Abstract
Therapeutic hypothermia has consistently been shown to be a robust neuroprotectant in many labs studying different models of neurological disease Although this therapy has shown great promise, there are still challenges at the clinical level that limit the ability to apply this routinely to each pathological condition. In order to overcome issues involved in hypothermia therapy, understanding of this attractive therapy is needed. We review methodological concerns surrounding therapeutic hypothermia, introduce the current status of therapeutic cooling in various acute brain insults, and review the literature surrounding the many underlying molecular mechanisms of hypothermic neuroprotection. Because recent work has shown that body temperature can be safely lowered using pharmacological approaches, this method may be an especially attractive option for many clinical applications. Since hypothermia can affect multiple aspects of brain pathophysiology, therapeutic hypothermia also could be viewed as a model of neuroprotection, which could be used to identify potential therapeutic targets. We discuss how research in this area carries the potential to improve outcome from a variety of neurological conditions such as hypoxia, ischemia, trauma and hemorrhage.
Keywords: Hypothermia, pharmacology induced hypothermia, stroke, traumatic brain injury, cardiac arrest, hypoxic-ischemic encephalopathy
1. INTRODUCTION
Based on promising results from numerous experimental studies from multiple different laboratories, hypothermia has been viewed as an attractive therapy for several acute neurological diseases. Therapeutic hypothermia (TH) has been extensively studied at the experimental level, and has shown benefit against a variety mechanisms of brain injury, including reduction in metabolic activity, glutamate release, inflammation, production of reactive oxygen species, and mitochondrial cytochrome c release [1,2]. In various experimental models of acute brain diseases, several laboratories have consistently demonstrated that hypothermia ameliorates the extent of brain injuries and improves neurologic function. Based on past experimental research, recent clinical studies have established that therapeutic cooling improves neurological outcome from various acute brain insults, including global ischemia after cardiac arrest [3,4], and neonatal hypoxia-ischemia [5–7]. For other acute cerebral insults, such as ischemic stroke [8,9] and traumatic brain injury [10,11], the beneficial effect in clinical settings is now under investigation [12–15], and its strong neuroprotective effect has been shown consistently by several laboratories in preclinical models [16–18].
We reviewed optimal conditions for hypothermic therapy, in terms of target temperature, therapeutic duration, and attaining target temperature. In addition, we will discuss cellular and molecular pathways which are influenced by cooling. Since TH has been shown in the laboratory to have a robust and multifaceted neuroprotective effect, this therapy could be viewed as a model of neuroprotection. Precise understanding of this therapy may aditionally be helpful in the development of novel therapeutics. Papers cited in this article were identified using PubMed, and papers were selected which were considered to be scientifically reliable, important, and current.
1. OPTIMAL CONDITION FOR THERAPEUTIC HYPOTHERMIA
Therapeutic hypothermia is defined as an intentionally induced, controlled reduction from a normal body temperature of 37~38°C. Generally, classifications differentiate hypothermia into mild (32~35°C), moderate (28~32°C), deep (20~28°C), and profound (≤20°C). In early studies, deep states of hypothermia were commonly used. However, because of numerous complications and difficulty in achieving and maintaining these temperatures, mild to moderate hypothermia have become more attractive alternatives [19]. In a meta-analysis of animal studies, the benefits of hypothermia were inversely related to the temperature attained. Hypothermia also reduced infarct size by >40% with temperatures of 34°C or below [16]. However, specific studies that directly compared different temperatures failed to show dose dependent neuroprotection comparing temperatures of 27°C vs 32°C [20] and 30°C vs 33°C [21].
This dose non-dependency may be explained by the detrimental effects of cooling itself, or by a lack of statistical power from the experimental models used. That is, experimental studies may not have had the statistical power to detect small differences in outcomes, compared to the larger differences seen with the normothermic control. Nevertheless, the optimal target temperature should be determined so as to maximize its beneficial effect and minimize any detrimental effect. In clinical settings, the current literature suggests that cooling to 32~34°C may be optimal .
Numerous laboratory studies have attempted to determine the optimal timing of cooling. Early initiation of cooling prior to brain injury seems to confer the most positive outcome, but this is generally not feasible in many clinical settings. In a prior review of ischemic stroke models [17,22], it was reported that reduction of infarct size is commonly observed when cooling is begun within 60 to 180 minutes of onset. In contrast, in models of global cerebral ischemia, neuroprotection was observed, even when hypothermia was initiated 6 hours after ischemia onset [23,24]. Thus, there is significant variation in the therapeutic time window depending on the type of brain injury.
The optimal duration of therapeutic hypothermia is also not well known. Some groups have used brief durations of hypothermia (0.5~5 hours), whereas others used longer periods (12~48 hours). In a few studies of ischemic stroke where the duration of hypothermia therapy was compared directly, durations of 1–3 hours appeared effective, whereas durations between 30 min to 1 hour were not [21,25]. In global cerebral ischemia, intra-ischemic hypothermia (rectal temperature 28~32°C) completely prevented hippocampal cell damage if continued for 4 or 6 hours, whereas 2 hours of hypothermia protected less well, and 1 hour or less did not protect at all [26]. Longer cooling durations may be necessary especially when the initiation of cooling is delayed. Previous reports have shown that prolonged hypothermia initiated 4~6 hours after ischemia and maintained for 24 hours can provide sustained functional and histological neuroprotection as far as 6 months onset [27]. These data indicate that prolonged cooling can provide neuroprotection when treatment initiation is delayed by a few hours [28].
2. Hypothermia therapy and pharmacological approaches
2.1. Pharmacological Induction of Hypothermia
There has been a recent surge of interest in the investigation of drug-induced hypothermia as a treatment option for acute brain injuries [29]. This approach has been proposed for the aim of maximizing the beneficial effect of hypothermia in clinical settings with less adverse reactions. Currently, there are eight classes of pharmacological agents which are capable of inducing hypothermia (Table 1). These agents are capable of affecting a multitude of systems including cannabinoid (CB), opioid, transient receptor potential vanilloid 1 (TRPV1), neurotensin, and thyroxine derivatives, dopamine, gas, and adenosine derivatives. Interestingly, some of these drugs such as those in the cannabinoid families, may not only provide neuroprotection through hypothermia induction but may provide neuroprotection through other mechanisms as well [29]. One study used a CB1 receptor agonist (WIN55212–2) and demonstrated that a low dose (1mg/kg), which does not decrease body temperature, can exert a neuroprotective effect in a murine stroke model [30]. Further, keeping temperature between 37 and 38°C, the CB1 receptor agonist did not abolish its neuroprotective effect [31]. This neuroprotective effect of the CB system may be due to reduced excitotoxicity via reduced glutamate release[32]. In addition, the CB1 receptor agonist can reduce inflammation and microglial activation as well as reduce BBB disruption after ischemic injury [33]. These neuroprotective effects in addition to lowering body temperature may suggest additional benefits due to synergistic effects[1].
Table 1.
Classificartion of pharmacological agents that can induce hypothermia
| Class | Representative drug | target of pharmacological action |
|---|---|---|
| Cannabioid (CB) System [30–33] |
WIN5512–2 | CB1 agonist |
| HU-210 | CB1 agonist | |
| TAK-937 | CB1/CB2 agonist | |
| TRPV-1 Receptor | Capsaicin | thermoregulator in peripheral nerves and hypothalamus |
| Opioid Receptors | U50, 488H | κ-opioid agonist |
| SNC-80 | δ-opioid agonist | |
| Neurotensin | NT69L | neurotensin analog |
| JMV449 | neurotensin analog | |
| Thyroxine Derivatives | T0AM | Trace amine-associated receptor |
| T1AM | Trace amine-associated receptor | |
| Dopamine Receptor Activators | Lisutide | D2 dopamine receptor agonist |
| Talipexole | D2 dopamine receptor agonist | |
| Gasseous Hypothermia | Xenone, Helium | Unknown |
| Adenosine and Adenine Nucleotides | AMP | A1 receptor agonist |
| ATP | Unknown | |
Another advantage of pharmacologically induced hypothermia is its effect on central thermoregulation at the level of the hypothalamus [29]. Several drugs, such as TPRV1 receptor, neurotensin and thyroxine families, have been shown to have effects on thermoregulatory control by decreasing the compensatory hypothermic response during cooling. This effect on thermoregulation could also reduce shivering and vasoconstriction, which often interferes with the therapeutic effect of hypothermia. Therefore, these agents might be efficacious in combination with physical hypothermia in reducing discomfort, hastening the cooling process, and prolonging tolerable cooling durations [29].
2.2. Synergistic effects of several neuroprotectants and Hypothermia therapy
Some drugs have been reported to provide synergistic neuroprotective effects when combined with hypothermia treatment (Summarized in Table 2) [34]. One such drug reported recently is glibenclamide (GBC), a sulfonyl urea receptor 1-transient receptor potential M4 (SUR1-TRPM4) channel inhibitor, which is already used as an antidiabetic drug [35–37]. Zhu et al. [35] revealed that the combination of GBC and TH exhibited synergistic effects in a rodent stroke model. Combination treatment was associated with greater reductions in brain edema, better neurological recovery, prevention of tight junction protein loss, and enhanced anti-inflammatory effects. Furthermore, in cardiac arrest model, combined therapy with GBC and HT also showed trends towards less histological injury [37].
Table 2.
Summary of therapeutic strategies and drugs tested in combination with hypothermia therapy.
| Concept of therapeutic strategy | phamacological target | Specified Treatment |
|---|---|---|
| Reducing excitotoxicitty | NMDA receptor antagonist | MK801 [38–40] |
| Selfotel [41, 42] | ||
| Mg2+ [43, 44] | ||
| Anti-inflammation | immunosuppressive | Tacrolimus [46] |
| Antibiotics | Minocycline [47, 48] | |
| Anti-oxidative stress | free radical scavenger | Edaravone [49] |
| Mannitol | ||
| Increase blood supply | Vasodilator | Statin |
| Angiogenesis | G-CSF | |
| Multiple protection | antidiabetics | glibenclamide [35–37] |
| trophic factors | Insulin-like growth factor (IGF-1) | |
| Brain derived neurotrophic factor (BDNF) | ||
| other | Albumin | |
Historically, this synergistic neuroprotection was first reported with MK801, which is antagonist of the NMDA receptor [38–40] . Several drugs have since been reported to possess neuroprotective effects in various acute brain injury models have been tested for synergistic effects when combined with TH. Reducing excitotoxicity with NMDA antagonists is one such approach. Synergistic effects of MK801 [38–40] , Selfotel (CGS-19755) [41,42] and magnesium ion (Mg2+) [43,44] were tested in combination with TH. Although MK801 did not show enhanced neuroprotective effects in some laboratories [38,39], Mg2+, and other NMDA receptor antagonists did [44]. In contrast, another study of stroke using a model of permanent MCAO failed to find a benefit with the combination of Mg2+ and TH[45]. Reasons for these discrepancies are not fully clear, but could be explained by differences in the models used, and other experimental factors such as when therapy was initiated, how long treatment continued, and the depth of cooling.
Diverse neuroprotective strategies in combination with hypothermia has also been investigated (Table 2). Anti-inflammatory strategies have been studied in in combination with hypothermia. FK506 (Tacrolimus) [46] and minocycline are representative drugs which have shown synergy with TH. Combining FK506 with hypothermia prolonged its therapeutic time window and led to decreased brain damage (lesion volume) than either treatment alone[46]. Another anti-inflammatory drug is minocycline, a second-generation, semi-synthetic, tetracycline, which possess inhibitory effects on inducible nitric oxide synthase (iNOS) and matrix metalloproteinase (MMPs)[47]. One study involving ischemic stroke model found a small, albeit non-significant, increase in therapeutic benefit when minocycline was combined with hypothermia relative to either treatment its own [48]. Another attractive strategy is reduction of oxidative stress. Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one) can scavenge excess free radicals. A previous report found that the combination of edaravone and hypothermia can reduce the damage in an ischemic stroke model, although hypothermia itself failed to show a significant beneficial effect [49].
While these compounds and strategies seem feasible for clinical translation, there are no reports that have proven their benefit in humans so far. Further research in both pre-clinical and clinical trials are still needed to clarify how such approaches could be applied.
3. THERAPEUTIC HYPOTHERMIA IN VARIOUS ACUTE BRAIN DISEASES
Current standpoint of hypothermia in each acute brain diseases are summarized in Table3.
Table 3.
Summary of the Therapeutic Efficacy of Hypothermia for Neurological Injury
| Pathological type of acute brain injury | Current status of therapeutic hypothermia |
|
|---|---|---|
| pre-clinical | clinical studies | |
| Cardiac Arrest | protectie [17,19] | beneficial [3,4] |
| neonatal hypoxic-ischemic encephalopathy (HIE) | protective [51] | beneficial [5,7] |
| Ischemic stroke | transient: protective [17,22] | controversial [14, 15, 19] |
| permanent: controversial | ||
| Intra cerebral hemmorhage (ICH) | controversial [55–60] | controversial [61–64] |
| Subarachnoid hemorrhage (SAH) | protective [65–67] | controversial [68–70] |
| Traumatic brain injury (TBI) | protective [73–78] | controversial [79–86] |
| Spinal cord injury (SCI) | protective [90–96] | controversial [87,88] |
3.1. Hypothermia for Cardiac Arrest
Several experimental studies have demonstrated the neuroprotective effects of mild or moderate hypothermia for cardiac arrest (global ischemia) [17,19]. These studies have shown the durability of this protective effect and have defined a temporal therapeutic window which can be lengthened provided cooling is prolonged [50]. Several studies in global cerebral ischemia models have found that cooling in the range of 30~34oC consistently leads to robust neuroprotection. Mild hypothermia for 12 h enhances neuroprotection of hippocampal CA1 after a 3 min insult in a global cerebral ischemia model in gerbils, whereas neuroprotection following a 5 min insult required cooling for 24 h [24]. Thus, longer cooling periods may be more suitable for more severe insults.
