Highlights
-
•
First study on patient acceptability of patient derived cell culture models.
-
•
First study to assess the feasibility of using patient derived kidney cancer 3D tumour models.
-
•
Study will inform the design of a clinical trial in personalised tumouroid response to therapy.
Keywords: Renal cell carcinoma, Precision medicine, In vitro techniques, Drug screening assays, Personalised cancer treatment, 3D in vitro cancer models
Abstract
Introduction
‘Personalised medicine’ aims to tailor interventions to the individual, and has become one of the fastest growing areas of cancer research. One of these approaches is to harvest cancer cells from patients and grow them in the laboratory, which can then be subjected to treatments and the response assessed. We have developed a 3D tumour model with a complex protein matrix that mimics the tumour stroma, cell to cell and cell-matrix interactions seen in vivo, called a tumouroid. In this study, we test the acceptability and feasibility of using this model to establish patient-derived tumouroids.
Methods and analysis
This is a first in-human study using prospective tissue and data collection of adult participants with confirmed or suspected renal cell carcinoma. The goals of the study are to assess patient acceptability to the use of patient-derived tumour models for future treatment decisions, and to assess the feasibility of generating patient-specific renal cancer tumouroids that can be challenged with drugs. These goals will be realised through the collection of tumour samples (expected n = 10), participant-completed questionnaires (expected n = 10), and in-depth semi-structured interviews with patients (expected n = 5). Collected multiregional tumour samples will be dissociated to isolate primary cells which are then expanded in vitro and incorporated into tumouroids. Drug challenge will ensue and the response will be categorised into “responder”, “weak responder”, and “non-responder”. Statistical analysis will be descriptive.
Ethics and dissemination
The study has ethical approval (REC reference 17/LO/1744). Findings will be made available to patients, clinicians, funders, and the National Health Service (NHS) through presentations at national and international meetings, peer-reviewed publications, social media and patient support groups.
Trial registration
Registered on ClinicalTrials.gov (NCT03300102).
Strengths and limitations
-
–
This is the first study to assess patient acceptability of using patient derived cell culture models as a personalised medicine method.
-
–
This is the first study to formally assess the feasibility of using patient derived kidney cancer 3D tumour models.
-
–
The tumouroid model may not capture the full extent of in vivo tumour heterogeneity.
-
–
This study will provide feasibility and aims to will inform the design and sample size of a clinical trial of comparing personalised tumouroid response to therapy versus patient response to standard of care.
1. Introduction
1.1. Context
More than three hundred thousand people are diagnosed with cancer each year in the UK [1], resulting in an NHS cancer-related expenditure of £6.7 billion during 2012/3 [2]. These costs will rise due to increases in incidence, longevity, and use of novel, more costly treatments. For example, per patient costs for immunotherapy (such as Nivolumab [2]) is approximately £68,000 per annum. In addition, failure of first line treatments requiring second line interventions will expend time and money and may lead to disease progression, which is costly to treat.
Although the diagnostic and treatment pathways for many cancers continue to improve, discrimination between responders and non-responders is less than optimal. There is a need to develop tools that allow for better disease characterisation and stratification. The ultimate goal is to develop personalised medicine to the level whereby prevention, diagnosis, and treatment of disease is tailored to the individual to reduce side effects, improve access to effective treatment, and decrease cost [3]. Enhancing the ability to predict response to treatment may be more cost-effective than designing new therapeutic options.
The Stratified Medicine Programme [4] represents the most coordinated approach to the problem of personalised medicine in the UK. However, to date, only limited success can be claimed. The two best examples are HER2 testing (breast cancer) and K-RAS testing (colon cancer); the latter results in an increased overall survival of 0.034 years resulting in an incremental cost-effectiveness ratio of approximately $650,000 per additional year of life [5], which is equivalent to £491,855 far in excess of the NICE threshold of £20–£30 K per QALY.
This predictive approach might not be sustainable, as the transition from a morphology-based to a genetics-based taxonomy of cancer has considerable challenges. For example, recently a number of morphology-agnostic trials have been conducted whereby patient selection is solely driven by molecular alterations present within the tumour (basket trials). Reported treatment responses have been lower than anticipated [6], possibly due to an over simplification of the factors that contribute to drug response.
