Abstract
The Philadelphia chromosome-negative myeloproliferative neoplasms (MPNs) share a common pathobiology of constitutive activation of the JAK and STAT pathway, despite having the 3 distinct phenotypes of essential thrombocythemia, polycythemia vera, and primary myelofibrosis. Targeting the JAK-STAT pathway has led to remarkable clinical benefit, including reduction in splenomegaly, amelioration of cytokine-driven symptoms, improvement in quality of life, and even some improvement in survival. However, targeting this pathway has not resulted in consistent disease modification by current metrics, including a reduction in mutant allele burden or reversal of fibrosis. Moreover, targeting JAK-STAT can lead to limiting treatment-emergent side effects, such as anemia and thrombocytopenia. Continued discovery points to a complex system of pathogenesis beyond JAK-STAT driving the formation and evolution of MPNs. This article reviews the successes and limitations of JAK-STAT inhibition, surveys the strategies behind emerging therapies, and discusses the challenges that are present in moving beyond JAK-STAT.
Learning Objectives
Recognize the benefit of treatment with JAK-SAT inhibition for MPNs, as well as recognize the shortcomings of this therapeutic approach
Identify new therapeutic targets beyond JAK-STAT inhibition in the treatment of MPNs
Clinical case
The patient is a 70-year-old man from New Jersey. As part of his annual physical with his primary care physician, he describes a 3- to 6-month history of increasing fatigue. He often travels for work and finds himself more and more exhausted after gigs. He adds that his appetite is down, but he does not notice any weight loss. On physical examination, his lungs are clear, and his heart rhythm is regular. He is found to have splenomegaly with the spleen tip being 8 cm below the left costal margin at the midclavicular line. His medical history includes medically controlled hypertension, and he is a former smoker. Laboratory studies show the following: hemoglobin, 9.1 g/dL; hematocrit, 28%; leukocyte count, 14 × 109/L; neutrophils, 68%; lymphocytes, 18%; band forms, 3%; monocytes, 3%; metamyelocytes, 2%; myelocytes, 1%; promyelocytes, 2%; eosinophils, 2%; basophils, 1%; platelet count, 115 × 109/L; and lactate dehydrogenase, 310 U/L.
He ultimately undergoes a bone marrow biopsy for unexplained anemia and leukocytosis. The biopsy is hypercellular with moderate reticulin fibrosis (MF-2). Concurrent cytogenetics reveal a normal male karyotype. Additional testing reveals the presence of the JAK2 V617F mutation.
Introduction
“I get up in the evening; And I ain’t got nothing to say. I come home in the morning; I go to bed feeling the same way.” Bruce Springsteen, “Dancing in the Dark”1
It is pretty unlikely when The Boss penned “Dancing in the Dark,” he was discussing the finer points of treatment of Philadelphia chromosome-negative myeloproliferative neoplasms (MPNs). Rather, under the upbeat tempo and synthesizer sounds, he was brooding on how the pressures of fame and struggle for perfection were weighing down on him. From a certain perspective, the treatment of MPNs today share some similarities to Bruce Springsteen in the early 1980s. However, just as he found his place in his art with time, by moving beyond the JAK-STAT pathway, the treatment of MPNs is ready to take the next step.
Targeting JAK-STAT
“You can’t start a fire; You can’t start a fire without a spark.”1
It is possible that the current pressure surrounding the development of new treatments for this family of diseases stems from the discovery of the JAK2 V617F mutation. After all, the MPNs went a long time without a hit song. William Dameshek, in his now famous Blood editorial in 1951, hypothesized that the Philadelphia chromosome-negative MPNs, essential thrombocythemia (ET), polycythemia vera (PV), and primary myelofibrosis (MF), having similar phenotypes, must have a similar pathogenesis driving them.2 Less than a decade later, chronic myelogenous leukemia (CML) began its meteoric rise to stardom with the discovery of the Philadelphia chromosome, and the subsequent targeting of the BCR-ABL1 fusion protein by imatinib, ushering in a new era of medicine. In 2004, when 4 groups independently discovered the JAK2 V617F mutation as a recurring mutation in MPNs, Dameshek’s hypothesis came to life.3 This mutation, present in 70% of MPNs, leads to the constitutive activation of the JAK-STAT pathway, resulting in myeloproliferation and cytokine production, the ultimate phenotype of MPNs. The discoveries of recurrent mutations in MPL and CALR, as well as how they lead to constitutive JAK-STAT activation, filled in the gap about how JAK2-negative MPNs can have the same phenotype as JAK2-mutated disease.4 Like a producer in the sound booth with the artist, is it fair to demand another “hit song” like imatinib by targeting the JAK-STAT pathway?
Development of JAK inhibitors
After the discovery of JAK2 V617F, targeting the aberrantly activated JAK-STAT pathway with inhibitors quickly came about. Like a fleet of ships launching, several JAK inhibitors were evaluated in preclinical and clinical testing. Although all of the JAK inhibitors in clinical development target the adenosine triphosphate binding site under the active conformation of the kinase domain,5 the main difference between them is the specificity for each of the JAKs. For example, ruxolitinib is primarily a JAK1 and JAK2 inhibitor,6 whereas itacitinib is a JAK1 inhibitor,7 and pacritinib is a JAK2 inhibitor.8 Like tyrosine kinase inhibitors for CML, the JAK inhibitors also act on non-JAK kinases, which may be of therapeutic benefit, such a fedratinib hitting fms-like tyrosine kinase 3 (FLT3)6 and momelotinib inhibiting activin A receptor type I (ACVR1).9 Along with targeting a mix of non-JAK kinases comes various side effects. For example, inhibition of FLT3 is often associated with the development of diarrhea.