The clinical benefit of hypothermia has also been demonstrated in two large-scale clinical studies based on data from multiple medical centers conducted in 2002 [3,4]. These clinical studies showed that mild hypothermia for 12~24h reduces mortality and improves functional recovery from cardiac attest [3,4]. Cooled patients had improved neurological outcome 6 months later, compared to those who were maintained at normal body temperature [4]. Since then, therapeutic cooling has been widely accepted as standard treatment for comatose survivors of cardiac arrest.
3.2. Hypothermia for neonatal hypoxic-ischemic encephalopathy (HIE)
Therapeutic hypothermia has also been shown to be effective in preventing perinatal brain injury from hypoxic-ischemic encephalopathy (HIE) [51]. There have been several clinical trials of newborns with HIE [5,7]. These studies have shown benefit in infants with moderate and severe HIE; however, long term, lifelong benefits are especially key in pediatric populations, and there are no reports of outcomes beyond 21 months of age. This condition has also been studied in the laboratory, although not as extensively as in adult models.
3.3. Hypothermia for Ischemic Stroke
In abundant experimental studies of experimental stroke (focal cerebral ischemia models), mild or moderate hypothermia has shown to be neuroprotective and improves neurological function when cooling was initiated within a few hours of ischemia onset. There is no doubt that hypothermia reduces infarct volume and improves neurological function in the range of 24°C to 33°C after onset in ischemic stroke models [17,22].
Recently, many experimental studies and large clinical trials demonstrated that recanalization of occluded vessels within specific time windows after ischemia (recanalization therapy) increases the likelihood of a good outcome [17]. In transient middle cerebral occlusion (tMCAO) models, hypothermia consistently showed neuroprotection, whereas the results of permanent MCAO (pMCAO) were conflicting. Thus, the role of therapeutic hypothermia in combination with recanalization therapy in clinical setting becomes very important [19].
As TH was shown to be neuroprotective in both cardiac arrest patients and neonatal HIE patients, there is hope that it will similarly reduce morbidity and mortality following ischemic stroke. Indeed, a few preclinical studies showed that mild therapeutic hypothermia initiated during acute ischemic stroke or after a short delay reduced infarct size and mitigated functional impairment in a recent meta-analysis [16,52]. In addition to these studies, another study showed feasibility and tolerability, and established regimens to prevent or reduce shivering in the typically awake stroke patient [9,53]. In a recent multicenter study, an endovascular cooling device was used in combination with the administration of recombinant tissue plasminogen activator (rt-PA) in acute stroke patients. Here, patients could be treated within 0–6 h of symptom onset followed by endovascular cooling to 33oC for 24 h. While the study was not designed to evaluate relative efficacy of TH, this regimen appeared well tolerated, although there was increased incidence of pneumonia amongst cooled patients [54]. However, these studies were all small, and larger prospective studies have yet to be published. A few clinical trials in ischemic stroke [14,15] showed promising effects by TH. These results may provide useful direction in the design of future clinical trials.
3.4. Hypothermia for Intracerebral Hemorrhage
Intracerebral hemorrhage (ICH) is a devastating stroke that can occur in patients with high blood pressure. The mortality and morbidity from ICH is often higher than ischemic stroke, and there is currently no specific therapy with proven benefits for this condition. Recent experimental studies have been directed at determining the pathophysiology of ICH and at identifying effective treatments, including the study of TH. In experimental reports, a few studies have shown that hypothermia reduced brain edema, inflammation, and blood-brain barrier (BBB) disruption in ICH models created by intra-striatal thrombin (to model edema associated with ICH) [55] or autologous whole blood [56–58] injection. However, this was not as consistent a finding across labs. In fact, some labs have observed that histological and functional benefits are not consistently found [57] and one report described increased bleeding in the brains of cooled animals [58]. Although this appears to depend upon the model, insult severity, and time of treatment, in another study, delayed mild hypothermia (48 h) after ICH failed to reduce lesion size when started soon after collagenase-induced ICH, whereas treatment delayed 12 h still showed neuroprotection [58]. In a recent meta-analysis of preclinical studies of TH in ICH [59], authors concluded that hypothermia can reduce edema, protect blood-brain barrier disruption, and can improve behavioral outcomes. However, one study found increased bleeding with early cooling, and some protection was observed only when cooling was delayed 12h [60]. The authors pointed out that hypothermia could affect critical pro-coagulant and thrombolytic systems, and predispose to bleeding in the acute period. The authors also suggested that it is also possible that hypothermia exacerbated complications of the initial increased blood pressure observed in these models. These findings indicate that further studies are needed to clarify the reasons for worsening in certain scenarios, and whether cooling might be detrimental if not applied in an optimal manner.
At the clinical level, application of therapeutic hypothermia in patients with ICH remains controversial [61]. Kollmar et al. [62] reported that 12 patients with large ICH were treated with hypothermia to 35°C for 10 days (initiated 3–12 h after symptoms onset) and these patients were compared to data from a local hemorrhage data bank. In the hypothermia group, edema volume remained stable during 14 days, whereas edema significantly increased in the control group. A recent systematic review and meta-analysis [61] revealed that hypothermia can reduce the incidence of delayed cerebral ischemia, although TH did not show significant differences in mortality and poor outcomes. Furthermore, on-going clinical trials Targeted temperature management after intracerebral hemorrhage (TTM-ICH) and Cooling in intracerebral hemorrhage (CINCH))explore the safety and efficacy of hypothermia in ICH patients [63,64].
3.5. Hypothermia for Subarachnoid hemorrhage
Subarachnoid hemorrhage (SAH) is often due to aneurysmal rupture, and hypothermia is occasionally used intraoperatively during aneurysm repair. There are several animal model studies of SAH that hypothermia exhibited neuroprotection. In one study, mild hypothermia applied for 2h led to improved post-hemorrhagic neurological deficits, reduced intracranial pressure and postoperative weight gain by 1–7 days if cooling was delayed for 3 h after SAH [65]. Another study demonstrated the neuroprotective effects of hypothermia on the acute changes after experimental SAH as evaluated by diffusion weighted MRI (DWI) and magnetic resonance spectroscopy (MRS) [66]. The investigators established that hypothermia improved early development of cytotoxic edema, lactate accumulation, and a general metabolic stress response after SAH a rat model. The mechanisms underlying this protective effect have not been explored as extensively as in brain ischemia models, but one study showed that hypothermia interrupted the early expression of genes associated with cellular stress, such as c-jun and Hsp 70 [67].
At the clinical level, Muroi and colleagues [68] assessed the neuroprotective effects of TH in combination with high-dose barbiturates. The inflammatory response in 7 patients with this intervention showed decreased systemic and cerebrospinal fluid levels of interleukin (IL)-1β, IL-6, and leukocyte counts compared to a group of 8 patients who received no intervention. Interestingly, while cooling suppressed most immune markers, cooling increased tumor necrosis factor alpha (TNF-α) [68]. In a large multi-center randomized study, mild intra-operative hypothermia during surgery for intracranial aneurysms failed to improve neurologic outcomes among favorable-grade patients with aneurysmal SAH (WFNS grade 1–3) [69]. In response to these results, more studies have focused on therapeutic hypothermia for patients with poor grade SAH. One clinical study conducted by Seule and colleagues [70] evaluated the feasibility and safety of mild hypothermia in patients with poor grade aneurysmal SAH who experience intracranial hypertension and/or cerebral vasospasm [70]. The findings led authors to conclude that prolonged systemic hypothermia may be considered a last-resort option for a carefully selected group of younger SAH patients with resistant intracranial hypertension or cerebral vasospasm. Thus, the clinical effectiveness of therapeutic cooling for SAH remains unclear. This may be due to many factors including fever, vasospasm, surgical technique, and excessive decompression by skull removal, that may affect outcomes in patients with SAH.
3.6. Hypothermia for traumatic brain injury
Traumatic brain injury (TBI) is the primary cause of significant morbidity and mortality in young populations. Similar to hypoxic and ischemic injury, it is known that TBI leads to a spectrum of secondary injury through ionic fluxes, excitotoxicity, necrotic and apoptotic cell death and inflammation [71,72]. Cell damage is not only produced by the trauma itself, but by a series of these pathophysiological mechanisms which may cause secondary injury. Because numerous preclinical studies have shown that cooling affects multiple pathological pathways, the use of TH has been tested in a variety of preclinical and clinical situations [2,71].
In the area of pre-clinical TBI research, Clifton et al. [73] first reported that mild hypothermia following a lateral fluid percussion brain injury in rats improved motor recovery. Subsequent preclinical studies using similar or different TBI models resulting in both focal and diffuse injury also demonstrated that early (within 5 minutes) posttraumatic hypothermia using systemic cooling strategies could reduce contusion volume and protect against patterns of neuronal vulnerability [74,75]. In addition, TH also attenuated the severity of diffuse axonal injury (DAI) and blood brain barrier (BBB) damage [76,77] Furthermore, recent studies revealed that TH can improve not only acute but also chronic behavioral outcome measures including sensorimotor and cognitive function [78].
Based on promising preclinical findings, several clinical trials had been carried out to test its efficacy in TBI patients. In 1997, Marion et al. [79] published a randomized study involving 84 patients with severe TBI who were treated using mild hypothermia (33°C for 24 h). There was a significantly better neurologic recovery at 3 and 6 months among the patients with GCS scores of 5–7 at the time of admission to the hospital [79]. Jian et al. [80] also reported the results from a randomized study of 215 patients with TBI which showed that prolonged hypothermia (5 days) is effective in improving neurological outcomes. In contrast, several randomized control studies showed no benefit on neurological outcome in severe TBI patients [81,82]. Although the efficacy of hypothermia in TBI is still controversial, several hypothermia trials in TBI have been carried out [83,84]. The European Study of Therapeutic Hypothermia Trial, an international multicenter randomized control trial examined the effect of titrated therapeutic hypothermia on intracranial pressure and neurological outcome. Recently, the trial carried out among 387 patients at 47 centers in 18 countries have been completed. The study reported that TH, while successfully reducing intracranial pressure, did not ultimately improve functional recovery [85,86]. Collectively, results from both pre-clinical and clinical studies of TH in TBI continues to evolve, and a number of factors including patient selection and the timing of the therapy appear to be critical in successful trial design.
3.7. Hypothermia for spinal cord injury
As well as other acute neurological diseases, spinal cord injury (SCI) may also benefit from TH[87,88]. Damage and pathology related to SCI are categorized into 2 phases: primary and secondary. Similar to related conditions, hypothermia for SCI focused on mitigating the secondary phase of damage, with the goal of preventing further damage and improving neurological outcomes.
Based on numerous experimental reports, there have been a wide range of reported histologic, biochemical, and pathophysiologic beneficial effects attributed to hypothermia[88]. These include reduction in level of excitotoxic metabolite glutamate in the cerebrospinal fluid[89], reduced vasogenic edema at the site of injury[90,91], decreased neutrophil invasion[92], and lack of reduction of blood flow after SCI[93] There is also evidence that hypothermia decreases tissue metabolism and energy requirements[94], oxidative stress[95], and reduces tissue hemorrhage[90,91] and apoptosis[96]. However, in clinical settings, the benefit of hypothermia in SCI is also still controversial [87,88]. Considering that SCI is a devastating injury with uncertain therapeutic options, except possibly acute methylprednisolone, large and well-designed prospective study are still lacking.
4. MECHANISMS OF HYPOTHERMIC NEUROPROTECTION
4.1. Acute Molecular Events
4.1. Cellular Metabolism
The neuroprotective effect of hypothermia has long been linked to decreases in metabolic rate and the reduction of cerebral blood flow [25,26]. Hypothermia decreases the brain metabolic rates of oxygen consumption and glucose metabolism [26]. Hypothermia, on average, decreases brain oxygen consumption by approximately 5%/°C fall in body temperature in the range of 22–37°C [26,97], and in anesthetized animals, oxygen consumption reduces linearly between 37 and 38°C [98]. Hypothermia can interrupt downstream consequences of increased lactate production due to dependence on anaerobic metabolism and the development of acidosis by preserving the brain’s metabolic stores [2]. Hypothermia conserves high-energy phosphate compounds, such as adenosine triphosphate (ATP), and maintains tissue pH, all mechanisms likely linked to its suppressive effect on brain metabolism, which conserves tissue ATP levels. ATP is needed to maintain ion gradients, and when these concentration gradients are disturbed, such as in the case of ischemic stroke, calcium influx occurs and leads to increased extracellular glutamate levels [27]. Several investigators also demonstrated that hypothermia also significantly decreases the release of excitotoxins and calcium influx due to cerebral ischemia [99,100]. The glutamate receptor 2 (GluR2) subunit of the α-smino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptor is thought to be responsible for limiting calcium influx. GluR2 suppression by ischemia is thought to lead to massive calcium entry which, in turn, activates neuronal cell death by activating numerous injurious processes such as proteases and lipases. In fact, some investigators have hypothesized that one potentially neuroprotective mechanism of hypothermia is that it could salvage neurons from delayed calcium influx via GluR 2 receptors [101]. In recent work, our group has established that hypothermia decreases ischemia-induced upregulation of calcium influx through a newly characterized calcium sensing receptor (CaSR) [102]. CaSR is thought to sense changes in extracellular calcium levels, but also appears to reciprocally down regulate GABA receptors, thereby decreasing inhibitory tone. Cerebral ischemia increases expression of CaSR while inhibiting GABA-B-R1 expression, but this is reversed by TH [102]. Thus, TH may have identified CaSR as a potential target for treatment of stroke and related conditions. Excitotoxic neurotransmitters are released early after ischemia onset, and it is well known that glutamate antagonists have a rather narrow temporal therapeutic window of 1–2 h. Since earlier cooling is superior to delayed cooling, this may explain some of the protective effect of hypothermia. However, cooling even after glutamate release has occurred is still protective [103].
4.2. Early Molecular Events
Hypothermia has also been shown to affect other acute processes associated with ischemia, including the induction of immediate early gene expression [104] and the cellular stress response [105]. Some reports showed that one of the stress related proteins, the 70kDa inducible heat shock protein (HSP70), is increased under hypothermic conditions [106], and this might be consistent with HSP70’s neuroprotective properties [105]. However, it is still unclear whether hypothermic neuroprotection is necessarily mediated through alterations in immediate early gene expression and the cellular stress response [2].
