Abstract
Chronic heart failure (HF) is a growing health problem, and it is associated with high morbidity and mortality. Left ventricular assist devices (LVADs) are nowadays an important treatment option for patients with end-stage HF not only as a bridging tool to heart transplantation but also, as a permanent therapy for end-stage HF (destination therapy). The use of LVAD is associated with a high risk for bleeding complications and thromboembolic events, including pump thrombosis and ischemic stroke. Bleeding is the most frequent complication, occurring in 30% to 60% of patients, both early and late after LVAD implantation. Although the design of LVADs has improved over time, bleeding complications are still the most common complication and occur very frequently. The introduction of an LVAD results in an altered hemostatic balance as a consequence of blood-pump interactions, changes in hemodynamics, acquired coagulation abnormalities, and the strict need for long-term anticoagulant treatment with oral anticoagulants and antiplatelet therapy. LVAD patients may experience an acquired coagulopathy, including platelet dysfunction and impaired von Willebrand factor activity, resulting in acquired von Willebrand syndrome. In this educational manuscript, the epidemiology, etiology, and pathophysiology of bleeding in patients with LVAD will be discussed. Because hematologist are frequently consulted in cases of bleeding problems in these individuals in a critical care setting, the observed type of bleeding complications and management strategies to treat bleeding are also reviewed.
Learning Objectives
Learn that bleeding is a frequent and severe complication after implantation of left ventricular assist devices (LVADs)
Understand that acquired von Willebrand syndrome (AVWS) is found in nearly all patients with LVAD implants and may influence bleeding episodes
Understand that recurrent gastrointestinal bleeding is frequently observed and may be caused by the combination of angiodysplasia and AVWS
Introduction
In patients admitted to the critical care unit, bleeding is a frequently encountered complication. Over the years, the causes of bleeding have changed, and nowadays, many of these bleeding episodes are observed in patients receiving new devices used to support the circulatory system or the pulmonary system, including left ventricular assist devices (LVADs) and extracorporal membrane oxygenation (ECMO). In this case-based article, the epidemiology, pathophysiology, and management of bleeding problems that are observed in patients in whom an LVAD has been implanted to improve cardiac function will be discussed.
Case 1
This is a 57-year-old male who developed cardiac failure owing to severely reduced left ventricular function after a large anterior myocardial. Four months after the infarction in 2015, he was transferred to our cardiology department, and a HeartMate II LVAD was implanted as a bridge to heart transplantation. Anticoagulation was initiated according to local protocols, including aspirin and vitamin K antagonists (acenocoumarol) (Table 1). A week after LVAD implantation, he developed regular nose bleeds, for which cautery by the otolaryngologist was performed. One month later, he was admitted with collapse, dizziness, and melena. During hospitalization, he developed hypotension and was transmitted to the intensive care unit (ICU). Endoscopy showed no bleeding focus in stomach or colon. He received several transfusions, and anticoagulation was temporarily stopped for a few days and resumed after cessation of bleeding. In the year after LVAD implantation, he was admitted 4 times with hemoglobin levels between 3.2 and 5.5 mmol/L owing to gastrointestinal (GI) bleeding, for which transfusion with packed red cells was needed. The bleeding could not be stopped by local measures because no bleeding focus was found.
Table 1.
Recommendations for the use of anticoagulation and postoperative management
| Time course and events | Management strategies |
|---|---|
| Intraoperative period | |
| If intraoperative extracorporeal life support or off-pump implantation is performed, administration of a reduced dose of heparin may be considered | |
| Early postoperative period | |
| Direct postoperative | Complete reversal of heparin |
| First 24 h | No action required, consider acetylsalicylic acid |
| Postoperative days 1 and 2 | IV heparin or alternative anticoagulation if no evidence of bleeding |
| Postoperative days 2 and 3 | Continue heparin and start warfarin and aspirin (81-325 mg daily) after removal of chest tubes; the use of LMWH for bridging during long-term support is recommended |
| During LVAD support | |
| A postoperative INR target between 2.0 and 3.0 is recommended | |
| Anticoagulation | Anticoagulation with warfarin to maintain an INR within a range as specified by each device’s manufacturer is recommended |
| Antiplatelet therapy | Chronic antiplatelet therapy with aspirin (81-325 mg daily) may be used in addition to warfarin, and additional antiplatelet therapy may be added according to the recommendations of specific device manufacturers |
| Complications | |
| Early postoperative bleeding | Urgently evaluate necessity of lowering, discontinuation, and/or reversal of anticoagulation and antiplatelet medications; in all cases of bleeding, exploration and treatment of a bleeding site should be considered |
| Gastrointestinal bleeding | Anticoagulation and antiplatelet therapy should be held in the setting of clinically significant bleeding; anticoagulation should be reversed in the setting of an elevated INR, and careful monitoring of the devices parameters is warranted |
| Neurologic event/deficit | Discontinuation or reversal of anticoagulation in the setting of hemorrhagic stroke is recommended |
| Hemolysis | Hemolysis in the presence of altered pump function should prompt admission for optimization of anticoagulation and antiplatelet management and possible pump exchange |
| Pump thrombosis | Heparin, GPIIb/IIIa inhibitors, and thrombolytics, either alone or in combination, have been proposed as treatment option for pump thrombosis; however, definitive therapy for pump stoppage is surgical pump exchange |
| Cessation of acetylsalicylic acid | After resolution of the first bleeding episode, discontinuation of long-term acetylsalicylic acid should be considered |
| DOAC | The use of novel oral anticoagulants is not recommended |
Modified from the 2013 International Society for Heart and Lung Transplantation guideline recommendations and the 2019 European Association for Cardio-Thoracic Surgery expert consensus on long-term mechanical circulatory support.
DOAC, direct oral anticoagulant; GPIIb/IIIa, glycoprotein IIb/IIIa; INR, international normalized ratio; IV, intravenous; LMWH, lower-molecular weight heparin.
This case illustrates the complicated course of recurrent GI bleeding in an LVAD recipient. In the course of this patient, the coagulation laboratory assessments performed were limited to platelet counts, international normalized ratio (INR), and activated partial thromboplastin time. Hematologists were not consulted despite the frequent bleeding episodes that required transfusion. A hematological cause (coagulopathy) for the bleeding has not been studied thus far. Fortunately, no bleeding has occurred in the last years.
Case 2
This concerns a 49-year-old male with severe heart failure (HF) after an acute myocardial infarction 16 months ago. After the myocardial infarction, he has been admitted 7 times in the last year with cardiac decompensation and HF. Seven months ago, he received a HeartMate 3 LVAD and a CardioMems device, and anticoagulants, including acenocoumarol and aspirin, were started. Three months later, he was admitted with a hemoglobin drop from 6.2 to 3.2 mmol/L. His INR was >4.2 at the time of admission, and acenocoumarol was temporarily stopped. Subsequently, the INR was targeted at 1.8 to 2.5 during admission, and he received 5 U packed red cells. In addition, he underwent endoscopy of the upper and lower GI tract. However, no bleeding source was found. Finally, aspirin was discontinued, and he was discharged (Table 1).
