Abstract
Objective:
Overexpression of human epidermal growth factor receptor-2 (HER2) in breast cancers provides promising opportunities for imaging and targeted therapy. Developing HER2 targeted positron emission tomography (PET) probes might be benefit for management of the disease. Small high-affinity scaffold proteins, affibodies, are ideal vectors for imaging HER2 overexpressed tumors. Despite of the initial success on development of 18F labeled ZHER2:342 affibody, the tedious synthesis producers, low yields and unfavorable pharmacokinetics may hinder the clinical use. 68Ga is an attractive positron emitter for PET imaging. A simple preparation of 68Ga labeled ZHER2:342 analog, 68Ga-NOTA-MAL-Cys-MZHER2:342, was reported in the study. The in vivo performances of the tracer for assessing HER2 status in breast cancers were also evaluated.
Methods:
NOTA-MAL conjugated Cys-MZHER2:342 was radiolabeled with 68Ga. The probe was evaluated by in vitro tests including stability and cell binding studies in breast cancer cells with different HER2 levels. In vivo evaluation was performed in mice bearing tumors using microPET imaging and biodistribution experiments. A PET/CT imaging study was initially performed in patients with breast cancers.
Results:
The tracer was synthesized in a straightforward chelation method with satisfactory non-decay corrected yield (81±5%) and radiochemical purity (>95%). In vivo micro-PET imaging showed that HER2 high levels expressed BT474 xenografts were more clear visualized than HER2 low levels expressed MCF-7 tumors (16.12 ± 2.69 ID%/g vs 1.32 ± 0.19 ID%/g at 1 h post-injection). The outcome was consistent with the immunohistochemical analysis. No significant radioactivity was accumulated in healthy tissues (less than 2% ID/g) except kidneys. In a preliminary clinical study, 68Ga-NOTA-MAL-Cys-MZHER2:342 PET imaging allowed more high-contrast detection of HER2 positive primary tumors (maximum standardized uptake value = 2.16±0.27) than those in HER2 negative primary focus (maximum standardized uptake value = 0.32±0.05). No detectable side-effects were found.
Conclusion:
In summary, this study indicates the significant efficiency of the 68Ga labeled HER2 affibody. Preclinical and clinical studies support the possibility of monitoring HER2 levels in breast cancers using 68Ga-NOTA-MAL-Cys-MZHER2:342.
Advances in knowledge:
The research investigated the feasibility of a 68Ga labeled HER2 affibody modified with a hydrophilic linker for breast cancer PET imaging. Favorable outcomes showed that the probe might be valuable for determining HER2 status of the disease.
Introduction
Breast cancer is a life-threatening disease in females. Overexpression of human epidermal growth factor receptor-2 (HER2)has been detected up to 20% cases of breast cancer with high recurrence rates and poor prognosis.1 Targeted treatment with specific HER2 agents, such as trastuzumab, significantly improves survival.2 A 12 months course of trastuzumab (~€31,600) chemotherapy treatment reduces the risk of death by a third.3 Assessing HER2 status is helpful for identifying patients to benefit from therapy and avoiding invalid costs as well as serious adverse effects.
Currently, biopsy is a predominant approach to evaluate the expression of HER2 through immunohistochemistry or fluorescence in situ hybridization.4 However, the invasive procedure may provide non-representative and mislead information mainly due to the heterogeneity of receptors. Clinical trials confirmed that even HER2 negative primary locus can develop HER2 positive metastases lesions.5 Moreover, multiple sampling for determination of receptor concentration is not feasible since it may cause patients’ discomfort and potential side-affects, including infection and hemorrhage etc. Hence, a safe and global method of quantifying HER2 expression is needed.
A non-invasive and whole body based imaging method, positron emission tomography (PET), can accurately measure the density of the receptor and show the heterogeneity. PET imaging permits a repeatable quantitative analysis method with high sensitivity and spatial resolution.6–8 First, antibody-based radioligands, e.g. 89Zr labeled trastuzumab and pertuzumab, have been chosen to image HER2-positive primary breast cancer or metastases and monitor treatment efficacy.9–11 Despite of the encouraging results, the favorable contrast between the target and non-target tissues was achieved after 3 or 7 days postinjection due to slow blood clearance and tumor penetration of probes with high molecular weights (~150 kDa). Moreover, high absorbed dose is another major problem to be solved for routine clinical use since it may lead to additional damages in the normal organs.12,13
Affibody, a type of engineered scaffold proteins, is a promising molecule for development of a HER2-targeting imaging agent. Affibody is originated from the B domain of Staphylococcus aureus protein A.14 Compared with antibody, the small size affibody quickly accumulates in the tumors and rapidly excretes from normal organs (blood, muscle etc.). Images with high contrast could be obtained only at few hours after administration.14,15 ZHER2:342 is composed with 58 amino acids and is a suitable HER2-binding affibody since it did not interact with trastuzumab and pertuzumab treatment due to different binding sites in the receptor.16–18
Several ZHER2:342 based PET tracers have been explored and investigated in the preclinic studies.19,20 18F is routinely applied PET radionuclide in clinic. 18F labeled ZHER2:342 and its derivatives have been initially synthesized for preclinical studies with satisfactory effects. For example, 18F-FBEM or 18F-FET conjugated ZHER2:342 specifically bound to HER2 receptors in tumor cells and may predict response to anti-HER2 monoclonal antibody.21–24 However, tedious synthesis producers (~2 h) might hinder the widely spread of the agent in clinic.21,24 Meanwhile, unspecific radioactivity accumulation in the liver may hinder the clinical use since metastases often occurred in the organ. Our previous study confirmed that maleimide–NOTA coupled ZHER2:342 analogs modified with a hydrophilic linker (GGGRDN), NOTA-MAL-Cys-MZHER2:342, enables facile labeling with 18FAl complexes.25 Preclinical experiments showed that the probe, 18FAl-NOTA-MAL-Cys-MZHER2:342, displayed specific binding to the receptor and satisfactory abdomen backgrounds.25 It also showed that the tracer might accurately diagnose HER2 levels since a significant relationship was found between the tumor uptake values and HER2 levels among different xenografts.25 Despite the favorable performance, low labeling yields (~10% non-decay-corrected) may partially limit its wide use.
Gallium-68 (68Ga) is an attractive positron emitter for PET imaging since it can be simply acquired from an economic 68Ge/68Ga generator without an onsite cyclotron.26–28 The long shelf-life generator [t1/2 (68Ge)=270 day] guarantees the steady source for medical centers. The isotope can be easily acquired by eluting the instrument again every 4 h in the same day, which facilitates the preparation of PET tracers. Meanwhile, biomolecules can be facilely labeled with 68Ga via macrocyclic chelators. Numerous DOTA coupled HER2 affibody derivatives were labeled with 68Ga (such as 68Ga-ABY002, 68Ga-ABY025 etc.) and the resulting probes displayed diagnostic sensitivity and specificity.29–31 Compared with DOTA, a triaza macrocycle, NOTA, owns high conformational and excellent size selectivity which might be more suitable for combining the small cation such as 68Ga etc. in the cavity.32
In this study, NOTA-MAL conjugated Cys-MZHER2:342 was labeled with 68Ga, and the perspectives of the probe in determining HER2 status was firstly explored in tumor models through microPET imaging and biodistribution experiments. To further investigate its clinical application, a preliminary clinical trial was performed in patients with breast cancers using PET/CT.