Micro RNAs (miRNAs), a subset of non-coding RNAs, have been a topic of recent investigation in brain injury models, where their expression has been observed to increase as early as 2h after onset. It is conceivable that they have an important role in pathogenesis of acute brain injury, and the roles of specific miRNAs are currently under investigation. A report in traumatic brain injury models showed that therapeutic hypothermia alters the expression of several miRNAs, as represented by miR-874 and miR-451. In particular, a few miRNAs, including miR-874 and miR-451 were most changed by cooling, with both being decreased by cooling at 7h, while miR-451 was increased by cooling at 24h compared to normothermia[107]. Further research is needed to better define their role in brain injury.
Cold-inducible (or ‘cold shock’) proteins are also of relevance to hypothermic neuroprotection [2]. Two of these genes, cold-inducible RNA-binding protein (CIRBP) and RNA binding motif protein 3 (RBM3), have been reported to be specifically induced by therapeutic hypothermia [2,108]. CIRBP has been speculated to protect and restore native RNA conformation during stress, and protects against apoptosis by upregulating ERK. RBM3 may also protect cells from death pathway by acting in a manner similar to X-linked inhibitor of apoptosis (XIAP) [109]. A few studies have begun to explore their significance in neuroprotection and potential mechanisms of protection by hypothermia. For instance, CIRBP mRNA was increased in rodent stroke models, with even higher increases in animal exposed to hypothermia [110]. RBM3 was also upregulated by hypothermia in neuronal cells, and hypothermia’s neuroprotective effect was eliminated when RBM3 expression was inhibited. [111]
4.3. Sub-acute Molecular Events
4.3.1. Apoptosis
In past studies of acute brain injury models, hypothermia was shown to prevent damage by interrupting both the intrinsic and extrinsic apoptotic pathways. The intrinsic pathway takes place within the cell at the level of the mitochondria [112], while the extrinsic pathway is triggered by engagement of a cell surface receptor by its ligand(s) [113]. Hypothermia can interrupt these pathways, but whether cooling has any effect on neuron survival depends on whether apoptosis actually occurs in a given model or paradigm. In models of global cerebral ischemia, hypothermia can interfere with changing the expression of Bcl2 family members. Some Bcl2 family members are pro-apoptotic, while others are anti-apoptotic. Thus, the relative balance of Bcl2 proteins could determine the cell’s fate. A few studies in hypothermia models have shown that cooling leads to suppression of pro-apoptotic Bax while increasing anti-apoptotic Bcl2 [114–117], reducing cytochrome c release [114,118]and decreasing caspase activation [119,120]. Delta-PKC (a protein kinase C isoform) has been shown to contribute to ischemic injury by inhibiting phosphorylation and thereby activation of pro-survival kinase Akt and facilitating cytosolic translocation of pro-apoptotic BAD [121]. Caspase-3 leads to transport of delta-PKC from the cytosol to the mitochondria and nucleus, where it interacts with other molecules to induce apoptosis [122]. In contrast, a different PKC isoform, epsilon-PKC is anti-apoptotic, and is degraded by caspases. While hypothermia did not appear to alter overall levels of delta-PKC [123], it blocked its translocation to the mitochondria and the nucleus and stimulated epsilon-PKC in brain ischemia models [124].
Extrinsic apoptotic pathways also appear to be involved in various acute brain injuries. The most widely studied apoptosis-inducing receptor and ligand pair are Fas and FasL, respectively. How FasL binds Fas is somewhat unclear, as many reports indicate that FasL must be present on the cell’s surface in order to engage Fas, but other reports indicate that FasL must first be cleaved from the surface by activated matrix metalloproteinases (MMPs) and solubilized prior to any interaction with Fas. Hypothermia seems to prevent FasL cleavage, as levels of soluble FasL are decreased in ischemic rodent brains exposed to TH, as are levels of several MMPs [125,126]. Decreased soluble FasL levels was also associated with decreased caspase 8 activation, an event which occurs downstream of Fas activation [127]. A recent study [128] used an experimental stroke model that showed that TH inhibits Fas trafficking through downregulation of dynamin, a guanine triphosphatase thought to transport Fas from the ER to the cell surface. This may suggest a novel therapeutic target in stroke as well as other acute neurological diseases. In models of severe ischemic stroke (MCAO of 2 hours or longer), hypothermia does not appear to affect Bcl-2 family members or caspase activation, but does prevent cytochrome c release [118]. These observations might be explained by yet a third apoptotic pathway which involves direct cell killing by mitochondrial AIF (apoptosis inducing factor) release, and is caspase independent [129]. Hypothermia was shown to reduce apoptotic cell death in a more severe model of ischemic stroke while suppressing AIF translocation [130].
Laboratory studies have also investigated other molecules implicated in apoptotic pathways which have been shown to be affected by hypothermia as well. Phosphatase and tensin homolog (PTEN) is a tumor suppressor molecule with pro-apoptotic functions. PTEN deletion has previously been shown to prevent brain injury [131]. However, PTEN phosphorylation leads to its deactivation, and is normally decreased in brain injury [132]. Under conditions of hypothermia where neuroprotection was observed, phosphorylated PTEN levels remained the same but reduced in non-neuroprotective conditions of hypothermia [123]. Thus, the deactivated form of this pro-apoptotic protein seems to correlate to hypothermic neuroprotection. The mechanisms underlying this correlation require further investigation.
4.3.2. Survival pathways
Interestingly, while hypothermia downregulates a majority of cell death pathways, it can also upregulate cell survival and growth pathways. Several neurotrophic factors in the brain have been reported with regard to their therapeutic potential in acute brain injuries. These factors play a key role in multiple neuronal cell processes such as synaptic function and plasticity and sustain neuronal cell differentiation, survival, and morphology. In ischemic models, exogenous administration of one or more of these factors seemed to improve functional neurological outcome without necessarily affecting lesion size. Hypothermia increased the brain-derived neurotrophic factor (BDNF) after ischemic brain insults [133,134], glial-derived neurotrophic factor (GDNF) [135] and neurotrophin [136]. Hypothermia also activated extracellular signal-regulated kinase-1/2 (ERK1/2) phosphorylation, a downstream element of BDNF signaling [133]. In other cases, hypothermia has been reported to induce protective effects of ERK1/2 and to interrupt the activation of ERK1/2 and suppresses inflammation and cell death [137].
Studies on pro-survival protein Akt have been reported. Akt is a serine/threonine protein kinase that regulates proliferation, apoptosis, glucose metabolism, transcription and cell migration. After phosphorylation by phosphoinositol 3-kinase (PI3K), activated Akt phosphorylates (that is, inactivates) proapoptotic proteins such as glycogen synthase-3beta (GSK-3beta) and Bcl-xL/Bcl-2-associated death promoter (BAD). In an animal model of ischemic stroke, hypothermia appeared to promote activation of Akt while reducing infarct size. This effect disappeared when hypothermia was combined with an Akt inhibitor [138].
4.3.3. Inflammation
Inflammatory accompanies a variety of acute neurological insults. These insults which lead to neuronal cell damage include a complex series of biochemical and molecular mechanisms [139]. Dying or dead cells trigger activation of several immune responses via microglia, a monocyte lineage cell that resides in the brain, as well as infiltrating immune cells from the circulation [140,141]. Further, there is evidence that brain cells not normally viewed as immunologic, including astrocytes and even neurons, are capable of elaborating immune molecules. Several animal studies have now shown that inhibiting various aspects of this immune response by hypothermia may be involved in the beneficial effects on neurological outcome following brain injuries [142]. Following ischemic stroke, inflammation can be detected within a few hours after the injury onset. Because of the unanticipated nature of acute brain insults, the ensuing immune response is most likely innate, rather than adaptive. The innate immune response is a triggered by a variety of signals that, unlike the adaptive immune response, do not require antigen recognition. Mild and moderate hypothermia affect several pathways of the inflammatory response at different time points [132]. Microglia play an important role in this innate response [143]. At rest, microglia display a ramified appearance, but when activated, undergo a series of morphologic changes often leading to an amoeboid morphology making them phenotypically indistinguishable from circulating macrophages and monocytes. Microglial activation is the initial step in the brain inflammatory response. Depending on the stimulus, this step may be followed by infiltration of circulating monocytes, neutrophils, and T-cells, and by reactive astrocytosis [144]. Microglial activation occurs through a complex series of events, with changes in morphology and gene expression which vary depending on the type, severity, and duration of the stimulus [145]. Hypothermia appears to decrease tissue density and activation of microglia. This effect leads to about 54% reduction in microglial activation after 3 days [146]. However, some phagocytic properties of microglia likely exerts beneficial effects in the long term [147]; thus, suppression of microglial function may not be ideal at later timepoints.
After ischemic stroke, endogenous immune stimulators are collectively referred to as damage-associated molecular patterns (DAMPs). DAMPs include hyaluronan, surfactant protein and uric acid. These substances bind to and stimulate microglia and other immune cells leading to the upregulation of many immune mediators by activating several pro-inflammatory transcription factors, including nuclear factor kappa B (NF-kB) [148], hypoxia inducible factor 1 (HIF-1), interferon regulator factor 1 and signal transducer and activator of transcription 3 (STAT3) [149]. Studies have also shown that hypothermia may suppresses inflammation at the transcriptional level, as it has been shown to suppress transcription factors such as NF-kB [150], MAPK [137,151] and JAK/STAT [152].
Cytokines were originally described as mediators involved in regulating the innate and adaptive immune systems. Cytokines are quickly and extensively upregulated in the brain in a variety of disease states [141,153]. The most studied cytokines related to inflammation in acute brain injury are tumor necrosis factor-α (TNF-α), several of the interleukins (IL) and transforming growth factor (TGF)-β. Among these cytokines, mild hypothermia can reduce the expression levels of pro-inflammatory cytokine (IL-1β) and TNF-α [138,141]; however, hypothermia also suppressed anti-inflammatory cytokines such as IL-10 and TGF-beta [154], indicating that hypothermia does not always result in a purely anti-inflammatory outcome.
Oxidative and nitrosative stresses may play a central role in this inflammatory response. Reactive oxygen species (ROS) are produced by injured brain cells, presumably because their mitochondria are no longer able to neutralize these reactive species [155]. ROS are also released by inflammatory cells, presumably as host defense against invading pathogens. The increase in ROS can then trigger more immune responses by leading to pro-inflammatory transcription factor activation, activation of endogenous immune molecules and can damage adjacent viable tissue that surround the area of injury [156]. Thus, ROS can participate in a rather vicious cycle of immune response activation and direct cytotoxicity. Several studies have now shown that hypothermia attenuates free radical formation and thus provides protection [157,158]. Hypothermia, by preventing the generation of ROS, has been shown to inhibit all of these downstream processes including the prevention of cytochrome c release, downregulation of pro-apoptotic factors and the prevention of caspase activation [159]. Similarly, nitrosative stresses include the increase in nitric oxide (NO) via the different nitric oxide synthase (NOS) isoforms, particularly the inducible NOS (iNOS) that is primarily found in immune cells [160]. Further, the ROS superoxide and NO can combine to form peroxynitrite, which is a particularly reactive species that is genotoxic as well. In various experimental models, hypothermia significantly attenuates increases in NOS isoforms.
The Mitogen-Activated Protein Kinase (MAPK) pathway is another important enzyme system in affecting inflammation, in a cell-type dependent manner. While there is no consensus on the role of ERK after ischemia stroke, there is also no consensus on the effect of hypothermia on ERK. Studies have shown that hypothermia suppresses ERK signaling in activated cultured microglia [137], while in an experimental stroke model, hypothermia activates ERK in brain endothelial cells. This latter finding seems to be associated with reduction of intercellular adhesion molecule-1 (ICAM-1) expression, which is one of many inflammatory genes regulated by the MAPK signaling pathway, and is one of a class of adhesion molecules involved in trafficking circulating inflammatory cells to the CNS [151].
In brief, the effect of hypothermia on inflammation is largely suppressive, and this anti-inflammatory property might serve as a major mechanism of hypothermic neuroprotection. Studies in a model of brain inflammation where cell death does not occur showed similar suppress in the immune response by hypothermia, and suggests that inflammatory responses are quite temperature sensitive [161].
4.3.4. Blood-Brain Barrier and Edema
The disruption of blood-brain barrier (BBB) after acute brain insults promote the secondary injuries by edema formation and hemorrhage, which is caused by structural and functional impairment of basement membrane tight junction proteins, transport proteins, endothelial cells, astrocytes and neurons. Studies have shown that mild and moderate hypothermia protects the BBB disruption [162] and decreases edema formation [163] by attenuating loss of vascular basement proteins [164]. Matrix metalloproteinases (MMPs) are recognized to break down the extracellular matrix, and can lead to disruption of the BBB causing further infiltration of circulating immune cells, serum proteins and hemorrhage. Inactivated MMPs are normally found in the cytosol, but in pathologic states, they can be transported extracellularly where they are cleaved to an active form and degrade substrates of the extracellular matrix [141]. MMP-2, −3 and −9 have been described in cerebral ischemia, but MMP-9 appears to be expressed in traditional immune cells. Neutrophilic MMP-9 expression after stroke correlates with worse outcome [165], and studies of bone marrow chimeras suggests that MMP-9 derived from circulating leukocytes contributes significantly to its pathology [166]. Hypothermia reduces proteolytic activities of MMPs and consequent degradation of vascular basement membrane proteins [164] and degradation the extracellular matrix [163]. It also prevents the degradation of extracellular matrix proteins agrin and laminin, both targets of activated MMPs [164]. In addition to suppressing MMPs, hypothermia has been shown to increase expression of endogenous MMP inhibitors, such as tissue inhibitor of metalloproteinase-2 (TIMP-2) [126].