Laboratory assessment performed several weeks after admission revealed a platelet count of 179 × 10e9/L (normal 150-370), INR of 2.1, APPT 40 seconds (normal 22-32 seconds), fibrinogen 4.0 g/L (normal 1.5-3.6 g/L), platelet function analyzer collagen-epinephrin 286 seconds (normal 82-150 seconds), and platelet function analyzer collagen- adenosine diphosphate 165 seconds (normal 62-130 seconds). factor VIII coagulation activity (FVIII:C) was 1.22 U/mL (normal 0.60-1.40 U/mL), von Willebrand factor (VWF):antigen (Ag) was 1.57 U/mL (normal 0.60-1.40 U/mL), VWF:ristocetin cofactor activity (RCo) was 1.14 U/mL (normal 0.60-1.40 U/mL), and VWF:RCo/Ag ratio was 0.7 (normal >0.6). Multimer pattern was not assessed. Platelet aggregation studies revealed normal aggregation patterns with ADP, ristocetin, collagen, and arachidonic acid; however, they were weak and reversible with thrombin. It was concluded that he suffered from platelet dysfunction, probably related to the LVAD in situ. Despite the slightly reduced VWF:RCo/Ag ratio (0.7), an acquired von Willebrand syndrome (AVWS), which is based on a ratio <0.6, could not be confirmed. No rebleeding occurred during follow-up without aspirin and on acenocoumarol (INR target 2.0-3.0), and he is currently on the waiting list for heart transplantation.
LVADs
Chronic HF is a rising global epidemic with an increasing incidence. It is estimated that >35 million people suffer from this disease worldwide.1,2 Despite the fact that heart transplantation remains the standard therapy for patients who progress to end-stage HF, only a limited number of patients eventually receive a donor heart.
Initially, LVADs were developed as a bridge to transplantation for patients at high risk for mortality on the waiting list for transplantation. However, HF treatment changed dramatically with the introduction of the first durable mechanical circulatory support (MCS) device, the HeartMate XVE (Thoratec Corporation, Pleasanton, CA), and its approval by the Food and Drug Administration (FDA) for destination therapy in 2003.3 Since then, the use of LVADs has become a regular therapy for end-stage HF patients. Based on large registries, the survival of LVAD patients is now ∼80% at 1 year and 70% at 2 years after implantation.4,5
In conjunction with a shortage of donor hearts for transplant and the increased incidence of HF in the elderly population, nowadays thousands of LVADs are placed every year in the United States and Europe.5,6 A number of different circulatory support devices are currently in use, including axial flow and centrifugal pump devices. For detailed information on the types of devices, we refer to a manuscript in the 2018 Hematology American Society of Hematology Education Program.7 Currently, all implanted LVADs are continuous flow devices; however, the pump mechanism differs between devices, including axial or centrifugal.5 The HeartMate II (Abbott Laboratories, Chicago, IL), a continuous flow LVAD with an axial rotor and mechanical contact bearings, is still the most used device worldwide.4,8 In 2008, the HeartMate II received FDA approval for short-term MCS (bridge to transplantation), and in 2010, it was approved for long-term MCS (as destination therapy).3,4,9 The second most used devices is the HeartWare Ventricular Assist Device (HVAD; HeartWare Inc., Framingham, MA), a continuous flow LVAD with a suspended rotor, which rotates in a passive magnetic and hydrodynamic bearing that eliminates friction, heat, and component wear.4,8 The HVAD received its FDA approvals in 2012 and 2017 for short-term and long-term MCS, respectively. The HeartMate 3 (Abbott Laboratories) is a continuous flow LVAD with a centrifugal pump containing a fully magnetically levitated rotor.10 It has an intrinsic pulse wave designed to avert stasis within the pump.11 In 2017 and subsequently, 2018, the HeartMate 3 was approved for short-term and long-term MCS, respectively. Although the stroke rates were not different between the HeartMate 3 and the HeartMate II device, the HeartMate 3 has better outcomes with respect to the combined end point of disabling stroke or reoperation.12 This difference was mainly owing to the significantly lower rate of reoperation for pump malfunction in the HeartMate 3 group.
Thromboembolic complications observed in LVAD patients
In the first years of development, the main complication of LVAD implantation was thrombosis in the mechanical system, leading to embolization of clots and cerebral infarction. Therefore, strict anticoagulant regimens were developed that reduced the risk of thrombotic complications; however, this increased the risk of bleeding. The devices that have been developed in recent years, including HeartMate 3 and HVAD, develop fewer pump thrombosis episodes; however, bleeding episodes are still a main complication observed in 40% to 60% of these patients.10,12
Pump thrombosis and cerebral ischemia are the most severe thromboembolic complications in LVAD patients, with mortality rates up to 50%. Because of the increased risk for LVAD failure and subsequently, life-threatening hemodynamic impairment, cardiogenic shock, and death, immediate action is required.13 The definitive treatment of pump thrombosis is pump exchange or urgent transplant if possible.14 However, glycoprotein (GP) inhibitors or unfractionated heparin could be used as medical therapy for pump thrombosis in selected cases.13 Lactate dehydrogenase increase is regarded as an early sign of thrombosis, and it is highly likely to predict a thrombotic complication, especially within the LVAD device or the system.15 Fortunately, the risk of pump thrombosis is very low with the newest LVAD systems.12 Compared with the HeartMate II, the reported incidence of pump thrombosis is substantially lower for the HeartMate 3 LVAD.16 Although not directly compared, the incidence rates of suspected and confirmed pump thrombosis at 2 years were 1.4% in the HeartMate 3 clinical trial and 6.4% in the HVAD trial.10,16
Both ischemic strokes and (subsequent) intracranial hemorrhages are neurological events occurring in LVAD patients. The neurologic event rate in a recent report including >18,000 LVADs was 13% in axial flow LVADs and 20% in centrifugal flow LVADs at 1-year post-LVAD implantation.5 In addition, the incidence also varies with the device implanted, with higher incidence in the HeartWare HVAD compared with the HeartMate II device.10,17,18 Although less frequently observed compared with other bleeding events, the outcome after a neurologic event can be devastating. In addition, the risk for a stroke or transient ischemic attack (TIA) is higher in patients recently implanted with the centrifugal flow devices compared with the older devices.5 Furthermore, neurologic events are the leading cause of death, with 20% of patients having a neurologic event as a primary cause of death.4,5,9,19 In addition, intracranial hemorrhages is associated with a higher (up to 5-fold increase) risk of mortality compared with ischemic stroke.20,21
What is the recommended anticoagulant treatment of LVAD patients?