At the time of the most recent American Society of Hematology Annual Meeting, ruxolitinib was the only agent approved by the US Food and Drug Administration for the treatment of a Philadelphia chromosome-negative MPN. By reducing phosphorylation of JAK2, STAT5, and ERK1/2, it decreases myeloproliferation, as well as reduces circulating cytokine levels, spleen size, and symptom burden.3 Ruxolitinib was quick off the starting line, swiftly moved through the development process, and was granted regulatory approval in 7 short years after the discovery of JAK2 V617F. To this day, it remains a central fixture in the treatment of MF (Figure 1). The initial approval of ruxolitinib was based on the randomized phase 3 COMFORT-I10 and COMFORT-II11 studies. Compared with placebo in COMFORT-I, or best available therapy in COMFORT-II, it resulted in superior spleen volume reduction, decrease in symptom burden, as measured by the total symptom score, and improved quality-of-life measures. Of note, ruxolitinib has similar efficacy in patients with MPL and CALR mutations to those with JAK2 mutations. This is due to the fact that it inhibits wild-type JAK1 and JAK2 and, thus, dampens JAK-STAT activation, irrespective of driver mutation. With 5 years’ worth of follow-up, the median duration of response was 3.2 years in both studies.12 By pooling the 5-year data from both studies, and accounting for cross-over with the rank-preserving structural failure time method, the median overall survival was 5.3 years for ruxolitinib vs 2.3 years for the control arm (hazard ratio, 0.35; 95% confidence interval, 0.23-0.59).12 After its approval for MF, ruxolitinib also gained approval for PV after hydroxyurea based on the RESPONSE study.13
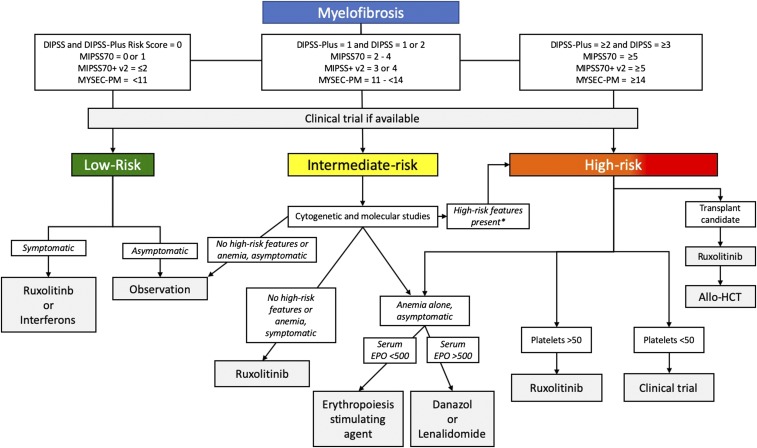
Figure 1.
Treatment algorithm for MF. *High-risk features include cytogenetic markers: complex karyotype, −7, i(17q), inv(3)/3q21, 12p-/12p11.2, 11q-/11q23, other autosomal trisomies not including +8 or +9, mutations in ASXL1, EZH2, SRSF2, IDH1/2, U2AF1, TP53, as well as aggressive clinical features (rapidly increasing circulating blasts or leukocytosis, severe symptoms, or splenomegaly). Allo-HCT, allogeneic hematopoietic cell transplantation; DIPSS, Dynamic International Prognostic Scoring System54; DIPSS-Plus55; EPO, erythropoietin level; MIPSS70, Mutation-Enhanced International Prognostic Score System56; MIPSS70-Plus v257; MYSEC-PM, MF secondary to PV and ET-prognostic Model.58
Several other compounds have been in development, each with a different twist on JAK inhibition. The JAK2/FLT3 inhibitor pacritinib may provide an opportunity to deliver JAK inhibition to patients with thrombocytopenia.8 Fedratinib, a JAK2 inhibitor, has demonstrated activity in patients previously exposed to ruxolitinib,14 even when applying more stringent criteria in defining relapse or refractoriness to prior JAK inhibition.15 In addition to inhibiting JAK1 and JAK2, momelotinib inhibits ACVR1/ALK2, decreases hepcidin production, and can improve anemia in transfusion-dependent patients.9 Even inhibitors with selectivity for JAK2 V167F have been developed with the intention that a more selective JAK inhibitor may lead to better responses and fewer side effects.16
Clinical case: part 2
Shortly after his diagnosis, the patient was started on ruxolitinib. Within a few days he began to feel better. At his next follow-up visit, he said that his appetite was improved, and on examination, his spleen size had decreased to 4 cm below the left costal margin at the midclavicular line. In the first 4 weeks of treatment his anemia worsened, with his hemoglobin dropping down to 8.2 g/dL, but then increasing back up to 9.1 g/dL 2 months later.
Approximately 4 years after starting ruxolitinib, he began to feel a return in his symptoms (ie, abdominal fullness and early satiety). He reported some night sweats and occasional bone pain. His spleen was now 10 cm below the left costal margin on examination, and in reviewing his laboratory results, his anemia (hemoglobin of 7.9 g/dL) and thrombocytopenia (platelet count of 84 × 109/L) worsened.
Where JAK inhibitors fall short
“Message keeps getting clearer; radio's on and I'm moving 'round the place. I check my look in the mirror; I want to change my clothes, my hair, my face. Man, I ain't getting nowhere.” 1
Despite successes, there are many limitations in the application of JAK inhibitors in the clinic. Although some may not be as myelosuppressive, or even promise to increase low blood counts, anemia and thrombocytopenia are common adverse events across the class. In a population of patients already burdened with cytopenias, this can be a big challenge. Also, the development of some JAK inhibitors have been slowed, or stopped altogether, by emergence of nonhematologic toxicity. Regulatory holds from clinical development have been issued, and then lifted, over concerns of bleeding and cardiac toxicity with pacritinib and Wernicke’s encephalopathy with fedratinib.
