Abstract
Subsequent to the development and global availability of BCR/ABL–targeted tyrosine kinase inhibitors (TKIs), the prognosis of patients with chronic myeloid leukemia (CML), at least those in the chronic phase, has markedly improved, and in the developed world, the average lifespan of these patients is now close to that of age- and sex-matched subjects without the disease. However, the situation in low- and middle-income countries (LMICs) may not be so rosy. Many important differences in hematological cancers, including CML, have been highlighted in various publications in LMICs vs developed countries. These include differences in incidence and prevalence rates, age and stage of disease at diagnosis, response rates, and survival. Some of the possible reasons proposed for these are varying socioeconomic milieu (impacting availability of effective drugs and essential monitoring), environmental factors (mainly exposure to viral infections and pesticides), nutritional factors with interplay of malnutrition and diet on drug absorption and blood levels, and possible unknown genetic factors. Although generic first-generation TKIs (imatinib) are available in many parts of the world, several challenges remain in providing optimal treatment to patients with CML in resource-poor countries. Some of these include availability of optimal and high-quality BCR/ABL testing, availability and expense related to use of second- and third-generation TKIs (nilotinib, dasatinib, bosutinib, and ponatinib) and hematopoietic stem cell transplantation, issues with compliance and toxicities of drugs, and ensuring a minimal standard-of-care treatment and monitoring for every patient diagnosed with CML. For the purpose of this article, the more objective country label—LMIC—coined by the World Bank will be used (gross national income per capita between $1026 and $3995; World Bank, June 2019). Some of these issues will be discussed in this article in greater detail by experts in the field in 3 different but interconnected sections.
Learning Objectives
Understand issues, problems, and pitfalls related to optimal treatment of patients with chronic myeloid leukemia (CML) on a global scale, especially in low- and middle-income countries (LMICs)
Discuss and understand innovative, low-cost interventions and efforts that could cost-effectively impact the monitoring, management, and outcomes of CML patients in LMICs
Highlight future global approaches and interventions needed to improve outcomes in LMICs with a resource-limited setting
Introduction and background
With the widespread availability of good-quality tyrosine kinase inhibitors (TKIs) in the developed world, the survival of patients with chronic myeloid leukemia (CML) in the chronic phase is approaching that of the general population.1-3 However, in low-income countries and low- and middle-income countries (LMICs), the ground reality is not so encouraging because of several reasons.4-7
CML is the most common adult leukemia in India (and possibly, in the other LMICs), being much more common than chronic lymphocytic leukemia and acute myeloid leukemia. Figures from various Indian cancer registries show a CML incidence of 0.8 to 2.2 per 100 000 population for men and 0.6 to 1.6 per 100 000 population for women. Given the good prognosis, the prevalence of cases of CML is expected to be huge in the coming decade (Figure 1). Health planners will need to plan resources in terms of both trained manpower and funds for medications and monitoring to deal with these numbers. The disease is seen in a younger population, with the median age at onset being between 30 to 40 years old. More than half of the patients present with intermediate and high Sokal and high and European Treatment and Outcomes Score (EUTOS) score.8-18 Both of these issues can be seen in our case. Complete hematological responses to imatinib are seen in >90% patients, but complete cytogenetic and molecular responses are documented in <60% patients after 1 year of treatment. These lower response rates in the LMICs vs the developed world are more likely to be owing to high disease burden at diagnosis—patients are diagnosed later in the chronic phase of the disease—rather than a different disease biology (Figure 2).
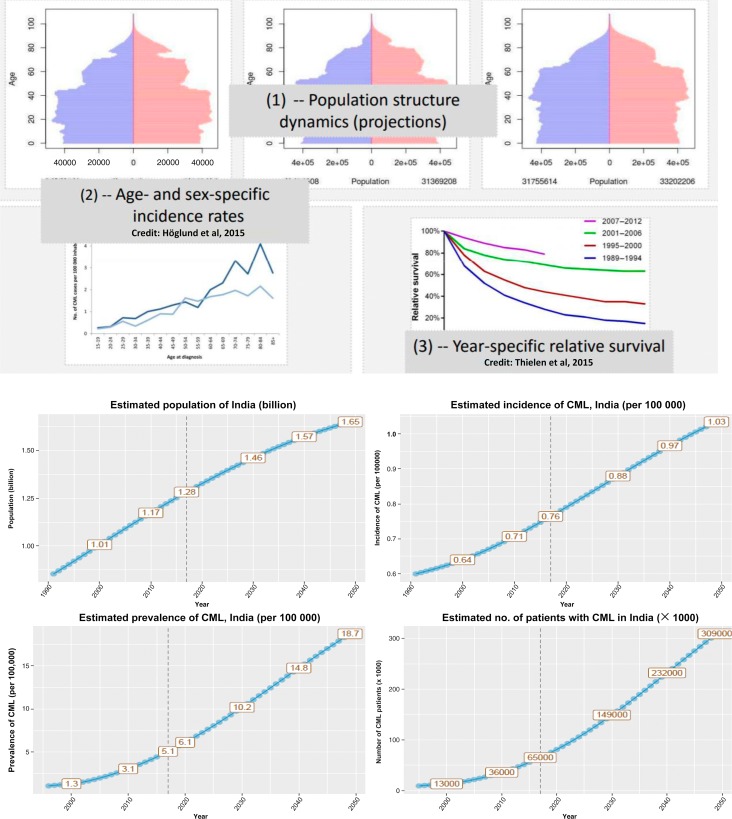
Figure 1.
To estimate the prevalence of any disease, 3 parameters are needed: (1) population structure dynamics, (2) age- and sex-specific incidence rates, and (3) year-specific relative survival (depicted in the top 2 rows). The bottom 2 rows show the population, incidence, and prevalence of CML and the expected number of cases. It is estimated that, by the year 2030, there will be >150 000 patients with CML in India. (Estimates are provided by Marc Delord, Université Paris 7, Institut Universitaire d'Hématologie, Hôpital Saint-Louis, Paris, France.)
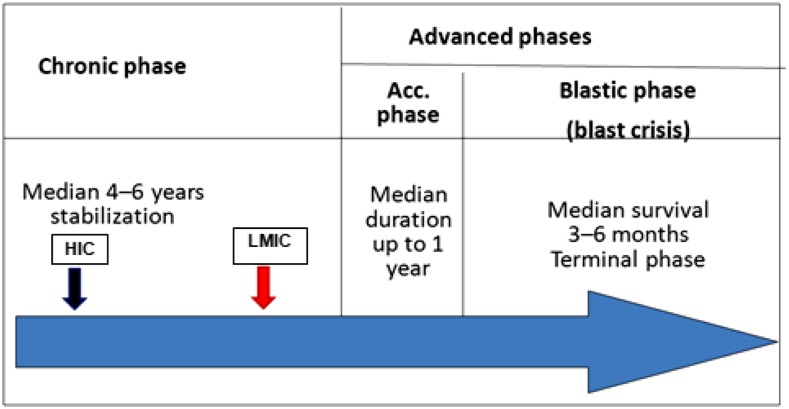
Figure 2.