Current regimens for anticoagulant therapy after LVAD implantation are based on the 2013 International Society for Heart and Lung transplantation guidelines reviewed by Muslem et al.22 In addition, a recent expert consensus on long-term MCS has been published.14 A summary of the recommendations regarding anticoagulation management is presented in Table 1. In brief, before LVAD implantation, normalization of coagulation is required to avoid postoperative complications, including bleeding, need of transfusion, and right HF. In addition, intraoperatively full anticoagulation is recommended, with full reversal and restoration of blood components and coagulation factors at the end, except for off-pump implantation or ECMO support.23 At day 1 after implantation, anticoagulation should be initiated with unfractionated heparin if there is no evidence of active bleeding. The target activated partial thromboplastin time is usually 40 to 50 seconds and can be increased to 50 to 60 seconds on days 2 to 3 if there is no evidence of bleeding.10 On days 2 to 3 after surgery, warfarin or another vitamin K antagonist is started and aimed at a target INR of >2.0 or >2.5, within a range that is specified by each manufacturer (summarized in ref. 22). Heparin is discontinued after a few days only if the INR is within the target range. Aspirin is started at a dose 81 to 325 mg/d on the day of surgery or on days 1 to 3 depending on the LVAD device that is implanted. Long-term anticoagulation with vitamin K antagonist is required for all LVAD devices, with a device-dependent INR target.22,24 For the most frequently implanted devices (HeartMate II, HeartMate 3, and HVAD), an INR between 2.0 and 3.0 is advised. For smaller devices, such as the HeartWare CircuLite (HeartWare Inc., Framingham, MA; advised INR of 2.5-3.0), MicroMed DeBakey (MicroMed Technology, Inc., Houston, TX; advised INR 2.5-3.5), and the Jarvik 2000 (Jarvik Heart Inc., New York, NY; advised INR 2.5-3.5), higher INR levels are advised. Additional antiplatelet therapy with clopidogrel may be initiated because of the possibility of thrombosis, shear-induced platelet dysfunction, and hemolysis. Activation of platelets has been observed in patients with MCS and may underline the ongoing increased risk of thromboembolic events.25 In the case of subtherapeutic INR, patients can be treated with additional low-molecular weight heparin temporarily to reduce the risk of thrombosis.26 The above-mentioned anticoagulant guidelines are strongly dependent on the LVAD implanted, and it is recommended to follow the instructions by the manufacturer of the implanted device.10
The use of direct oral anticoagulants in LVAD patients is not recommended because of the increased risk of thromboembolic events. In a recent small, prospective, randomized, open-label, single-center study on the use of dabigatran, a direct oral thrombin inhibitor, vs phenprocoumon after LVAD implantation, a high rate of thromboembolic events on dabigatran was observed (4 of 8 patients experienced thrombosis, of whom 3 had pump thrombosis and 1 had a TIA) and led to early termination of the trial after inclusion of 16 patients.27 These findings are in line with the RE-ALIGN study, a randomized, phase 2 study to evaluate dabigatran compared with warfarin in patients after mechanical heart valve replacement. This study was also stopped prematurely because of excess bleeding and thrombotic events in the dabigatran-treated group.28
What kind of bleeding complications occur in LVAD patients?
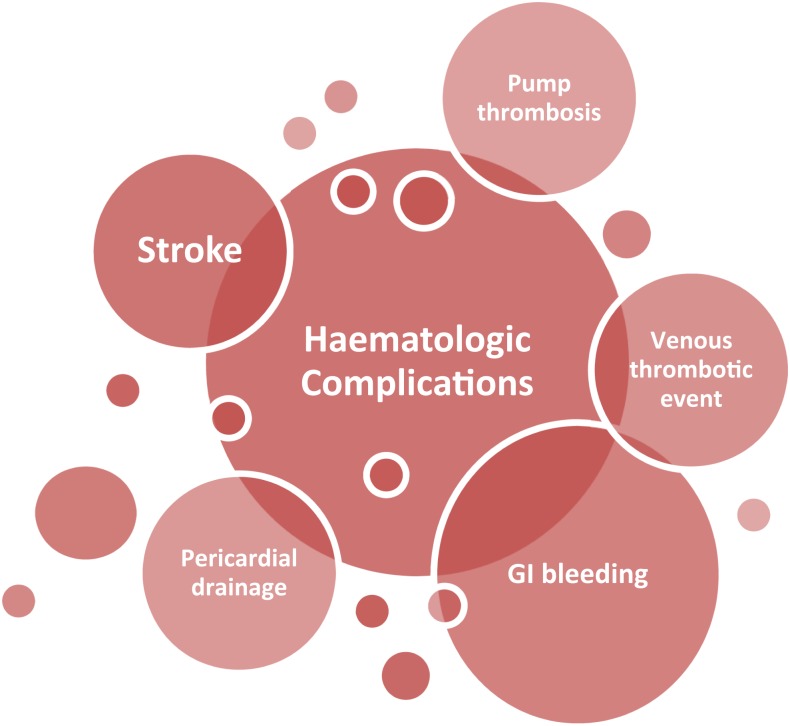
Bleeding is the most common complication in LVAD patients, with an incidence ranging between 20% and 60%, and it is associated with high morbidity and mortality.29,30 Early bleeding is mainly surgery related and may be persistent wound bleeding or bleeding from drains or cardiac tamponade. It is associated with a nearly 20% increased risk for mortality.31 Late bleeding events are nonsurgical bleeding events, mainly GI bleeding or epistaxis.32,33 In addition, patients who experience GI bleeding have a high risk for rebleeding events, requiring more frequent readmissions, and they develop more frequent nondevice-related infections (Figure 1).32,34
Figure 1.
Hematological complications after LVAD implantation.
Bleeding shortly after LVAD implantation
As with other surgical procedures, bleeding may complicate the implantation of an LVAD. Patients are at high risk of major bleeding events in the postoperative period, which leads to a surgical re-exploration in 20% to 30% of the patients.35,36 In a recent retrospective study in our center, we observed an early bleeding event in 47% of patients in a cohort of 83 patients after LVAD implantation.31 Another recent single-center study by Angleitner et al37 showed that 14% of patients in their center underwent a surgical revision for bleeding after LVAD implantation. Patients undergoing a bleeding revision had significantly reduced survival and increased morbidity during LVAD support, including stroke. They concluded that an altered hematological state, including thrombocytopenia and an increased mechanical disruption of VWF multimers, was the major mechanism underlying mediastinal bleeding. In addition, bleeding at the time of revision was diffuse, and in only a minority of the cases of bleeding, a surgical cause, mainly bleeding from the LVAD outflow graft anastomosis or thoracic wall, was identified. In addition, thrombocytopenia was found often in patients before bleeding revision.37 This was also found by our group in a larger number of patients, in whom platelet counts <150 × 10e9/L were associated with a nearly 5-fold increased risk of an early bleeding event.31 In the large randomized trial by Rogers et al10 that compared the HeartWare HVAD with a control device, a bleeding rate of 60% was found. In the HVAD group, 15% of all patients needed reintervention because of bleeding, and also, 15% needed blood transfusion of ≥4 packed cells within 1 week after implantation.10 This emphasizes the need for close monitoring of the hematological and hemodynamic conditions of the patient during the postoperative readmission at the ICU.
GI bleeding
GI bleeding is a frequently occurring and concerning complication in patients supported with an LVAD. In addition, a significant increase in the event rate of GI bleeding has been observed in patients with a continuous flow (CF)-LVAD compared with pulsatile devices.38-40 The pathophysiology remains partly understood, and the treatment is frequently unsuccessful. GI bleeding episodes can occur at any time point after LVAD implantation, with a mean time to bleeding ranging from 10 to 154 days.38,41 In epidemiological studies, GI bleeding occurs in up to 60% of patients with an LVAD.42,43 In the recent large, randomized, controlled trial on HeartMate 3, GI bleeds still occurred in 35% of patients, which was similar to the observed bleeding in the control device groups. This illustrates that, despite differences in device design, GI bleeding remains a common and important bleeding problem.12 GI bleeding is the most common cause for hospital readmissions (23% of the causes of 30-day readmission), and it is associated with morbidity and increased health care costs.44 The pooled event rate for all-cause mortality in patients with GI bleeding is 23% (95% confidence interval, 16%-32%). Nonetheless, despite the severe burden for the patients, GI bleeding does not impact the survival of LVAD patients.38,43,45
GI bleeds are associated with a longer support time, which makes it one of the most important and recurrent complications in LVAD patients. In a recently reported large cohort of 526 LVAD patients, 140 (27%) had a GI bleeding event, of whom 34% had recurrent bleeding.45 Bleeding occurred evenly from the upper and lower GI tracts. The most frequently observed etiology was an arteriovenous malformation (50 of 72 events) occurring 300 days after the implantation. However, in a large proportion of patients, no bleeding source could be detected by endoscopy.
