Highlights
-
•
Patients undergoing relaparotomies are underrepresented in clinical trials.
-
•
The study has three steps: health care research, translational research, and RCT.
-
•
The clinical course will be followed prospectively.
-
•
Main outcomes are postoperative complications, incisional hernias and adhesions.
-
•
The ReLap study will gain evidence for best care in relaparotomies.
Abbreviations: POD, Postoperative day; SDGC, Study Center of the German Surgical Society
Keywords: Relaparotomy, Abdominal surgery, Adhesions, Fascial closure
Abstract
Background
Patients undergoing relaparotomies are underrepresented in clinical trials. Standard of care, relative outcomes compared to primary laparotomy, and the ideal fascial closure technique are unknown.
Objective
The ReLap study has three objectives: First, to determine standard of care and gain evidence of intra-/postoperative outcomes for patients undergoing relaparotomy compared to patients undergoing primary laparotomy. Second, to gain evidence of an association between biomarkers and adhesion grade in a clinical-translational approach in patients undergoing relaparotomy or primary laparotomy. Third, to gain evidence of the feasibility and comparative effectiveness of fascial closure after relaparotomy using the small stitches technique with Monomax 2–0 versus the large stitches technique with PDS 1 loop.
Methods
The ReLap study is a monocentric, prospective, mixed-methods, exploratory study with three steps: health care research, translational research, and randomized controlled trial. All patients scheduled for elective laparotomies or relaparotomies at the University of Heidelberg will be screened for eligibility. There will be five study visits during the hospital stay and one study visit one year after surgery. The clinical course will be followed and outcomes necessary to answer the study objectives will be captured prospectively. Relaparotomy patients eligible for closure with the small and large stitches technique will be randomized intraoperatively to one technique.
Discussion
The ReLap study will bridge a significant knowledge gap regarding patients undergoing relaparotomy. Differences in the standard of care between relaparotomies and primary laparotomies will be determined. The relation between biomarkers and manifestation of adhesions will be explored and evidence for the comparative effectiveness of fascial closure after relaparotomy will be gained.
1. Introduction
Accessing the abdomen via an open incision (laparotomy) is a standard surgical procedure for diseases of the abdominal cavity. Patients may undergo primary laparotomies, as well as repeat laparotomies (relaparotomies). The indications for relaparotomy are manifold, e.g., local, peritoneal or lymphatic recurrence of a malignant disease or infection in patients that have had prior abdominal surgery. The reopening of the abdomen, including the separation of adhesions and, if necessary, the resection of recurrence formation represent a technical challenge in comparison to a primary laparotomy.
The currently available literature does not provide sufficient high-quality data to allow for an evidence-based approach to relaparotomy. Therefore, it is unclear to what extent patients undergoing relaparotomy suffer from a higher risk of short-term postoperative complications, surgical site infections or burst abdomen than patients undergoing primary laparotomy. Moreover, it is unclear to what extent the time required to open and close the abdomen differs between primary laparotomy and relaparotomy, or if there should be a different standard of care between these groups. What is known is that relaparotomies carry a higher risk for long-term complications such as incisional hernia [1]. Since patients undergoing relaparotomy are often excluded from trials investigating fascial closure, the most effective closure technique for this population remains uncertain.
In contrast, multiple studies on abdominal adhesions, which develop as a reaction to surgical manipulation, ischemia, infection or foreign material, already exist [2]. While adhesions are a natural part of the healing process, they also cause abdominal discomfort, chronic abdominal pain, and bowel obstruction and hinder conditions for relaparotomies. One particular problem in these cases is accidental enterotomy during adhesiolysis [3]. Adhesions manifest in 67–93% of adults after abdominal surgery [4]. So far, many studies have investigated the topic of adhesion prophylaxis; however, the problem remains unsolved [5]. Therefore, a prospective mixed-methods study was designed to address the previously-described knowledge gaps.