An alternative to prediction is verification or authentication. Cancer cells can be harvested from patients and grown in animal models, which can then be subjected to treatments and response assessed. This information can be used to inform care. A sample obtained from the patient’s tumour is implanted into mice, and allowed to grow; the implanted tumour is then tested against an array of clinically relevant agents. However, the process can take over 6 months to obtain data on tumour responsiveness, and is expensive (US$20,000 per patient) [7]. In addition, there are concerns about the ability of animal models to predict behaviour of human tumour; this was reinforced by the European Medicine Agency in 2012: ‘the absence of [non-clinical models with good predictive properties] is considered to constitute the greatest hurdle for efficient drug development within the foreseeable future’ [8].
Another approach, explored here, involves the use of 3D in vitro tumour models. Cancer cells can be harvested from patients and grown in the lab, using a system that provides an extra level of complexity to 2D in vitro studies. With these models, it is possible to recreate tumour characteristics unavailable in 2D, such as interactions between cancer cells and stromal cells, cell-matrix interaction, or hypoxia. The advantages of using 3D tumour models over xenografts could be a faster timeline to obtain data on tumour responsiveness, coupled with decreased cost and less ethical concerns. This promising new technique needs to undergo clinical validation in order to assess its use in routine care.
1.2. Preliminary work
A 3D complex tumour model – named “tumouroid”- has been developed in our laboratories. Our preliminary work used two different kidney cancer cell lines (Caki-2 and 786-O) to establish tumouroids and challenge them with therapeutic agents. This work enabled us to define ideal growth conditions, the best timings of drug and dose challenge, and how to assess drug response. A commercially available protocol was employed with modifications to increase the density of the supporting matrix (RAFT, Lonza, UK [9]). Proteins commonly found in renal tumours (collagen type I, collagen type IV, laminin and fibronectin) were added to mimic the microenvironment as closely as possible to human tumour tissue, by taking into account cell to cell and cell-matrix interactions more accurately. We then progressed to establishing patient-derived tumouroids using renal cell carcinoma samples obtained after surgery.
2. Methods and design
2.1. Study design
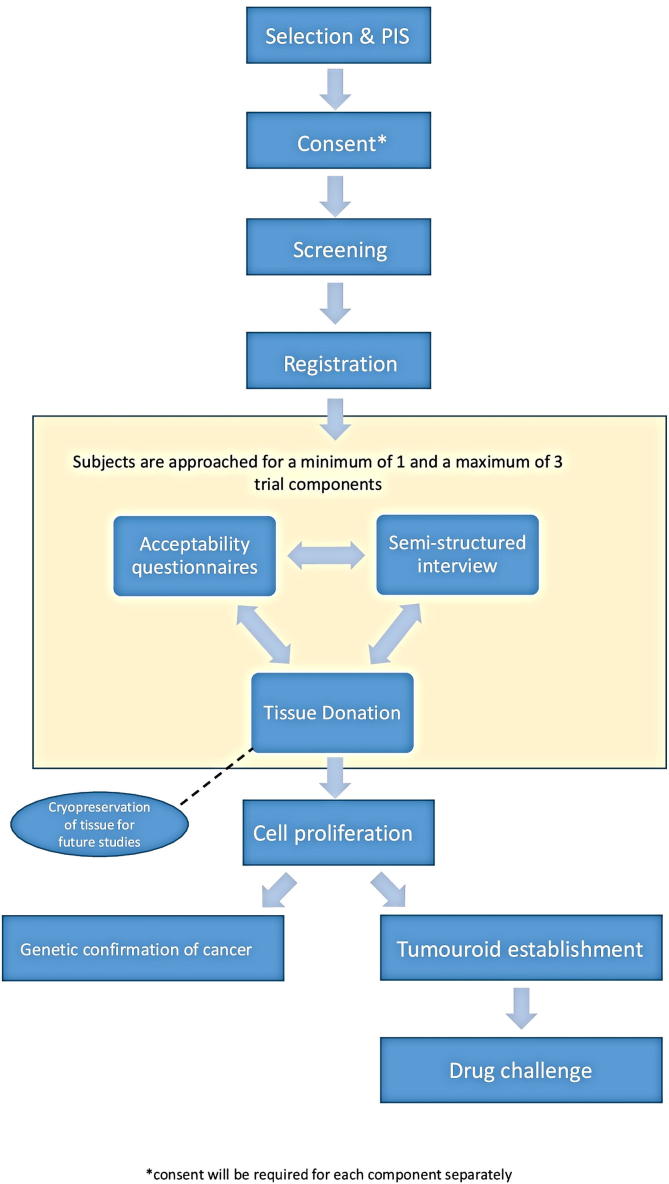
A first-in-human study using prospective tissue and data collection of participants with confirmed or suspected renal cell carcinoma in combination with questionnaires and interviews to assess acceptability (Fig. 1). Table 1 summarises and outlines the assessment points within the Tumouroids study.