However, the main limitation with JAK inhibition is the lack of disease modification and eventual formation of resistance, although the definition of disease modification is prone to debate itself. The median survival of patients with MF treated with ruxolitinib is longer than that of patients treated with best available therapy or placebo; thus, the drugs are disease modifying by a broad definition. However, this is likely due, in large part, to patients living better as a result of lower cytokine levels, fewer symptoms and cachexia, as well as improved functional status. In clinical studies, treatment with JAK inhibitors seems to have little effect on the malignant clone. They have not led to consistent reductions in mutant allele burden and only rare molecular remissions.13,17 With the caveat that fibrosis can be difficult to measure consistently, they seem to stabilize marrow fibrosis at best, rather than displaying significant antifibrotic activity.10,11 At some point, patients on a JAK inhibitor will have a return of their symptoms and splenomegaly, worsening marrow failure, or progression to accelerated or blast phase. For example, patients whose disease responded to ruxolitinib in the COMFORT-II study had a <50% chance (hazard ratio, 0.48; 95% confidence interval, 0.35-0.60) of maintaining that response at 5 years.18 Also, in PV, the median duration of treatment with ruxolitinib in controlled trials is not indefinite. Of the patients randomized to ruxolitinib in the phase 3 RESPONSE trial, 60% were under hematocrit control by 32 weeks, and the probability of maintaining that control for another 48 weeks was 89%.19 Several mechanisms by which MPNs move past JAK inhibition have been identified. These include upregulation of parallel pathways that lead to myeloproliferation despite downregulation of JAK-STAT, heterodimerization of activated JAK2 with other JAK kinases, point mutations in the kinase domain of JAK2 akin to resistance mechanisms in CML, and clonal evolution and the acquisition of secondary mutations in other genes, such as ASXL1, relevant to the pathogenesis of myeloid malignancies.20-23
Nonetheless, JAK inhibitors have been a huge success and topped the charts, even though they have not been the next imatinib. Their arrival has improved the lives of many patients and radically changed the treatment landscape of MPNs. There is no doubt that they are the spark that started the fire.
Other pathways and targets in MPNs
“There's something happening somewhere. Baby, I just know that there is.”1
The MPNs are not a 1-hit wonder, and looking to other pathways is the next step in building on the success of JAK inhibitors. Several agents, both alone and as a dance partner for ruxolitinib in the setting of combination therapy, have been explored (Table 1). The following is by no means an exhaustive list, but rather a taste of things to come.
Table 1.
Current and new targets in the treatment of MPNs
| Aim of therapy | Target—therapeutic mechanism of action | Agent | |
|---|---|---|---|
| Where we are now | Reduce myeloproliferation | Ribonucleoside diphosphate reductase inhibition | Hydroxyurea |
| Reduce symptom burden, splenomegaly, and myeloproliferation. | JAK1/JAK2 inhibition | Ruxolitinib | |
| Reduce symptom burden, splenomegaly, and myeloproliferation; reduce allele burden and fibrosis (in early disease). | Apoptosis and immune modulation | Interferons | |
| Alleviating anemia | EPO receptor - early erythroid maturation | Darbepoetin, EPO | |
| Suppression of inflammatory cytokines and angiogenesis; enhancement of erythropoietin signaling. | Lenalidomide, thalidomide | ||
| Androgen, mechanism unknown. | Danazol | ||
| Reduce malignant clone | DNA methylation | Azacitidine, decitabine | |
| Maintenance-free remission (cure) | Alloreactive T cells eliminating the malignant clone | Allo-HCT | |
| Just ahead | Alleviating anemia | Activin receptor IIA ligand trap—late erythroid maturation | Luspatercept, sotatercept |
| Reduce symptom burden and splenomegaly and alleviate anemia | JAK2/ACVR1—reduction in hepcidin levels | Momelotinib | |
| Reduce symptom burden and splenomegaly in the setting of thrombocytopenia | JAK2/FLT3 inhibition | Pacritinib | |
| Reduce symptom burden and splenomegaly after ruxolitinib | JAK2/FLT3 inhibition | Fedratinib | |
| Down the road | Reverse fibrosis | Differentiation of monocytes into fibrocytes | PRM-151 |
| TGF-β ligand trap | AVID200 | ||
| Reverse fibrosis and clonal hematopoiesis | Hedgehog-smoothened inhibitor | Glasdegib, sonidegib | |
| Reduce symptom burden, splenomegaly, and myeloproliferation. | PI3K—suppress neoplastic clonal hematopoiesis via cell cycle arrest and apoptosis | Buparlisib, parsaclisib | |
| Reduce clonal hematopoiesis and potentially fibrosis | SMAC (activation)—increase apoptosis | LCL161 | |
| MDM2—increase apoptosis | Idasanutlin, KRT-232 | ||
| Aurora kinase A—increase apoptosis | Alisertib | ||
| Telomerase | Imetelstat | ||
| Bromodomain and extraterminal proteins—reduction in inflammatory cytokine production | CPI-0610 | ||
| LSD1—epigenetic reprogramming | Bomedemstat (IMG-7289) | ||
| On the horizon | Reduction in mutant allele burden | JAK2—type II inhibitor | CHZ868 |
| Reduction in myeloproliferation | Mutant CALR trap | ? | |
| Clonal eradication | CAR T cells | ? | |
| Delay of progression in early disease | ? | ? | |
| Cure without allo-HCT | ? | ? |
allo-HCT, allogeneic hematopoietic cell transplantation; CAR, chimeric antigen receptor; EPO, erythropoietin; MDM2, mouse double minute 2 homolog; SMAC, second mitochondria-derived activator of caspases; ?, unknown.