BBB disruption increases brain edema through breakdown of the brain’s water balance. A water channel family, the aquaporins, facilitates water flux through the plasma membrane of many cell types. In rodent brain, several studies have demonstrated the presence of different types of aquaporins. Among these aquaporins, aquaprin-4 (AQP4) is the predominant subtype in the CNS microvasculature, and is present on astrocytic end-feet in contact with brain vessels. AQP4 expression is increased in reactive astrocytes in cerebral lesions [115,167], and its deficiency has been shown to reduce brain edema [168]. Mild hypothermia also reduces brain edema by suppressing AQP4 expression in models of intracerebral hemorrhage [56] and cardiac arrest [169]. In brief, by preserving structural proteins and cells constituting the BBB, and inhibiting the activation of damaging proteases and preventing the opening of water channels, hypothermia prevents secondary brain injury from brain edema and hemorrhage.
In addition to water channel regulation, hypothermia also has been shown to affect molecular transport across the BBB. One study demonstrated that hypothermia decreased multi-drug resistance protein-1 (MDR-1) mediated transport but did not show any effect on passive diffusion and paracellular transport [2]. MDR-1 is a type of transport protein that mobilizes drugs and metabolites through transcellular pathways. The finding that hypothermia can affect drug transport points to a need for further pharmacokinetic studies under hypothermic conditions, since transport of drugs may be impacted by cooling. In particular, drugs that may be co-administered during cooling should be studied for any temperature dependent changes in pharmacokinetics.
4.4. Late Phase Molecular Events
Studies investigating the long-term impact of hypothermia include observations made weeks to months post cooling, well after cooling has ceased. Studies addressing hypothermia’s lasting effects has specifically examined on-going recovery and repair mechanisms particularly in focal cerebral ischemia and traumatic brain injury models [170]. Though research has yet to reach a consensus on the matter, studies have identified correlations between therapeutic hypothermia and the injured brain’s regenerative capacity, stem-cell retention, neuronal synaptic connectivity repair, and neurogenesis as well as gliogenesis and angiogenesis.
Neurons in the injured brain are known to change morphology and lose synaptic connectivity as they undergo cell death [171]. In parallel, endogenous recovery mechanisms are also activated after injury, leading to some neurogenesis and synaptogenesis. Though neurogenesis appears rarely in the injured brain [172], rodent studies have shown that acute brain insults initiate proliferation of neural stem cells in the subventricular and subgranular zones [171]. Although spontaneous recovery by neurogenesis is limited following brain injury, there is an obvious need to develop strategies to improve regenerative processes including proliferation of neuronal precursor cells, migration of precursor cells to the injury area, differentiation into mature neurons and the re-establishment of connections between neurons [2]. While these endogenous responses are evidently not sufficient to fully restore neurological function following various brain insults, selective enhancement of some of these processes may serve as a useful therapeutic approach.
To date, the relationship between hypothermia and neurogenesis has only been studied by a few groups and is far from clear. Studies examining mild hypothermia in cultured neural stem cells showed decreased apoptosis, increased nestin-positive cells, and inhibition of stem cell differentiation into astrocytes [173], suggesting an overall inductive role for hypothermia in neurogenesis. However, the effects of hypothermia on neurogenesis have also been shown to vary according to conditions such as brain age, injured vs. non-injured state, and the severity or duration of hypothermia. One study in the developing brain showed that cooling to 30°C for 21 hours decreased numbers of proliferating cells in the subgranular zone of the hippocampus but not in the periventricular zone [174]. Following hypoxic-ischemic injury, cooling the developing brain to 33°C showed an increase in neural progenitor cell differentiation in the striatum, as well as protection of proliferating neural stem cells in response to ischemic stimuli [175]. Studies in global cerebral ischemia models showed that mild hypothermia increased newborn neurons in the dentate gyrus relative to those of normothermic injured animals [176]. Another study involving global cerebral ischemia in adult rats showed that hypothermia had no effect on neurogenesis [177]. This latter study employed a similar model of global cerebral ischemia, but used a shorter cooling duration (33°C for 45 minutes). The findings in the adult brain suggest that hypothermia may only influence neurogenesis within specific time windows, but these exact time windows remain poorly defined.
Reports of hypothermia’s effects on endogenous cell genesis in injured relative to uninjured brains have been inconsistent. There are conflicting reports as to whether hypothermia suppresses stem cell proliferation [174,178] or induces it [173,176]. Some investigators even suggest hypothermia may preferentially promote cell differentiation toward neurogenesis over gliogenesis [173,175]. Studies also indicate that the effect of hypothermia on gliogenesis is dependent upon cooling temperature. Cooling to temperatures lower than 30°C has been shown to induce apoptosis/necrosis and cell cycle arrest as a result of reduced energy supply, thereby suppressing cell proliferation [174,179]. In contrast, mild hypothermia has been shown to protect against progenitor cell death [173,178].
Brain injury studies have found that gliogenesis and angiogenesis contributes to brain recovery following various insults [171,180]. However, the role of newborn astrocytes and vascular cells post insult has not been studied extensively. Astrocytes comprise the largest population of cells in the brain [181], and glial scar formation following injury is thought to obstruct new neurite outgrowth [182,183]. However, the inhibition of astrocytic activation and related downstream processes can exacerbate injury responses including inflammation [183]. Mild hypothermia has also been observed to increase angiogenic signals in stroke [184], spinal cord injury [185], and traumatic brain injury models [186], but the significance of these observations has yet to be clarified.
Although less understood, oligodendrocytes are known to respond to brain injury in a manner similar to neurons. Hypothermia has also been shown to attenuate trauma-induced oligodendrocyte death, demyelination, and circuit disruption [187]. Hypothermia improved survival in primary cultures of mouse oligodendrocyte precursor cells [188], demonstrating that cooling can help the prenatal brain retain greater numbers of actively replicating, less-differentiated oligodendrocyte precursor cells.
Few studies have examined the role of hypothermia on neuronal circuit repair. Repair of synaptic connectivity is crucial to functional recovery after brain injury. Deep hypothermia (17°C) showed neurite and axonal outgrowth in brain slices [137,189], suggesting that cooling may have a restorative effect on cell morphology. Mild hypothermia has also been shown to significantly alter hippocampal gene expression to favor synpaptogenesis in rat brains after traumatic brain injury. One study identified 133 transcripts altered by brain injury whose expression profiles were statistically different between hypothermic and normothermic groups. Of the 57 transcripts upregulated by hypothermia, especially prominent increases were observed among genes related to synapse organization and biogenesis.
While the full effect of cooling in brain repair is still unclear, current research suggests that therapeutic hypothermia may have a beneficial role under specific conditions, whether by protecting stem cells, promoting their proliferation and differentiation, increasing growth-factor signaling, and encouraging recovery of neural circuitry.
5. THERAPEUTIC HYPOTHERMIA AS A MODEL OF NEUROPROTECTION
Although TH is a promising therapeutic starategy shown to improve neurological outcome for certain clinical conditions, it is not always practical or feasible to cool many patients with acute cerebral injuries. However, TH might also be described as a ‘model of neuroprotection’ where therapeutic strategies could be identified, taking into consideration some of the benefits of hypothermia without the risks or challenges.
Protective mechanism of hypothermia appears to affect multiple aspects of ischemia pathogenesis. Therefore, it may behoove investigators to identify drugs that may also have similar multi-faceted properties [2,47]. Representative of such multi-faceted drugs are minocycline and fingolimod .
Like TH, minocycline, which is semi-synthetic tetracycline antibiotic, confers robust neuroprotective effects through similar mechanisms as cooling. Minocyline has been shown to attenuate neuro-inflammation, reduction of the matrix metalloproteinases (MMPs) and apoptosis [190–194]. Minocycline is also available in an oral form, and there is already abundant clinical experience in terms of dosing and safety. A few small studies of minocycline in stroke patients was shown to improve neurological outcome [50,195]. The efficacy of minocycline in combination with alteplase (rt-PA) in acute ischemic stroke patients is ongoing. An early phase dose-finding trial, the “Minocycline to improve Neurological Outcome in Stroke trial” (MINOS) [196,197], showed that intravenous minocycline was safe and well tolerated when administrated alone or concurrently with rt-PA. In addition to these trials, there is an ongoing trial [198] of minocycline plus rt-PA in acute ischemic stroke. One clinical trial investigating the efficacy of minocycline in patients with cerebral hemorrhage is also on-going[199]. However, the efficacy of minocycline in various cerebral injuries requires further investigation in larger phase III clinical trials.
Another representative drug which can provide neuroprotection through multifaceted effects is fingolimod. Fingolimod is an immune modulator that non-selectively targets the sphingosine-1-phosphate (S1P) receptor, and has been approved for the treatment of multiple sclerosis by the US Food and Drug Administration [200]. In brain injury models, there appears to be a similar beneficial effect of fingolimod treatment [201–204]. Its protective mechanism is thought to be related to the stabilization of the BBB [205], inhibition of lymphocyte migration [206] and reduction of neuronal apoptosis with upregulation of survival pathways [207]. In ischemic stroke patients, Fu et al. [208,209] carried out a series of trials to explore the efficacy of fingolimod. They found that fingolimod exhibited neuroprotective effects when combined with rt-PA, and led to decreased infarct volume and improved neurological function. Moreover, smaller hemorrhage volumes resulted in patients treated with rt-PA plus fingolimod. However, its long-term effects are still unclear, and any adverse effects due to immunosuppression or cardiac effects need to be clarified. Large-scale trials are warranted in stroke and related conditions. With increasing availability of gene profiling technologies, it is possible to identify large numbers of genes and changes in those genes in a relatively short period of time. In work by Kobayashi and colleagues [210], rats were subjected to cerebral ischemic injury under normothermic, hypothermic or hyperthermic conditions. Brain samples were subjected to gene profiling which identified 33 genes that were temperature sensitive. Many of these genes and their gene products had been studied in the scientific literature previously, and cooling seemed to lead to the suppression of genes, most of which have been described as being neurotoxic. However, cooling also led to the upregulation of several cytoprotective genes. Interestingly, many of the genes altered by hypothermia were similarly changed by preconditioning, an endogenous defense mechanism. Thus, future research may focus on the significance of these genes in acute cerebral insults, and whether their modulation represents a potential therapeutic target by the development of novel drugs.
However, not all protein and gene changes observed by cooling translate into an instant therapeutic target. One example of this is the 70 kD heat shock protein (HSP70), which is known as stress related protein [106]. While HSP70 is upregulated in the various acute cerebral injuries and is known to protect brain from its injuries [211–213], previous studies reported that hypothermia down-regulated its expression [214,215]. Thus, validation of the significance of observed changes by hypothermia would be obviously needed.
6. CONCLUSION
Hypothermia has long been known to be a potent neuroprotective intervention that preserves tissues and limits injury after various acute cerebral insults. Experimental evidence and clinical experience show that induced hypothermia affects nearly every metabolic, molecular and cellular event in cell death to promote tissue preservation. The multiple ways therapeutic hypothermia affects protection has shown that the goal of neuroprotection requires multi-target approaches. It may also be possible to extend the therapeutic window for other neuroprotective treatments by hypothermia, and combination therapies with neuroprotective drugs such as anti-inflammatory, anti-apoptotic and thrombolytic agents. This effect of hypothermia may lead to the re-examination of the many failed neuroprotectant drugs at the clinical level, since many drugs may not have been studied under optimal conditions.
In spite of the robust protective effect demonstrated in the laboratory, there are still clinical obstacles to overcome, including effective cooling in humans and prevention of harmful side-effects. There is also a need to develop more sophisticated translational research tools in the lab. Animal models and the method of cooling used in the laboratory are quite different from those employed clinically. Thus, an effort to simulate the clinical condition more precisely might provide solutions for better and wider application of TH in patients.
Figure. Phase specific effect of hypothermia therapy.
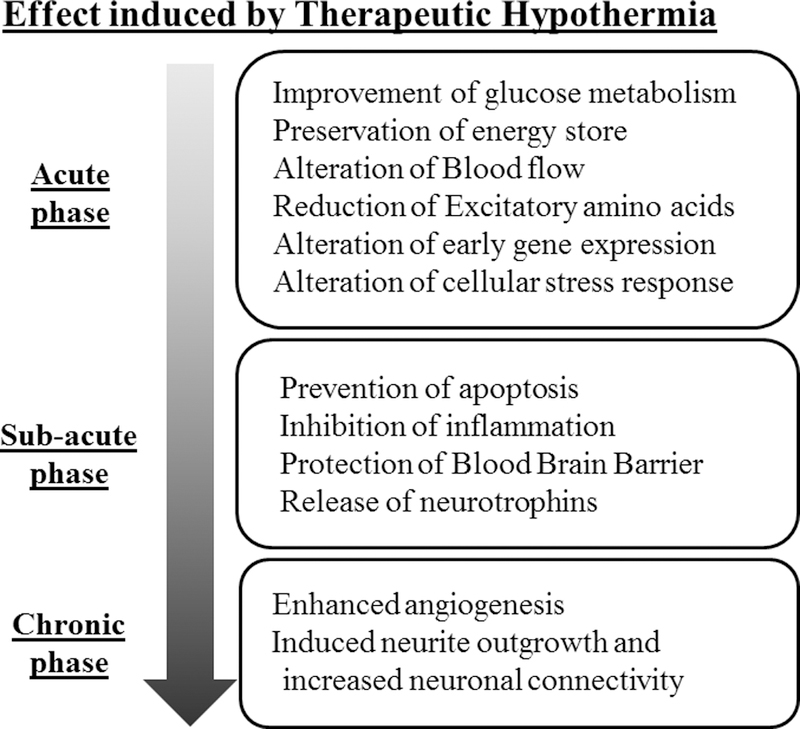
Diagram shows the different phases of neurological insults and the corresponding pathological processes that occur during that phase. Therapeutic cooling has been shown to each of these phases by influencing the pathways described.
ACKNOWLEDGEMENTS
This work was supported by grants from the Veterans Affairs Merit Program (I01 BX000589 to MY), NIH NINDS (R03 NS101246 to MY), Uehara Foundation (2016 Postdoctoral Fellowship, to KK), and National Research Foundation of Korea (NRF-2018R1C1B6006159, to JSY). Grants to MY were administered by the Northern California Institute for Research and Education, and supported by resource of the Veterans Affairs Medical Center, San Francisco, California.
Footnotes
CONFLICT OF INTEREST
There are no conflicts of interest to declare.