2. Aim of the study
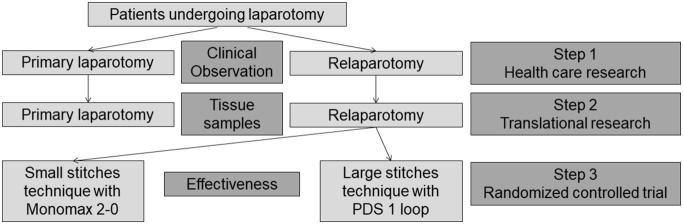
The ReLap study is a monocentric, prospective, mixed-methods (health care research, translational research and randomized controlled trial), exploratory study in three steps. Each of the steps has a specific aim:
Step 1 (Health care research): To gain evidence for the standard of care and intra-/postoperative outcomes of patients undergoing relaparotomy compared to patients undergoing primary laparotomy.
Step 2 (Translational research): To gain evidence for an association between biomarkers and adhesion grade in a clinical-translational approach in patients undergoing relaparotomy or primary laparotomy.
Step 3 (Randomized controlled trial): To gain evidence for feasibility and comparative effectiveness of fascial closure after relaparotomy comparing the small stitches technique with Monomax 2–0 with the large stitches technique with PDS 1 loop.
3. Methods
3.1. Study population
The study population will consist of adult patients undergoing relaparotomy or primary laparotomy at the Department of General, Visceral, and Transplantation Surgery at the University of Heidelberg. All patients undergoing relaparotomy or primary laparotomy will be included, regardless of underlying disease type. The following eligibility criteria were chosen to achieve a broad sample representative of high-volume surgical centres (Table 1). All patients will be informed about the study orally and in writing.
Table 1.
Eligibility criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| ● Age ≥ 18 | ● Primary reason for relaparotomy is an incisional hernia or presence of a laparostoma |
| ● Elective relaparotomy or primary laparotomy | ● Emergency operation |
| ● Written informed consent | ● Retroperitoneal operations without transperitoneal access ● incompliant patient |
3.2. Type of study
A mixed-methods study design (health care research, translational research, and randomized controlled trial) will be used to reach the defined aims (Fig. 1). The first and second steps (health care research and translational research) will involve a prospective cohort comparison of patients undergoing relaparotomy, with those undergoing primary laparotomy serving as a control group. For the third step (randomized controlled trial), patients undergoing relaparotomy fascial closure will be randomly allocated to either the small stitches technique, using Monomax 2–0, or the large stitches technique, using PDS 1 loop (step 3).
Fig. 1.
Study Flow chart.
Due to the exploratory nature of the study, there will be no sample size calculation. Recruitment will end after 100 patients undergoing relaparotomy are randomized. For each of these, a patient undergoing primary laparotomy will be included in a 2:1 ratio (50 patients), as a control group. For quality reasons, prospective (rather than historically collected) control data are preferable. However, to save resources the sample size of the control group is limited to 50 patients. None of the 50 patients in the control group of primary laparotomies will be randomized.
3.3. Study specific interventions
Step 1 (health care research):
During the first step, no study-specific intervention will be performed and the standard of care as outlined below will be recorded.
Step 2 (translational research):
During the second step, a peritoneal tissue sample from the left middle abdomen will be taken from patients in both groups. Additionally, tissue samples from adhesions of different grades will be taken from the relaparotomy group.
Step 3 (randomized controlled trial):
During the third step, patients will be allocated randomly to either the small stitches technique, using Monomax 2–0, or the large stitches technique, using PDS 1 loop. Both techniques and materials have a long history of use at the investigating institution. If both techniques are considered feasible by the operating surgeon, intraoperative randomization will take place directly before the fascial closure. The pre-randomization selection is important to determine in which proportion of relaparotomy patients both techniques would be feasible.
3.3.1. Abdominal closure with Monomax 2–0
Monomax 2–0 is a slow-absorbing monofilament suture with a thread size of 2–0. The first stitch must be anchored cranially and caudally of the incision with a knot. The distance to the edge of the fascia should be 5 mm and the distance between the two stitches should be 2–5 mm. Overall, two sutures are needed; one from cranially and the other from caudally, closing the wound centrally (Fig. 2).
Fig. 2.