Anemia is common in patients with MF; in fact, it is one of the minor diagnostic criteria. It contributes significant morbidity and is associated with increased mortality.24 Therapies that are available to ameliorate anemia have modest benefit, and improved treatments for MF-associated anemia represent a practical and achievable short-term goal. Sotatercept is an activin receptor IIA ligand trap that improves anemia by sequestering transforming growth factor-β (TGF-β) superfamily ligands. In a phase 2 study of MF patients with anemia, 6 of 17 patients (35%) treated with sotatercept alone and 1 of 8 patients (12.5%) treated with sotatercept in combination with ruxolitinib had an erythroid response.25 Hypertension and limb pain were the most common side effects observed. A phase 2 study of luspatercept alone or in combination with ruxolitinib in anemic MF patients is nearing completion (NCT03194542).
There is more to the TGF-β story though, because it plays a major role in the formation of fibrosis. This cytokine is expressed at high levels in the bone marrow in animal models and patient samples, produced largely by the megakaryocytes in MPN patients.26 AVID200 is a TGF-β ligand trap that inhibits the TGF-β1 and TGF-β3 isoforms, the putative drivers of fibrosis formation. It spares the TGF-β2 isoform, which promotes hematopoiesis. A multicenter phase 1/1b study is underway through the Myeloproliferative Neoplasm Research Consortium (NCT03895112).sd
As with many biologic systems, the formation of fibrosis is more complex than just simply an increase in TGF-β leading to scar tissue production. This opens up the opportunity for other ways to target fibrosis formation to modify disease in MPNs. Although originally developed to treat pulmonary fibrosis, PRM-151 has been studied in MF. It is a recombinant form of pentraxin 2, an endogenous protein that regulates the differentiation of monocytes into fibrocytes that has been shown to reverse fibrosis formation in preclinical models. Twenty-seven patients were enrolled in a phase 2 study of PRM-151 given alone or in combination with ruxolitinib. Of the 26 evaluable patients, 6 had a bone marrow fibrosis response (reduction of ≥1 grade).27 The long-term follow-up of the 18 patients in the extension study showed sustained improvement in bone marrow fibrosis grade, as well as spleen and symptom responses.
In addition to its role in normal hematopoiesis, the hedgehog-smoothened signaling pathway has been linked to the pathobiology of MPNs, in particular the formation of fibrosis. In preclinical models of MF, combined inhibition of the hedgehog pathway and JAK inhibition reduced JAK2 mutant allele burden, bone marrow fibrosis, and white blood cell and platelet counts.28 Although approved for the treatment of acute myeloid leukemia, a phase 1/2 study of glasdegib in patients with MF showed modest activity as a single agent.28 When ruxolitinib was combined with sonidegib in 27 JAK inhibitor–naive MF patents, 15 patients (56%) achieved a ≥35% reduction in spleen volume at any time on treatment.29 Of note, 17 patients (63%) required dose adjustment or interruption because of adverse events.
Along with constitutive activation of the JAK-STAT pathway, mutations in JAK2, CALR, or MPL also lead to activation of the mTOR pathway, providing another mechanism of resistance and a therapeutic target. Inhibitors of phosphatidylinositol 3-kinase (PI3K), AKT, and mTOR have been evaluated in the preclinical and clinical trial settings. The phase 1 HARMONY study evaluated the combination of ruxolitinib and the pan-PI3K inhibitor buparlisib in patients with MF.30 The spleen response rate was similar to that of ruxolitinib alone, with ∼40% of the patients having a spleen response. An ongoing study is looking at the addition of the selective PI3Kδ inhibitor parsaclisib to ruxolitinib as an add-back strategy to regain responses lost in the setting of ruxolitinib failure. Interim results have been presented,31 and efforts are underway to identify the optimal dosing strategy for the combination.
Several approaches are looking at reversing dysregulated apoptosis signaling, yet another key factor in the pathobiology of MPNs. Second mitochondria-derived activator of caspases (SMAC) is a mitochondrial‐derived proapoptotic protein that ultimately leads to apoptosis via degradation of cellular inhibitor of apoptosis protein 1 (cIAP1), which is overexpressed in MF cell.32 Thus, second mitochondria-derived activator of caspases mimetics work by activating the inhibitor of the inhibitor of apoptosis. LCL161 is being evaluated in the setting of a single-agent phase 2 study in MF patients previously treated with a JAK inhibitor. Interim results from the first 43 patients demonstrated a response rate of 30%, including 5 patients with improved anemia.32 In addition to increased expression of cIAP1, increased expression of mouse double minute 2 homolog (MDM2) has been observed in MPN mononuclear cells harboring wild-type TP53.33 MDM2 is a negative regulator of p53; therefore, inhibiting MDM2 would be inhibiting the inhibitor of apoptosis. A single-center phase 1 study evaluated the MDM2 inhibitor idasanutlin in previously treated patients: 11 patients with PV and 1 patient with ET. The overall response rate was 75% by cycle 6, with minimal toxicity and on-target P53 pathway activation.34 A multicenter phase 2 study of idasanutlin for hydroxyurea-resistant or intolerant PV is underway (NCT03287245). Another MDM2 inhibitor, KRT-232 (AMG 232), is being evaluated in patients with MF after prior treatment with a JAK inhibitor (NCT03662126) and in phlebotomy-dependent PV after treatment with hydroxyurea or interferon (NCT03669965), with additional combination-therapy studies being planned for accelerated and blast-phase MPN.