Fig. 1.
Flow diagram of the Tumouroid study design.
Table 1.
Study assessments within the tumouroids study.
| Visit | Before surgery/Baseline | Surgery | After surgery/Exit |
|---|---|---|---|
| Screening | X | ||
| Informed consent | X | ||
| Demographic data, medical history, other clinical history | X | ||
| Tissue collection | X | ||
| Tumouroid establishment and drug challenge | X | ||
| Pathology assessment confirming the diagnosis of kidney cancer | X |
Participants who consent to complete either the structured questionnaire and/or to be interviewed will be asked to provide this data within 12 weeks post consent.
2.2. Study aims and outcomes
The primary objective of this study is to assess patient acceptability for the use of personalised lab-based tumour models for future treatment decision.
The secondary objective is to assess the feasibility of generating patient-specific renal cancer tumouroids to be used as platforms to test drug response, including defining the following:
-
–
Rate of success of establishing kidney cancer tumouroids from fresh tumour tissue, defined as number of participants where patient-specific tumouroids were established;
-
–
The ideal dose(s) of drug to be used when challenging the kidney cancer patient-derived tumouroids;
-
–
Number of kidney cancer tumouroids that were successfully therapeutically challenged;
-
–
The expected timeline from tissue collection to therapeutic response assessment in kidney cancer patient-derived tumouroids.
2.3. Trial population
Recruitment will be conducted over a period of eight months. Patients with suspected or confirmed renal cancer who are undergoing partial or radical nephrectomy, identified during the weekly multidisciplinary team meeting or at the time of pre-operative counselling clinics, will be approached. Only patients who have agreed to surgery as part of standard of care will be approached – no surgery will be carried out for research purposes. The eligibility criteria are as follows:
2.3.1. Inclusion criteria
-
•
Adult patients (≥18 years old), of either gender, able to provide consent;
-
•
Suspected or confirmed renal cell carcinoma;
-
•
Signed informed consent by patient.
2.3.2. Exclusion criteria
-
•
Non-English speaker;
-
•
Inability to provide informed consent.
2.4. Sample size
This pilot study will include 10 participants with confirmed or suspected renal cell carcinoma for tissue donation. Ten participants will also complete a Likert scale non-validated questionnaire looking at acceptability, and five will complete a semi-structured interview. The three groups may overlap.
2.5. Screening and enrolment
A screening log will be maintained of subjects who potentially meet the eligibility criteria identified by the clinical research team at multidisciplinary cancer team meetings and at clinic appointments. These subjects will be approached by a member of the clinical research team to have an initial conversation about the study, either face to face in clinic, over the telephone or by letter. Subjects who express an interest in participation will then be given a copy of the REC approved patient information sheet (PIS) and given time to consider the study.
Those who confirm willingness to participate will be approached to give informed consent. The subject must be given ample time (without breaching the NHS cancer waiting/treatment times) and opportunity to inquire and ask questions about the trial and to decide whether or not to participate. The right of the subject to refuse to participate in the trial, with or without giving a reason, must be respected.
Subjects who are screened but are then deemed ineligible, or those who choose not to participate in the study, will be recorded in the screening log.
2.6. Consent
Participants who sign the study consent form are deemed to be recruited into the study and will then be assigned a unique subject number. The original signed consent form will be filed in the investigator's site file, a copy given to the participant and a copy filed in the hospital case notes.
