AJR Am J Roentgenol 2014; 202: 1187–95. doi: 10.2214/AJR.13.12294 [DOI] [PubMed] [Google Scholar]
- 6.Panebianco V, Narumi Y, Altun E, Bochner BH, Efstathiou JA, Hafeez S, et al. Multiparametric magnetic resonance imaging for bladder cancer: development of VI-RADS (vesical Imaging-Reporting and data system. Eur Urol 2018; 74: 294–306. doi: 10.1016/j.eururo.2018.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woo S, Suh CH, Kim SY, Cho JY, Kim SH. Diagnostic performance of MRI for prediction of muscle-invasiveness of bladder cancer: a systematic review and meta-analysis. Eur J Radiol 2017; 95: 46–55. doi: 10.1016/j.ejrad.2017.07.021 [DOI] [PubMed] [Google Scholar]
- 8.Ashby D. Practical statistics for medical research. Douglas G. Altman, Chapman and Hall, London, 1991. No. of Pages: 611. price: £32.00. Stat Med 1991; 10: 1635–6. doi: 10.1002/sim.4780101015 [DOI] [Google Scholar]
- 9.Shariat SF, Palapattu GS, Karakiewicz PI, Rogers CG, Vazina A, Bastian PJ, et al. Discrepancy between clinical and pathologic stage: impact on prognosis after radical cystectomy. Eur Urol 2007; 51: 137–51. doi: 10.1016/j.eururo.2006.05.021 [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi M, Sasaki S, Naiki T, Kawai N, Kohri K, Hara M, et al. Mr imaging of urinary bladder cancer for T-staging: a review and a pictorial essay of diffusion-weighted imaging. J Magn Reson Imaging 2013; 38: 1299–309. doi: 10.1002/jmri.24227 [DOI] [PubMed] [Google Scholar]
- 11.Abou-El-Ghar ME, El-Assmy A, Refaie HF, El-Diasty T. Bladder cancer: diagnosis with diffusion-weighted MR imaging in patients with gross hematuria. Radiology 2009; 251: 415–21. doi: 10.1148/radiol.2503080723 [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi S, Koga F, Yoshida S, Masuda H, Ishii C, Tanaka H, et al. Diagnostic performance of diffusion-weighted magnetic resonance imaging in bladder cancer: potential utility of apparent diffusion coefficient values as a biomarker to predict clinical aggressiveness. Eur Radiol 2011; 21: 2178–86. doi: 10.1007/s00330-011-2174-7 [DOI] [PubMed] [Google Scholar]
- 13.Tekes A, Kamel I, Imam K, Szarf G, Schoenberg M, Nasir K, et al. Dynamic MRI of bladder cancer: evaluation of staging accuracy. AJR Am J Roentgenol 2005; 184: 121–7. doi: 10.2214/ajr.184.1.01840121 [DOI] [PubMed] [Google Scholar]
- 14.Ahmed Hafez Afifi TASAM, El-noueam KI, Ataa MA, Abdallah DM. Multiparametric MRI as a comprehensive study in evaluation. characterization & local staging of urinary bladder carcinomas The Egyptian Journal of Radiology and Nuclear Medicine 2017; 48: 493–507. [Google Scholar]
- 15.Watanabe H, Kanematsu M, Kondo H, Goshima S, Tsuge Y, Onozuka M, et al. Preoperative T staging of urinary bladder cancer: does diffusion-weighted MRI have supplementary value? AJR Am J Roentgenol 2009; 192: 1361–6. doi: 10.2214/AJR.08.1430 [DOI] [PubMed] [Google Scholar]
- 16.Gupta N, Sureka B, Kumar MM, Malik A, Bhushan TB, Mohanty NK, et al. Comparison of dynamic contrast-enhanced and diffusion weighted magnetic resonance image in staging and grading of carcinoma bladder with histopathological correlation. Urol Ann 2015; 7: 199–204. doi: 10.4103/0974-7796.150480 [DOI] [PMC free article] [PubMed] [Google Scholar]