Methods and materials
General
Cys-MZHER2:342 peptide was purchased from Apeptide Co., Ltd. (Shanghai, China). NOTA-MAL-Cys-MZHER2:342 was prepared according to the reported methods and the chemical purity was greater than 95%.25 68Ge/68Ga generator was purchased from ITG, Germany. Other reagents were analytical grade.
Ethical statement
All procedures were complied with the ethical standards of the institutional or national research committee. The clinical study was approved by the Ethics Committee of Wuxi No. 4 People’s Hospital (LS2018001). The use of animals was approved by the animal research committee in Jiangsu Institute of Nuclear Medicine.
Preparation of 68Ga-NOTA-MAL-Cys-MZHER2:342
Fresh 68GaCl3 (1 ml, 370MBq) eluted from the generator with 0.05M HCl was added to a solution of NOTA-MAL-Cys-MZHER2:342 (100 µg, 12.8 nmol) in 250 µL 0.25M sodium acetate buffer. The mixture was heated for 10 min at 100°C. After diluted with 10 ml deionized water, the complex was transferred to a BOND ELUT C18 cartridge (Varian) . The labeled affibody was obtained by eluting the cartridge with 200 µL 10 mM HCl in ethanol. The eluent was diluted with 3 ml saline and filtered via a 0.22 µm Millipore filter assembled on a sterile vial. The radiochemical purity of the product was determined by reversed-phase-high performance liquid chromatography (RP-HPLC).33,34
In vitro stability
10 µL 370KBq 68Ga-NOTA-MAL-Cys-MZHER2:342 in saline (the amount of labeled peptide was determined to be less than 0.016 nmol) was incubated in 200 µL phosphate buffered saline (PBS) or mouse serum for 30, 60 and 120 min at 37°C respectively. The radiochemical purity of the tracer in PBS was measured by HPLC at the corresponding time points.
To precipitate the proteins, 200 µL acetonitrile was added to the serum. Subsequently, the supernatant was harvested after centrifugation and the radiochemical purity was determined by HPLC.35
Cell culture
Human breast carcinoma cell lines (BT474 and MCF7) were obtained from Cell Bank of Shanghai Institutes for Biological Sciences. The cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (GIBCO) and grown in a humidified atmosphere containing 5% CO2 at 37°C.
Cell uptakes
BT474 and MCF7 cells were seeded in 24 well plates (1 × 105 cells per well) for overnight cultures. Cells were washed with PBS, then incubated with 370 KBq 68Ga-NOTA-MAL- Cys-MZHER2:342 (the amount of labeled peptide was determined to be less than 0.016 nmol)at 37°C for 15, 30, 60 and 120 min respectively. Blocking studies were performed in the presence of unlabeled 1 µM Cys-MZHER2:342. The cells were washed with ice-cold PBS three times and lysed via 0.5 ml of 1M NaOH. The radioactivity of the cells was measured in a γ-counter (Perkin–Elmer). Cell uptakes were expressed as the percentage of added radioactive dose per 105 cells (%AD/105 cells). Experiments were conducted in triplicates.
Animal models
Xenograft tumor models were established by subcutaneously implanting 5 × 106 BT474 or MCF7 cells into the right front shoulder region of 4 weeks old female athymic nude mice (SLAC Laboratory Animal Co. Ltd., China). When the tumor volumes were determined to be 100–300 mm3, the mice were allowed to be used for the animal studies.
MicroPET imaging
Under isoflurane anesthesia, mice bearing BT474 or MCF7 tumors (n = 4 per group) were injected intravenously with 100 µL 3.7 MBq 68Ga-NOTA-MAL-Cys-MZHER2:342 (the amount of labeled peptide was determined to be less than 0.16 nmol) for micro-PET imaging. 5 min static PET scans were performed using an Inveon Micro-PET scanner (Siemens Medical Solutions) at 30, 60 and 120 min after administration respectively. For blocking study, unlabeled Cys-MZHER2:342 peptide (10 mg/kg body weight) and 3.7 MBq 68Ga labeled tracers were coinjected into four mice bearing BT474 tumors. Subsequently, animals were scanned for 5 min at 30, 60 and 120 min after injection using the Inveon MicroPET scanner respectively. The quantification analysis of PET images was performed using the reported method.36
Biodistribution studies
68Ga-NOTA-MAL-Cys-MZHER2:342 (740 KBq, the amount of labeled peptide was determined to be less than 0.032 nmol) were injected into the mice via the lateral tail vein in the presence or absence of excessive unlabeled Cys-MZHER2:342 peptide and sacrificed at 30, 60 and 120 min (n = 5 per group). Tumor and normal organs were dissected and weighed. Radioactivity uptake in the tissues were measured in a γ-counter and expressed as percentage injected dose per gram of tissue (% ID/g).
Histopathology
After microPET imaging or biodistribution studies, xenograft tumors were harvested and stored at −70℃. After radioactivity decaying for at least 48 h, the frozen tissues were sectioned and stained for HER2 evaluation using reported procedures.23 After primary antibody (biotinylated monoclonal anti-HER2 antibody) incubation, the slides were incubated with secondary antibody then visualization was performed using peroxidase. Images were obtained with an epifluorescence microscope (Olympus, X81,Japan).
PET/CT imaging in patients
All patients signed informed consent. Exclusion criteria were allergy to radiotracer; mental illness; severe liver or kidney disorder; fear to the PET/CT scanning; pregnancy or breast feeding.
Two patients with proved breast cancers were enrolled in this study. They were not pregnant or lactating and did not suffer from severe liver or kidney dysfunction. Without specific preparation such as fasting, PET/CT scans were performed using a Biograph 64 PET/CT scanner (Siemens Medical Solutions, Nuremberg, Germany). Patients were positioned in the supine position on the scanner bed after administrating 74 MBq 68Ga-NOTA-MAL-Cys-MZHER2:342. Scan was performed at 60 min post-injection and analyzed following the reported literature.37 Regions of interest were drawn in the tumors or normal organs under the guidance of CT images by two experienced nuclear medicine physicians. The results were expressed as maximum standardized uptake value (SUVmax).37
Biopsy
Biopsies were performed by immunohistochemistry (HercepTest) to assess the HER2 levels of primary tumors in patients with breast cancers. HER2 positive and HER2 negative expressions were defined when the HER2 staining intensity were scored IHC 3+ and IHC 1+ respectively.