Patients in LMICs are diagnosed later in the chronic phase of the disease (red arrow) compared with their high-income country (HIC) counterparts (black arrow), and this is probably the cause of lower cytogenetic and molecular responses.
Clinical case 1
A 38-year-old male, a farmer by occupation, presented to us in January 2013 with a 4-month history of malaise, weakness, anorexia, and lump on the left side of his abdomen. On clinical examination, he was found to have pallor, marked sternal tenderness, palpable liver 7 cm below costal margin in the right midclavicular line, and a 20-cm splenomegaly below the left coastal margin extending beyond the umbilicus. Routine investigations showed the following: hemoglobin 9.2 g/dL, total leukocyte count 320 × 109/L, platelet count 650 × 109/L, peripheral blood film showed left shift with many metamyelocytes, myelocytes, 2% blasts, 2% basophils, and 4% eosinophils. Hepatic and renal function tests were normal except for raised serum uric acid. Serum lactate dehydrogenase was 2890 (about 6× the upper limit of normal). With a provisional diagnosis of myeloproliferative neoplasm-CML, further investigations were ordered, and the patient was found to have very low leukocyte alkaline phosphatase score of 4, hypercellular bone marrow with accelerated granulopoiesis, and left shift with 4% blasts and 3% promyelocytes. Philadelphia chromosome was detected in 20 of 20 metaphases [46,XY,t(9;22)(q34;q11.2)], and his B-cell receptor (BCR)/ABL1:ABL1 by real-time polymerase chain reaction (PCR) on peripheral blood (on International Scale [IS], P210, transcript b3a2) was 68.9226%.
With a final diagnosis of CML chronic phase, high EUTOS and Sokal risk, he was counseled about his disease, and considering his economic status and documented inability to afford a second-generation TKI, he was enrolled in an imatinib access program and started on Glivec (imatinib) 400 mg/d with hydroxyurea and antitumor lysis measures for the initial couple of weeks. He went into complete hematological remission within 6 weeks of starting treatment, became asymptomatic, and started regular work in his fields. He was requested to get complete blood counts (CBCs) done every month and the BCR/ABL1 real-time PCR done after 3 months of starting imatinib, which he refused citing economic reasons. However, we managed to persuade him to get the test done after 6 months of imatinib, and the result was 2.6872%. He was continued on imatinib with 2- to 3-month CBC monitoring and BCR/ABL testing every 6 months.
He achieved a major molecular response (MMR) after 12 months of treatment with a BCR/ABL1:ABL1 of 0.1344% IS and remained in MMR for 3 years. The BCR/ABL1:ABL1 test done in May 2016 showed a value of 1.3246 (>1 log increase); repeat test after 1 month (done without charge on personal request to the laboratory) was 1.7446% IS. On careful and persistent questioning of the patient and his wife, noncompliance was confirmed (he admitted to omitting about 5-7 doses of imatinib per month over 5-6 months; the reason was that he was feeling well and wanted to see if he could stop the medication!). After detailed counseling, compliance was ensured, and MMR was achieved again in the test done 3 months later (September 2016).
This case highlights several important facts related to CML in LMICs.
Most patients at presentation are younger and present in the fourth decade of life rather than in the sixth/seventh decade.5,7,10,13 This may have implications related to fertility and childbearing in female patients and also, considerations for keeping “treatment-free remission” as one of the goals of treatment in the newly diagnosed patients.
The majority of patients have high disease bulk and advanced stage at diagnosis but are not candidates for upfront more potent second-generation TKI because of economic considerations.
Although several low-cost generic imatinib brands are freely available in the market, significant numbers of patients are on support programs (like the Glivec International Assistance Program [GIPAP]) or government supply of free imatinib.
Optimal and regular disease monitoring at time points as described in various guidelines is adhered to by only a minority of patients, again primarily because of economic reasons.
Drug noncompliance, either because of a good clinical response that quickly makes the patient symptom free or because of minor toxicities, is quite prevalent (ranges from 20% to 40%), and it is one of the main reasons for suboptimal response/loss of response.4,11-14
Support programs
Approximately 60% to 70% of all patients with CML in India today are on the GIPAP or Novartis Oncology Access (NOA), ongoing in India since May 2002. Today, >15 000 patients are accessing Glivec through GIPAP or NOA because of a lack of affordability, despite the fact that several low-cost Indian generic versions of imatinib are freely available in the Indian market. Our patient was also approved to receive free of charge Glivec through one of these programs. Most patients who do not qualify for the access programs are on Indian generic imatinib as are patients diagnosed after the program stopped enrolling new patients since April 2016.
As per revised World Health Organization (WHO) guidelines, imatinib has been included in the essential drug list of some developing countries19; however, it is not provided by most governments in sub-Saharan Africa, low-income countries in Asia, or Central America. In these countries, imatinib is provided free of cost to patients through The Max Foundation’s Max Access Solutions (MAS). MAS also provides second- and third-line treatment in most of these countries. BCR/ABL testing is not provided for free to patients in most countries, and despite the preferential pricing offered by The Max Foundation and Cepheid in LMICs, most patients and institutions are not able to afford testing as per international guidelines.
Generic TKI availability and quality
The escalating cost of cancer treatment is a matter of concern the world over in high-income countries as well as LMICs. The price of drugs for CML has been reviewed by an elegant publication with inputs from global experts.20 Questions regarding quality of the generic imatinib have been addressed in several Indian studies; some have also looked at the trough imatinib blood levels after Glivec vs generic imatinib, and there are no significant differences in response rates or hematological, cytogenetic, or molecular21,22 findings as well as blood level between the 2.23,24
Clinical case 1—revisited
Our patient started to lose his MMR in January 2018 (1.2228) and April 2018 (2.3104). He swore that this time he was 100% compliant with his medication. He was diagnosed as having molecular relapse to imatinib, and he was advised to BCR/ABL1 kinase domain mutation studies, which he refused because of economic reasons. He was offered the option of starting one of the second-generation TKIs (nilotinib or dasatinib), which again, he refused because of lack of funds. The dose of imatinib was increased to 600 mg/d and then, 800 mg/d, which had to be brought down back to 600 mg/d because of toxicity (grade 3 musculoskeletal pains and grade 3 thrombocytopenia); 3 and 6 months later, he still had not achieved an MMR. After counseling and consenting, pioglitazone 30 mg/d was added to imatinib. He responded well and achieved an MMR within 6 months with no or minimal side effects. Presently, he is on imatinib 600 mg/d and pioglitazone 30 mg/d, and he is tolerating both drugs well and is in deep molecular remission (BCR/ABL1 of 0.0388).