A high level of aurora kinase A (AURKA) is a common feature of hematopoietic cells in MF and is mediated by increased c-MYC expression as a downstream consequence of JAK-STAT activation.35 When AURKA is inhibited in preclinical models, it leads to megakaryocyte differentiation and subsequent apoptosis, resulting in reduction of marrow fibrosis. In a phase 1 study of higher-risk MF patients, treatment with the AURKA inhibitor alisertib led to a spleen response in 29% (4 of 14), transfusion independence in 8% (1 of 13), and ≥50% symptom improvement in 23% (5 of 22) of patients.36 In addition to restoring GATA1 expression in megakaryocytes, a 1-grade reduction if fibrosis score was observed in 4 of 6 patients with paired samples.
The oligonucleotide imetelstat is a potent telomerase inhibitor; it was evaluated in a phase 2 study of 107 MF patients previously treated with a JAK inhibitor.37 With a median treatment duration of 6.2 months, (range, 0.0-27.2), 10% of evaluable patients had a spleen response, and 38% had a symptom response (reduction in total symptom score ≥ 50%) at week 24. Of note, survival of this high-risk population was longer than expected based on historical controls, and the spleen response rate was higher in patients with a high-risk mutation (ASXL1, EZH2, SRSF2, or IDH1/2), suggesting disease modification in this adverse-risk group.
Bromodomain and extraterminal protein inhibitors, such as CPI-0610, are another class of compounds being developed in MPNs (NCT02158858).38 JAK-STAT signaling in MPNs also leads to aberrant NF-κB signaling and cytokine-independent myeloproliferation through epigenetic modification.39 In diseases other than MPNs, the histone lysine reader BRD4 is a key mediator of NF-κB–driven chronic inflammation.40 In preclinical models of MPN, bromodomain and extraterminal inhibition reduced inflammatory cytokine production and marrow fibrosis.39,41 Moreover, the combination of JAK and BET inhibition resulted in added efficacy, both reversing fibrosis and reducing allele burden.39
Epigenetic modification with hypomethylating agents (eg, azacitidine) or histone deacetylase inhibitors (eg, panobinostat), as well as treatment with immunomodulatory drugs (IMiDs; thalidomide, lenalidomide, pomalidomide), has been extensively explored and expertly reviewed in the American Society of Hematology Education Program and other publications.42-45 Allogeneic hematopoietic cell transplantation remains the only proven immunotherapy leading to sustained maintenance-free remissions; however, other immunotherapies, such as checkpoint inhibitors and chimeric antigen receptor T cells, are still on the distant horizon (Table 1).
Discovery is driving innovation in the field. New concepts of how mutant CALR (mCALR) leads to the MPN phenotype have emerged. These mechanisms are unique to this driver mutation and open the door for new therapeutics. One explanation is that secreted mCALR can act as a rogue cytokine, activating any cell carrying thrombopoietin receptor46 and making mCALR trap a possible therapeutic strategy. Also, mCALR still acts as a chaperone and can transport thrombopoietin receptor that would otherwise not make it to the surface, such as in the setting of incomplete glycosylation, making it available for activation.47 Because the mCALR protein is still present with the receptor on the surface of the cell, this could be a new target for immune or cellular therapy.
Moving beyond JAK-STAT inhibition
“I’ll shake this world off my shoulders.”1
A whirlwind of scientific discovery is unlocking the mysteries of what makes MPNs tick, with the aim of identifying new treatment. Despite arriving with great interest, many candidates have fallen to the sidelines; even the future of imetelstat and PRM-151 is unclear, and only 1 therapeutic strategy outside of allogeneic hematopoietic cell transplantation has hit the big time: JAK-STAT inhibition. There are likely a number of reasons for this. One reason is that the pathobiology of MPNs is complicated. As surveyed above, there are numerous biologic pathways that are deranged in MPN cells, and it is not completely clear which ones represent the Achilles’ heel. This is in stark contrast to CML. In addition to the extensive diversity in the types and numbers of molecular lesions that drive disease, patients with similar genotypic lesions can have divergent phenotypes. MPNs are a myeloid malignancy of blasts and cytopenias, as well as a disease of cytokines and splenomegaly; more aggressive therapies are not always well tolerated in a population set up for frailty. Long-term events, such as thrombosis, bleed, and disease progression to MF or blast phase, occur less frequently in PV and ET. Therefore, to design studies to evaluate these end points, large numbers of patients may need to be followed for a long period of time. Even lower-risk patients with MF have a median survival of more than a decade, as predicted by current prognostic models.
Perhaps a bigger question is as follows: “Should we should be moving away from JAK-STAT inhibition?” JAK-STAT activation has proven to be fundamental and universal to the pathogenesis of the MPNs. Should we be focusing on building better JAK inhibitors? Inhibitors with activity against the mutant JAK2 V617F have been tested.16 Ruxolitinib, as well as all of the JAK inhibitors tested in clinical trials to date, are type I inhibitors that bind to the active conformation of the kinase.5 Type II inhibitors that bind to the inactive conformation of JAK2 have shown significant activity in preclinical models, including reductions in allele burden not seen with type I inhibitors.48 Also, if JAK-STAT activation is so essential, should we be combining JAK inhibition with new therapies as they are being developed? If so, which JAK inhibitor should be used? These are difficult questions to answer, and the development of many new compounds based on targets beyond JAK-STAT do include JAK inhibition in combination with the new agent. Even combinations with older agents, such as interferons, have been explored.49 Although this can make for a more complicated assessment of clinical benefit, it may be difficult for patients whose disease has a suboptimal response to JAK inhibition to fully stop that treatment. Therefore an “add-back” strategy is desired. Because the pathogenesis of the MPNs involves numerous pathways, for the time being, rational combinations with JAK inhibitors will be a strategy often reached for in drug development.