2.7. Interventions & outcomes
2.7.1. Tissue collection
The surgical specimen removed at nephrectomy will be delivered fresh (i.e. without any tissue preservatives such as formalin) from theatre to the Pathology Department at Royal Free Hospital. After assessment by a trained pathologist to ensure diagnosis is not compromised, samples of tumour and of adjacent normal kidney tissue will be extracted using a blade or a biopsy punch. These tissue samples will be delivered to the designated laboratory of the UCL Division of Surgery & Interventional Science located at Royal Free Hospital where they will be mechanically and chemically digested to generate a suspension of single cells, then transferred to common tissue culture plates to allow for cell proliferation. After up to 8 weeks of expansion, patient-specific tumouroids will be established and maintained. If cell expansion is deemed unsuccessful at 8 weeks, the experiment will be terminated without mamufacturing patient-specific tumouroids. At day 10 after tumouroid establishment, drug challenge with pazopanib will be carried out, followed by assessment of drug response at 72 h using a commercially available assay – Cell-Titer Glo 3D (Promega, USA) – that determines cell metabolism and viability. Immunofluorescent imaging will also be used to assess cell morphology. As this platform and the results of its drug challenge are experimental and have not been clinically validated, individual patient level results will not be disclosed to participants or the clinical team.
If participants have also consented to donate tissue samples of tumour and adjacent normal kidney to be used in other current or future ethically approved studies, one or more of these samples may be cryopreserved, and stored at the UCL/Royal Free Hospital biobank facility.
All procedures will follow steps described in a number of laboratory standard operating procedures (SOPs).
Each phase of the tissue model production (tumouroid establishment, therapeutic challenge, and assessment of drug response) will be scored as completed, partially completed or incomplete. Tumouroids that achieve viability and are subjected to in vitro therapeutic challenge will be scored as responder” (typically 50% cell death after drug challenge), “weak responder” (around 25% cell death after drug challenge), and “non-responder” (typically 0% but with an upper threshold of 10% cell death after drug challenge). These ranges were established using renal cancer cell line tumouroids, treated with pazopanib, using a range of different pharmacological protocols (unpublished data).
Given the diversity of cells present within tumours, assurance is required to confirm that isolated and proliferating cells are malignant. As such, after cell expansion (and prior to tumouroid establishment), DNA and/or RNA will be extracted for genetic analysis. Stored tissue will undergo the same process for comparison with the grown cells, as appropriate. The purpose of genetically testing cells before tumouroid establishment is solely to confirm that in vitro expanded cells harbour renal cancer-related mutations and thus are not expanded benign/interstitial cells. Genetic analysis will not guide the tumouroid drug challenge. The results obtained from this genetic testing may not fully represent the true genetic landscape of the tumour as it was in vivo prior to nephrectomy. This will be due, in part, to intratumoural heterogeneity [10] and because the cancer cells will have already been grown in the laboratory (thus exposed to ex vivo selective pressures that may have contributed to changing the genetic landscape of the proliferated cells). For the above reasons, patients and the clinical team will not be informed of any genetic testing results.
2.7.2. Questionnaire
Acceptability will be elicited using a Likert scale non-validated questionnaire. Questionnaire responses will be coded prior to descriptive analysis.
2.7.3. Interview
A subgroup of participants will be invited to a semi-structured interview in which the views and preferences relating to the acceptability of the laboratory-based personalised tumour models and their impact on future decision making will be explored. The interview could take place over the telephone, or face to face in a quiet clinic room, either before or after a clinic appointment, or at a time that suits the patient even if this is not on a clinic day. All transcripts will be pseudo-anonymised, the first transcripts from the semi-structured interviews will be analysed and coded by two independent researchers for themes and patterns.
2.8. Participant withdrawal
Participants may be withdrawn from the study for the following reasons:
-
•
Participant choice;
-
•
Pathology analysis after harvesting proving histologic diagnosis other than renal cell carcinoma;
-
•
Inability to collect sample due to risk of compromising pathologic staging during tissue collection.
2.9. Patient and public involvement
Patients and members of the public (including those who have had cancer) have been involved in the design of the study, and will participate in the management of the research, and analysis and dissemination of the findings.
2.10. Data collection
Baseline clinical data and demographics will be collected. After tissue collection, the pathology report and staging will be obtained. Responsibility for data collection will be taken by nominated individuals. Data will be collected using an electronic case report form (eCRF). Data will be stored centrally by the Surgical & Interventional Trials Unit (SITU) at UCL.