Which factors may contribute to bleeding in these patients?
As mentioned above, the factors that contribute to bleeding are strongly dependent on the type of bleeding concerned. Postsurgical bleeding may be related to the preoperative condition of the patients, the surgical procedure itself, and the initiation of anticoagulant and antiplatelet therapy. There are various underlying mechanisms that contribute to the risk of the above-mentioned hematological complications, including device hemocompatibility, GI angiodysplasia, vascular malformations, and acquired coagulopathies, such as von Willebrand disease (VWD) or platelet dysfunction.32,46-49 It may also be owing to the presence of thrombocytopenia and comorbidity, including renal failure, further impairing platelet function, and previous need for ECMO support pre-LVAD implantation. This may represent a subset of patients in a poor condition who require carefully monitoring during the initial admission at the ICU postimplantation.31 Also, systemic infections do occur, leading to coagulation activation and disseminated intravascular coagulation. An overview of the factors associated with the risk of bleeding is presented in Table 2.
Table 2.
Factors that are associated with bleeding in LVAD patients
| Factors |
|---|
| Use of anticoagulant drugs |
| Unfractionated heparin, low-molecular weight heparin, vitamin K antagonists |
| Antiplatelet drugs |
| Aspirin, clopidogrel |
| Platelet dysfunction |
| Thrombocytopenia |
| Acquired von Willebrand syndrome |
| Arteriovenous malformations/angiodysplasia |
| Impaired renal function |
| Infectious complications (disseminated intravascular coagulation) |
| ECMO support pre-LVAD implantation |
What kind of hemostatic abnormalities are found in these patients?
AVWS
VWF is a multimeric large GP with various domains that have specific functions (eg, binding of factor VIII, GPIbα, and GPIIb/IIIa on platelets and binding of collagen).50-52 The size of the multimers strongly determines the hemostatic function of VWF. Ultralarge VWF multimers have the most procoagulant activity.53 VWF is mainly synthesized and secreted by endothelial cells, especially at sites of high shear rate. VWF mediates platelet adhesion and aggregation, thereby forming a platelet plug and achieving hemostasis.52 By being a carrier protein for factor VIII, VWF protects factor VIII from proteolytic degradation.52,54 Therefore, most patients with severe VWF deficiency also have low FVIII levels contributing to the bleeding phenotype.55 The procoagulant activity of VWF is mainly mediated by the VWF cleaving protease A Disintegrin and Metalloprotease with Thrombospondin type 1 repeat 13 (ADAMTS13).56 This metalloprotease has as its only known function the cleavage of hemostatically very active (ultra-)large molecular multimers into less prothrombotic smaller multimers. Already in 1958, Heyde57 noted the clinical association between aortic stenosis, intestinal angiodysplasia, and GI bleeding.57 Decades later, this observation was attributed to the fact that patients with aortic stenosis have an acquired reduced VWF activity because of the lack of high-molecular weight multimers: AVWS.58 Later, Sadler59 suggested that AVWS was owing to cleavage of uncoiled VWF, thereby exposing the VWF cleaving site in the A1 domain by ADAMTS13. The relationship with angiodysplasia was established by showing that VWF has antiangiogenic properties.60 Reduction of high molecular multimers of VWF in the circulation, both inherited or acquired, results in increased angiogenesis by upregulation of vascular endothelial growth factor (VEGF) and thereby, VEGF-dependent vascular proliferation and formation of angiodysplasia in the intestinal mucosa.61-63 Because of the hemostatic dysfunction and the occurrence of angiodysplasia, patients may develop recurrent GI bleeding. The first description of AVWS in LVAD patients was by Geisen et al,64 who described LVAD patients with nonsurgical bleeding who had a qualitatively abnormal VWF. Despite an increase of both VWF:Ag and VWF:RCo, the ratio VWF:RCo/VWF:Ag was strongly reduced because of the loss of large VWF multimers, indicative of AVWS. Because of the high shear stress in LVAD, VWF, which is (under normal conditions) circulating in a coiled conformation, uncoils, by which the A1 and A2 domains becomes exposed for cleavage by ADAMTS13.64-67 The shear stress in LVAD patients is a consequence of the continuous flow pump design and cardiac conditions.58,65,66 In the laboratory, this can easily be detected by an abnormal VWF:RCo/VWF:Ag ratio (<0.6). However, because of HF, endothelial dysfunction, and the presence of shear stress, the concentration of VWF (VWF:Ag) may even be above the normal range, which may also be the case for VWF:RCo. Therefore, the reduced VWF:RCo/VWF:Ag ratio has been hypothesized to be associated with a strongly decreased hemostatic efficacy of VWF and subsequently, may lead to bleeding problems. However, an association has not been found between the level of VWF:Ag or VWF:RCo and bleeding frequency. This is probably because of the fact that almost all patients with an LVAD experienced AVWS, yet only 60% had a bleeding event.4,38 Of importance, a recent study by Bansal et al68 showed that the HeartMate 3 patients exhibited significantly greater preservation of the VWF large multimers ratio than those supported with the HeartMate II device at 90 days after implantation. The other laboratory values for all VWF assays did not differ significantly between the devices at 90 days after implantation. In addition, this was the first study to show an association between lower VWF large multimers and risk of bleeding. Laboratory evaluation of VWF multimers consists of agarose gel electrophoresis, which shows a loss of the large multimers.69 Also, a reduced binding of VWF to collagen (measured by VWF:collagen binding [CB] activity) is present as is a reduced VWF:CB/VWF:Ag ratio.49,70 The reduced VWF:RCo activity is already observed early in the postoperative period after LVAD implantation. It persists during LVAD support and resolves after the LVAD has been explanted.70-73 In addition, binding of VWF multimers to platelets also leads to an increased proteolytic cleavage by ADAMTS13.74 The loss of VWF activity occurs in >90% of all LVAD patients and nearly, if not, all CF-LVAD types.67,70,75
Platelet dysfunction
Thrombocytopenia is frequently observed in cardiac surgery because of consumption of platelets. On LVAD implantation, platelets are activated through increased shear stress, hemolysis, and contact with foreign bodies.76,77 Activated platelets may bind to VWF or fibrinogen on the surface of the pump that is exposed to blood. This may lead to platelet aggregation and formation of a platelet plug, which eventually may grow and lead to occlusion of the device.76
However, platelets dysfunction may increase the risk of bleeding events in patients supported with an LVAD.48,78-80 Several studies have shown that platelet aggregation is decreased in the majority of LVAD patients.48,80
The use of anticoagulant treatment, AVWS, and platelet dysfunction contribute to the risk for bleeding in LVAD patients and require different management strategies to prevent and reduce bleeding complications.
What laboratory diagnostics are helpful in determining the cause of bleeding?