For many of the new treatments being applied in MPNs, the ultimate goal is disease modification; however, it is unclear how to define this. In some instances, it is simply defined as a treatment that leads to a longer survival than the natural history of the disease. In the COMFORT studies, MF patients who received ruxolitinib lived longer than those who received placebo or best available therapy12; however, many do not consider JAK inhibitors to be disease modifying.45 A stricter definition would include delaying or reversing the underlying pathological disease processes along with improving the clinical symptoms. Common approaches to measure disease modification with this definition in mind include reversal of marrow fibrosis or reduction in mutant allele burden of the driver mutation (JAK2, CALR, or MPL). Bone marrow fibrosis grade can be prognostic50; however, it can also be patchy in the marrow and difficult to measure consistently.51 Although it may seem intuitive, the association between a reduction in driver mutation allele burden with therapy and long-term clinical outcomes has not been fully linked. In the future, better ways of measuring disease modification may allow us to identify more successful treatments earlier with smaller studies.
Some of the challenge lies in the way in which we are trying to study the clinical effects of new therapies. Many new therapies are being evaluated in the second-line setting. Although there are consensus criteria for hydroxyurea failure in PV and MF,52 there are no such criteria for ruxolitinib failure, resulting in varying populations enrolled into post-JAK inhibitor studies.
Although consensus criteria for response to treatment have been established for MPNs,52 they can be complex, opening up the door for missing the signal of a benefit. These response criteria were developed mainly for use in clinical trials, and clinical benefit may not reach the level of consensus response criteria. For acute leukemia, a complete remission is having a normal percentage of bone marrow blasts along with the restoration of normal blood counts, and many commercially available and experimental agents reach that threshold. The International Working Group-Myeloproliferative Neoplasms Research and Treatment and European LeukemiaNet response criteria for complete remission include normalization of bone marrow blasts and blood counts, as well as regression of marrow fibrosis. However, many current therapies, including ruxolitinib, fall short of this lofty goal, even though patients are deriving measurable benefit. Recognizing this, the MPN community is coming together to better measure benefit of current and emerging therapies. For example, new criteria to balance the long- and short-term goals of treatment of ET have been proposed.53 These criteria are a composite of blood count normalization, spleen and symptom control, thrombotic events, and mutant allele burden reduction.
The same goalposts are often set for treatment in dissimilar settings. For example, in MF the COMFORT studies set the goal of a spleen volume reduction ≥ 35% and a reduction in total symptom score ≥ 50% as measures of success. Subsequent studies with new treatments for patients before, after, and sometimes long after initial JAK inhibitor therapy have set the same lofty goals, but many have fallen short. Perhaps a closer goalpost of ≥15% spleen volume reduction or ≥30% symptom reduction is still a meaningful outcome for patients who have already been exposed to 1 or 2 lines of therapy. Additional studies linking these responses with quality of life and other patient-centered outcomes will help to fill this knowledge gap.
Conclusions
The discovery of JAK-STAT activation and targeting the pathway have transformed the care of patients with MPNs. Likewise, the continuous discovery of other pathogenic insights is opening up the door on new and exciting treatments. These new treatments, along with established treatments and rational combinations, are positioning the field for another quantum leap forward. While “sitting 'round here trying to write this book,”1 the anticipation of something big around the corner is palpable. “This gun’s for hire, even if we’re just dancing in the dark.”1
Correspondence
Aaron T. Gerds, 9500 Euclid Ave, CA60, Cleveland, OH 44195; e-mail: gerdsa@ccf.org.
References
- 1.Springsteen B. Dancing in the dark. Born in the USA. New York, NY: Columbia Records; 1984. [Google Scholar]
- 2.Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951;6(4):372-375. [PubMed] [Google Scholar]
- 3.Shammo JM, Stein BL. Mutations in MPNs: prognostic implications, window to biology, and impact on treatment decisions. Hematology (Am Soc Hematol Educ Program). 2016;2016(1):552-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vainchenker W, Kralovics R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood. 2017;129(6):667-679. [DOI] [PubMed] [Google Scholar]
- 5.Leroy E, Constantinescu SN. Rethinking JAK2 inhibition: towards novel strategies of more specific and versatile Janus kinase inhibition [published correction appears in Leukemia. 2017;31(12):2853]. Leukemia. 2017;31(5):1023-1038. [DOI] [PubMed] [Google Scholar]
- 6.Zhou T, Georgeon S, Moser R, Moore DJ, Caflisch A, Hantschel O. Specificity and mechanism-of-action of the JAK2 tyrosine kinase inhibitors ruxolitinib and SAR302503 (TG101348) [published correction appears in Leukemia. 2014;28(2):471-472]. Leukemia. 2014;28(2):404-407. [DOI] [PubMed] [Google Scholar]