Learning points from the revisited case are as follows.
It is critical to follow-up all patients with periodic BCR/ABL testing by real-time PCR preferably every 3 months and definitely every 6 months to detect early molecular relapse.
Kinase domain mutation analysis should be asked for to be able to choose the right second- or third-generation TKI only for patients ready and willing for switch to a second- or third-generation agent.
For patients with molecular relapse postimatinib, one of the second-generation TKIs, either nilotinib or dasatinib, remains the treatment of choice, However, increasing the dose of imatinib remains an option for the nonaffording patients, which form the majority in LMICs.
For patients who are not candidates for second- or third-generation TKIs and allogeneic stem cell transplantation, experimental treatments with newer combinations or enrollment in a clinical trial remain viable options.
Second- and third-generation TKIs and novel combinations
Second-generation TKIs are approved and available for first- as well as second-line use in India and some other LMICs, but they are accessible to a much smaller number of eligible patients because of economic reasons. It may be noted that the second-generation TKIs are not registered or available in most LMICs and some high-income countries as well. Because suboptimal responders do not have the option of second-generation TKI (because of economic reasons or nonavailability), several innovative approaches have been tried. These include the addition of pioglitazone, a peroxisome proliferator-activated receptor γ agonist, to imatinib, and the combination can lead to a significant number of patients achieving a deep molecular response.25,26 There is also some data on the synergistic effect of the combination of imatinib with curcumin, a traditional Indian herb used in ayurvedic medicine for centuries, both in cell line in vitro models as well as a small clinical study that we did at our center.27
All patients get the first-line imatinib approach
In LMICs, where most patients are not candidates for upfront, more expensive second-generation TKIs, the early assessment of molecular response at 3 and/or 6 months may be even more important. One approach could be to administer first-line imatinib to all patients of CML-chronic phase, and only those who have a suboptimal response at 3 and/or 6 months may then be considered for a change to the more expensive second-line agents or an enhanced dose of imatinib—the TIDEL-II (Therapeutic Intensification in De Novo Leukaemia-II) trial approach.28 Prospective studies testing this hypothesis are ongoing.
Summary and conclusions
In LMICs, like India, with a population of >1.2 billion, where 85% of expenditure on health happens from noninsured, out-of-pocket spending, it is important to keep economics in the forefront of all treatment and research initiatives. In the LMICs, to improve outcomes of patients with CML, several suggestions can be considered. Accurate and subsidized molecular testing for BCR/ABL transcripts and also, kinase domain mutations needs to be made available at designated academic institutions with easy accessibility for practicing physicians, hematologists, and oncologists. Drug support, especially for the second (nilitinib and dasatinib) and third (busutinib and ponatinib) generation TKIs for which generics are not easily available, needs to be thought of and provided either by the government or through industry-supported/sponsored programs, like the GIPAP.
Can BCR/ABL testing be done on RNA obtained from a dried blood spot on a piece of filter paper? This would eliminate to a large extent the issue of transporting samples from the patient bedside to the reference testing laboratory. Clinical trials involving stopping of imatinib after prolonged complete molecular remission in the LMIC setting would also be important and relevant, and they could result in significant savings for the individual and/or the health care system.29 The lower response rates to imatinib seen in these resource-constrained regions in addition to other factors could also be owing to suboptimal absorption of the drug with lower serum levels because of dietary and/or genetic factors. This needs to be studied. Interventions to improve molecular response rates, like drug combinations of imatinib with interferon and use of new leukemic stem cell–targeting agents (like the mammalian target of rapamycin inhibitors, hedgehog pathway inhibitors, and JAK2 inhibitors), need to be studied in the laboratory and in the clinic. Pioglitazone, a peroxisome proliferator-activated receptor γ agonist, in combination with a BCR/ABL TKI may be a cost-effective way to eliminate residual stem cells and convert a suboptimal response into a deep molecular response. Can one of the traditional Indian/Chinese medicinal plants/herbs in combination with a TKI have an additive effect on CML control? There is some preliminary evidence of synergy with curcumin and imatinib. These are the trials that need to be done in LMICs.
In India, such as in other LMICs, there is a pressing need to identify and bring together physicians who have an interest in CML management. They can then form a nucleus and look at issues particularly relevant to the wants and needs related to CML specific to the region. They can identify and ask LMIC-specific questions; design and conduct studies to address and answer these questions; and develop, collate, and publish local treatment guidelines that can guide optimal local practices.
We have a group of physicians interested in CML—the Indian CML Study Group—who collectively take care of >30 000 patients with CML and see >1000 new cases of CML every month. The International CML Foundation is a major international effort by world leaders in the field to get together CML experts and advocates from across the world and address issues related to treatment and education of patients, families, and caregivers.
BCR/ABL monitoring strategies in resource-poor countries
Introduction
The vast majority of cancer occurs in areas of the world where scarcity of resources makes obtaining drugs or diagnostics impossible. Here, we are not talking about access to molecular assays, cytogenetics, or morphological examinations—we mean even basic needs, such as needles, blood tubes, means of travel to clinics, and electricity. It is easy to be overwhelmed at the obstacles to diagnosis and care in low-resource settings. In this section, we will highlight lessons learned in trying to bring diagnostics to areas of low resources.
Obstacles and potential solutions
There are several strategies to test in the developing world that are summarized in Table 1. Each strategy has distinctive pros and cons.
Table 1.
Pros and cons of different approaches to BCR-ABL testing in low-resource areas
| Strategy | Pros | Cons |
|---|---|---|
| Home brew assay | Optimized to specific laboratory needs, locally sensitive and reproducible | Highly technical training, expensive machines and reagents, often performs worse at new locations |
| (Semi-)automated assays | Less technical training needed, reagents self-contained, can be run without batching, quick | Machines and cartridges are expensive to buy and maintain |
| Shipping to specialized centers | Standardized testing often means better assay performance | Shipping is very expensive, samples can be compromised, reliance on others |
Establishment of “home brew” assays
Assays developed at individual laboratories are optimized to individualized test characteristics (sensitivity, speed, etc), cost, and the capabilities of the persons actually performing the assays. In addition, there are always local “style” issues that are often poorly documented. Taken together, this explains why home brew assays often do not travel well, performing less smoothly and suffering worse performance than advertised (Figure 3).