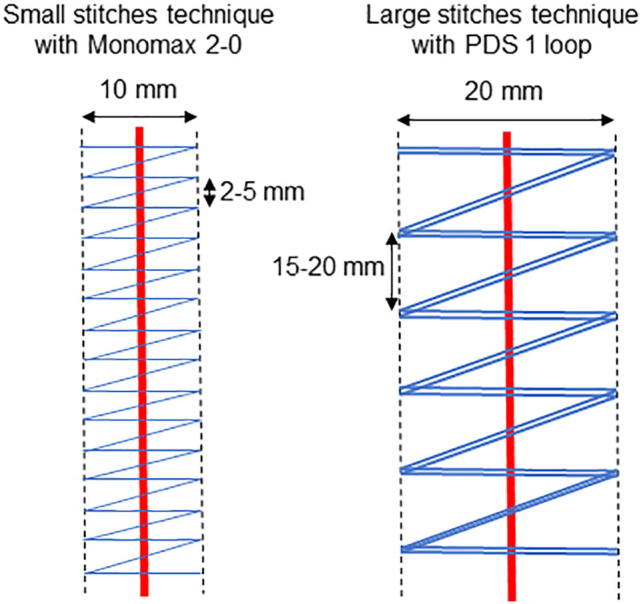
Graphic comparison of the small stitches technique using Monomax 2–0 vs. the large stitches technique using PDS 1 loop.
3.3.2. Abdominal closure with PDS 1-loop
This monofilament suture is also slow absorbing, with a thread size of 1, and is formed as a loop. The first stitch must be anchored cranially and caudally to the incision. The needle is pulled through the loop, so a knot is not necessary. The distance to the edge of the fascia should be a maximum of 10 mm and the distance between the two stitches should be 15–20 mm. Overall, two sutures are needed; one from cranially and the other from caudally, closing the wound centrally (Fig. 2).
Neither subcutaneous closure nor subcutaneous drainage is performed in either group. Skin closure is performed using skin clips. All patients will be treated within the standardized fast-track concept, which includes physiotherapy-assisted early mobilization and early transition to a normal diet.
3.4. Data capture and outcomes
For all patients, gender, age, height, comorbidities (in order to calculate the Charlson comorbidity index [6]), current or previous abdominal surgeries, ASA-Score, previous radio- or chemotherapy, current weight and weight loss over the last 3 months, and experience level of the surgeon closing the abdomen will be captured. Moreover, abdominal symptoms like pain, nausea, vomiting, constipation, and infertility (female patients only) will be recorded.
For patients in the relaparotomy group, the number of previous laparotomies, reasons for laparotomies, and days since the last laparotomy will be recorded. Moreover, the presence of an abdominal separation or incisional hernia will be recorded.
To evaluate the standard of care for step 1 (health care research) in primary and relaparotomies, the steps of opening and closing the abdomen will be closely monitored and the following aspects will be recorded: skin incision with electric scalpel or other device, cutting out of a previous scar, and type of subcutaneous and fascial incision. Further, the length of the skin and fascial incision, as well as incidental incisions of the rectus sheath, enterotomies, and the presence of occult hernias will be evaluated. Furthermore, the total time required for opening (from skin incision to installation of the supporting frame) and closing the abdomen (from final instrument count to skin closure) will be captured.
For step 2 (translational research), adhesion formation according to the Peritoneal adhesion index [7] will be captured. The Peritoneal adhesion index divides the abdomen into 10 areas and asks to rate each area with a number from 0 (no adhesions) to 3 (strong adhesions). These ratings are summed to produce an index between 0 and 30. In a translational approach, associations between the manifestation and grades of adhesions with expression of biomarkers in the tissue samples will be evaluated. Among others, the following factors, which collectively modulate adhesion formation in vivo [8], will be investigated: interleukin-6 (IL-6), fibroblast growth factor 1 (FGF-1), tumor necrosis factor alpha (TNF-alpha), transforming growth factor beta (TGF-β), hypoxia-inducible factors (HIF-1, HIF-2), and HIF-downstream targets such as plasminogen activator inhibitor (PAI-1), vascular endothelial growth factor (VEGF), Twist2, and Snail1.