Screening tests for primary hemostasis function, using PFA-200 (Siemens AG) or Multiplate (Roche Diagnostics International), are not very informative of the cause of primary hemostasis dysfunction due to the combined platelet dysfunction, the reduced VWF:RCo activity, and the (double-)antiplatelet therapy given to these patients.81 More specific platelet function tests, including platelet aggregation tests using various agonists, are cumbersome and time consuming, and they may reveal limited information. Platelet aggregation will be disturbed by thrombocytopenia because of the use of antiplatelet drugs and the activation of platelets that induce an acquired storage pool disease.
VWF testing should be performed, including VWF:Ag, VWF:RCo activity, VWF:CB activity, VWF:RCo/VWF:Ag ratio, and FVIII:C. In addition, VWF multimer patterns should be determined by agarose gel electrophoresis and densitometry to quantify VWF multimers.82 One should be aware that, despite (high) normal VWF:RCo or VWF:Ag, the functional activity of VWF may be reduced as can be established by calculating the ratio between VWF:RCo and VWF:Ag.
Thromboelastometry (ROTEM Tem International, Munich, Germany) or thomboelastography may provide more insight into the global hemostatic capacity. Studies regarding risk prediction for bleeding and thrombosis in LVAD patients based on these global hemostasis tests are limited and so far, have not been successful.83,84
Therefore, we suggest, based on the recent findings of Bansal et al68 in addition to other case series showing that VWF parameters may be predictive of bleeding, to measure VWF:Ag and VWF:RCo to calculate the VWF:RCo/VWF:Ag ratio to assess the presence of AVWS. In addition, INR should be performed to assess whether vitamin k antagonist reversal is needed.
How do we manage bleeding in patients with LVADs?
Interestingly, despite the fact that nearly all patients with LVADs develop AVWS, not all of them experience bleeding complications.69 It has been suggested that there is a 2-hit model requiring additional abnormalities for arteriovascular malformations to become symptomatic and result in bleeding. This second hit could be sepsis, gut hypoxia, or high levels of angiopoietin-2.69 Unfortunately, only limited longitudinal studies on the treatment of bleeding in LVAD patients have been performed. No randomized, controlled trials have been performed to study the optimal treatment options in case of bleeding. Desmopressin (DDAVP), which is frequently used to treat bleeding in VWD, has only sporadically been reported in LVAD patients. This may be because of the fact that DDAVP is contraindicated in patients with cardiovascular disease or because of the fact that it is not useful given the rapid degradation of the secreted VWF. AVWS can be temporarily treated by the infusion of plasma-derived VWF or FVIII/VWF concentrate.85,86 It has been suggested that the lack of high-molecular weight multimers of VWF is a risk factor for bleeding, although evidence is limited.68,87 However, because of the LVAD in situ, the exogenous VWF will also be degraded, and it will have a short half-life. Only 1 patient has been reported who was treated successfully for intractable GI bleeding for a period of several weeks with pure VWF concentrate 2 to 3 times per week to prevent new bleeding episodes.88 Therefore, long-term prophylaxis of patients with recurrent bleeding associated with LVAD-induced AVWS is not yet recommended.
Inhibition of VWF proteolysis by ADAMTS13 may represent a novel treatment option. An ex vivo study using whole blood exposed to LVAD-like supraphysiological shear stress revealed that inhibition of ADAMTS13 by doxycycline decreased VWF proteolysis and improved VWF function.89 However, this has not yet been applied in LVAD patients and needs additional clinical investigations.
In the case of GI bleeding, endoscopy of both the upper and lower GI tract should be performed, because the locations of GI bleeds are equally distributed over the lower and upper GI tract.34 Actively bleeding vascular malformations can be clipped or treated with argon plasma coagulation. However, in a considerable number of LVAD patients, no actively angiodysplastic bleeding lesions can be found on endoscopy. Furthermore, it has been suggested that omega-3 possesses anti-inflammatory and antiangiogenic properties and that it was associated with significantly lower GI bleeding at 1 year in a retrospective study.90 Patients receiving 4 g/d omega-3 therapy for nearly a year had a 1-year GI bleeding free rate of 97% vs 73%, compared with patients in the control group. Future prospective, randomized, controlled trials have yet to confirm this observation.
In patients with severe bleeding complications, anticoagulant therapy should be adjusted or discontinued. Vitamin K antagonists can be immediately reversed by administration of prothrombincomplex concentrate.91 Only a very small group of patients has been described in the literature with permanent cessation of anticoagulation (vitamin K antagonist) because of recurrent bleeding events after LVAD implantation. More importantly, all of these patients had impaired platelet function and AVWS.92 Unfractionated heparin or low molecular heparin may be temporarily disrupted or in the case of severe bleeding, counteracted by protamine sulfate; however, as soon as the bleeding is stopped and controlled, the anticoagulants should be resumed to prevent thrombotic complications. It has been shown that patients with GI bleeding are also at higher risk for thromboembolic events, including pump thrombosis and ischemic stroke, because of adjustment or interruption of the anticoagulant regimen.93 In the case of mucocutaneous bleeding, tranexamic acid could be added to the treatment (orally 1 g 3 or 4 times daily). The LVAD-associated hemostatic abnormalities, including platelet dysfunction and thrombocytopenia, can be treated with platelet transfusion.
Several case series have been reported in which GI bleeding associated with angiodysplasia in patients suffering from hereditary VWD were treated by antiangiogenic drugs, including octreotide, thalidomide, and statins.94 Despite the fact that, through the antiangiogenic pathway, thalidomide and atorvastatin reduce the rate of bleeding from arterio venous malformations and the transfusion need in VWD patients, these have only been used sporadically in LVAD patients.95 Large prospective, randomized trials should be performed to define its role in preventing LVAD-associated bleeding complications. In addition, these agents are not registered for use in LVAD patients.
Conclusion
LVADs have a major positive impact on the life expectancy and quality of life of patients with end-stage left ventricular failure. Despite these improvements, several important complications may occur after LVAD implantation, including bleeding and thromboembolic events. These bleeding episodes are major complications occurring in the majority of LVAD patients both immediately after implantation and in the long term. Acquired coagulopathies are seen in nearly all LVAD patients. Because not all patients with these laboratory abnormalities experience a bleeding episode, a definite association with bleeding events has yet to be established. AVWS is diagnosed using specific VWF assays; however, it is mainly characterized by a low VWF:RCo/VWF:Ag ratio and lack of high-molecular weight VWF multimers. Because the bleeding frequency remains high, despite improvements of LVAD systems, additional studies are needed on prevention and management of bleeding complications in these patients.
Correspondence
F. W. G. Leebeek, Department of Hematology, Room Na822, Erasmus University Medical Center Rotterdam, Doctor Molewaterplein 40, 3015 GD Rotterdam, The Netherlands; e-mail: f.leebeek@erasmusmc.nl.