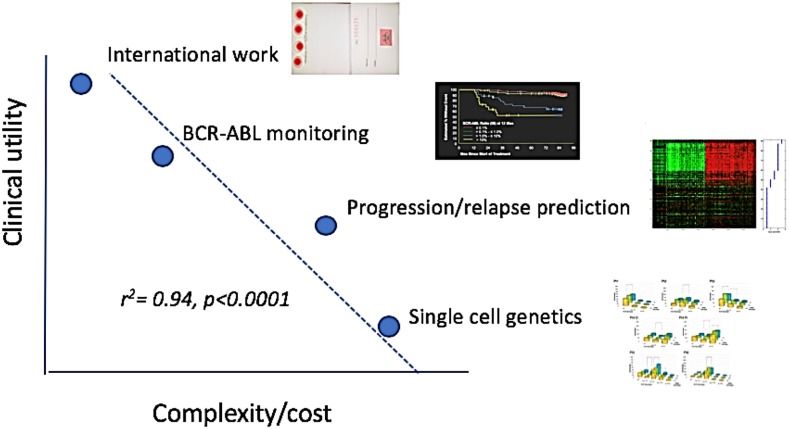
Figure 3.
Work and reward. This is a primitive attempt to make qualitative judgment look semiquantitative. The graph emphasizes the tradeoffs between the creative, the arcane, and the psychological rewards of work judged by potential clinical impact as of 2019. (Note to grant reviewers: this does not imply that single-cell genetics will not be of huge clinical import a few years from now.) Not to scale.
The “pro” of the home brew test is that someone has optimized it, and it is often relatively cheap. The con, other than that noted above, is that there are many features of a home brew assay that cannot be approached in a low-resource setting. First, highly trained technicians are the assays most important ingredient (remember this when it is time for the yearly raise). Finding technicians in low-resource settings can be difficult, and training is expensive and time consuming. Second, getting the components of the assay to the low-resource area laboratory is hard—even shipping molecular reagent–quality water is not possible to some locations. Refrigeration can be iffy both in transit and at its destination. Third, in many cases, the actual hardware needed (eg, a thermocycler) is not available, and purchasing it is beyond budgets.
The use of “automated” systems
Mostly self-contained systems, such as that designed by Cepheid, perform nucleic acid isolation and target amplification in a separate cartridge.30 There is very little hands-on wet laboratory time, and thus, training is relatively easier than for a home brew assay. There are further advantages in controlling amplimer cross-contamination and rapid (same day) result turnaround, a very positive feature in cases where patients must travel long distances to undergo testing. The availability of the Cepheid BCR-ABL test was greatly accelerated by the WHO enthusiasm toward Cepheid’s infectious disease assays (eg, tuberculosis and HIV), which has led to machines placed in many regional centers.31 Because the box is agnostic to what is being assayed, this allows CML patients to potentially to run alongside of cases testing for other diseases.
Although the pros of using this system are obvious, so too are the cons: the system is relatively costly compared with the home brew assay and relies on electricity.
Use of centralized testing centers
If home brew assays or automated assays are largely unavailable, what is the alternative? The mail. Sending a blood sample to a centralized center has the advantage of having the assay done at a dedicated center. The disadvantages are many. Sending the blood by express air courier places the sample at risk of serious degradation, especially if not adequately cooled (obviously a challenge in many areas of the world). Even worse, this option is horribly expensive. For example, it costs roughly $500 to send a single blood tube from Africa to Seattle. Moreover, unless the sending clinic is fantastically organized, the opportunity to batch samples to decrease the per unit cost of shipping is impossible.
An attractive option is dried blood spots. DNA is fairly stable on commercially available paper, whereas RNA is not. However, it seems that, although RNA on paper degrades, enough persists after weeks in the mail that an accurate BCR-ABL measurement can be obtained.32 Moreover, samples from several days can be batched, further driving down costs. RNA and DNA can be obtained from the same paper (which contains 4 spot locations), allowing for BCR-ABL quantification, and if clinically indicated, ABL mutation testing.
Electricity-free systems and point of care devices
The WHO has recently introduced the ASSURED standards for developing assays for low-resource areas.33 The acronym is instructive and outlines the challenges well. The WHO recommendations are affordable (check), sensitive (good), specific (sure), user friendly (logical), rapid and robust (no complaints), equipment free (what?), and deliverable to end users (fine but what about that “E”?).
What the WHO is looking for in “equipment free” is no electricity required. There are solutions more creative and practical than running a thermocycler on 1000 or so AAA batteries. Nature has provided a solution to end the dependence on a reliable electrical grid, and some polymerases can amplify nucleic acid without thermocycling. This isothermal PCR allows great creative freedom to design simpler assay methods that require little in the way of training and equipment, perfect for the use as point of care devices.34,35 One of the biggest obstacles to care in low-resource settings is actually getting the patient to the clinic. This can be expensive at the onset, and if the travel takes several days, it can be economically untenable. Why not take the test to the patient?
Infectious applications are myriad. For example, in fact, the testing for bird flu at airports was done by isothermal assays.36 Detection of the BCR-ABL fusion transcript has been performed by 2 slightly different isothermal methods: nuclei acid sequence-based amplification (NASBA)37 and loop-mediated isothermal amplification (LAMP).38 NASBA works by directly amplifying RNA molecules by first utilizing reverse transcriptase in a series of initial steps to create a double-stranded DNA molecular and then, utilizing T7 RNA polymerase to continuously amplify complementary RNA transcripts. In contrast, LAMP utilizes specialized primers and the Bst polymerase enzyme, a combination that allows for increased strand displacement and high replication activity, thereby increasing the amount of DNA produced that can be detected by DNA stains or ultraviolet light. Studies using LAMP to detect the promyelocytic leukemia/retinoic acid receptor α transcript in acute promyelocytic leukemia reliably demonstrate the feasibility of isothermal PCR for rapid and simple diagnostics.39
Conclusion
Other diseases than CML obviously need attacking, and the approaches noted above can potentially be used for other diseases (examples from our laboratory include performing molecular assays on biopsy material smears, JAK2 mutation testing, rapid tests for promyelocytic leukemia/retinoic acid receptor α, etc). As more oral targeted therapies are developed, we will need as a community to quickly “MacGyver” technical diagnostic solutions so that these drugs can get to the appropriate patients.
The cost-benefit of performing low-cost diagnostics is obvious by some simple math. Let us return to CML as an example. Say that we can diagnose a case for $100 (shipping, labor, and reagent costs). The diagnosis of CML gives the patient access to a TKI, which if treated in chronic phase, will give the patient a near-normal expected lifespan. If we add jut 10 years of life and the patient receives (in US dollars) roughly $100 000 of TKI per year, the return on the investment of diagnostic testing is 10 000 to 1! One does not need to be a health economist, certified professional accountant, or banker to recognize a pretty amazing yield on a minimal investment. Additionally, this calculation ignores what must be our prime concern, giving care to the ill, which is the ultimate benefit.