Statistical analysis
Statistical analyses were conducted using GraphPad Prism (v. 5.0). Differences between groups were assessed using the unpaired,two-tailed Student's t test. The significant differences was defined as p < 0.05 for the tests.
Results
Radiochemistry
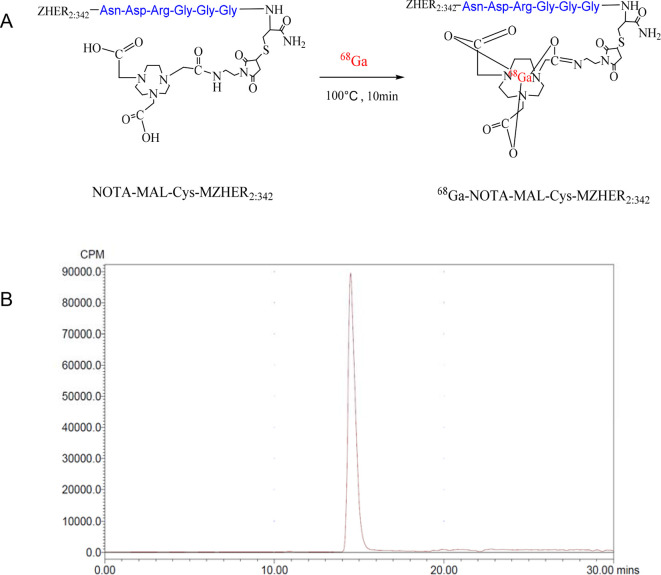
The average non-decay corrected yield after C18 column purification for 68Ga-NOTA-MAL-Cys-MZHER2:342 was 81±5%. HPLC analysis showed that the radiochemical purity was greater than 95% with a single peak at 14 min. (Figure 1) The specific activity of the radiolabeled peptide was determined to be at least 23 GBq/μmol.
Figure 1.
Radiolabeling of NOTA-MAL-Cys-MZHER2:342 with 68Ga (A) and HPLC chromatograms of 68Ga-NOTA-MAL-Cys-MZHER2:342 (B). HPLC, high-performance liquidchromatography.
In vitro stability
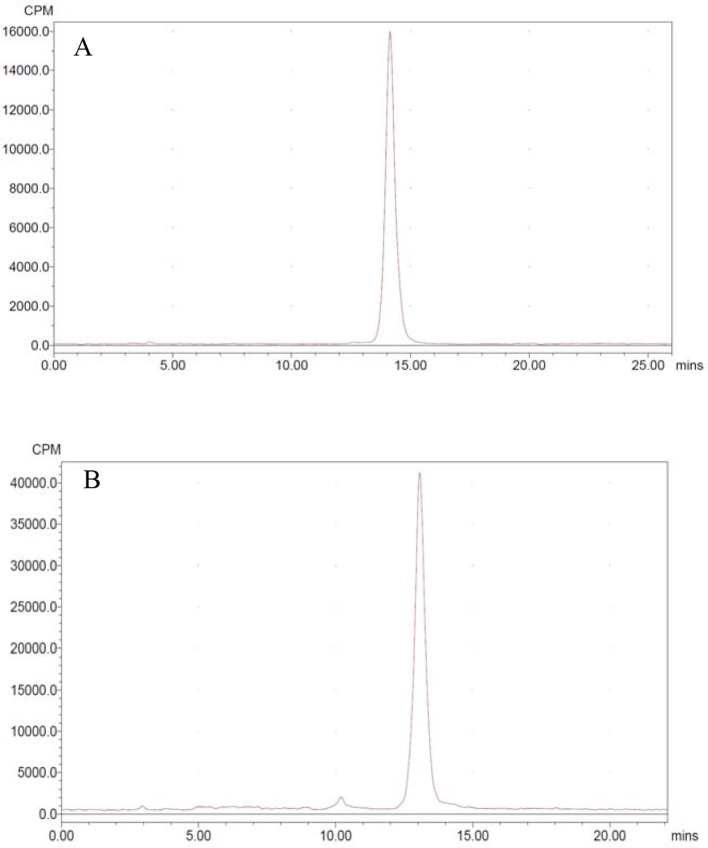
To test stability, 68Ga labeled tracer was incubated in PBS or mouse serum at 37℃ respectively. It indicated that the radiochemical purity of the probe kept consistent (>95%) after 2 h incubation (Figure 2).
Figure 2.
HPLC chromatograms of 68Ga-NOTA-MAL-Cys-MZHER2:342 in PBS (A) and serum (B) at 37°C for 2 h respectively. HPLC, high-performance liquidchromatography; PBS, phosphate buffered saline.
Cell uptake studies
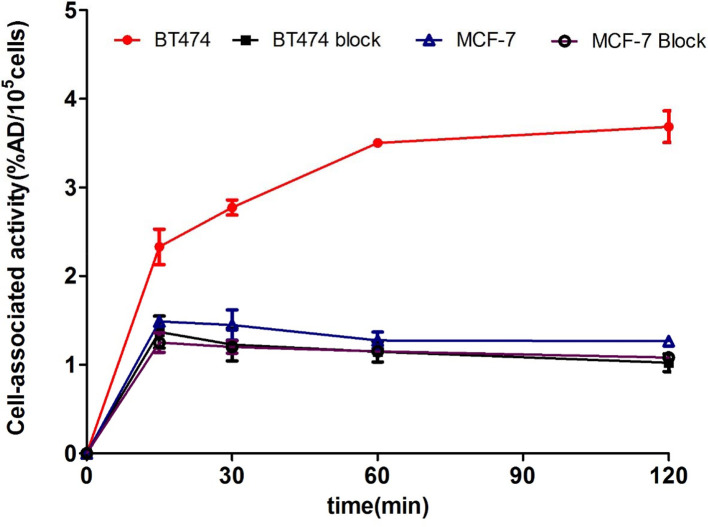
Cell uptake levels for 68Ga-NOTA-MAL-Cys-MZHER2:342 are shown in Figure 3 respectively. The tracer quickly accumulated in BT474 cells and reached a plateau (3.50±0.03%AD/105 cells) at 60 min incubation and remained stable upto 2 h (3.68±0.17%AD /105 cells). By contrast, the uptakes in MCF-7 cells were 1.26±0.05%AD/105 cells after 2 h incubation (p < 0.0001). When the probes were incubated with excess Cys-MZHER2:342 , the uptake values in BT474 and MCF-7 cells were determined to be 1.22 ± 0.18, 1.14 ± 0.11, 1.02 ± 0.09%AD/105 cells and 1.20 ± 0.07, 1.15 ± 0.13, 1.08 ± 0.02%AD/105 cells for incubating 30, 60 and 120 min at 37°C respectively.
Figure 3.