REFERENCES
- [1].Kurisu K, Yenari MA: Therapeutic hypothermia for ischemic stroke; pathophysiology and future promise. Neuropharmacology 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yenari MA, Han HS: Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci 2012;13:267–278. [DOI] [PubMed] [Google Scholar]
- [3].Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K: Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. The New England journal of medicine 2002;346:557–563. [DOI] [PubMed] [Google Scholar]
- [4].Hypothermia after Cardiac Arrest Study G: Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. The New England journal of medicine 2002;346:549–556. [DOI] [PubMed] [Google Scholar]
- [5].Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, Kapellou O, Levene M, Marlow N, Porter E, Thoresen M, Whitelaw A, Brocklehurst P, Group TS: Moderate hypothermia to treat perinatal asphyxial encephalopathy. The New England journal of medicine 2009;361:1349–1358. [DOI] [PubMed] [Google Scholar]
- [6].Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ: Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: Multicentre randomised trial. Lancet 2005;365:663–670. [DOI] [PubMed] [Google Scholar]
- [7].Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH, National Institute of Child H, Human Development Neonatal Research N: Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. The New England journal of medicine 2005;353:1574–1584. [DOI] [PubMed] [Google Scholar]
- [8].van der Worp HB, Macleod MR, Bath PM, Demotes J, Durand-Zaleski I, Gebhardt B, Gluud C, Kollmar R, Krieger DW, Lees KR, Molina C, Montaner J, Roine RO, Petersson J, Staykov D, Szabo I, Wardlaw JM, Schwab S, Euro HYPi: Eurohyp-1: European multicenter, randomized, phase iii clinical trial of therapeutic hypothermia plus best medical treatment vs. Best medical treatment alone for acute ischemic stroke. Int J Stroke 2014;9:642–645. [DOI] [PubMed] [Google Scholar]
- [9].Lyden P, Hemmen T, Grotta J, Rapp K, Ernstrom K, Rzesiewicz T, Parker S, Concha M, Hussain S, Agarwal S, Meyer B, Jurf J, Altafullah I, Raman R, Collaborators: Results of the ictus 2 trial (intravascular cooling in the treatment of stroke 2). Stroke 2016;47:2888–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chiu AW, Hinson HE: Future directions for hypothermia following severe traumatic brian injury. Semin Respir Crit Care Med 2017;38:768–774. [DOI] [PubMed] [Google Scholar]
- [11].Watson HI, Shepherd AA, Rhodes JKJ, Andrews PJD: Revisited: A systematic review of therapeutic hypothermia for adult patients following traumatic brain injury. Crit Care Med 2018 [DOI] [PubMed] [Google Scholar]
- [12].Meinert E, Bell MJ, Buttram S, Kochanek PM, Balasubramani GK, Wisniewski SR, Adelson PD, Pediatric Traumatic Brain Injury Consortium: Hypothermia I: Initiating nutritional support before 72 hours is associated with favorable outcome after severe traumatic brain injury in children: A secondary analysis of a randomized, controlled trial of therapeutic hypothermia. Pediatr Crit Care Med 2018;19:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Davies AR: Hypothermia improves outcome from traumatic brain injury. Crit Care Resusc 2005;7:238–243. [PubMed] [Google Scholar]
- [14].Hong JM, Lee JS, Song HJ, Jeong HS, Choi HA, Lee K: Therapeutic hypothermia after recanalization in patients with acute ischemic stroke. Stroke 2014;45:134–140. [DOI] [PubMed] [Google Scholar]
- [15].Hwang YH, Jeon JS, Kim YW, Kang DH, Kim YS, Liebeskind DS: Impact of immediate post-reperfusion cooling on outcome in patients with acute stroke and substantial ischemic changes. J Neurointerv Surg 2017;9:21–25. [DOI] [PubMed] [Google Scholar]
- [16].van der Worp HB, Macleod MR, Kollmar R, European Stroke Research Network for H: Therapeutic hypothermia for acute ischemic stroke: Ready to start large randomized trials? Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2010;30:1079–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Krieger DW, Yenari MA: Therapeutic hypothermia for acute ischemic stroke: What do laboratory studies teach us? Stroke 2004;35:1482–1489. [DOI] [PubMed] [Google Scholar]
- [18].Wu TC, Grotta JC: Hypothermia for acute ischaemic stroke. Lancet Neurol 2013;12:275–284. [DOI] [PubMed] [Google Scholar]
- [19].Lyden PD, Krieger D, Yenari M, Dietrich WD: Therapeutic hypothermia for acute stroke. Int J Stroke 2006;1:9–19. [DOI] [PubMed] [Google Scholar]
- [20].Huh PW, Belayev L, Zhao W, Koch S, Busto R, Ginsberg MD: Comparative neuroprotective efficacy of prolonged moderate intraischemic and postischemic hypothermia in focal cerebral ischemia. Journal of neurosurgery 2000;92:91–99. [DOI] [PubMed] [Google Scholar]
- [21].Maier CM, Ahern K, Cheng ML, Lee JE, Yenari MA, Steinberg GK: Optimal depth and duration of mild hypothermia in a focal model of transient cerebral ischemia: Effects on neurologic outcome, infarct size, apoptosis, and inflammation. Stroke 1998;29:2171–2180. [DOI] [PubMed] [Google Scholar]
- [22].Clark DL, Penner M, Orellana-Jordan IM, Colbourne F: Comparison of 12, 24 and 48 h of systemic hypothermia on outcome after permanent focal ischemia in rat. Exp Neurol 2008;212:386–392. [DOI] [PubMed] [Google Scholar]
- [23].Lawrence EJ, Dentcheva E, Curtis KM, Roberts VL, Siman R, Neumar RW: Neuroprotection with delayed initiation of prolonged hypothermia after in vitro transient global brain ischemia. Resuscitation 2005;64:383–388. [DOI] [PubMed] [Google Scholar]
- [24].Colbourne F, Corbett D: Delayed and prolonged post-ischemic hypothermia is neuroprotective in the gerbil. Brain research 1994;654:265–272. [DOI] [PubMed] [Google Scholar]
- [25].Shackelford RT, Hegedus SA: Factors affecting cerebral blood flow--experimental review: Sympathectomy, hypothermia, co2 inhalation and pavarine. Ann Surg 1966;163:771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hagerdal M, Harp J, Nilsson L, Siesjo BK: The effect of induced hypothermia upon oxygen consumption in the rat brain. J Neurochem 1975;24:311–316. [DOI] [PubMed] [Google Scholar]
- [27].Lee JM, Zipfel GJ, Choi DW: The changing landscape of ischaemic brain injury mechanisms. Nature 1999;399:A7–14. [DOI] [PubMed] [Google Scholar]
- [28].Colbourne F, Li H, Buchan AM: Indefatigable ca1 sector neuroprotection with mild hypothermia induced 6 hours after severe forebrain ischemia in rats. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 1999;19:742–749. [DOI] [PubMed] [Google Scholar]
- [29].Liu K, Khan H, Geng X, Zhang J, Ding Y: Pharmacological hypothermia: A potential for future stroke therapy? Neurol Res 2016;38:478–490. [DOI] [PubMed] [Google Scholar]
- [30].Fernandez-Lopez D, Faustino J, Derugin N, Wendland M, Lizasoain I, Moro MA, Vexler ZS: Reduced infarct size and accumulation of microglia in rats treated with win 55,212–2 after neonatal stroke. Neuroscience 2012;207:307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bonfils PK, Reith J, Hasseldam H, Johansen FF: Estimation of the hypothermic component in neuroprotection provided by cannabinoids following cerebral ischemia. Neurochemistry international 2006;49:508–518. [DOI] [PubMed] [Google Scholar]
- [32].Gerdeman G, Lovinger DM: Cb1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. Journal of neurophysiology 2001;85:468–471. [DOI] [PubMed] [Google Scholar]
- [33].Chi OZ, Barsoum S, Grayson J, Hunter C, Liu X, Weiss HR: Effects of cannabinoid receptor agonist win 55,212–2 on blood-brain barrier disruption in focal cerebral ischemia in rats. Pharmacology 2012;89:333–338. [DOI] [PubMed] [Google Scholar]
- [34].Zhang Z, Zhang L, Ding Y, Han Z, Ji X: Effects of therapeutic hypothermia combined with other neuroprotective strategies on ischemic stroke: Review of evidence. Aging and disease 2018;9:507–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhu S, Gao X, Huang K, Gu Y, Hu Y, Wu Y, Ji Z, Wang Q, Pan S: Glibenclamide enhances the therapeutic benefits of early hypothermia after severe stroke in rats. Aging and disease 2018;9:685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nakayama S, Taguchi N, Isaka Y, Nakamura T, Tanaka M: Glibenclamide and therapeutic hypothermia have comparable effect on attenuating global cerebral edema following experimental cardiac arrest. Neurocritical care 2018;29:119–127. [DOI] [PubMed] [Google Scholar]
- [37].Huang K, Wang Z, Gu Y, Hu Y, Ji Z, Wang S, Lin Z, Li X, Xie Z, Pan S: Glibenclamide is comparable to target temperature management in improving survival and neurological outcome after asphyxial cardiac arrest in rats. Journal of the American Heart Association 2016;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Green EJ, Pazos AJ, Dietrich WD, McCabe PM, Schneiderman N, Lin B, Busto R, Globus MY, Ginsberg MD: Combined postischemic hypothermia and delayed mk-801 treatment attenuates neurobehavioral deficits associated with transient global ischemia in rats. Brain research 1995;702:145–152. [DOI] [PubMed] [Google Scholar]
- [39].Dietrich WD, Lin B, Globus MY, Green EJ, Ginsberg MD, Busto R: Effect of delayed mk-801 (dizocilpine) treatment with or without immediate postischemic hypothermia on chronic neuronal survival after global forebrain ischemia in rats. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 1995;15:960–968. [DOI] [PubMed] [Google Scholar]
- [40].Alkan T, Kahveci N, Buyukuysal L, Korfali E, Ozluk K: Neuroprotective effects of mk 801 and hypothermia used alone and in combination in hypoxic-ischemic brain injury in neonatal rats. Archives of physiology and biochemistry 2001;109:135–144. [DOI] [PubMed] [Google Scholar]
- [41].Shuaib A, Waqar T, Wishart T, Kanthan R: Post-ischemic therapy with cgs-19755 (alone or in combination with hypothermia) in gerbils. Neuroscience letters 1995;191:87–90. [DOI] [PubMed] [Google Scholar]
- [42].Shuaib A, Ijaz S, Mazagri R, Senthilsevlvan A: Cgs-19755 is neuroprotective during repetitive ischemia: This effect is significantly enhanced when combined with hypothermia. Neuroscience 1993;56:915–920. [DOI] [PubMed] [Google Scholar]
- [43].Campbell K, Meloni BP, Knuckey NW: Combined magnesium and mild hypothermia (35 degrees c) treatment reduces infarct volumes after permanent middle cerebral artery occlusion in the rat at 2 and 4, but not 6 h. Brain research 2008;1230:258–264. [DOI] [PubMed] [Google Scholar]
- [44].Song W, Wu YM, Ji Z, Ji YB, Wang SN, Pan SY: Intra-carotid cold magnesium sulfate infusion induces selective cerebral hypothermia and neuroprotection in rats with transient middle cerebral artery occlusion. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 2013;34:479–486. [DOI] [PubMed] [Google Scholar]
- [45].Meloni BP, Cross JL, Brookes LM, Clark VW, Campbell K, Knuckey NW: Fast-mag protocol with or without mild hypothermia (35 degrees c) does not improve outcome after permanent mcao in rats. Magnesium research 2013;26:67–73. [DOI] [PubMed] [Google Scholar]
- [46].Nito C, Kamiya T, Ueda M, Arii T, Katayama Y: Mild hypothermia enhances the neuroprotective effects of fk506 and expands its therapeutic window following transient focal ischemia in rats. Brain research 2004;1008:179–185. [DOI] [PubMed] [Google Scholar]
- [47].Zhou H, Huang S, Sunnassee G, Guo W, Chen J, Guo Y, Tan S: Neuroprotective effects of adjunctive treatments for acute stroke thrombolysis: A review of clinical evidence. Int J Neurosci 2017;127:1036–1046. [DOI] [PubMed] [Google Scholar]
- [48].Nagel S, Su Y, Horstmann S, Heiland S, Gardner H, Koziol J, Martinez-Torres FJ, Wagner S: Minocycline and hypothermia for reperfusion injury after focal cerebral ischemia in the rat: Effects on bbb breakdown and mmp expression in the acute and subacute phase. Brain research 2008;1188:198–206. [DOI] [PubMed] [Google Scholar]
- [49].Nito C, Kamiya T, Amemiya S, Katoh K, Katayama Y: The neuroprotective effect of a free radical scavenger and mild hypothermia following transient focal ischemia in rats. Acta neurochirurgica Supplement 2003;86:199–203. [DOI] [PubMed] [Google Scholar]
- [50].Amiri-Nikpour MR, Nazarbaghi S, Hamdi-Holasou M, Rezaei Y: An open-label evaluator-blinded clinical study of minocycline neuroprotection in ischemic stroke: Gender-dependent effect. Acta Neurol Scand 2015;131:45–50. [DOI] [PubMed] [Google Scholar]
- [51].Zhu C, Wang X, Xu F, Qiu L, Cheng X, Simbruner G, Blomgren K: Intraischemic mild hypothermia prevents neuronal cell death and tissue loss after neonatal cerebral hypoxia-ischemia. The European journal of neuroscience 2006;23:387–393. [DOI] [PubMed] [Google Scholar]
- [52].van der Worp HB, Sena ES, Donnan GA, Howells DW, Macleod MR: Hypothermia in animal models of acute ischaemic stroke: A systematic review and meta-analysis. Brain 2007;130:3063–3074. [DOI] [PubMed] [Google Scholar]
- [53].De Georgia MA, Krieger DW, Abou-Chebl A, Devlin TG, Jauss M, Davis SM, Koroshetz WJ, Rordorf G, Warach S: Cooling for acute ischemic brain damage (cool aid): A feasibility trial of endovascular cooling. Neurology 2004;63:312–317. [DOI] [PubMed] [Google Scholar]