- 7.Mascarenhas JO, Talpaz M, Gupta V, et al. Primary analysis of a phase II open-label trial of INCB039110, a selective JAK1 inhibitor, in patients with myelofibrosis. Haematologica. 2017;102(2):327-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mascarenhas J, Hoffman R, Talpaz M, et al. Results of the Persist-2 phase 3 study of pacritinib (PAC) versus best available therapy (BAT), including ruxolitinib (RUX), in patients (pts) with myelofibrosis (MF) and platelet counts <100,000/µl. Blood. 2016;128(22):LBA-5. [Google Scholar]
- 9.Oh ST, Talpaz M, Gerds AT, et al. Hepcidin suppression by momelotinib is associated with increased iron availability and erythropoiesis in transfusion-dependent myelofibrosis patients. Blood. 2018;132(suppl 1):4282. [Google Scholar]
- 10.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787-798. [DOI] [PubMed] [Google Scholar]
- 12.Verstovsek S, Gotlib J, Mesa RA, et al. Long-term survival in patients treated with ruxolitinib for myelofibrosis: COMFORT-I and -II pooled analyses. J Hematol Oncol. 2017;10(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372(5):426-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison CN, Schaap N, Vannucchi AM, et al. Janus kinase-2 inhibitor fedratinib in patients with myelofibrosis previously treated with ruxolitinib (JAKARTA-2): a single-arm, open-label, non-randomised, phase 2, multicentre study. Lancet Haematol. 2017;4(7):e317-e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison CN, Schaap N, Vannucchi AM, et al. Fedratinib (FEDR) in myelofibrosis (MF) patients previously treated with ruxolitinib (RUX): a reanalysis of the JAKARTA-2 study. J Clin Oncol. 2019;37(15 suppl):7057. [Google Scholar]
- 16.Berdeja J, Palandri F, Baer MR, et al. Phase 2 study of gandotinib (LY2784544) in patients with myeloproliferative neoplasms. Leuk Res. 2018;71:82-88. [DOI] [PubMed] [Google Scholar]
- 17.Deininger M, Radich J, Burn TC, Huber R, Paranagama D, Verstovsek S. The effect of long-term ruxolitinib treatment on JAK2p.V617F allele burden in patients with myelofibrosis. Blood. 2015;126(13):1551-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison CN, Vannucchi AM, Kiladjian JJ, et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis [published correction appear in Leukemia. 2017;31(3):775]. Leukemia. 2016;30(8):1701-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verstovsek S, Vannucchi AM, Griesshammer M, et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica. 2016;101(7):821-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer SC, Levine RL. Molecular pathways: molecular basis for sensitivity and resistance to JAK kinase inhibitors. Clin Cancer Res. 2014;20(8):2051-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newberry KJ, Patel K, Masarova L, et al. Clonal evolution and outcomes in myelofibrosis after ruxolitinib discontinuation. Blood. 2017;130(9):1125-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel KP, Newberry KJ, Luthra R, et al. Correlation of mutation profile and response in patients with myelofibrosis treated with ruxolitinib. Blood. 2015;126(6):790-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pardanani A, Abdelrahman RA, Finke C, et al. Genetic determinants of response and survival in momelotinib-treated patients with myelofibrosis. Leukemia. 2015;29(3):741-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbui T, Tefferi A, Vannucchi AM, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32(5):1057-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bose P, Daver N, Pemmaraju N, et al. Sotatercept (ACE-011) alone and in combination with ruxolitinib in patients (pts) with myeloproliferative neoplasm (MPN)-associated myelofibrosis (MF) and anemia. Blood. 2017;130(suppl 1):255. [Google Scholar]
- 26.Rossi C, Zini R, Rontauroli S, et al. ; AGIMM (AIRC-Gruppo Italiano Malattie Mieloproliferative) investigators. Role of TGF-β1/miR-382-5p/SOD2 axis in the induction of oxidative stress in CD34+ cells from primary myelofibrosis. Mol Oncol. 2018;12(12):2102-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verstovsek S, Mesa RA, Foltz LM, et al. Phase 2 trial of PRM-151, an anti-fibrotic agent, in patients with myelofibrosis: stage 1 results. Blood. 2014;124(21):713. [Google Scholar]
- 28.Gerds AT, Tauchi T, Ritchie E, et al. Phase 1/2 trial of glasdegib in patients with primary or secondary myelofibrosis previously treated with ruxolitinib [published correction appears in Leuk Res. 2019;81:105]. Leuk Res. 2019;79:38-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta V, Harrison CN, Hasselbalch H, et al. Phase 1b/2 study of the efficacy and safety of sonidegib (LDE225) in combination with ruxolitinib (INC424) in patients with myelofibrosis. Blood. 2015;126(23):825.26473195 [Google Scholar]
- 30.Durrant ST, Nagler A, Guglielmelli P, et al. Results from HARMONY: an open-label, multicentre, 2-arm, phase 1b, dose-finding study assessing the safety and efficacy of the oral combination of ruxolitinib and buparlisib in patients with myelofibrosis [published online ahead of print 9 May 2019]. Haematologica. doi:10.3324/haematol.2018.209965.haematol.2018.209965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daver NG, Kremyanskaya M, O’Connell C, et al. A phase 2 study of the safety and efficacy of INCB050465, a selective PI3Kδ inhibitor, in combination with ruxolitinib in patients with myelofibrosis. Blood. 2018;132(suppl 1):353. [Google Scholar]
- 32.Pemmaraju N, Carter BZ, Kantarjian HM, et al. LCL161, an oral Smac mimetic/IAP antagonist for patients with myelofibrosis (MF): novel translational findings among long-term responders in a phase 2 clinical trial. Blood. 2018;132(suppl 1):687-687. [Google Scholar]