In vitro cell uptake assays of 68Ga-NOTA-MAL-Cys-MZHER2:342.
Xenografted tumor models PET imaging
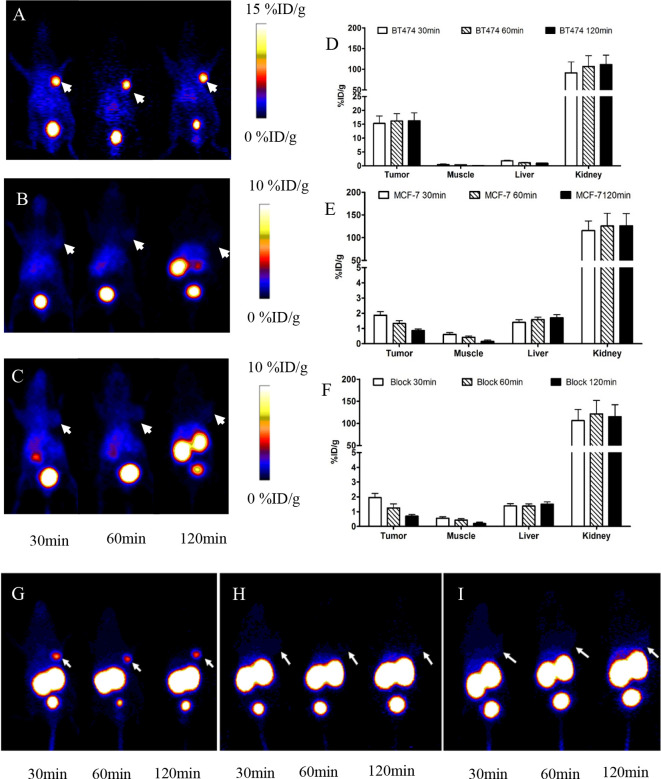
PET images acquired during 30 min to 2 h after injection are shown in Figure 4. BT474 xenografts were clearly visible with favorable contrast. However, MCF-7 xenografts signals were faint. The BT474 tumor uptake of the probe was determined to be 15.26 ± 2.73, 16.12 ± 2.69, 16.23 ± 2.89% ID/g at 30, 60 and 120 min. At the same time points, the corresponding values in MCF-7 tumor were 1.87 ± 0.23, 1.32 ± 0.19 and 0.86 ± 0.11% ID/g respectively (p < 0.0001). Following a blocking dose with Cys-MZHER2:342, the BT474 tumor uptake decreased to 1.38 ± 0.15% ID/g at 60 min post-injection respectively (p < 0.0001).
Figure 4.
Decay-corrected whole-body PET images of mice bearing BT474 (A) , MCF-7 xenografts (B) after injection of 68Ga-NOTA-MAL-Cys-MZHER2:342 without block; (C) PET images of mice bearing BT474 xenografts injection of the tracer under block. Quantification of 68Ga-NOTA-MALCys- MZHER2:342 in BT474 xenografts models in the absence (D) or presence of excess block agents (F) and in MCF-7 xenografts models (E). Maximum intensity projects of mice bearing BT474 (G), MCF-7 xenografts (H) after injection of 68Ga-NOTA-MAL-Cys-MZHER2:342 without block and mice bearing BT474 xenografts under block (I) respectively. Tumors are indicated by arrows. PET,positron emission tomography.
Quantification of PET images showed that the tumor to liver and tumor to muscle uptakes ratios increased from 8.73 ± 1.92 and 17.96 ± 3.35 to 35.94 ± 10.36 and 195.67 ± 26.83 respectively in mice bearing BT474 xenografts at 30 min and 2 h time point after administration. Excessive radioactivity uptake was also observed in the kidneys, which varied from 106.80 ± 26.15% ID/g to 125.45 ± 28.23% ID/g in mice bearing BT474 and MCF-7 xenografts after 1 h post-injection respectively.
Biodistribution studies
Biodistribution data of 68Ga-NOTA-MAL-Cys-MZHER2:342 in mice bearing tumors were shown in Table 1. The uptake of the tracer was 15.87 ± 2.30% ID/g at 30 min and kept stable to 13.47 ± 1.84% ID/g in BT474 xenografts at 2 h postinjection. The tumor to blood and tumor to muscle uptake ratios increased from 12.33 ± 4.00 and 21.87 ± 2.56 at 30 min postinjection to 34.57 ± 8.67 and 74.58 ± 3.40 at 2 h postinjection in mice bearing BT474 tumors respectively. Co-injection with unlabeled HER2 affibody significantly reduced the tumor uptake of the probe to 1.87 ± 0.15% ID/g at 1 h postinjection (p < 0.0001). Also, the corresponding tumor to blood and tumor to muscle uptake ratios decreased to 1.36 ± 0.63 and 4.73 ± 1.55 respectively. At the same time point, uptakes in MCF-7 xenografts were determined to be 1.43 ± 0.34% ID/g. The corresponding tumor to blood and tumor to muscle uptake ratios were 1.41 ± 0.47 and 7.31 ± 0.55 respectively. No significant radioactivity was accumulated in healthy tissues (less than 2% ID/g) except kidneys.
Table 1.