Data will be held according to the Data Protection Act, 2018 and General Data Protection Regulation (GDPR). Data will be pseudo-anonymised as necessary. The key to the pseudo-anonymised data will be held at site, on a secure NHS computer or if a paper file is preferred it will be kept in a fire-proof lockable cabinet. Each participant will be given a subject number and subject identifier and this will be used on all of their study records. The subject number and subject identifier will be known to site staff and the SITU team. All clinical information required will be kept in study records and analysed at the end of the study. The records will be kept in a secure manner in the research offices with access available to named individuals from the study group only. The paper records will be retained for a minimum of three years after publication of the study.
2.11. Data items
-
a)
Demographics and clinical information
-
•
Age;
-
•
Gender;
-
•
If previous diagnosis of renal cancer: date of diagnosis, staging at time of diagnosis (TNM), any previous local treatment (type and date);
-
•
Family history of first degree relatives with same cancer type and/or presence of previous diagnosis of hereditary syndrome associated with malignancies;
-
•
Current clinical staging (TNM);
-
•
Pathology report and staging.
-
b)
Feasibility-related information:
-
•
Number of tumour tissue samples collected;
-
•
If adjacent normal tissue also collected, number of tissue samples;
-
•
Gross morphology, weight and size of each sample;
-
•
Date and time of tissue collection;
-
•
Date and time of arrival to laboratory;
-
•
Date and time of tissue processing;
-
•
Method of cell isolation;
-
•
Cell count after isolation;
-
•
Cell growth method and time;
-
•
Characterisation of cells;
-
•
Date of tumouroid establishment;
-
•
Date of drug challenge;
-
•
Drug concentrations tested;
-
•
Duration of drug exposure;
-
•
Drug challenge response.
-
c)
Acceptability-related information:
-
•
Questionnaire responses (coded);
-
•
Semi-structured interview responses (coded).
2.12. Statistical analyses
Analyses will be descriptive; continuous variables will be summarised using the mean, standard deviation, median, and interquartile range; categorical variables will be summarised as frequencies and proportions; all estimates will include confidence intervals.
2.13. Trial funding, organisation and administration
The study funding has been reviewed by the sponsor and deemed sufficient to cover the requirements of the study. NHS costs will be supported via Royal Free London NHS Foundation Trust.
The research costs for the study are supported by National Institute of Health Research i4i Invention for innovation grant (reference number: II-LA-0813-20002; funding amount: £1,170,986.00; date of award: 1st July 2014).
2.14. Ethics and dissemination
The study has ethical approval from the London Central Research Ethics Committee (REC reference 17/LO/1744). The results of the study will be published in peer-reviewed publications and will be presented at relevant national and international conferences. We will work with our patient panel to develop plain English reports to disseminate research findings to patient groups and the clinical teams at participating sites.
2.15. Availability of data
The Surgical & Interventional Trials Unit (SITU) will control the final trial dataset and any requests for access must adhere to the current SITU data sharing policy, subject to existing contractual arrangements with the funders. The protocol, sample case report forms and participant information are available on upon request to the corresponding author.
2.16. Trial status
The trial opened to recruitment on 10 January 2018. Amendments were reviewed and approved by the sponsor, and the National Research Ethics Service Committee. Protocol amendments are disseminated to relevant parties by the Surgical & Interventional Trials Unit.
2.17. Discussion
Personalised medicine has the potential to improve health outcomes in the NHS and globally. It is estimated that half the UK population will receive a cancer diagnosis within their lifetime [11]. Treatments are improving, but they are also becoming more expensive, particularly in relation to new drugs. However, tools to discriminate between responders from non-responders are limited and so all patients will be offered a trial of therapy. It is only by giving treatments that are destined to work that we can maximise cost-effectiveness and minimise harm.
If proven acceptable to patients and feasible, in vitro-directed therapy could be a very useful personalised approach to guide treatment decisions. The development of patient-derived tumouroids could overcome the current over-simplified strategies that focus on gene or protein markers as predictors of response, and ignore epigenetic and functional alterations within cancer cells and the influence of tumour stroma on treatment sensitivity. Limitations to this approach could still be not capturing the full extent of intratumoural heterogeneity and inducing in vitro selective pressures that are not present in vivo and could alter treatment response. The use of multiregional tumour sampling and short culture timeframes are two strategies used to limit these potential shortfalls. In addition, patient-derived tumouroids could be used as a standalone platform or as a verification tool coupled with other personalised medicine approaches.