If we are lucky, we get to do work that interests and fulfills us. In health care, this can be direct patient care, data analysis, or even the more esoteric (eg, our laboratory plunged into single-cell genomics for the last decade). This is fine—we should do what we are passionate and creative about. However, as a personal testimony, the time that our laboratory has spent trying to do some good for those who our medical revolution has forgotten, neglected, or abandoned has been unusually enriching and sustaining.
Challenges with availability and accessibility of drugs on resource-poor countries: the problem and some suggested solutions
Introduction
The world of oncology treatment is at a very exciting time, with many new innovative therapies being developed and brought to market on a regular basis. However, in parallel, the cancer divide is larger than ever, with 95% of the world resources for cancer available to a small proportion of the world’s population.40 Moreover, the development of targeted and personalized therapies put patients in LMICs at a disadvantage because of lack of availability of molecular diagnostics and lack of universal health care or cancer control plans.
In this manuscript, I relate the experience of providing access to treatment of CML in LMICs as a proof of concept of a model that could be applied in other areas of oncology.
CML
The advent of TKIs in the early 2000s transformed the treatment of CML from a once fatal disease to a chronic condition with relatively good quality of life, and people living with CML can now expect a close to normal life expectancy. However, today, of the 5 TKIs approved in the western world for the treatment of CML, most are either not registered or not commercially available in many of the LMICs, and in the countries where those are commercially available, they are not reimbursed and thus, are unaffordable by most patients.
In 2001, soon after the Food and Drug Administration approval of Glivec (imatinib), Novartis launched the GIPAP in partnership with The Max Foundation. The GIPAP was an innovative access model that made Novartis’ breakthrough oral TKI therapy, Glivec (imatinib), available to individual patients who met program criteria in 80 LMICs around the world.41 The Max Foundation administered the program, working closely with a global network of >1500 trained physicians in these countries.
The GIPAP was the first oncology patient–oriented program of its kind, a direct-to-patient donation program where the manufacturer makes the treatment available to specific patients based on prescriptions from approved hematologists and following specific medical and financial criteria. Patients qualified for GIPAP if they were diagnosed for an indication for which the drug was approved and as long as they had no insurance, no reimbursement, and no capacity to afford the treatment. The Max Foundation as the program administrator had the role of the independent third party that protected the identity of the patients, performed screening of patients, and liaised with the approved physician and the donor company, informing the company to deliver supply for each approved patient to their treating physician on a 3-months basis. Figure 4 shows the lifecycle of a patient on the GIPAP.42
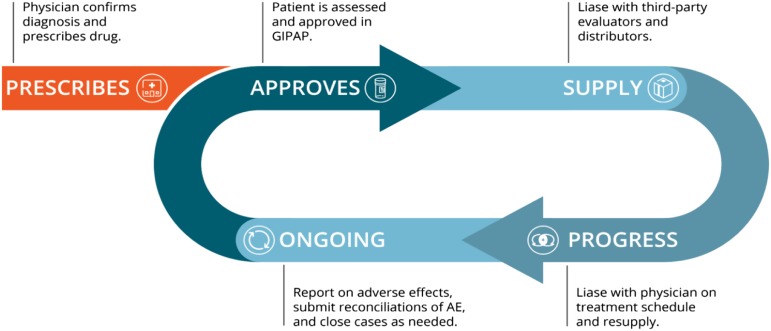
Figure 4.
GIPAP model. Initial diagnosis is done by the approved CML physician, who then submits an application on behalf of the patient to the independent NGO administrator. The administrator verifies diagnosis as well as medical and financial criteria and approves the application, enrolling the patient in the program. The administrator informs the program owner to send 4 months of supply to that physician for that specific patient. The physician is required to periodically reassess the patient and seek continuation of treatment or close the case if there is progression or unacceptable adverse effects of the treatment. AE, adverse events; NGO, nongovernmental organization.
The GIPAP ran for 15 consecutive years from 2002 to 2017, providing ongoing imatinib treatment to >75 000 patients in Africa, Asia, the Commonwealth of Independent States region, and Latin America. In many of the GIPAP countries, especially sub-Saharan Africa, the GIPAP provided treatment to all known diagnosed CML patients in the country. In a few countries, especially Latin America, the GIPAP served a subset of patients, providing access to patients not covered by social security programs.
As successful as the GIPAP was in providing access to treatment to imatinib,41,42 it had the limitation of being centered on the drug instead of around the needs of the patient and did not support patients who were intolerant or resistant to imatinib. In 2015, The Max Foundation envisioned a new model with a similar patient lifecycle but working with multiple manufacturers. This new model, MAS, required The Max Foundation to take on the international delivery and distribution of the products, acting as a clearing house of donated pharmaceuticals. In this model, The Max Foundation requests donations of specific products and independently distributes them to qualified institutions for approved patients under the umbrella of its MAS.
Two manufacturers of TKIs for CML joined the MAS initiative starting in 2015. In 2017, Novartis and The Max Foundation initiated the transition of the GIPAP to MAS. By 2018, Novartis, Pfizer, Bristol-Myers Squibb, Takeda, and Incyte had established similar collaborations with The Max Foundation under the umbrella of MAS, making available 8 compounds, 4 of which provide second- and third-line treatments for CML.