- 33.Lu M, Xia L, Li Y, Wang X, Hoffman R. The orally bioavailable MDM2 antagonist RG7112 and pegylated interferon α 2a target JAK2V617F-positive progenitor and stem cells. Blood. 2014;124(5):771-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mascarenhas J, Lu M, Kosiorek H, et al. Oral idasanutlin in patients with polycythemia vera [published online ahead of print 5 June 2019]. Blood. doi:10.1182/blood.2018893545.blood.2018893545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mascarenhas J, Lu M, Virtgaym E, et al. Open label phase I study of single agent oral RG7388 (idasanutlin) in patients with polycythemia vera and essential thrombocythemia. Blood. 2017;130(suppl 1):254. [Google Scholar]
- 36.Gangat N, Stein BL, Marinaccio C, et al. Alisertib (MLN8237), an oral selective inhibitor of aurora kinase A, has clinical activity and restores GATA1 expression in patients with myelofibrosis. Blood. 2018;132(suppl 1):688. [Google Scholar]
- 37.Mascarenhas J, Komrokji RS, Cavo M, et al. Imetelstat is effective treatment for patients with intermediate-2 or high-risk myelofibrosis who have relapsed on or are refractory to janus kinase inhibitor therapy: results of a phase 2 randomized study of two dose levels. Blood. 2018;132(suppl 1):685. [Google Scholar]
- 38.Kremyanskaya M, Hoffman R, Mascarenhas J, et al. A phase 2 study of Cpi-0610, a bromodomain and extraterminal (BET) inhibitor, in patients with myelofibrosis (MF). Blood. 2018;132(suppl 1):5481. [Google Scholar]
- 39.Kleppe M, Koche R, Zou L, et al. Dual targeting of oncogenic activation and inflammatory signaling increases therapeutic efficacy in myeloproliferative neoplasms. Cancer Cell. 2018;33(1):29-43.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown JD, Lin CY, Duan Q, et al. NF-κB directs dynamic super enhancer formation in inflammation and atherogenesis. Mol Cell. 2014;56(2):219-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sashida G, Wang C, Tomioka T, et al. The loss of Ezh2 drives the pathogenesis of myelofibrosis and sensitizes tumor-initiating cells to bromodomain inhibition. J Exp Med. 2016;213(8):1459-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vannucchi AM, Harrison CN. Emerging treatments for classical myeloproliferative neoplasms. Blood. 2017;129(6):693-703. [DOI] [PubMed] [Google Scholar]
- 43.Harrison CN, McLornan DP. Current treatment algorithm for the management of patients with myelofibrosis, JAK inhibitors, and beyond. Hematology (Am Soc Hematol Educ Program). 2017;2017(1):489-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scherber RM, Mesa RA. Managing myelofibrosis (MF) that “blasts” through: advancements in the treatment of relapsed/refractory and blast-phase MF. Hematology (Am Soc Hematol Educ Program). 2018;2018(1):118-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pardanani A, Tefferi A. How I treat myelofibrosis after failure of JAK inhibitors. Blood. 2018;132(5):492-500. [DOI] [PubMed] [Google Scholar]
- 46.Pecquet C, Balligand T, Chachoua I, et al. Secreted mutant calreticulins as rogue cytokines trigger thrombopoietin receptor activation specifically in CALR mutated cells: perspectives for MPN therapy. Blood. 2018;132(suppl 1):4.29976778 [Google Scholar]
- 47.Pecquet C, Chachoua I, Roy A, et al. Calreticulin mutants as oncogenic rogue chaperones for TpoR and traffic-defective pathogenic TpoR mutants. Blood. 2019;133(25):2669-2681. [DOI] [PubMed] [Google Scholar]
- 48.Meyer SC, Keller MD, Chiu S, et al. CHZ868, a type II JAK2 inhibitor, reverses type I JAK inhibitor persistence and demonstrates efficacy in myeloproliferative neoplasms. Cancer Cell. 2015;28(1):15-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kiladjian J-J, Soret-Dulphy J, Resche-Rigon M, et al. Ruxopeg, a multi-center Bayesian phase 1/2 adaptive randomized trial of the combination of ruxolitinib and pegylated interferon alpha 2a in patients with myeloproliferative neoplasm (MPN)-associated myelofibrosis. Blood. 2018;132(suppl 1):581. [Google Scholar]
- 50.Gianelli U, Vener C, Bossi A, et al. The European Consensus on grading of bone marrow fibrosis allows a better prognostication of patients with primary myelofibrosis. Mod Pathol. 2012;25(9):1193-1202. [DOI] [PubMed] [Google Scholar]
- 51.Kvasnicka HM, Beham-Schmid C, Bob R, et al. Problems and pitfalls in grading of bone marrow fibrosis, collagen deposition and osteosclerosis - a consensus-based study. Histopathology. 2016;68(6):905-915. [DOI] [PubMed] [Google Scholar]
- 52.Mesa R, Jamieson C, Bhatia R, et al. Myeloproliferative neoplasms, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(12):1572-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mesa RA, Zimmerman C, Kang LL, et al. Proposal of an endpoint for a phase III clinical study of essential thrombocythemia: balancing between short term effects and long term benefits. J Clin Oncol. 2019;37(15 suppl):7055. [Google Scholar]
- 54.Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood. 2010;115(9):1703-1708. [DOI] [PubMed] [Google Scholar]
- 55.Gangat N, Caramazza D, Vaidya R, et al. DIPSS plus: a refined dynamic international prognostic scoring system for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29(4):392-397. [DOI] [PubMed] [Google Scholar]
- 56.Guglielmelli P, Lasho TL, Rotunno G, et al. MIPSS70: mutation-enhanced international prognostic score system for transplantation-age patients with primary myelofibrosis. J Clin Oncol. 2018;36(4):310-318. [DOI] [PubMed] [Google Scholar]
- 57.Tefferi A, Guglielmelli P, Lasho TL, et al. MIPSS70+ version 2.0: mutation and karyotype-enhanced international prognostic scoring system for primary myelofibrosis. J Clin Oncol. 2018;36(17):1769-1770. [DOI] [PubMed] [Google Scholar]
- 58.Passamonti F, Giorgino T, Mora B, et al. A clinical-molecular prognostic model to predict survival in patients with post polycythemia vera and post essential thrombocythemia myelofibrosis. Leukemia. 2017;31(12):2726-2731. [DOI] [PubMed] [Google Scholar]