References
- 1.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8(1):30-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13(6):368-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rose EA, Gelijns AC, Moskowitz AJ, et al. ; Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345(20):1435-1443. [DOI] [PubMed] [Google Scholar]
- 4.Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34(12):1495-1504. [DOI] [PubMed] [Google Scholar]
- 5.Kormos RL, Cowger J, Pagani FD, et al. The Society of Thoracic Surgeons Intermacs Database annual report: evolving indications, outcomes, and scientific partnerships. Ann Thorac Surg. 2019;107(2):341-353. [DOI] [PubMed] [Google Scholar]
- 6.de By TMMH, Mohacsi P, Gahl B, et al. ; EUROMACS members. The European Registry for Patients with Mechanical Circulatory Support (EUROMACS) of the European Association for Cardio-Thoracic Surgery (EACTS): second report. Eur J Cardiothorac Surg. 2018;53(2):309-316. [DOI] [PubMed] [Google Scholar]
- 7.Kreuziger LB, Massicotte MP. Adult and pediatric mechanical circulation: a guide for the hematologist. Hematology Am Soc Hematol Educ Program. 2018;2018:507-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de By TM, Mohacsi P, Gummert J, et al. ; EUROMACS members. The European Registry for Patients with Mechanical Circulatory Support (EUROMACS): first annual report. Eur J Cardiothorac Surg. 2015;47(5):770-776. [DOI] [PubMed] [Google Scholar]
- 9.Slaughter MS, Rogers JG, Milano CA, et al. ; HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241-2251. [DOI] [PubMed] [Google Scholar]
- 10.Rogers JG, Pagani FD, Tatooles AJ, et al. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med. 2017;376(5):451-460. [DOI] [PubMed] [Google Scholar]
- 11.Netuka I, Sood P, Pya Y, et al. Fully magnetically levitated left ventricular assist system for treating advanced HF: a multicenter study. J Am Coll Cardiol. 2015;66(23):2579-2589. [DOI] [PubMed] [Google Scholar]
- 12.Mehra MR, Uriel N, Naka Y, et al. ; MOMENTUM 3 Investigators. A fully magnetically levitated left ventricular assist device - final report. N Engl J Med. 2019;380(17):1618-1627. [DOI] [PubMed] [Google Scholar]
- 13.Luc JGY, Tchantchaleishvili V, Phan K, Dunlay SM, Maltais S, Stulak JM. Medical therapy as compared to surgical device exchange for left ventricular assist device thrombosis: a systematic review and meta-analysis. ASAIO J. 2019;65(4):307-317. [DOI] [PubMed] [Google Scholar]
- 14.Potapov EV, Antonides C, Crespo-Leiro MG, et al. 2019 EACTS Expert Consensus on long-term mechanical circulatory support. Eur J Cardiothorac Surg. 2019;56(2):230-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowger JA, Romano MA, Shah P, et al. Hemolysis: a harbinger of adverse outcome after left ventricular assist device implant. J Heart Lung Transplant. 2014;33(1):35-43. [DOI] [PubMed] [Google Scholar]
- 16.Mehra MR, Goldstein DJ, Uriel N, et al. ; MOMENTUM 3 Investigators. Two-year outcomes with a magnetically levitated cardiac pump in heart failure. N Engl J Med. 2018;378(15):1386-1395. [DOI] [PubMed] [Google Scholar]
- 17.Lalonde SD, Alba AC, Rigobon A, et al. Clinical differences between continuous flow ventricular assist devices: a comparison between HeartMate II and HeartWare HVAD. J Card Surg. 2013;28(5):604-610. [DOI] [PubMed] [Google Scholar]
- 18.Willey JZ, Gavalas MV, Trinh PN, et al. Outcomes after stroke complicating left ventricular assist device. J Heart Lung Transplant. 2016;35(8):1003-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirklin JK, Naftel DC, Stevenson LW, et al. INTERMACS database for durable devices for circulatory support: first annual report. J Heart Lung Transplant. 2008;27(10):1065-1072. [DOI] [PubMed] [Google Scholar]
- 20.Cho SM, Moazami N, Frontera JA. Stroke and intracranial hemorrhage in HeartMate II and HeartWare left ventricular assist devices: a systematic review. Neurocrit Care. 2017;27(1):17-25. [DOI] [PubMed] [Google Scholar]
- 21.Shahreyar M, Bob-Manuel T, Khouzam RN, et al. Trends, predictors and outcomes of ischemic stroke and intracranial hemorrhage in patients with a left ventricular assist device. Ann Transl Med. 2018;6(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muslem R, Caliskan K, Leebeek FWG. Acquired coagulopathy in patients with left ventricular assist devices. J Thromb Haemost. 2018;16(3):429-440. [DOI] [PubMed] [Google Scholar]
- 23.Pagano D, Milojevic M, Meesters MI, et al. 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53(1):79-111. [DOI] [PubMed] [Google Scholar]
- 24.Sandner SE, Riebandt J, Haberl T, et al. Low-molecular-weight heparin for anti-coagulation after left ventricular assist device implantation. J Heart Lung Transplant. 2014;33(1):88-93. [DOI] [PubMed] [Google Scholar]
- 25.Matsubayashi H, Fastenau DR, McIntyre JA. Changes in platelet activation associated with left ventricular assist system placement. J Heart Lung Transplant. 2000;19(5):462-468. [DOI] [PubMed] [Google Scholar]
- 26.Cook JL, Colvin M, Francis GS, et al. ; American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Radiology and Intervention; and Council on Cardiovascular Surgery and Anesthesia. Recommendations for the use of mechanical circulatory support: ambulatory and community patient care: a scientific statement from the American Heart Association. Circulation. 2017;135(25):e1145-e1158. [DOI] [PubMed] [Google Scholar]
- 27.Andreas M, Moayedifar R, Wieselthaler G, et al. Increased Thromboembolic events with dabigatran compared with vitamin K antagonism in left ventricular assist device patients: a randomized controlled pilot trial. Circ Heart Fail. 2017;10(5):e003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eikelboom JW, Connolly SJ, Brueckmann M, et al. ; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369(13):1206-1214. [DOI] [PubMed] [Google Scholar]
- 29.Kirklin JK, Naftel DC, Kormos RL, et al. Third INTERMACS Annual Report: the evolution of destination therapy in the United States. J Heart Lung Transplant. 2011;30(2):115-123. [DOI] [PubMed] [Google Scholar]
- 30.Jabbar HR, Abbas A, Ahmed M, et al. The incidence, predictors and outcomes of gastrointestinal bleeding in patients with left ventricular assist device (LVAD). Dig Dis Sci. 2015;60(12):3697-3706. [DOI] [PubMed] [Google Scholar]
- 31.Muslem R, Caliskan K, van Thiel R, et al. Incidence, predictors and clinical outcome of early bleeding events in patients undergoing a left ventricular assist device implant. Eur J Cardiothorac Surg. 2018;54(1):176-182. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein DJ, Aaronson KD, Tatooles AJ, et al. ; ADVANCE Investigators. Gastrointestinal bleeding in recipients of the HeartWare Ventricular Assist System. JACC Heart Fail. 2015;3(4):303-313. [DOI] [PubMed] [Google Scholar]