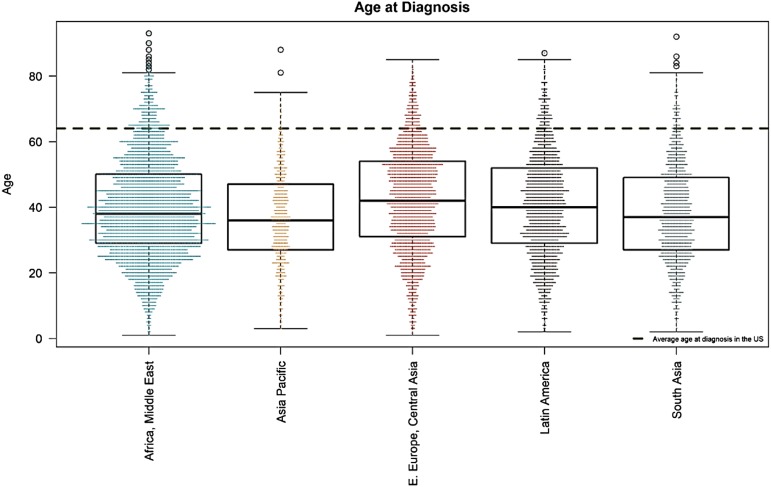
MAS for CML runs in 72 LMICs. Figure 5 shows the map of countries where MAS is available. Table 2 displays patient demographics (unpublished). MAS has helped 14 408 patients since 2017, most of whom (91.7%) were treated with imatinib. The Max Foundation has distributed 6.3 million defined daily doses of TKI since July 2017.43 The median age at diagnosis of patients enrolled in MAS is 39 years old (Figure 6), similar to the median age of patients in GIPAP,44 with 94% of patients being diagnosed at 64 years old or younger, the median age of diagnosis in the United States.45
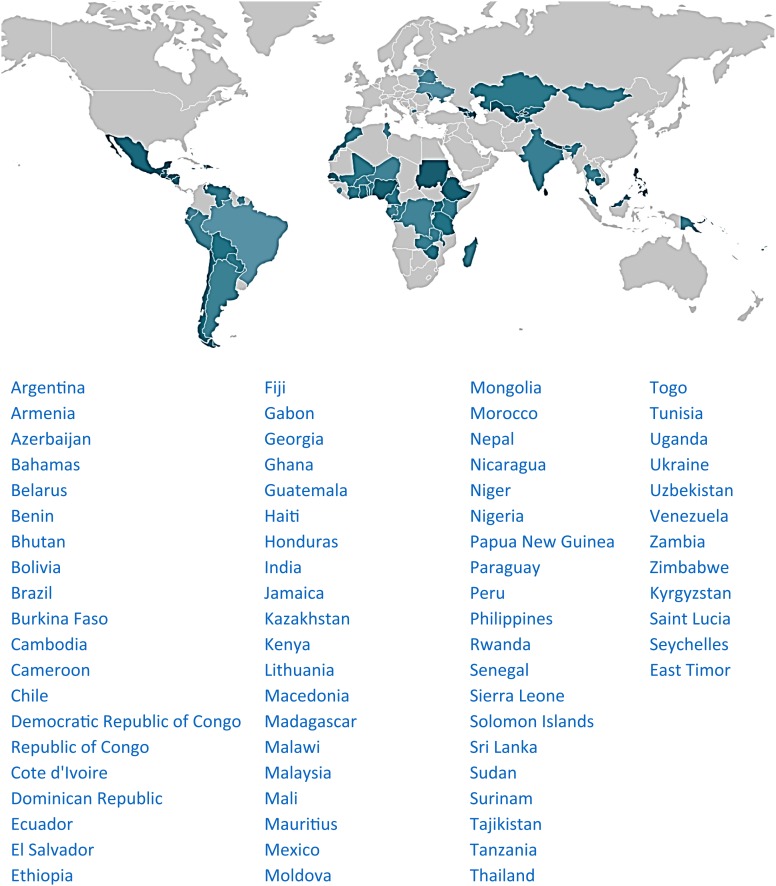
Figure 5.
Global scope of MAS for CML. Countries with access to at least 1 TKI are in green—darker color denotes a larger number of patients helped. The Max Foundation does not operate in areas shown in light gray.
Table 2.
Demographics of patients on the GIPAP
| All | Africa and Middle East | Asia Pacific | Eastern Europe, Central Asia | Latin America | South Asia | |
|---|---|---|---|---|---|---|
| Years | 2001-2019 | 2001-2019 | 2001-2019 | 2001-2019 | 2001-2019 | 2003-2019 |
| Patients, n | 14c408 | 5904 | 850 | 3141 | 2629 | 1884 |
| Age, median [range] | 39 [1-93] | 38 [1-93] | 36 [3-88] | 42 [1-85] | 40 [2-87] | 37 [2-92] |
| Male, n | 7c739 (55.8%) | 3307 (57.2%) | 456 (57.1%) | 1464 (48.6%) | 1410 (55.7%) | 1102 (62.7%) |
| Phase, n | ||||||
| Accelerated | 932 (6.7%) | 268 (4.6%) | 56 (7.0%) | 420 (13.9%) | 140 (5.5%) | 48 (2.7%) |
| Blast crisis | 144 (1.0%) | 42 (0.7%) | 25 (3.1%) | 29 (1.0%) | 22 (0.9%) | 26 (15%) |
| Chronic | 12c581 (91.7%) | 5363 (92.8%) | 686 (86.0%) | 2540 (84.3%) | 2356 (93.1%) | 1636 (93.1%) |
| Remission | 177 (1.3%) | 107 (1.9%) | 1 (0.1%) | 20 (0.7%) | 8 (0.3%) | 41 (2.3%) |
| Treatment, n | ||||||
| Bosutinib | 118 (0.8%) | 49 (0.3%) | 49 (6.1%) | 0 (0.0%) | 6 (0.0%) | 14 (0.1%) |
| Imatinib | 13c225 (91.8%) | 5632 (97.4%) | 561 (69.8%) | 2937 (97.2%) | 2461 (97.7%) | 1634 (92.5%) |
| Ponatinib | 303 (2.1%) | 27 (0.3%) | 145 (17.8%) | 46 (0.8%) | 7 (0.2%) | 78 (3.9%) |
| Dasatinib | 430 (3.0%) | 178 (1.7%) | 88 (6.3%) | 25 (0.4%) | 56 (1.2%) | 83 (1.6%) |
| Nilotinib | 332 (2.3%) | 18 (0.2%) | 7 (0.0%) | 133 (1.7%) | 99 (0.8%) | 75 (1.9%) |
This table describes the distribution and demographics of patients enrolled in the GIPAP program across various regions of the world. Also, the phase of disease at diagnosis and percentage of second- and third-generation TKIs is provided.
Figure 6.
Mean age at diagnosis of patients in different areas of the world where The Max Foundation operates (mean age of 39 years old). The dotted transverse line is the mean age of patients in the west (mean of 64 years old).
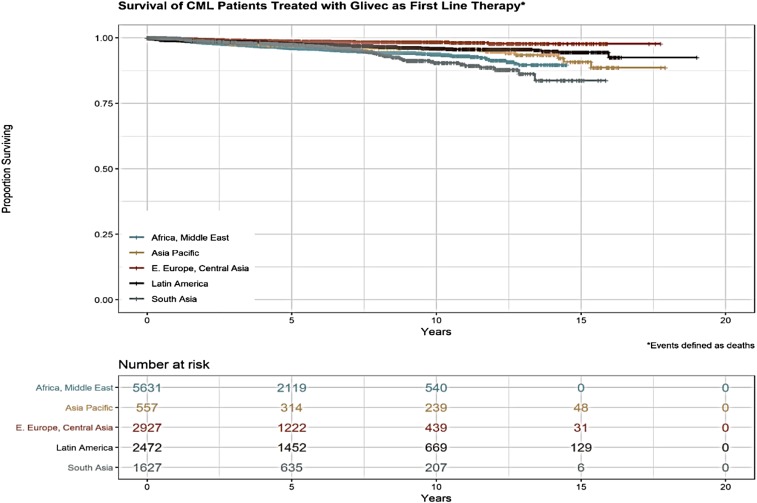
Figure 7 shows that the estimated overall survival of CML patients in MAS by region is ∼80% (unpublished data), quite similar that to patients in industrialized countries. These patients include patients who accessed imatinib through the GIPAP and whose care transitioned to MAS in 2017 and 2018 as well as new patients accrued by MAS after the transition.