- [54].Hemmen TM, Raman R, Guluma KZ, Meyer BC, Gomes JA, Cruz-Flores S, Wijman CA, Rapp KS, Grotta JC, Lyden PD, Investigators IC- L: Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ictus-l): Final results. Stroke 2010;41:2265–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kawai N, Kawanishi M, Okauchi M, Nagao S: Effects of hypothermia on thrombin-induced brain edema formation. Brain research 2001;895:50–58. [DOI] [PubMed] [Google Scholar]
- [56].Dai DW, Wang DS, Li KS, Mao Y, Zhang LM, Duan SR, Sheng L: [effect of local mild hypothermia on expression of aquaporin-4 following intracerebral hemorrhage in rats]. Zhonghua Yi Xue Za Zhi 2006;86:906–910. [PubMed] [Google Scholar]
- [57].Fingas M, Clark DL, Colbourne F: The effects of selective brain hypothermia on intracerebral hemorrhage in rats. Exp Neurol 2007;208:277–284. [DOI] [PubMed] [Google Scholar]
- [58].MacLellan CL, Davies LM, Fingas MS, Colbourne F: The influence of hypothermia on outcome after intracerebral hemorrhage in rats. Stroke 2006;37:1266–1270. [DOI] [PubMed] [Google Scholar]
- [59].Melmed KR, Lyden PD: Meta-analysis of pre-clinical trials of therapeutic hypothermia for intracerebral hemorrhage. Therapeutic hypothermia and temperature management 2017;7:141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Preston E, Webster J: A two-hour window for hypothermic modulation of early events that impact delayed opening of the rat blood-brain barrier after ischemia. Acta neuropathologica 2004;108:406–412. [DOI] [PubMed] [Google Scholar]
- [61].Yao Z, You C, He M: Effect and feasibility of therapeutic hypothermia in patients with hemorrhagic stroke: A systematic review and meta-analysis. World neurosurgery 2018;111:404–412 e402. [DOI] [PubMed] [Google Scholar]
- [62].Kollmar R, Staykov D, Dorfler A, Schellinger PD, Schwab S, Bardutzky J: Hypothermia reduces perihemorrhagic edema after intracerebral hemorrhage. Stroke 2010;41:1684–1689. [DOI] [PubMed] [Google Scholar]
- [63].Rincon F, Friedman DP, Bell R, Mayer SA, Bray PF: Targeted temperature management after intracerebral hemorrhage (ttm-ich): Methodology of a prospective randomized clinical trial. Int J Stroke 2014;9:646–651. [DOI] [PubMed] [Google Scholar]
- [64].Kollmar R, Juettler E, Huttner HB, Dorfler A, Staykov D, Kallmuenzer B, Schmutzhard E, Schwab S, Broessner G, investigators C: Cooling in intracerebral hemorrhage (cinch) trial: Protocol of a randomized german-austrian clinical trial. Int J Stroke 2012;7:168–172. [DOI] [PubMed] [Google Scholar]
- [65].Torok E, Klopotowski M, Trabold R, Thal SC, Plesnila N, Scholler K: Mild hypothermia (33 degrees c) reduces intracranial hypertension and improves functional outcome after subarachnoid hemorrhage in rats. Neurosurgery 2009;65:352–359; discussion 359. [DOI] [PubMed] [Google Scholar]
- [66].Schubert GA, Poli S, Mendelowitsch A, Schilling L, Thome C: Hypothermia reduces early hypoperfusion and metabolic alterations during the acute phase of massive subarachnoid hemorrhage: A laser-doppler-flowmetry and microdialysis study in rats. Journal of neurotrauma 2008;25:539–548. [DOI] [PubMed] [Google Scholar]
- [67].Kawamura Y, Yamada K, Masago A, Katano H, Matsumoto T, Mase M: Hypothermia modulates induction of hsp70 and c-jun mrna in the rat brain after subarachnoid hemorrhage. Journal of neurotrauma 2000;17:243–250. [DOI] [PubMed] [Google Scholar]
- [68].Muroi C, Frei K, El Beltagy M, Cesnulis E, Yonekawa Y, Keller E: Combined therapeutic hypothermia and barbiturate coma reduces interleukin-6 in the cerebrospinal fluid after aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol 2008;20:193–198. [DOI] [PubMed] [Google Scholar]
- [69].Todd MM, Hindman BJ, Clarke WR, Torner JC, Intraoperative Hypothermia for Aneurysm Surgery Trial I: Mild intraoperative hypothermia during surgery for intracranial aneurysm. The New England journal of medicine 2005;352:135–145. [DOI] [PubMed] [Google Scholar]
- [70].Seule MA, Muroi C, Mink S, Yonekawa Y, Keller E: Therapeutic hypothermia in patients with aneurysmal subarachnoid hemorrhage, refractory intracranial hypertension, or cerebral vasospasm. Neurosurgery 2009;64:86–92; discussion 92–83. [DOI] [PubMed] [Google Scholar]
- [71].Dietrich WD, Bramlett HM: Therapeutic hypothermia and targeted temperature management in traumatic brain injury: Clinical challenges for successful translation. Brain research 2016;1640:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Andresen M, Gazmuri JT, Marin A, Regueira T, Rovegno M: Therapeutic hypothermia for acute brain injuries. Scand J Trauma Resusc Emerg Med 2015;23:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Clifton GL, Jiang JY, Lyeth BG, Jenkins LW, Hamm RJ, Hayes RL: Marked protection by moderate hypothermia after experimental traumatic brain injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 1991;11:114–121. [DOI] [PubMed] [Google Scholar]
- [74].Bramlett HM, Dietrich WD, Green EJ, Busto R: Chronic histopathological consequences of fluid-percussion brain injury in rats: Effects of post-traumatic hypothermia. Acta neuropathologica 1997;93:190–199. [DOI] [PubMed] [Google Scholar]
- [75].Dietrich WD, Alonso O, Busto R, Globus MY, Ginsberg MD: Post-traumatic brain hypothermia reduces histopathological damage following concussive brain injury in the rat. Acta neuropathologica 1994;87:250–258. [DOI] [PubMed] [Google Scholar]
- [76].Bramlett HM, Dietrich WD: The effects of posttraumatic hypothermia on diffuse axonal injury following parasaggital fluid percussion brain injury in rats. Therapeutic hypothermia and temperature management 2012;2:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ma M, Matthews BT, Lampe JW, Meaney DF, Shofer FS, Neumar RW: Immediate short-duration hypothermia provides long-term protection in an in vivo model of traumatic axonal injury. Exp Neurol 2009;215:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Dietrich WD, Bramlett HM: The evidence for hypothermia as a neuroprotectant in traumatic brain injury. Neurotherapeutics 2010;7:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Marion DW, Penrod LE, Kelsey SF, Obrist WD, Kochanek PM, Palmer AM, Wisniewski SR, DeKosky ST: Treatment of traumatic brain injury with moderate hypothermia. The New England journal of medicine 1997;336:540–546. [DOI] [PubMed] [Google Scholar]
- [80].Jiang JY, Xu W, Li WP, Gao GY, Bao YH, Liang YM, Luo QZ: Effect of long-term mild hypothermia or short-term mild hypothermia on outcome of patients with severe traumatic brain injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2006;26:771–776. [DOI] [PubMed] [Google Scholar]
- [81].Adelson PD, Wisniewski SR, Beca J, Brown SD, Bell M, Muizelaar JP, Okada P, Beers SR, Balasubramani GK, Hirtz D, Paediatric Traumatic Brain Injury C: Comparison of hypothermia and normothermia after severe traumatic brain injury in children (cool kids): A phase 3, randomised controlled trial. Lancet Neurol 2013;12:546–553. [DOI] [PubMed] [Google Scholar]
- [82].Beca J, McSharry B, Erickson S, Yung M, Schibler A, Slater A, Wilkins B, Singhal A, Williams G, Sherring C, Butt W, Pediatric Study Group of the A, New Zealand Intensive Care Society Clinical Trials G: Hypothermia for traumatic brain injury in children-a phase ii randomized controlled trial. Crit Care Med 2015;43:1458–1466. [DOI] [PubMed] [Google Scholar]
- [83].Lei J, Gao G, Mao Q, Feng J, Wang L, You W, Jiang J, collaborators LTHt: Rationale, methodology, and implementation of a nationwide multicenter randomized controlled trial of long-term mild hypothermia for severe traumatic brain injury (the lth-1 trial). Contemp Clin Trials 2015;40:9–14. [DOI] [PubMed] [Google Scholar]
- [84].Nichol A, Gantner D, Presneill J, Murray L, Trapani T, Bernard S, Cameron P, Capellier G, Forbes A, McArthur C, Newby L, Rashford S, Rosenfeld JV, Smith T, Stephenson M, Varma D, Walker T, Webb S, Cooper DJ: Protocol for a multicentre randomised controlled trial of early and sustained prophylactic hypothermia in the management of traumatic brain injury. Crit Care Resusc 2015;17:92–100. [PubMed] [Google Scholar]
- [85].Andrews PJ, Harris BA, Murray GD: Hypothermia for intracranial hypertension after traumatic brain injury. The New England journal of medicine 2016;374:1385. [DOI] [PubMed] [Google Scholar]
- [86].Flynn LM, Rhodes J, Andrews PJ: Therapeutic hypothermia reduces intracranial pressure and partial brain oxygen tension in patients with severe traumatic brain injury: Preliminary data from the eurotherm3235 trial. Therapeutic hypothermia and temperature management 2015;5:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Martirosyan NL, Patel AA, Carotenuto A, Kalani MY, Bohl MA, Preul MC, Theodore N: The role of therapeutic hypothermia in the management of acute spinal cord injury. Clinical neurology and neurosurgery 2017;154:79–88. [DOI] [PubMed] [Google Scholar]
- [88].Ahmad FU, Wang MY, Levi AD: Hypothermia for acute spinal cord injury--a review. World neurosurgery 2014;82:207–214. [DOI] [PubMed] [Google Scholar]
- [89].Yamamoto K, Ishikawa T, Sakabe T, Taguchi T, Kawai S, Marsala M: The hydroxyl radical scavenger nicaraven inhibits glutamate release after spinal injury in rats. Neuroreport 1998;9:1655–1659. [DOI] [PubMed] [Google Scholar]
- [90].Yu WR, Westergren H, Farooque M, Holtz A, Olsson Y: Systemic hypothermia following spinal cord compression injury in the rat: An immunohistochemical study on map 2 with special reference to dendrite changes. Acta neuropathologica 2000;100:546–552. [DOI] [PubMed] [Google Scholar]
- [91].Yu WR, Westergren H, Farooque M, Holtz A, Olsson Y: Systemic hypothermia following compression injury of rat spinal cord: Reduction of plasma protein extravasation demonstrated by immunohistochemistry. Acta neuropathologica 1999;98:15–21. [DOI] [PubMed] [Google Scholar]
- [92].Chatzipanteli K, Yanagawa Y, Marcillo AE, Kraydieh S, Yezierski RP, Dietrich WD: Posttraumatic hypothermia reduces polymorphonuclear leukocyte accumulation following spinal cord injury in rats. Journal of neurotrauma 2000;17:321–332. [DOI] [PubMed] [Google Scholar]
- [93].Westergren H, Farooque M, Olsson Y, Holtz A: Spinal cord blood flow changes following systemic hypothermia and spinal cord compression injury: An experimental study in the rat using laser-doppler flowmetry. Spinal cord 2001;39:74–84. [DOI] [PubMed] [Google Scholar]
- [94].Zager EL, Ames A 3rd: Reduction of cellular energy requirements. Screening for agents that may protect against cns ischemia. Journal of neurosurgery 1988;69:568–579. [DOI] [PubMed] [Google Scholar]
- [95].Ji X, Luo Y, Ling F, Stetler RA, Lan J, Cao G, Chen J: Mild hypothermia diminishes oxidative DNA damage and pro-death signaling events after cerebral ischemia: A mechanism for neuroprotection. Frontiers in bioscience : a journal and virtual library 2007;12:1737–1747. [DOI] [PubMed] [Google Scholar]
- [96].Ohmura A, Nakajima W, Ishida A, Yasuoka N, Kawamura M, Miura S, Takada G: Prolonged hypothermia protects neonatal rat brain against hypoxic-ischemia by reducing both apoptosis and necrosis. Brain & development 2005;27:517–526. [DOI] [PubMed] [Google Scholar]
- [97].Hagerdal M, Harp J, Siesjo BK: Effect of hypothermia upon organic phosphates, glycolytic metabolites, citric acid cycle intermediates and associated amino acids in rat cerebral cortex. J Neurochem 1975;24:743–748. [PubMed] [Google Scholar]
- [98].Ehrlich MP, McCullough JN, Zhang N, Weisz DJ, Juvonen T, Bodian CA, Griepp RB: Effect of hypothermia on cerebral blood flow and metabolism in the pig. Ann Thorac Surg 2002;73:191–197. [DOI] [PubMed] [Google Scholar]
- [99].Matsumoto M, Scheller MS, Zornow MH, Strnat MA: Effect of s-emopamil, nimodipine, and mild hypothermia on hippocampal glutamate concentrations after repeated cerebral ischemia in rabbits. Stroke 1993;24:1228–1234. [DOI] [PubMed] [Google Scholar]
- [100].Busto R, Dietrich WD, Globus MY, Valdes I, Scheinberg P, Ginsberg MD: Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 1987;7:729–738. [DOI] [PubMed] [Google Scholar]
- [101].Colbourne F, Grooms SY, Zukin RS, Buchan AM, Bennett MV: Hypothermia rescues hippocampal ca1 neurons and attenuates down-regulation of the ampa receptor glur2 subunit after forebrain ischemia. Proc Natl Acad Sci U S A 2003;100:2906–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Kim JY, Kim N, Yenari MA, Chang W: Mild hypothermia suppresses calcium-sensing receptor (casr) induction following forebrain ischemia while increasing gaba-b receptor 1 (gaba-b-r1) expression. Transl Stroke Res 2011;2:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Ginsberg MD, Sternau LL, Globus MY, Dietrich WD, Busto R: Therapeutic modulation of brain temperature: Relevance to ischemic brain injury. Cerebrovasc Brain Metab Rev 1992;4:189–225. [PubMed] [Google Scholar]