Biodistribution of 68Ga-NOTA-MAL-Cys-MZHER2:342 in mice bearing BT474 and MCF-7 xenografts respectively (mean ± SD, n = 5)
| Organ(%ID/g) | BT474 | BT474 Block | MCF-7 | ||
| 30 min | 60 min | 120 min | 60 min | 60 min | |
| blood | 1.43 ± 0.22 | 0.93 ± 0.13 | 0.27 ± 0.15 | 1.12 ± 0.22 | 1.42 ± 0.59 |
| brain | 0.32 ± 0.12 | 0.42 ± 0.12 | 0.27 ± 0.00 | 0.36 ± 0.06 | 0.18 ± 0.02 |
| heart | 1.75 ± 0.09 | 1.01 ± 0.19 | 0.58 ± 0.05 | 0.85 ± 0.08 | 0.94 ± 0.06 |
| liver | 1.74 ± 0.17 | 1.87 ± 0.30 | 1.53 ± 0.25 | 1.97 ± 0.58 | 1.66 ± 0.12 |
| spleen | 1.35 ± 0.78 | 1.36 ± 0.23 | 0.96 ± 0.13 | 1.03 ± 0.14 | 1.27 ± 0.27 |
| lung | 1.33 ± 0.35 | 1.51 ± 0.26 | 0.97 ± 0.12 | 1.20 ± 0.11 | 1.29 ± 0.30 |
| kidney | 138.85 ± 22.74 | 168.30 ± 21.06 | 169.45 ± 12.13 | 150.82 ± 26.89 | 134.71 ± 10.66 |
| stomach | 1.82 ± 0.54 | 1.51 ± 0.54 | 0.77 ± 0.44 | 1.27 ± 0.08 | 1.21 ± 0.04 |
| intestine | 1.40 ± 0.51 | 1.32 ± 0.56 | 0.42 ± 0.22 | 1.23 ± 0.17 | 1.25 ± 0.29 |
| muscle | 0.73 ± 0.10 | 0.21 ± 0.04 | 0.14 ± 0.04 | 0.26 ± 0.09 | 0.26 ± 0.40 |
| pancreas | 1.23 ± 0.72 | 0.64 ± 0.09 | 0.35 ± 0.05 | 0.44 ± 0.00 | 1.01 ± 0.10 |
| bone | 1.23 ± 0.18 | 1.10 ± 0.29 | 0.85 ± 0.25 | 1.15 ± 0.37 | 1.59 ± 0.23 |
| tumor | 15.87 ± 2.30 | 14.49 ± 2.29 | 13.47 ± 1.84 | 1.87 ± 0.15 | 1.43 ± 0.34 |
| Ratios | |||||
| Tumor/blood | 12.33 ± 4.00 | 19.17 ± 0.70 | 34.57 ± 8.67 | 1.36 ± 0.63 | 1.41 ± 0.47 |
| Tumor/muscle | 21.87 ± 2.56 | 53.82 ± 6.40 | 74.58 ± 3.40 | 4.73 ± 1.55 | 7.31 ± 0.55 |
| Tumor/liver | 9.87 ± 2.87 | 8.01 ± 0.37 | 8.95 ± 2.27 | 0.40 ± 0.09 | 1.13 ± 0.17 |
| Tumor/kidney | 0.11 ± 0.00 | 0.08 ± 0.01 | 0.06 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Tumor/lung | 11.35 ± 1.99 | 9.94 ± 2.25 | 11.90 ± 2.68 | 0.99 ± 0.13 | 1.50 ± 0.46 |
| Tumor/spleen | 6.88 ± 2.93 | 10.47 ± 2.54 | 12.14 ± 2.85 | 1.24 ± 0.36 | 1.51 ± 0.43 |
| Tumor/bone | 11.64 ± 2.47 | 13.06 ± 0.34 | 14.95 ± 1.16 | 2.49 ± 0.11 | 1.17 ± 0.07 |
SD, standard deviation.
Histopathology
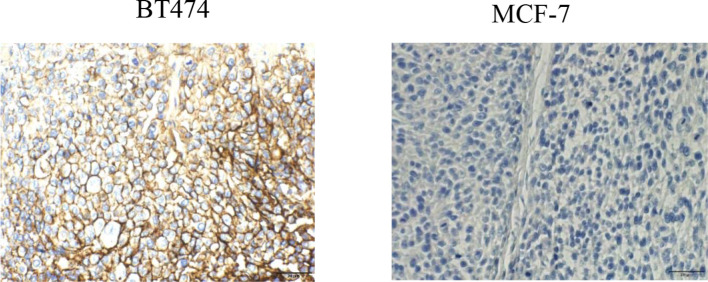
Results of HER2 expression in representative tumor crysections are shown in Figure 5. HER2 was abundantly expressed in BT474 xenografts, but significantly weaker in MCF-7 tumors.
Figure 5.
Immunohistochemical visualization of HER2 receptors in breast cancer tissues (BT474 and MCF-7)(×400).
Patient study
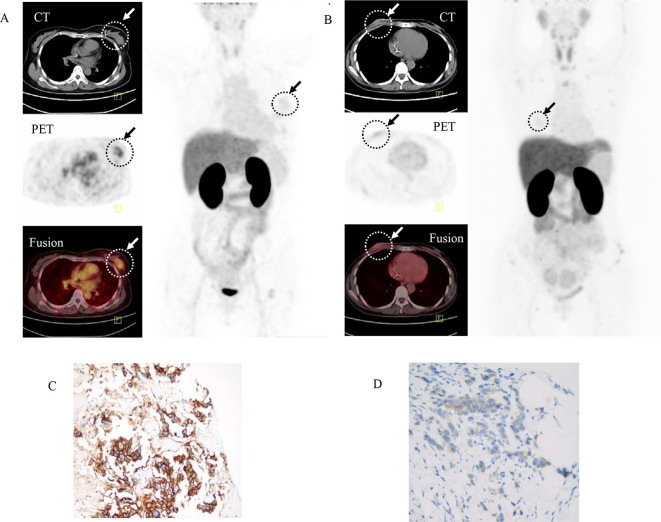
HER2 positive recurrent breast cancers in Patient 1 was clearly identified by 68Ga-NOTA-MAL-Cys-MZHER2:342 PET/CT imaging, which was confirmed by the corresponding biopsy (Figure 6). The SUVmax of the loci was 2.16 ± 0.27. In contrast, HER2 negative primary breast cancer determined by fluorescencein situhybridization in Patient 2 showed weak retention values (SUVmax = 0.32±0.05, p < 0.0001). The corresponding SUVmax of contralateral normal mammary gland was 0.07 ± 0.04. The SUVmax of normal organs such as lung, heart, liver, kidney in patients 1 and 2 were 0.11 ± 0.02, 0.44 ± 0.07, 1.63 ± 0.14. 12.15 ± 2.74 and 0.12 ± 0.01, 0.29 ± 0.04, 1.81 ± 0.17. 10.27 ± 2.29 respectively. No adverse events were reported.
Figure 6.
Representative whole body images and transaxial PET/CT fusion image of Patient 1 (A) and Patient 2 (B) with breast cancer obtained 60 min after intravenous injection of 68Ga labeled affibody. (C)Primary tumor from Patient 1 showed immunohistochemistry 3+ staining. (D) Primary tumor from Patient 2 showed immunohistochemistry 1+ staining. Arrows indicated tumors. PET,positron emission tomography.
Biopsy
Biopsies taken from the original primary tumor tissue of Patient 1 and Patient 2 were shown in Figure 6. More than 10% of the tumor cells from Patient 1 were scored 3+. The biopsy samples from Patient 2 was determined to be 1+ score by the immunohistochemistry analysis.
Discussion
Overexpression of HER2 in aggressive tumors provides promising opportunities for imaging and targeted therapy by radiolabeled HER2 affibody analogs. It might have a significant impact on patient care. 68Ga is utilized in radiopharmaceuticals for oncology diagnostics due to short scanning time and available iterative examinations.29,30 Our previous studies showed that NOTA conjugated ZHER2:342 affibody analog, 18FAl-NOTA-MAL-Cys-MZHER2:342, owned satisfactory pharmacokinetics.25 Thus, we designed a new radiotracer suitable for PET imaging by replacing 18FAl with 68Ga. Following an integrated bench-to-patient approach, the resulting PET probe, 68Ga-NOTA-MAL-Cys-MZHER2:342, was evaluated first in animal tumor models and then in patients with breast cancers.