- 33.Genovese EA, Dew MA, Teuteberg JJ, et al. Incidence and patterns of adverse event onset during the first 60 days after ventricular assist device implantation. Ann Thorac Surg. 2009;88(4):1162-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welden CV, Truss W, McGwin G, Weber F, Peter S. Clinical predictors for repeat hospitalizations in left ventricular assist device (LVAD) patients with gastrointestinal bleeding. Gastroenterol Res. 2018;11(2):100-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller LW, Pagani FD, Russell SD, et al. ; HeartMate II Clinical Investigators. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357(9):885-896. [DOI] [PubMed] [Google Scholar]
- 36.Schaffer JM, Arnaoutakis GJ, Allen JG, et al. Bleeding complications and blood product utilization with left ventricular assist device implantation. Ann Thorac Surg. 2011;91(3):740-747. [DOI] [PubMed] [Google Scholar]
- 37.Angleitner P, Simon P, Kaider A, et al. Impact of bleeding revision on outcomes after left ventricular assist device implantation. Ann Thorac Surg. 2019;108(2):517-523. [DOI] [PubMed] [Google Scholar]
- 38.Draper KV, Huang RJ, Gerson LB. GI bleeding in patients with continuous-flow left ventricular assist devices: a systematic review and meta-analysis. Gastrointest Endosc. 2014;80(3):435-446.e1. [DOI] [PubMed] [Google Scholar]
- 39.Crow S, John R, Boyle A, et al. Gastrointestinal bleeding rates in recipients of nonpulsatile and pulsatile left ventricular assist devices. J Thorac Cardiovasc Surg. 2009;137(1):208-215. [DOI] [PubMed] [Google Scholar]
- 40.Islam S, Cevik C, Madonna R, et al. Left ventricular assist devices and gastrointestinal bleeding: a narrative review of case reports and case series. Clin Cardiol. 2013;36(4):190-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nascimbene A, Neelamegham S, Frazier OH, Moake JL, Dong JF. Acquired von Willebrand syndrome associated with left ventricular assist device. Blood. 2016;127(25):3133-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stern DR, Kazam J, Edwards P, et al. Increased incidence of gastrointestinal bleeding following implantation of the HeartMate II LVAD. J Card Surg. 2010;25(3):352-356. [DOI] [PubMed] [Google Scholar]
- 43.Kataria R, Jorde UP. GI bleeding during CF-LVAD support: state of the field [published online ahead of print 9 May 2018]. Cardiol Rev. doi:10.1097/CRD.0000000000000212. [Google Scholar]
- 44.Akhter SA, Badami A, Murray M, et al. Hospital readmissions after continuous-flow left ventricular assist device implantation: incidence, causes, and cost analysis. Ann Thorac Surg. 2015;100(3):884-889. [DOI] [PubMed] [Google Scholar]
- 45.Kawabori M, Kurihara C, Critsinelis AC, et al. Gastrointestinal bleeding after HeartMate II or HVAD implantation: incidence, location, etiology, and effect on survival [published online ahead of print 4 April 2019]. ASAIO J. doi:10.1097/MAT.0000000000000998. [DOI] [PubMed] [Google Scholar]
- 46.Crow S, Chen D, Milano C, et al. Acquired von Willebrand syndrome in continuous-flow ventricular assist device recipients. Ann Thorac Surg. 2010;90(4):1263-1269, discussion 1269. [DOI] [PubMed] [Google Scholar]
- 47.Demirozu ZT, Radovancevic R, Hochman LF, et al. Arteriovenous malformation and gastrointestinal bleeding in patients with the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2011;30(8):849-853. [DOI] [PubMed] [Google Scholar]
- 48.Steinlechner B, Dworschak M, Birkenberg B, et al. Platelet dysfunction in outpatients with left ventricular assist devices. Ann Thorac Surg. 2009;87(1):131-137. [DOI] [PubMed] [Google Scholar]
- 49.Uriel N, Pak SW, Jorde UP, et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol. 2010;56(15):1207-1213. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi Y, Kalafatis M, Girma JP, Sewerin K, Andersson LO, Meyer D. Localization of a factor VIII binding domain on a 34 kilodalton fragment of the N-terminal portion of von Willebrand factor. Blood. 1987;70(5):1679-1682. [PubMed] [Google Scholar]
- 51.Tsai HM. Physiologic cleavage of von Willebrand factor by a plasma protease is dependent on its conformation and requires calcium ion. Blood. 1996;87(10):4235-4244. [PubMed] [Google Scholar]
- 52.Springer TA. von Willebrand factor, Jedi knight of the bloodstream. Blood. 2014;124(9):1412-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Budde U, Pieconka A, Will K, Schneppenheim R. Laboratory testing for von Willebrand disease: contribution of multimer analysis to diagnosis and classification. Semin Thromb Hemost. 2006;32(5):514-521. [DOI] [PubMed] [Google Scholar]
- 54.Choi H, Aboulfatova K, Pownall HJ, Cook R, Dong JF. Shear-induced disulfide bond formation regulates adhesion activity of von Willebrand factor. J Biol Chem. 2007;282(49):35604-35611. [DOI] [PubMed] [Google Scholar]
- 55.Leebeek FW, Eikenboom JC. von Willebrand’s disease. N Engl J Med. 2016;375(21):2067-2080. [DOI] [PubMed] [Google Scholar]
- 56.Dong JF, Moake JL, Nolasco L, et al. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100(12):4033-4039. [DOI] [PubMed] [Google Scholar]
- 57.Heyde E. Gastrointestinal bleeding in aortic stenosis. N Engl J Med. 1958;259(4):196. [Google Scholar]
- 58.Warkentin TE, Moore JC, Morgan DG. Aortic stenosis and bleeding gastrointestinal angiodysplasia: is acquired von Willebrand’s disease the link? Lancet. 1992;340(8810):35-37. [DOI] [PubMed] [Google Scholar]
- 59.Sadler JE. Aortic stenosis, von Willebrand factor, and bleeding. N Engl J Med. 2003;349(4):323-325. [DOI] [PubMed] [Google Scholar]
- 60.Starke RD, Ferraro F, Paschalaki KE, et al. Endothelial von Willebrand factor regulates angiogenesis. Blood. 2011;117(3):1071-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lenting PJ, Casari C, Christophe OD, Denis CV. von Willebrand factor: the old, the new and the unknown. J Thromb Haemost. 2012;10(12):2428-2437. [DOI] [PubMed] [Google Scholar]
- 62.Randi AM, Laffan MA. Von Willebrand factor and angiogenesis: basic and applied issues. J Thromb Haemost. 2017;15(1):13-20. [DOI] [PubMed] [Google Scholar]
- 63.Groeneveld DJ, Sanders YV, Adelmeijer J, et al. Circulating angiogenic mediators in patients with moderate and severe von Willebrand disease: a multicentre cross-sectional study. Thromb Haemost. 2018;118(1):152-160. [DOI] [PubMed] [Google Scholar]
- 64.Geisen U, Heilmann C, Beyersdorf F, et al. Non-surgical bleeding in patients with ventricular assist devices could be explained by acquired von Willebrand disease. Eur J Cardiothorac Surg. 2008;33(4):679-684. [DOI] [PubMed] [Google Scholar]
- 65.Loscalzo J. From clinical observation to mechanism--Heyde’s syndrome. N Engl J Med. 2012;367(20):1954-1956. [DOI] [PubMed] [Google Scholar]
- 66.Le Tourneau T, Susen S, Caron C, et al. Functional impairment of von Willebrand factor in hypertrophic cardiomyopathy: relation to rest and exercise obstruction. Circulation. 2008;118(15):1550-1557. [DOI] [PubMed] [Google Scholar]