Figure 7.
Overall survival (OS) of CML patients supported by MAS. OS seems to be lower in Africa, the Middle East, and South Asia compared with Eastern European and Central Asian countries.
Lessons learned
The efforts of the past 18 years to provide access to TKI therapy to patients in LMICs shows us that it is possible to run a humanitarian access program for an oncology product, extending survival of patients in these countries and achieving overall survival close to that of patients in the western world.
The experience also shows how the original impact of 1 original donor can foster an environment where other stakeholders are more likely to join the efforts.
Being able to treat CML patients has provided an opportunity to physicians working in these countries to successfully treat cancer patients and strengthened not only prescribing practices but also, clinic management, dispensation practices, and diagnostic practices.
Access to diagnostics and molecular testing in these regions has been a major barrier to optimizing treatment, and efforts to overcome this barrier should be described elsewhere.
Still, only few governments are active partners in these efforts. Long-term sustainable access to treatment will only be achieved after we engage with Ministries of Health and other government bodies in each of the countries to recognize the impact of cancer treatment and cancer survival and invest in their patients.
Acknowledgments
H.M. acknowledges the patience and courage of the authors’ patients and their families in dealing with this disease and sticking to the treatment for years and, sometimes, decades. H.M. also acknowledges the advanced research laboratory of the Sawai Man Singh Medical College, Jaipur, especially the head of the laboratory, and his better half, Bharti Malhotra, and her team of dedicated laboratory workers for helping with most of the laboratory work done in their published and unpublished studies. J.R. acknowledges the hard work, persistence, and patience of his laboratory family, friends/colleagues at the Max Foundation and the International CML Foundation, industry partners, doctors, patient advocates, and of course, the patients themselves. P.G.-G. acknowledges 310 implementing partner physicians in 118 cancer treating institutions who volunteer their time for their dedication to their patients; 58 distribution partners that are part of the supply chain into each of these institutions for their efforts to overcome daily barriers to importation; 77 partner patient organizations focused on CML that provide support and education that allow CML patients to be informed partners in their treatment; 14 408 patients and their families for whom the free of charge treatment is not free, for investing in continuously attending hospital visits, covering their monitoring tests, and paying for transportation and other costs of treatment; 5 drug manufacturers that provide in-kind gifts of product as well as funding; and 68 members of the Max Foundation’s team, its Board of Directors, advisors, and donors.
H.M. acknowledges the International CML Foundation, the Indian Cooperative Oncology Network, and the Indian CML Study Group for constant and consistent funding, help, and support for his research.
Correspondence
Hemant Malhotra, Sri Ram Cancer Center, Mahatma Gandhi Medical College Hospital, C-70, Ram Marg, Vijay Path, Tilak Nagar, Jaipur 302004, India; e-mail: drmalhotrahemant@gmail.com.
References
- 1.Gunnarsson N, Sandin F, Höglund M, et al. Population-based assessment of chronic myeloid leukemia in Sweden: striking increase in survival and prevalence. Eur J Haematol. 2016;97(4):387-392. [DOI] [PubMed] [Google Scholar]
- 2.Björkholm M, Ohm L, Eloranta S, et al. Success story of targeted therapy in chronic myeloid leukemia: a population-based study of patients diagnosed in Sweden from 1973 to 2008. J Clin Oncol. 2011;29(18):2514-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TM. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34(24):2851-2857. [DOI] [PubMed] [Google Scholar]
- 4.Ganesan P, Ganesan TS, Radhakrishnan V, et al. Chronic myeloid leukemia: long-term outcome data in the imatinib era. Indian J Hematol Blood Transfus. 2019;35(1):37-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganesan P, Kumar L. Chronic myeloid leukemia in India. J Glob Oncol. 2016;3(1):64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malhotra H, Sharma R, Singh Y, et al. Report of chronic myeloid leukemia SMS Medical College Hospital, Jaipur. Ind J Med Paed Oncol. 2013;34(3):177-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldman JM. Chronic myeloid leukemia in India. Indian J Med Paediatr Oncol. 2013;34(3):147-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tapela N, Nzayisenga I, Sethi R, et al. Treatment of chronic myeloid leukemia in rural Rwanda: promising early outcomes. J Glob Oncol. 2016;2(3):129-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kayastha GK, Ranjitkar N, Gurung R, et al. Treating Philadelphia chromosome/BCR-ABL1 positive patients with Glivec (Imatinib mesylate): 10 years’ experience at Patan Hospital, Nepal. Br J Haematol. 2017;177(6):991-999. [DOI] [PubMed] [Google Scholar]
- 10.Au WY, Caguioa PB, Chuah C, et al. Chronic myeloid leukemia in Asia. Int J Hematol. 2009;89(1):14-23. [DOI] [PubMed] [Google Scholar]
- 11.Unnikrishnan R, Veeraiah S, Mani S, et al. Comprehensive evaluation of adherence to therapy, its associations, and its implications in patients with chronic myeloid leukemia receiving imatinib. Clin Lymphoma Myeloma Leuk. 2016;16(6):366-371.e3. [DOI] [PubMed] [Google Scholar]
- 12.Menon H. Non-adherence to CML therapy and its clinical implications in India. Natl Med J India. 2017;30(3):142-147. [PubMed] [Google Scholar]
- 13.Yanamandra U, Malhotra P, Sahu KK, et al. Variation in adherence measures to imatinib therapy. J Glob Oncol. 2018;4:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cantú-Rodríguez OG, Sánchez-Cárdenas M, Gutiérrez-Aguirre CH, et al. Cultural factors related to adherence to imatinib in CML: a Mexican perspective. Hematology. 2015;20(2):72-76. [DOI] [PubMed] [Google Scholar]
- 15.Sokal JE, Cox EB, Baccarani M, et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood. 1984;63(4):789-799. [PubMed] [Google Scholar]
- 16.Hoffmann VS, Baccarani M, Lindoerfer D, et al. The EUTOS prognostic score: review and validation in 1288 patients with CML treated frontline with imatinib. Leukemia. 2013;27(10):2016-2022. [DOI] [PubMed] [Google Scholar]
- 17.Deb P, Nath U, Aich R, et al. EUTOS score predicts long-term outcome in chronic myeloid leukemia patients transitioning from pre-imatinib era to imatinib upon availability. Blood. 2015;126(23):4023. [Google Scholar]
- 18.Ali MD, Badi AI, Al-Zebari SS, Al-Allawi NA. Response to tyrosine kinase inhibitors in chronic myeloid leukemia: experience from a west Asian developing country. Int J Hematol. 2014;100(3):274-280. [DOI] [PubMed] [Google Scholar]
- 19.WHO. WHO essential drug list 2017. https://www.who.int/medicines/publications/essentialmedicines/20th_EML2017.pdf. Accessed 8 August 2019.