68Ga-NOTA-MAL-Cys-MZHER2:342 could be easily prepared in nearly 20 min with good radiochemical purity. Compared with 18FAl labeled counterpart, the yields and specific activity were both significantly increased (~80% vs ~10% and~23GBq/μmol vs ~9 GBq/μmol respectively). Also, the yield was greater than that of another 68Ga labeled HER2 affibody such as 68Ga-ABY-025 (61.3 ± 6.7%) (p < 0.001).38
Stability studies showed the tracer could be stored up to 2 h with excellent purity in PBS or serum. It might be the results of a thermodymically stable complex formed between NOTA and gallium (III).
BT474 and MCF-7 cell lines are luminal A and luminal B subtypes of breast cancer cells respectively. Both of them expresses estrogen receptor and the difference lies in HER2 status. Higher levels of HER2 was found in BT474 cells than those in MCF-7 cells.39 In the literature, two types of tumors have been employed for evaluating the biologic characters of radiolabeled HER2 affibody.40,41 Thus, the HER2 targeting properties of 68Ga labeled modified affibody was initially determined using BT474 and MCF-7 tumor models.
In vitro experiments showed that 68Ga-NOTA-MAL-Cys-MZHER2:342 exhibited receptor-binding specificity for human HER2 since higher retention values was found in HER2 high levels expressed BT474 cells than those of HER2 low levels expressed MCF-7 cells. In vivo micro-PET imaging revealed that the tracer could be used to discriminate tumors with different HER2 status. High uptake of 68Ga-NOTA-MAL-Cys-MZHER2:342 (greater than 10% ID/g) was observed in BT474 xenografts at 30 min post-injection, and nearly 80% of the radioactivity remained in the tumor at 2 h after injection. In contrast, less radioactivity (~2% ID/g) was found in the MCF-7 control tumors. Meanwhile, it also displayed that >80% uptakes in the BT474 tumors were significantly blocked in the presence of excessive unlabeled HER2 affibody. These results confirmed 68Ga-NOTA-MAL-Cys-MZHER2:342 specifically bound to HER2.
It was also found that the favorable receptor targeting specificity of 68Ga-NOTA-MAL-Cys-MZHER2:342 was similar with reported 68Ga labeled HER2 affibody. The uptake values of 68Ga-NOTA-MAL-Cys-MZHER2:342 in BT474 tumors at 1 h post-injection (~16% ID/g) was similar with other 68Ga labeled affibody in HER2 overexpressed tumors. For example, the accumulation levels of 68Ga-DOTA-ZHER2:2891, 68Ga-DOTA-ZHER2:342, 68Ga-NODAGA-ZHER2:2395 and 68Ga-DOTA-ZHER2:2395 in BT474 or SKOV3 xenografts at 1 h or 45 min post-injection was 31 ± 7.0%, 8.9 ± 1.0%,15 ± 8% and 15 ± 2% ID/g respectively.40,42,43
Similar with the reported 18FAl labeled affibody, 68Ga-NOTA-MAL-Cys-MZHER2:342 exhibits faint accumulations in most low HER2 expression normal organs except kidney.25 It may lead to reduce background radioactivity especially in abdomen. Highest radioactivities in kidneys is mainly attributed to the responsibility of the organs for metabolism and clearance. This phenomenon was similar with those of radiometal labeled peptides.44–46 Since renal lesions was rarely detected in breast cancers, excessive radioactivity in kidney might not be a major diagnostic limitation.
The attractive preclinical profile prompted us to further evaluate 68Ga-NOTA-MAL-Cys-MZHER2:342 in a clinical study with breast cancer patients. Two patients with different HER2 levels in primary lesions was selected to primarily investigate the imaging proprieties of the probe in clinic. PET/CT imagings showed that lesions confirmed with high receptor expression by IHC were positively identified by the tracer. Although the absolute SUV of 68Ga-NOTA-MAL-Cys-MZHER2:342 in HER2 positive and HER2 negative breast tumors was less than those of 68Ga-ABY-025 at 1 h postinjection (2.1 vs 10 and 0.3 vs 2.5 respectively), the contrast factor between two types of tumors were similar (nearly 4 for 68Ga-ABY-025 and nearly 7 for 68Ga-NOTA-MAL-Cys-MZHER2:342 respectively).30 These results implied that the probe might have prognostic value for accurate detecting HER2 levels in breast cancers.
It was also observed that SUV values in the normal liver was significantly lower than reported 68Ga-ABY-025 and 68Ga-ABY-002 (~2 vs 10 and 15.6) at 60 or 95 min postinjection respectively.29,30 It confirmed that modification of ZHER2:342 with a hydrophilic linker was benefit for improving the in vivo pharmacokinetics performances. Low background in liver might be helpful in detecting the potential HER2 positive liver metastasis.
There also existed some limitations in this study including the relatively small number of patients investigated. Larger scale clinical investigations are warranted by the accurate diagnosis value of the 68Ga labeled affibody.
Conclusion
A 68Ga labeled HER2 affibody, 68Ga-NOTA-MAL-Cys-MZHER2:342, was prepared in a simple straightforward labeling procedures for clinical routine application. Preclinical experience revealed that the tracer is suitable for imaging HER2 receptor expression in tumors. Clinical data suggested that the probe might be valuable for identification HER2 status and theranostic management.
Footnotes
Acknowledgment: This work was partially supported by National Natural Science Foundation (31971316,31671035, 51803082), National
Significant New Drugs Creation Program (2017ZX09304021), National Key Research and Development Program (2016YFC1306602),Jiangsu Provincial Medical Innovation Team (CXTDA2017024), Jiangsu Provincial Natural Science Foundation ( BK20170204, BE2016632), Jiangsu Health International Exchange Program (JSH-2018–015), Jiangsu talent projects (LGY2017088), Jiangsu Provincial Commission of Health and Family Planning Fundation (H2017031, QNRC2016628), Wuxi Commission of Health and Family Planning Fundation (Q201729).
Contributor Information
Yuping Xu, Email: xuyuping@jsinm.org.
Lizhen Wang, Email: wanglizhen@jsinm.org.
Donghui Pan, Email: pandonghui@jsinm.org.
Chunjing Yu, Email: ycj_wxd1978@163.com.
Baoming Mi, Email: mibaoming@163.com.
Qianhuan Huang, Email: 393422212@qq.com.
Jie Sheng, Email: shengjie456123@163.com.
Junjie Yan, Email: yanjunjie@jsinm.org.
Xinyu Wang, Email: wangxinyu@jsinm.org.
Runlin Yang, Email: yangrunlin@jsinm.org.