- 67.Netuka I, Kvasnička T, Kvasnička J, et al. Evaluation of von Willebrand factor with a fully magnetically levitated centrifugal continuous-flow left ventricular assist device in advanced heart failure. J Heart Lung Transplant. 2016;35(7):860-867. [DOI] [PubMed] [Google Scholar]
- 68.Bansal A, Uriel N, Colombo PC, et al. Effects of a fully magnetically levitated centrifugal-flow or axial-flow left ventricular assist device on von Willebrand factor: a prospective multicenter clinical trial. J Heart Lung Transplant. 2019;38(8):806-816. [DOI] [PubMed] [Google Scholar]
- 69.Rauch A, Susen S, Zieger B. Acquired von Willebrand syndrome in patients with ventricular assist device. Front Med (Lausanne). 2019;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heilmann C, Geisen U, Beyersdorf F, et al. Acquired von Willebrand syndrome is an early-onset problem in ventricular assist device patients. Eur J Cardiothorac Surg. 2011;40(6):1328-1333. [DOI] [PubMed] [Google Scholar]
- 71.Heilmann C, Trummer G, Beyersdorf F, et al. Acquired von Willebrand syndrome in patients on long-term support with HeartMate II. Eur J Cardiothorac Surg. 2017;51(3):587-590. [DOI] [PubMed] [Google Scholar]
- 72.Wu T, Lin J, Cruz MA, Dong JF, Zhu C. Force-induced cleavage of single VWFA1A2A3 tridomains by ADAMTS-13. Blood. 2010;115(2):370-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schneider SW, Nuschele S, Wixforth A, et al. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc Natl Acad Sci USA. 2007;104(19):7899-7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shim K, Anderson PJ, Tuley EA, Wiswall E, Sadler JE. Platelet-VWF complexes are preferred substrates of ADAMTS13 under fluid shear stress. Blood. 2008;111(2):651-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meyer AL, Malehsa D, Budde U, Bara C, Haverich A, Strueber M. Acquired von Willebrand syndrome in patients with a centrifugal or axial continuous flow left ventricular assist device. JACC Heart Fail. 2014;2(2):141-145. [DOI] [PubMed] [Google Scholar]
- 76.de Biasi AR, Manning KB, Salemi A. Science for surgeons: understanding pump thrombogenesis in continuous-flow left ventricular assist devices. J Thorac Cardiovasc Surg. 2015;149(3):667-673. [DOI] [PubMed] [Google Scholar]
- 77.Linneweber J, Dohmen PM, Kertzscher U, Affeld K, Nosé Y, Konertz W. The effect of surface roughness on activation of the coagulation system and platelet adhesion in rotary blood pumps [published correction appears in Artif Organs. 2007;31(8):661]. Artif Organs. 2007;31(5):345-351. [DOI] [PubMed] [Google Scholar]
- 78.Mondal NK, Li T, Chen Z, et al. Mechanistic insight of platelet apoptosis leading to non-surgical bleeding among heart failure patients supported by continuous-flow left ventricular assist devices [published correction appears in Mol Cell Biochem. 2017;433(1-2):139]. Mol Cell Biochem. 2017;433(1-2):125-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu J, Mondal NK, Sorensen EN, et al. Platelet glycoprotein Ibα ectodomain shedding and non-surgical bleeding in heart failure patients supported by continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2014;33(1):71-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baghai M, Heilmann C, Beyersdorf F, et al. Platelet dysfunction and acquired von Willebrand syndrome in patients with left ventricular assist devices. Eur J Cardiothorac Surg. 2015;48(3):421-427. [DOI] [PubMed] [Google Scholar]
- 81.Koltai K, Kesmarky G, Feher G, Tibold A, Toth K. Platelet aggregometry testing: molecular mechanisms, techniques and clinical implications. Int J Mol Sci. 2017;18(8):E1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Federici AB, Budde U, Castaman G, Rand JH, Tiede A. Current diagnostic and therapeutic approaches to patients with acquired von Willebrand syndrome: a 2013 update. Semin Thromb Hemost. 2013;39(2):191-201. [DOI] [PubMed] [Google Scholar]
- 83.Tarzia V, Buratto E, Bortolussi G, et al. Hemorrhage and thrombosis with different LVAD technologies: a matter of flow? Ann Cardiothorac Surg. 2014;3(6):582-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Piche SL, Nei SD, Frazee E, et al. Baseline thromboelastogram as a predictor of left ventricular assist device thrombosis. ASAIO J. 2019;65(5):443-448. [DOI] [PubMed] [Google Scholar]
- 85.Hollis IB, Chen SL, Chang PP, Katz JN. Inhaled desmopressin for refractory gastrointestinal bleeding in a patient with a HeartMate II left ventricular assist device. ASAIO J. 2017;63(4):e47-e49. [DOI] [PubMed] [Google Scholar]
- 86.Federici AB, Rand JH, Bucciarelli P, et al. ; Subcommittee on von Willebrand Factor. Acquired von Willebrand syndrome: data from an international registry. Thromb Haemost. 2000;84(2):345-349. [PubMed] [Google Scholar]
- 87.Tiede A, Rand JH, Budde U, Ganser A, Federici AB. How I treat the acquired von Willebrand syndrome. Blood. 2011;117(25):6777-6785. [DOI] [PubMed] [Google Scholar]
- 88.Fischer Q, Huisse MG, Voiriot G, et al. Von Willebrand factor, a versatile player in gastrointestinal bleeding in left ventricular assist device recipients? Transfusion. 2015;55(1):51-54. [DOI] [PubMed] [Google Scholar]
- 89.Bartoli CR, Kang J, Restle DJ, et al. Inhibition of ADAMTS-13 by doxycycline reduces von Willebrand factor degradation during supraphysiological shear stress: therapeutic implications for left ventricular assist device-associated bleeding. JACC Heart Fail. 2015;3(11):860-869. [DOI] [PubMed] [Google Scholar]
- 90.Imamura T, Nguyen A, Rodgers D, et al. Omega-3 therapy is associated with reduced gastrointestinal bleeding in patients with continuous-flow left ventricular assist device. Circ Heart Fail. 2018;11(10):e005082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jennings DL, Rimsans J, Connors JM. Prothrombin complex concentrate for warfarin reversal in patients with continuous-flow left ventricular assist devices: a narrative review [published online ahead of print 5 June 2019]. ASAIO J. doi:10.1097/MAT.0000000000001021. [DOI] [PubMed] [Google Scholar]
- 92.Zayat R, Khattab MA, Grottke O, et al. Survival of HeartMate II patients despite cessation of anticoagulation—outcomes and hemostatic analysis. Circ J. 2018;82(5):1309-1318. [DOI] [PubMed] [Google Scholar]
- 93.Stulak JM, Lee D, Haft JW, et al. Gastrointestinal bleeding and subsequent risk of thromboembolic events during support with a left ventricular assist device. J Heart Lung Transplant. 2014;33(1):60-64. [DOI] [PubMed] [Google Scholar]
- 94.Shah KB, Gunda S, Emani S, et al. Multicenter evaluation of octreotide as secondary prophylaxis in patients with left ventricular assist devices and gastrointestinal bleeding. Circ Heart Fail. 2017;10(11):e004500. [DOI] [PubMed] [Google Scholar]
- 95.Molina TL, Krisl JC, Donahue KR, Varnado S. Gastrointestinal bleeding in left ventricular assist device: octreotide and other treatment modalities. ASAIO J. 2018;64(4):433-439. [DOI] [PubMed] [Google Scholar]