- 20.Experts in Chronic Myeloid Leukemia. The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood. 2013;121(22):4439-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madhav D. Generic imatinib in chronic myeloid leukemia: survival of the cheapest. Blood. 2016;128:630. [DOI] [PubMed] [Google Scholar]
- 22.Mathews V. Generic imatinib: the real-deal or just a deal? Leuk Lymphoma. 2014;55(12):2678-2680. [DOI] [PubMed] [Google Scholar]
- 23.Malhotra H, Sharma P, Bhargava S, Rathore OS, Malhotra B, Kumar M. Correlation of plasma trough levels of imatinib with molecular response in patients with chronic myeloid leukemia. Leuk Lymphoma. 2014;55(11):2614-2619. [DOI] [PubMed] [Google Scholar]
- 24.Natarajan H, Kumar L, Bakhshi S, et al. Imatinib trough levels: a potential biomarker to predict cytogenetic and molecular response in newly diagnosed patients with chronic myeloid leukemia. Leuk Lymphoma. 2019;60(2):418-425. [DOI] [PubMed] [Google Scholar]
- 25.Rousselot P, Prost S, Guilhot J, et al. ; French CML Group. Pioglitazone together with imatinib in chronic myeloid leukemia: a proof of concept study. Cancer. 2017;123(10):1791-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malhotra H, Malhotra B, Yadav A, et al. 358P - PPAR GAMMA Agonist in combination with BCR/ABL TKI in patients of CML-CP with suboptimal molecular response. Ann Oncol. 2016;27(suppl 9):ix104-ix111. [Google Scholar]
- 27.Bhargava S, Malhotra H, Rathore OS, et al. Anti-leukemic activities of alcoholic extracts of two traditional Indian medicinal plants. Leuk Lymphoma. 2015;56(11):3168-3182. [DOI] [PubMed] [Google Scholar]
- 28.Yeung DT, Osborn MP, White DL, et al. ; Australasian Leukaemia and Lymphoma Group. TIDEL-II: first-line use of imatinib in CML with early switch to nilotinib for failure to achieve time-dependent molecular targets. Blood. 2015;125(6):915-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elias F, Gebran A, Said C, Beker RV, Ammar W. Budget impact of treatment-free remission in treating chronic-phase Philadelphia-positive CML in Lebanon. J Glob Oncol. 2019;5:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winn-Deen ES, Helton B, Van Atta R, et al. Development of an integrated assay for detection of BCR-ABL RNA. Clin Chem. 2007;53(9):1593-1600. [DOI] [PubMed] [Google Scholar]
- 31.CDC. Availability of an assay for detecting mycobacterium tuberculosis, including rifampin-resistantstrains, and considerations for its use. MMWR Morb Mortal Wkly Rep. 2013;62(41):821-824. [PMC free article] [PubMed] [Google Scholar]
- 32.Sala Torra O, Beppu L, Smith JL, et al. Paper or plastic? BCR-ABL1 quantitation and mutation detection from dried blood spots. Blood. 2016;127(22):2773-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drain PK, Hyle EP, Noubary F, et al. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect Dis. 2014;14(3):239-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens WS, Scott LE, Crowe SM. Quantifying HIV for monitoring antiretroviral therapy in resource-poor settings. J Infect Dis. 2010;201(suppl 1):S16-S26. [DOI] [PubMed] [Google Scholar]
- 35.Petti CA, Polage CR, Quinn TC, Ronald AR, Sande MA. Laboratory medicine in Africa: a barrier to effective health care. Clin Infect Dis. 2006;42(3):377-382. [DOI] [PubMed] [Google Scholar]
- 36.Postel A, Letzel T, Frischmann S, Grund C, Beer M, Harder T. Evaluation of two commercial loop-mediated isothermal amplification assays for detection of avian influenza H5 and H7 hemagglutinin genes. J Vet Diagn Invest. 2010;22(1):61-66. [DOI] [PubMed] [Google Scholar]
- 37.Sooknanan R, Malek L, Wang XH, Siebert T, Keating A. Detection and direct sequence identification of BCR-ABL mRNA in Ph+ chronic myeloid leukemia. Exp Hematol. 1993;21(13):1719-1724. [PubMed] [Google Scholar]
- 38.Dugan LC, Hall S, Kohlgruber A, Urbin S, Torres C, Wilson P. Detection of BCR-ABL Fusion mRNA Using Reverse Transcriptase Loop-mediated Isothermal Amplification. Livermore, CA: Lawrence Livermore National Lab; 2011. Report no. LLNL-TR-520096. [Google Scholar]
- 39.Hashimoto Y, Hatayama Y, Kojima N, et al. Development of reverse transcription loop-mediated isothermal amplification for simple and rapid detection of promyelocytic leukemia-retinoic acid receptor α mRNA. Yonago Acta Med. 2016;59(4):262-269. [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization. Global Health Observatory, 2018. who.int/gho/database/en/. Accessed 8 August 2019.
- 41.Kanavos P, Vandoros S, Garcia-Gonzalez P. Benefits of global partnerships to facilitate access to medicines in developing countries: a multi-country analysis of patients and patient outcomes in GIPAP. Global Health. 2009;5(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Gonzalez P, Boultbee P, Epstein D. Novel humanitarian aid program: the Glivec International Patient Assistance Program—lessons learned from providing access to breakthrough targeted oncology treatment in low-and middle-income countries. J Glob Oncol. 2015;1(1):37-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.The Max Foundation. The Max Foundation. www.themaxfoundation.org. Accessed May 15, 2019.
- 44.American Cancer Society. American Cancer Society statistics. https://www.cancer.org/cancer/chronic-myeloid-leukemia/about/statistics.html. Accessed May 15, 2019.
- 45.Mendizabal AM, Garcia-Gonzalez P, Levine PH. Regional variations in age at diagnosis and overall survival among patients with chronic myeloid leukemia from low and middle income countries. Cancer Epidemiol. 2013;37(3):247-254. [DOI] [PubMed] [Google Scholar]