Min Yang, Email: yangmin@jsinm.org.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A, Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin 2018; 2018: 7–30. [Google Scholar]
- 2.Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. Herceptin adjuvant (HERA) trial study Team. Lancet 2017; 389: 1195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leung W, Kvizhinadze G, Nair N, Blakely T. Adjuvant trastuzumab in HER2-positive early breast cancer by age and hormone receptor status: a cost-utility analysis. PLoS Med 2016; 13: e1002067. doi: 10.1371/journal.pmed.1002067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varga Z, Noske A. Impact of modified 2013 ASCO/CAP guidelines on HER2 testing in breast cancer. one year experience. PLoS One 2015; 10: e0140652. doi: 10.1371/journal.pone.0140652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zardavas D, Irrthum A, Swanton C, Piccart M. Clinical management of breast cancer heterogeneity. Nat Rev Clin Oncol 2015; 12: 381–94. doi: 10.1038/nrclinonc.2015.73 [DOI] [PubMed] [Google Scholar]
- 6.Provost J, Garofalakis A, Sourdon J, Bouda D, Berthon B, Viel T, et al. Simultaneous positron emission tomography and ultrafast ultrasound for hybrid molecular, anatomical and functional imaging. Nat Biomed Eng 2018; 2: 85–94. doi: 10.1038/s41551-018-0188-z [DOI] [PubMed] [Google Scholar]
- 7.Virgolini I, Decristoforo C, Haug A, Fanti S, Uprimny C. Current status of theranostics in prostate cancer. Eur J Nucl Med Mol Imaging 2018; 45: 471–95. doi: 10.1007/s00259-017-3882-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson IM, Scott PJH, Thompson S. Clinical applications of radiolabeled peptides for PET. Semin Nucl Med 2017; 47: 493–523. doi: 10.1053/j.semnuclmed.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 9.Ulaner GA, Hyman DM, Ross DS, Corben A, Chandarlapaty S, Goldfarb S, et al. Detection of HER2-positive metastases in patients with HER2-negative primary breast cancer using 89Zr-Trastuzumab PET/CT. Journal of Nuclear Medicine 2016; 57: 1523–8. doi: 10.2967/jnumed.115.172031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulaner GA, Hyman DM, Lyashchenko SK, Lewis JS, Carrasquillo JA. 89Zr-Trastuzumab PET/CT for detection of human epidermal growth factor receptor 2-positive metastases in patients with human epidermal growth factor receptor 2-Negative primary breast cancer. Clin Nucl Med 2017; 42: 912–7. doi: 10.1097/RLU.0000000000001820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laforest R, Lapi SE, Oyama R, Bose R, Tabchy A, Marquez-Nostra BV, et al. 89 Zr Trastuzumab: Evaluation of Radiation Dosimetry, Safety, and Optimal Imaging Parameters in Women with HER2-Positive Breast Cancer. Mol Imaging Biol 2016; 18: 952–9. doi: 10.1007/s11307-016-0951-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dehdashti F, Wu N, Bose R, Naughton MJ, Ma CX, Marquez-Nostra BV, et al. Evaluation of [89 Zr]trastuzumab-PET/CT in differentiating HER2-positive from HER2-negative breast cancer. Breast Cancer Res Treat 2018; 169: 523–30. doi: 10.1007/s10549-018-4696-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulaner GA, Lyashchenko SK, Riedl C, et al. First-In-Human HER2-targeted imaging using 89Zr-pertuzumab PET/CT: dosimetry and clinical application in patients with breast cancer. J Nucl Med 2018; 59: 900–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ståhl S, Gräslund T, Eriksson Karlström A, Frejd FY, Nygren Per-Åke, Löfblom J, et al. Affibody molecules in biotechnological and medical applications. Trends Biotechnol 2017; 35: 691–712. doi: 10.1016/j.tibtech.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 15.Frejd FY, Kim K-T. Affibody molecules as engineered protein drugs. Exp Mol Med 2017; 49: e306: e306. doi: 10.1038/emm.2017.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westerlund K, Altai M, Mitran B, Konijnenberg M, Oroujeni M, Atterby C, et al. Radionuclide therapy of HER2-Expressing human xenografts using Affibody-Based peptide nucleic acid-mediated pretargeting: in vivo proof of principle. J Nucl Med 2018; 59: 1092–8. doi: 10.2967/jnumed.118.208348 [DOI] [PubMed] [Google Scholar]
- 17.Eigenbrot C, Ultsch M, Dubnovitsky A, Abrahmsén L, Härd T. Structural basis for high-affinity HER2 receptor binding by an engineered protein. Proc Natl Acad Sci U S A 2010; 107: 15039–44. doi: 10.1073/pnas.1005025107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Zhao X, Wang S, Wang N, Han J, Jia L, et al. Monitoring therapeutic response of human ovarian cancer to trastuzumab by SPECT imaging with (99 m)Tc-peptide-Z(HER2:342. Nucl Med Biol 2015; 42: 541–6. doi: 10.1016/j.nucmedbio.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 19.Henry KE, Ulaner GA, Lewis JS. Human epidermal growth factor receptor 2-Targeted PET/Single- photon emission computed tomography imaging of breast cancer: noninvasive measurement of a biomarker integral to tumor treatment and prognosis. PET Clin 2017; 12: 269–88. doi: 10.1016/j.cpet.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glaser M, Iveson P, Hoppmann S, Indrevoll B, Wilson A, Arukwe J, et al. Three methods for 18F labeling of the HER2-Binding affibody molecule ZHER2:2891 including preclinical assessment. Journal of Nuclear Medicine 2013; 54: 1981–8. doi: 10.2967/jnumed.113.122465 [DOI] [PubMed] [Google Scholar]
- 21.Kramer-Marek G, Kiesewetter DO, Martiniova L, Jagoda E, Lee SB, Capala J, et al. 18 FBEM-Z(HER2:342)-Affibody molecule-a new molecular tracer for in vivo monitoring of HER2 expression by positron emission tomography. Eur J Nucl Med Mol Imaging 2008; 35: 1008–18. doi: 10.1007/s00259-007-0658-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer-Marek G, Bernardo M, Kiesewetter DO, Bagci U, Kuban M, Aras O, Omer A, et al. Pet of HER2-positive pulmonary metastases with 18F-ZHER2:342 affibody in a murine model of breast cancer: comparison with 18F-FDG. J Nucl Med 2012; 53: 939–46. doi: 10.2967/jnumed.111.100354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kramer-Marek G, Gijsen M, Kiesewetter DO, Bennett R, Roxanis I, Zielinski R, et al. Potential of PET to predict the response to trastuzumab treatment in an ErbB2-positive human xenograft tumor model. J Nucl Med 2012; 53: 629–37. doi: 10.2967/jnumed.111.096685 [DOI] [PubMed] [Google Scholar]