- [104].Kamme F, Campbell K, Wieloch T: Biphasic expression of the fos and jun families of transcription factors following transient forebrain ischaemia in the rat. Effect of hypothermia. The European journal of neuroscience 1995;7:2007–2016. [DOI] [PubMed] [Google Scholar]
- [105].Yenari MA, Liu J, Zheng Z, Vexler ZS, Lee JE, Giffard RG: Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann N Y Acad Sci 2005;1053:74–83. [DOI] [PubMed] [Google Scholar]
- [106].Terao Y, Miyamoto S, Hirai K, Kamiguchi H, Ohta H, Shimojo M, Kiyota Y, Asahi S, Sakura Y, Shintani Y: Hypothermia enhances heat-shock protein 70 production in ischemic brains. Neuroreport 2009;20:745–749. [DOI] [PubMed] [Google Scholar]
- [107].Truettner JS, Alonso OF, Bramlett HM, Dietrich WD: Therapeutic hypothermia alters microrna responses to traumatic brain injury in rats. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2011;31:1897–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Tang XN, Yenari MA: Hypothermia as a cytoprotective strategy in ischemic tissue injury. Ageing Res Rev 2010;9:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Holcik M, Lefebvre C, Yeh C, Chow T, Korneluk RG: A new internal-ribosome-entry-site motif potentiates xiap-mediated cytoprotection. Nat Cell Biol 1999;1:190–192. [DOI] [PubMed] [Google Scholar]
- [110].Liu A, Zhang Z, Li A, Xue J: Effects of hypothermia and cerebral ischemia on cold-inducible rna-binding protein mrna expression in rat brain. Brain research 2010;1347:104–110. [DOI] [PubMed] [Google Scholar]
- [111].Chip S, Zelmer A, Ogunshola OO, Felderhoff-Mueser U, Nitsch C, Buhrer C, Wellmann S: The rna-binding protein rbm3 is involved in hypothermia induced neuroprotection. Neurobiol Dis 2011;43:388–396. [DOI] [PubMed] [Google Scholar]
- [112].Green DR, Reed JC: Mitochondria and apoptosis. Science 1998;281:1309–1312. [DOI] [PubMed] [Google Scholar]
- [113].Ashkenazi A, Dixit VM: Death receptors: Signaling and modulation. Science 1998;281:1305–1308. [DOI] [PubMed] [Google Scholar]
- [114].Prakasa Babu P, Yoshida Y, Su M, Segura M, Kawamura S, Yasui N: Immunohistochemical expression of bcl-2, bax and cytochrome c following focal cerebral ischemia and effect of hypothermia in rat. Neuroscience letters 2000;291:196–200. [DOI] [PubMed] [Google Scholar]
- [115].Slikker W 3rd, Desai VG, Duhart H, Feuers R, Imam SZ: Hypothermia enhances bcl-2 expression and protects against oxidative stress-induced cell death in chinese hamster ovary cells. Free Radic Biol Med 2001;31:405–411. [DOI] [PubMed] [Google Scholar]
- [116].Zhang Z, Sobel RA, Cheng D, Steinberg GK, Yenari MA: Mild hypothermia increases bcl-2 protein expression following global cerebral ischemia. Brain Res Mol Brain Res 2001;95:75–85. [DOI] [PubMed] [Google Scholar]
- [117].Inamasu J, Suga S, Sato S, Horiguchi T, Akaji K, Mayanagi K, Kawase T: Postischemic hypothermia attenuates apoptotic cell death in transient focal ischemia in rats. Acta neurochirurgica Supplement 2000;76:525–527. [DOI] [PubMed] [Google Scholar]
- [118].Yenari MA, Iwayama S, Cheng D, Sun GH, Fujimura M, Morita-Fujimura Y, Chan PH, Steinberg GK: Mild hypothermia attenuates cytochrome c release but does not alter bcl-2 expression or caspase activation after experimental stroke. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2002;22:29–38. [DOI] [PubMed] [Google Scholar]
- [119].Phanithi PB, Yoshida Y, Santana A, Su M, Kawamura S, Yasui N: Mild hypothermia mitigates post-ischemic neuronal death following focal cerebral ischemia in rat brain: Immunohistochemical study of fas, caspase-3 and tunel. Neuropathology 2000;20:273–282. [DOI] [PubMed] [Google Scholar]
- [120].Xu L, Yenari MA, Steinberg GK, Giffard RG: Mild hypothermia reduces apoptosis of mouse neurons in vitro early in the cascade. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2002;22:21–28. [DOI] [PubMed] [Google Scholar]
- [121].Bright R, Raval AP, Dembner JM, Perez-Pinzon MA, Steinberg GK, Yenari MA, Mochly-Rosen D: Protein kinase c delta mediates cerebral reperfusion injury in vivo. J Neurosci 2004;24:6880–6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Raval AP, Dave KR, Prado R, Katz LM, Busto R, Sick TJ, Ginsberg MD, Mochly-Rosen D, Perez-Pinzon MA: Protein kinase c delta cleavage initiates an aberrant signal transduction pathway after cardiac arrest and oxygen glucose deprivation. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2005;25:730–741. [DOI] [PubMed] [Google Scholar]
- [123].Lee SM, Zhao H, Maier CM, Steinberg GK: The protective effect of early hypothermia on pten phosphorylation correlates with free radical inhibition in rat stroke. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2009;29:1589–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Shimohata T, Zhao H, Steinberg GK: Epsilon pkc may contribute to the protective effect of hypothermia in a rat focal cerebral ischemia model. Stroke 2007;38:375–380. [DOI] [PubMed] [Google Scholar]
- [125].Hamann GF, Burggraf D, Martens HK, Liebetrau M, Jager G, Wunderlich N, DeGeorgia M, Krieger DW: Mild to moderate hypothermia prevents microvascular basal lamina antigen loss in experimental focal cerebral ischemia. Stroke 2004;35:764–769. [DOI] [PubMed] [Google Scholar]
- [126].Lee JE, Yoon YJ, Moseley ME, Yenari MA: Reduction in levels of matrix metalloproteinases and increased expression of tissue inhibitor of metalloproteinase-2 in response to mild hypothermia therapy in experimental stroke. Journal of neurosurgery 2005;103:289–297. [DOI] [PubMed] [Google Scholar]
- [127].Liu L, Kim JY, Koike MA, Yoon YJ, Tang XN, Ma H, Lee H, Steinberg GK, Lee JE, Yenari MA: Fasl shedding is reduced by hypothermia in experimental stroke. J Neurochem 2008;106:541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Kim JY, Kim N, Lee JE, Yenari MA: Hypothermia identifies dynamin as a potential therapeutic target in experimental stroke. Therapeutic hypothermia and temperature management 2017;7:171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G: Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999;397:441–446. [DOI] [PubMed] [Google Scholar]
- [130].Zhao H, Wang JQ, Shimohata T, Sun G, Yenari MA, Sapolsky RM, Steinberg GK: Conditions of protection by hypothermia and effects on apoptotic pathways in a rat model of permanent middle cerebral artery occlusion. Journal of neurosurgery 2007;107:636–641. [DOI] [PubMed] [Google Scholar]
- [131].Shi GD, OuYang YP, Shi JG, Liu Y, Yuan W, Jia LS: Pten deletion prevents ischemic brain injury by activating the mtor signaling pathway. Biochem Biophys Res Commun 2011;404:941–945. [DOI] [PubMed] [Google Scholar]
- [132].Zhao H, Steinberg GK, Sapolsky RM: General versus specific actions of mild-moderate hypothermia in attenuating cerebral ischemic damage. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2007;27:1879–1894. [DOI] [PubMed] [Google Scholar]
- [133].Vosler PS, Logue ES, Repine MJ, Callaway CW: Delayed hypothermia preferentially increases expression of brain-derived neurotrophic factor exon iii in rat hippocampus after asphyxial cardiac arrest. Brain Res Mol Brain Res 2005;135:21–29. [DOI] [PubMed] [Google Scholar]
- [134].D’Cruz BJ, Fertig KC, Filiano AJ, Hicks SD, DeFranco DB, Callaway CW: Hypothermic reperfusion after cardiac arrest augments brain-derived neurotrophic factor activation. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2002;22:843–851. [DOI] [PubMed] [Google Scholar]
- [135].Schmidt KM, Repine MJ, Hicks SD, DeFranco DB, Callaway CW: Regional changes in glial cell line-derived neurotrophic factor after cardiac arrest and hypothermia in rats. Neuroscience letters 2004;368:135–139. [DOI] [PubMed] [Google Scholar]
- [136].Boris-Moller F, Kamme F, Wieloch T: The effect of hypothermia on the expression of neurotrophin mrna in the hippocampus following transient cerebral ischemia in the rat. Brain Res Mol Brain Res 1998;63:163–173. [DOI] [PubMed] [Google Scholar]
- [137].Schmitt KR, Diestel A, Lehnardt S, Schwartlander R, Lange PE, Berger F, Ullrich O, Abdul-Khaliq H: Hypothermia suppresses inflammation via erk signaling pathway in stimulated microglial cells. J Neuroimmunol 2007;189:7–16. [DOI] [PubMed] [Google Scholar]
- [138].Zhao H, Shimohata T, Wang JQ, Sun G, Schaal DW, Sapolsky RM, Steinberg GK: Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci 2005;25:9794–9806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Mehta SL, Manhas N, Raghubir R: Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev 2007;54:34–66. [DOI] [PubMed] [Google Scholar]
- [140].Kim JY, Kawabori M, Yenari MA: Innate inflammatory responses in stroke: Mechanisms and potential therapeutic targets. Curr Med Chem 2014;21:2076–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Wang Q, Tang XN, Yenari MA: The inflammatory response in stroke. J Neuroimmunol 2007;184:53–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Ceulemans AG, Zgavc T, Kooijman R, Hachimi-Idrissi S, Sarre S, Michotte Y: The dual role of the neuroinflammatory response after ischemic stroke: Modulatory effects of hypothermia. J Neuroinflammation 2010;7:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Rivest S: Regulation of innate immune responses in the brain. Nat Rev Immunol 2009;9:429–439. [DOI] [PubMed] [Google Scholar]
- [144].Zheng Z, Yenari MA: Post-ischemic inflammation: Molecular mechanisms and therapeutic implications. Neurol Res 2004;26:884–892. [DOI] [PubMed] [Google Scholar]
- [145].Ransohoff RM: Immunology: Barrier to electrical storms. Nature 2009;457:155–156. [DOI] [PubMed] [Google Scholar]
- [146].Van Hemelrijck A, Vermijlen D, Hachimi-Idrissi S, Sarre S, Ebinger G, Michotte Y: Effect of resuscitative mild hypothermia on glutamate and dopamine release, apoptosis and ischaemic brain damage in the endothelin-1 rat model for focal cerebral ischaemia. J Neurochem 2003;87:66–75. [DOI] [PubMed] [Google Scholar]
- [147].Patel AR, Ritzel R, McCullough LD, Liu F: Microglia and ischemic stroke: A double-edged sword. Int J Physiol Pathophysiol Pharmacol 2013;5:73–90. [PMC free article] [PubMed] [Google Scholar]
- [148].Ghosh S, May MJ, Kopp EB: Nf-kappa b and rel proteins: Evolutionarily conserved mediators of immune responses. Annu Rev Immunol 1998;16:225–260. [DOI] [PubMed] [Google Scholar]
- [149].Yilmaz G, Granger DN: Cell adhesion molecules and ischemic stroke. Neurol Res 2008;30:783–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Yenari MA, Han HS: Influence of hypothermia on post-ischemic inflammation: Role of nuclear factor kappa b (nfkappab). Neurochemistry international 2006;49:164–169. [DOI] [PubMed] [Google Scholar]
- [151].Choi JS, Park J, Suk K, Moon C, Park YK, Han HS: Mild hypothermia attenuates intercellular adhesion molecule-1 induction via activation of extracellular signal-regulated kinase-1/2 in a focal cerebral ischemia model. Stroke Res Treat 2011;2011:846716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Tong G, Krauss A, Mochner J, Wollersheim S, Soltani P, Berger F, Schmitt KRL: Deep hypothermia therapy attenuates lps-induced microglia neuroinflammation via the stat3 pathway. Neuroscience 2017;358:201–210. [DOI] [PubMed] [Google Scholar]
- [153].Trendelenburg G: Acute neurodegeneration and the inflammasome: Central processor for danger signals and the inflammatory response? Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2008;28:867–881. [DOI] [PubMed] [Google Scholar]
- [154].Matsui T, Kakeda T: Il-10 production is reduced by hypothermia but augmented by hyperthermia in rat microglia. Journal of neurotrauma 2008;25:709–715. [DOI] [PubMed] [Google Scholar]
- [155].Sugawara T, Chan PH: Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxid Redox Signal 2003;5:597–607. [DOI] [PubMed] [Google Scholar]
- [156].Wong CH, Crack PJ: Modulation of neuro-inflammation and vascular response by oxidative stress following cerebral ischemia-reperfusion injury. Curr Med Chem 2008;15:1–14. [DOI] [PubMed] [Google Scholar]
- [157].Globus MY, Busto R, Lin B, Schnippering H, Ginsberg MD: Detection of free radical activity during transient global ischemia and recirculation: Effects of intraischemic brain temperature modulation. J Neurochem 1995;65:1250–1256. [DOI] [PubMed] [Google Scholar]
- [158].Maier CM, Sun GH, Cheng D, Yenari MA, Chan PH, Steinberg GK: Effects of mild hypothermia on superoxide anion production, superoxide dismutase expression, and activity following transient focal cerebral ischemia. Neurobiol Dis 2002;11:28–42. [DOI] [PubMed] [Google Scholar]
- [159].Liu L, Yenari MA: Therapeutic hypothermia: Neuroprotective mechanisms. Frontiers in bioscience : a journal and virtual library 2007;12:816–825. [DOI] [PubMed] [Google Scholar]
- [160].Moro MA, Cardenas A, Hurtado O, Leza JC, Lizasoain I: Role of nitric oxide after brain ischaemia. Cell Calcium 2004;36:265–275. [DOI] [PubMed] [Google Scholar]