Subject to successful testing, establishing tumouroids as a personalised platform to predict patient response to treatment could represent a more cost-efficient and ethical tool than animal platforms. This proof-of-concept study will assess if patient-derived tumouroids can be therapeutically challenged in a clinically relevant timeframe, and if patients are willing to accept such a method to guide clinical treatment decision making. The results will be used to inform the sample size, design and conduct of a subsequent early phase development programme comparing personalised tumouroid response to therapy versus patient response to standard of care.
3. Declarations
Ethical approval
REC approval was given on 20 November 2017 by London Central. Ref: 17/NI/0189.
Funding
JBN’s post was funded by Kidney Cancer UK.
This report is independent research funded by the National Institute for Health Research (NIHR Invention for Innovation Grant (i4i)(II-LA-0813-20002)).
Author contribution
Conception and design of the study: MGBT, JBN, KS, PR, AC, CBG, NW, UC, ML, ME.
Protocol/Patient Information Sheet: MGBT, JBN, KS, CBG, NW, JDG, ML, ME.
Writing of manuscript: all authors.
Conflict of interest statement
None to declare.
Guarantor
Mark Emberton.
Research registration number
Registered on clinicaltrials.gov:
ClinicalTrials.gov Identifier: NCT03300102.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.isjp.2019.03.019.
Contributor Information
Maxine G.B. Tran, Email: m.tran@ucl.ac.uk.
Jack Grierson, Email: situ.tumouroid@ucl.ac.uk.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Cancer Research UK, http://www.cancerresearchuk.org/health-professional/cancer-statistics (accessed November 2015).
- 2.https://bnf.nice.org.uk/medicinal-forms/nivolumab.html.
- 3.Jameson J.L., Longo D.L. Precision medicine – personalized, problematic, and promising. NEJM. 2015;372(23):2229–2234. doi: 10.1056/NEJMsb1503104. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Research UK, http://www.cancerresearchuk.org/funding-for-researchers/how-we-deliver-research/our-research-partnerships/stratified-medicine-programme (accessed November 2015).
- 5.Behl A.S., Goddard K.A., Flottemesch T.J., Veenstra D., Meenan R.T., Lin J.S., Maciosek M.V. Cost-effectiveness analysis of screening for KRAS and BRAF mutations in metastatic colorectal cancer. J. Natl. Cancer Inst. 2012;104(23):1785–1795. doi: 10.1093/jnci/djs433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyman D.M., Puzanov I., Subbiah V., Faris J.E., Chau I., Blay J.Y., Wolf J., Raje N.S., Diamond E.L., Hollebecque A., Gervais R., Elez-Fernandez M.E., Italiano A., Hofheinz R.D., Hidalgo M., Chan E., Schuler M., Lasserre S.F., Makrutzki M., Sirzen F., Veronese M.L., Tabernero J., Baselga J. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N. Engl. J. Med. 2015;373(8):726–736. doi: 10.1056/NEJMoa1502309. PubMed PMID: 26287849; PubMed Central PMCID: PMC4971773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Champions oncology, www.championsoncology.com (accessed November 2015).
- 8.Guideline on the evaluation of anticancer medicinal products in man, http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/01/WC500137128.pdf (accessed November 2015).
- 9.http://www.lonza.com/raft-3d-culture.
- 10.Gerlinger M., Santos C.R., Spencer-Dene B., Martinez P., Endesfelder D., Burrell R.A., Vetter M., Jiang M., Saunders R.E., Kelly G., Dykema K., Rioux-Leclercq N., Stamp G., Patard J.J., Larkin J., Howell M., Swanton C. Genome-wide RNA interference analysis of renal carcinoma survival regulators identifies MCT4 as a Warburg effect metabolic target. J Pathol. 2012;227(2):146–156. doi: 10.1002/path.4006. Epub 2012 Apr 18. Erratum in: J Pathol. 2015 Aug;236(4):531. PubMed PMID: 22362593; PubMed Central PMCID: PMC3504091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmad A.S., Ormiston-Smith N., Sasieni P.D. Trends in the lifetime risk of developing cancer in Great Britain: comparison of risk for those born from 1930 to 1960. Br. J. Cancer. 2015;112:943–947. doi: 10.1038/bjc.2014.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Surgical & Interventional Trials Unit (SITU) will control the final trial dataset and any requests for access must adhere to the current SITU data sharing policy, subject to existing contractual arrangements with the funders. The protocol, sample case report forms and participant information are available on upon request to the corresponding author.