- 24.Yanai A, Harada R, Iwata R, et al. Site-Specific Labeling of F-18 Proteins Using a Supplemented Cell-Free Protein Synthesis System and O-2-[18F]Fluoroethyl-L-Tyrosine: [18 F]FET-HER2 Affibody Molecule. Mol Imaging Biol 2018;. [DOI] [PubMed] [Google Scholar]
- 25.Xu Y, Bai Z, Huang Q, Pan Y, Pan D, Wang L, et al. PET of HER2 Expression with a Novel 18 FAl Labeled Affibody. J Cancer 2017; 8: 1170–8. doi: 10.7150/jca.18070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Velikyan I. 68Ga-Based radiopharmaceuticals: production and application relationship. Molecules 2015; 20: 12913–43. doi: 10.3390/molecules200712913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bodei L, Ambrosini V, Herrmann K, Modlin I. Current Concepts in 88 Ga-DOTATATE Imaging of Neuroendocrine Neoplasms: Interpretation, Biodistribution, Dosimetry, and Molecular Strategies. J Nucl Med 2017; 58: 1718–26. doi: 10.2967/jnumed.116.186361 [DOI] [PubMed] [Google Scholar]
- 28.Yan J, Lu Y, Chen G, Yang M, Gu Z, et al. Advances in liquid metals for biomedical applications. Chem Soc Rev 2018; 47: 2518–33. doi: 10.1039/C7CS00309A [DOI] [PubMed] [Google Scholar]
- 29.Baum RP, Prasad V, Müller D, Schuchardt C, Orlova A, Wennborg A, et al. Molecular imaging of HER2-expressing malignant tumors in breast cancer patients using synthetic 111In- or 68Ga-labeled affibody molecules. J Nucl Med 2010; 51: 892–7. doi: 10.2967/jnumed.109.073239 [DOI] [PubMed] [Google Scholar]
- 30.Sörensen J, Velikyan I, Sandberg D, Wennborg A, Feldwisch J, Tolmachev V, et al. Measuring HER2-Receptor Expression In Metastatic Breast Cancer Using [ 68Ga]ABY-025 Affibody PET/CT. Theranostics 2016; 6: 262–71. doi: 10.7150/thno.13502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandström M, Lindskog K, Velikyan I, Wennborg A, Feldwisch J, Sandberg D, et al. Biodistribution and radiation dosimetry of the anti-HER2 affibody molecule 68Ga-ABY-025 in breast cancer patients. Journal of Nuclear Medicine 2016; 57: 867–71. doi: 10.2967/jnumed.115.169342 [DOI] [PubMed] [Google Scholar]
- 32.Deng H, Wang H, Li Z. Matching chelators to Radiometals for positron emission tomography Imaging- guided targeted drug delivery. Curr Drug Targets 2015; 16: 610–24. doi: 10.2174/1389450116666150707100702 [DOI] [PubMed] [Google Scholar]
- 33.Pan D, Liu G, Xu Y, et al. Pet imaging of FSHR expression in tumors with 68Ga-Labeled FSH1 peptide. Contrast Media Mol Imaging 2017; 2674502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu C, Xu Q, Pan D, Xu Y, Liu P, Yang R, et al. Prostate cancer imaging of FSHR antagonist modified with a hydrophilic linker. Contrast Media Mol Imaging 2016; 11: 99–105. doi: 10.1002/cmmi.1662 [DOI] [PubMed] [Google Scholar]
- 35.Kiesewetter DO, Guo N, Guo J, Gao H, Zhu L, Ma Y, et al. Evaluation of an [18 F]AlF-NOTA Analog of Exendin-4 for Imaging of GLP-1 Receptor in Insulinoma. Theranostics 2012; 2: 999–1009. doi: 10.7150/thno.5276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Q, Zhu C, Xu Y, Pan D, Liu P, Yang R, et al. Preliminary evaluation of [ 18 F]AlF-NOTA-MAL-Cys 39 -exendin-4 in insulinoma with PET. J Drug Target 2015; 23: 813–20. doi: 10.3109/1061186X.2015.1020808 [DOI] [PubMed] [Google Scholar]
- 37.Yu C, Pan D, Mi B, Xu Y, Lang L, Niu G, et al. 18 F-Alfatide II PET/CT in healthy human volunteers and patients with brain metastases. Eur J Nucl Med Mol Imaging 2015; 42: 2021–8. doi: 10.1007/s00259-015-3118-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Velikyan I, Wennborg A, Feldwisch J, et al. Good manufacturing practice production of [(68)Ga]Ga-ABY-025 for HER2 specific breast cancer imaging. AJNMMI 2016; 6: 135–53. [PMC free article] [PubMed] [Google Scholar]
- 39.Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res 2011; 13: 215. doi: 10.1186/bcr2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kramer-Marek G, Shenoy N, Seidel J, Griffiths GL, Choyke P, Capala J, et al. 68Ga-DOTA-Affibody molecule for in vivo assessment of HER2/neu expression with PET. Eur J Nucl Med Mol Imaging 2011; 38: 1967–76. doi: 10.1007/s00259-011-1810-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kramer-Marek G, Kiesewetter DO, Capala J, et al. Changes in HER2 expression in breast cancer xenografts after therapy can be quantified using PET and 18F-labeled affibody molecules. Journal of Nuclear Medicine 2009; 50: 1131–9. doi: 10.2967/jnumed.108.057695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tolmachev V, Velikyan I, Sandström M, Orlova A, et al. A HER2-binding affibody molecule labelled with 68Ga for PET imaging: direct in vivo comparison with the 111In-labelled analogue. Eur J Nucl Med Mol Imaging 2010; 37: 1356–67. doi: 10.1007/s00259-009-1367-7 [DOI] [PubMed] [Google Scholar]
- 43.Altai M, Strand J, Rosik D, Selvaraju RK, Eriksson Karlström A, Orlova A, et al. Influence of Nuclides and Chelators on Imaging Using Affibody Molecules: Comparative Evaluation of Recombinant Affibody Molecules Site-Specifically Labeled with 68 Ga and 111 In via Maleimido Derivatives of DOTA and NODAGA. Bioconjug Chem 2013; 24: 1102–9. doi: 10.1021/bc300678y [DOI] [PubMed] [Google Scholar]
- 44.De Silva RA, Kumar D, Lisok A, Chatterjee S, Wharram B, Venkateswara Rao K, et al. Peptide-Based 68 Ga-PET Radiotracer for Imaging PD-L1 Expression in Cancer. Mol Pharm 2018; 15: 3946–52. doi: 10.1021/acs.molpharmaceut.8b00399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tolmachev V, Yim CB, Rajander J, et al. Comparative evaluation of anti-HER2 affibody molecules labeled with 64Cu using NOTA and NODAGA. Contrast Media Mol Imaging 2017; 8565802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stangl S, Tei L, De Rose F, Reder S, Martinelli J, Sievert W, et al. Preclinical Evaluation of the Hsp70 Peptide Tracer TPP-PEG 24-DFO[89 Zr] for Tumor-Specific PET/CT Imaging. Cancer Res 2018; 78: 6268–81. doi: 10.1158/0008-5472.CAN-18-0707 [DOI] [PubMed] [Google Scholar]