- [161].Deng H, Han HS, Cheng D, Sun GH, Yenari MA: Mild hypothermia inhibits inflammation after experimental stroke and brain inflammation. Stroke 2003;34:2495–2501. [DOI] [PubMed] [Google Scholar]
- [162].Dietrich WD, Busto R, Halley M, Valdes I: The importance of brain temperature in alterations of the blood-brain barrier following cerebral ischemia. J Neuropathol Exp Neurol 1990;49:486–497. [DOI] [PubMed] [Google Scholar]
- [163].Kawanishi M, Kawai N, Nakamura T, Luo C, Tamiya T, Nagao S: Effect of delayed mild brain hypothermia on edema formation after intracerebral hemorrhage in rats. J Stroke Cerebrovasc Dis 2008;17:187–195. [DOI] [PubMed] [Google Scholar]
- [164].Baumann E, Preston E, Slinn J, Stanimirovic D: Post-ischemic hypothermia attenuates loss of the vascular basement membrane proteins, agrin and sparc, and the blood-brain barrier disruption after global cerebral ischemia. Brain research 2009;1269:185–197. [DOI] [PubMed] [Google Scholar]
- [165].Montaner J, Alvarez-Sabin J, Molina C, Angles A, Abilleira S, Arenillas J, Gonzalez MA, Monasterio J: Matrix metalloproteinase expression after human cardioembolic stroke: Temporal profile and relation to neurological impairment. Stroke 2001;32:1759–1766. [DOI] [PubMed] [Google Scholar]
- [166].Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD, Chan PH, Park TS: Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol 2005;289:H558–568. [DOI] [PubMed] [Google Scholar]
- [167].Kurisu K, Abumiya T, Nakamura H, Shimbo D, Shichinohe H, Nakayama N, Kazumata K, Shimizu H, Houkin K: Transarterial regional brain hypothermia inhibits acute aquaporin-4 surge and sequential microvascular events in ischemia/reperfusion injury. Neurosurgery 2016;79:125–134. [DOI] [PubMed] [Google Scholar]
- [168].Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS: Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med 2000;6:159–163. [DOI] [PubMed] [Google Scholar]
- [169].Xiao F, Arnold TC, Zhang S, Brown C, Alexander JS, Carden DL, Conrad SA: Cerebral cortical aquaporin-4 expression in brain edema following cardiac arrest in rats. Acad Emerg Med 2004;11:1001–1007. [DOI] [PubMed] [Google Scholar]
- [170].Kernie SG, Parent JM: Forebrain neurogenesis after focal ischemic and traumatic brain injury. Neurobiol Dis 2010;37:267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Font MA, Arboix A, Krupinski J: Angiogenesis, neurogenesis and neuroplasticity in ischemic stroke. Curr Cardiol Rev 2010;6:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Shruster A, Melamed E, Offen D: Neurogenesis in the aged and neurodegenerative brain. Apoptosis 2010;15:1415–1421. [DOI] [PubMed] [Google Scholar]
- [173].Saito K, Fukuda N, Matsumoto T, Iribe Y, Tsunemi A, Kazama T, Yoshida-Noro C, Hayashi N: Moderate low temperature preserves the stemness of neural stem cells and suppresses apoptosis of the cells via activation of the cold-inducible rna binding protein. Brain research 2010;1358:20–29. [DOI] [PubMed] [Google Scholar]
- [174].Kanagawa T, Fukuda H, Tsubouchi H, Komoto Y, Hayashi S, Fukui O, Shimoya K, Murata Y: A decrease of cell proliferation by hypothermia in the hippocampus of the neonatal rat. Brain research 2006;1111:36–40. [DOI] [PubMed] [Google Scholar]
- [175].Xiong M, Cheng GQ, Ma SM, Yang Y, Shao XM, Zhou WH: Post-ischemic hypothermia promotes generation of neural cells and reduces apoptosis by bcl-2 in the striatum of neonatal rat brain. Neurochemistry international 2011;58:625–633. [DOI] [PubMed] [Google Scholar]
- [176].Silasi G, Colbourne F: Therapeutic hypothermia influences cell genesis and survival in the rat hippocampus following global ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2011;31:1725–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Lasarzik I, Winkelheide U, Thal SC, Benz N, Lorscher M, Jahn-Eimermacher A, Werner C, Engelhard K: Mild hypothermia has no long-term impact on postischemic neurogenesis in rats. Anesth Analg 2009;109:1632–1639. [DOI] [PubMed] [Google Scholar]
- [178].Bennet L, Roelfsema V, George S, Dean JM, Emerald BS, Gunn AJ: The effect of cerebral hypothermia on white and grey matter injury induced by severe hypoxia in preterm fetal sheep. J Physiol 2007;578:491–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Matijasevic Z, Snyder JE, Ludlum DB: Hypothermia causes a reversible, p53-mediated cell cycle arrest in cultured fibroblasts. Oncol Res 1998;10:605–610. [PubMed] [Google Scholar]
- [180].Gopurappilly R, Pal R, Mamidi MK, Dey S, Bhonde R, Das AK: Stem cells in stroke repair: Current success and future prospects. CNS Neurol Disord Drug Targets 2011;10:741–756. [DOI] [PubMed] [Google Scholar]
- [181].Li L, Harms KM, Ventura PB, Lagace DC, Eisch AJ, Cunningham LA: Focal cerebral ischemia induces a multilineage cytogenic response from adult subventricular zone that is predominantly gliogenic. Glia 2010;58:1610–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Hawthorne AL, Hu H, Kundu B, Steinmetz MP, Wylie CJ, Deneris ES, Silver J: The unusual response of serotonergic neurons after cns injury: Lack of axonal dieback and enhanced sprouting within the inhibitory environment of the glial scar. J Neurosci 2011;31:5605–5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Trendelenburg G, Dirnagl U: Neuroprotective role of astrocytes in cerebral ischemia: Focus on ischemic preconditioning. Glia 2005;50:307–320. [DOI] [PubMed] [Google Scholar]
- [184].Xie YC, Li CY, Li T, Nie DY, Ye F: Effect of mild hypothermia on angiogenesis in rats with focal cerebral ischemia. Neuroscience letters 2007;422:87–90. [DOI] [PubMed] [Google Scholar]
- [185].Kao CH, Chio CC, Lin MT, Yeh CH: Body cooling ameliorating spinal cord injury may be neurogenesis-, anti-inflammation- and angiogenesis-associated in rats. J Trauma 2011;70:885–893. [DOI] [PubMed] [Google Scholar]
- [186].Kuo JR, Lo CJ, Chang CP, Lin HJ, Lin MT, Chio CC: Brain cooling-stimulated angiogenesis and neurogenesis attenuated traumatic brain injury in rats. J Trauma 2010;69:1467–1472. [DOI] [PubMed] [Google Scholar]
- [187].Lotocki G, de Rivero Vaccari J, Alonso O, Molano JS, Nixon R, Dietrich WD, Bramlett HM: Oligodendrocyte vulnerability following traumatic brain injury in rats: Effect of moderate hypothermia. Therapeutic hypothermia and temperature management 2011;1:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Imada S, Yamamoto M, Tanaka K, Seiwa C, Watanabe K, Kamei Y, Kozuma S, Taketani Y, Asou H: Hypothermia-induced increase of oligodendrocyte precursor cells: Possible involvement of plasmalemmal voltage-dependent anion channel 1. J Neurosci Res 2010;88:3457–3466. [DOI] [PubMed] [Google Scholar]
- [189].Schmitt KR, Boato F, Diestel A, Hechler D, Kruglov A, Berger F, Hendrix S: Hypothermia-induced neurite outgrowth is mediated by tumor necrosis factor-alpha. Brain Pathol 2010;20:771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Wang X, Zhu S, Drozda M, Zhang W, Stavrovskaya IG, Cattaneo E, Ferrante RJ, Kristal BS, Friedlander RM: Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of huntington’s disease. Proc Natl Acad Sci U S A 2003;100:10483–10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Tikka TM, Koistinaho JE: Minocycline provides neuroprotection against n-methyl-d-aspartate neurotoxicity by inhibiting microglia. J Immunol 2001;166:7527–7533. [DOI] [PubMed] [Google Scholar]
- [192].Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J: Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci 2001;21:2580–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J: A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A 1999;96:13496–13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Murata Y, Rosell A, Scannevin RH, Rhodes KJ, Wang X, Lo EH: Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke 2008;39:3372–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Lampl Y, Boaz M, Gilad R, Lorberboym M, Dabby R, Rapoport A, Anca-Hershkowitz M, Sadeh M: Minocycline treatment in acute stroke: An open-label, evaluator-blinded study. Neurology 2007;69:1404–1410. [DOI] [PubMed] [Google Scholar]
- [196].Fagan SC, Waller JL, Nichols FT, Edwards DJ, Pettigrew LC, Clark WM, Hall CE, Switzer JA, Ergul A, Hess DC: Minocycline to improve neurologic outcome in stroke (minos): A dose-finding study. Stroke 2010;41:2283–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Switzer JA, Hess DC, Ergul A, Waller JL, Machado LS, Portik-Dobos V, Pettigrew LC, Clark WM, Fagan SC: Matrix metalloproteinase-9 in an exploratory trial of intravenous minocycline for acute ischemic stroke. Stroke 2011;42:2633–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Blacker DJ, Prentice D, Alvaro A, Bates TR, Bynevelt M, Kelly A, Kho LK, Kohler E, Hankey GJ, Thompson A, Major T: Reducing haemorrhagic transformation after thrombolysis for stroke: A strategy utilising minocycline. Stroke Res Treat 2013;2013:362961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Chang JJ, Kim-Tenser M, Emanuel BA, Jones GM, Chapple K, Alikhani A, Sanossian N, Mack WJ, Tsivgoulis G, Alexandrov AV, Pourmotabbed T: Minocycline and matrix metalloproteinase inhibition in acute intracerebral hemorrhage: A pilot study. Eur J Neurol 2017;24:1384–1391. [DOI] [PubMed] [Google Scholar]
- [200].Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, Burtin P, Group FS: A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. The New England journal of medicine 2010;362:387–401. [DOI] [PubMed] [Google Scholar]
- [201].Brait VH, Tarrason G, Gavalda A, Godessart N, Planas AM: Selective sphingosine 1-phosphate receptor 1 agonist is protective against ischemia/reperfusion in mice. Stroke 2016;47:3053–3056. [DOI] [PubMed] [Google Scholar]
- [202].Liu J, Zhang C, Tao W, Liu M: Systematic review and meta-analysis of the efficacy of sphingosine-1-phosphate (s1p) receptor agonist fty720 (fingolimod) in animal models of stroke. Int J Neurosci 2013;123:163–169. [DOI] [PubMed] [Google Scholar]
- [203].Gao C, Qian Y, Huang J, Wang D, Su W, Wang P, Guo L, Quan W, An S, Zhang J, Jiang R: A three-day consecutive fingolimod administration improves neurological functions and modulates multiple immune responses of cci mice. Mol Neurobiol 2017;54:8348–8360. [DOI] [PubMed] [Google Scholar]
- [204].Zhang L, Ding K, Wang H, Wu Y, Xu J: Traumatic brain injury-induced neuronal apoptosis is reduced through modulation of pi3k and autophagy pathways in mouse by fty720. Cell Mol Neurobiol 2016;36:131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Campos F, Qin T, Castillo J, Seo JH, Arai K, Lo EH, Waeber C: Fingolimod reduces hemorrhagic transformation associated with delayed tissue plasminogen activator treatment in a mouse thromboembolic model. Stroke 2013;44:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Massberg S, von Andrian UH: Fingolimod and sphingosine-1-phosphate--modifiers of lymphocyte migration. The New England journal of medicine 2006;355:1088–1091. [DOI] [PubMed] [Google Scholar]
- [207].Hasegawa Y, Suzuki H, Sozen T, Rolland W, Zhang JH: Activation of sphingosine 1-phosphate receptor-1 by fty720 is neuroprotective after ischemic stroke in rats. Stroke 2010;41:368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Fu Y, Zhang N, Ren L, Yan Y, Sun N, Li YJ, Han W, Xue R, Liu Q, Hao J, Yu C, Shi FD: Impact of an immune modulator fingolimod on acute ischemic stroke. Proc Natl Acad Sci U S A 2014;111:18315–18320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Zhu Z, Fu Y, Tian D, Sun N, Han W, Chang G, Dong Y, Xu X, Liu Q, Huang D, Shi FD: Combination of the immune modulator fingolimod with alteplase in acute ischemic stroke: A pilot trial. Circulation 2015;132:1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Kobayashi MS, Asai S, Ishikawa K, Nishida Y, Nagata T, Takahashi Y: Global profiling of influence of intra-ischemic brain temperature on gene expression in rat brain. Brain Res Rev 2008;58:171–191. [DOI] [PubMed] [Google Scholar]
- [211].Kelly S, Yenari MA: Neuroprotection: Heat shock proteins. Curr Med Res Opin 2002;18 Suppl 2:s55–60. [PubMed] [Google Scholar]
- [212].Kim JY, Kim N, Zheng Z, Lee JE, Yenari MA: 70-kda heat shock protein downregulates dynamin in experimental stroke: A new therapeutic target? Stroke 2016;47:2103–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Kim JY, Kim N, Zheng Z, Lee JE, Yenari MA: The 70 kda heat shock protein protects against experimental traumatic brain injury. Neurobiol Dis 2013;58:289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Kumar K, Wu X, Evans AT, Marcoux F: The effect of hypothermia on induction of heat shock protein (hsp)-72 in ischemic brain. Metab Brain Dis 1995;10:283–291. [DOI] [PubMed] [Google Scholar]
- [215].Lee BS, Jung E, Lee Y, Chung SH: Hypothermia decreased the expression of heat shock proteins in neonatal rat model of hypoxic ischemic encephalopathy. Cell Stress Chaperones 2017;22:409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]