For step 3 (randomized controlled trial), the feasibility of fascial closure prior to randomization will be evaluated and reasons for infeasibility as judged by the operating surgeon and actual closure technique will be reported.
For steps 1 (health care research) and 3 (randomized controlled trial), the outcomes described below will be evaluated. For step 1, outcomes between patients undergoing primary laparotomy and relaparotomy will be compared; for step 3, outcomes of patients undergoing abdominal closure with the small stitches technique using Monomax 2–0 vs. the large stitches technique using PDS 1 loop will be compared.
The outcomes of interest are first bowel movement, concomitant therapies (opiates, laxatives, physiotherapy), length of hospital stay, length of stay on intensive care unit, presence of incisional hernia at 12 months, and quality of life (QLQ 5 [9]: pre-operative, day of discharge, and at 12 months). Further, all postoperative complications fulfilling the criteria of the Clavien-Dindo classification [10] or a specific definition (Table 2) will be recorded. The comprehensive complication index of all complications will be calculated [11].
Table 2.
Assessment of postoperative complications.
| Burst abdomen/Incisional hernia |
| Anastomotic leakage [12] |
| Surgical Site Infection [13] |
| Other Infections and Sepsis [14] |
| Postoperative liver failure [15] |
| Bile leak [16] |
| Postoperative haemorrhage [17] |
| Delayed gastric emptying [18] |
| Postoperative pancreatic fistula [19] |
| Chyle leak [20] |
| Serious adverse event [21] |
3.5. Participant timeline and study visits
All patients entering the Department of General, Visceral, and Transplantation Surgery at the University Hospital of Heidelberg for elective operations will be screened. Eligible patients will be informed about the study’s purpose and procedures. After giving written informed consent, patients will be questioned and examined (Visit 1).
The clinical course will be followed prospectively (Table 3). Therefore, six planned visits will be performed. Preoperative screening will constitute visit 1, visit 2 will be the operation itself, visit 3 will be performed on postoperative day (POD) 3 to 7, visit 4 on POD 10 to 14, and visit 5 on the day of discharge or on POD 30. However, if the patient is longer in the hospital or is re-admitted within 10 days after discharge the course will be followed until definitive hospital discharge. Lastly, visit 6 will consist of a phone call to the family doctor or the patient one year after surgery.
Table 3.
Study visits of the ReLap study.
| Visit | V1/Screening | V2/operation | V3 (POD 3–7) | V4 (POD 10–14) | V5 (POD 30/Discharge) | V6 (1 year post op) |
|---|---|---|---|---|---|---|
| Personal data | X | |||||
| Informed consent | X | |||||
| Preoperative Physical examination | X | |||||
| Surgical procedure | X | |||||
| Adhesions incl. tissue sample | X | |||||
| Other intraoperative Data | X | |||||
| Postoperative complications | X | X | X | |||
| Bowel movements | X | X | X | |||
| Concomitant therapy | X | X | X | |||
| Length of hospital stay and CCI | X | |||||
| Quality of life | X | X | X | |||
| Incisional hernia | X |
Abbr.: CCI: Comprehensive complication index.
3.6. Data management and monitoring
All information required by the protocol will be recorded on a paper-based case report form. After the last visit, data will be entered into a password-protected and validated relational database (SQL Server 2008 Express). After the last patient has his or her last visit, the database will be soft locked. A monitoring will be performed of 100% of data necessary to evaluate the comprehensive complication index and the peritoneal adhesion index. Moreover, a randomly-selected 20% of the remaining data will be monitored. Finally, the database will be closed and made available for statistical analysis.
3.7. Statistical analysis
The ReLap study is a mixed-methods study with several steps; no primary endpoint has been defined. Therefore, only exploratory statistics will be used. Results will be presented either as mean with standard deviation or as rate. A descriptive p-value will be determined by chi-squared test for binary data or student’s t-test for continuous data. For steps 1 and 2, the outcomes between patients undergoing primary laparotomy and relaparotomy will be compared, and for step 3, the outcomes between patients undergoing abdominal closure with the small stitches technique using Monomax 2–0 vs. the large stitches technique using PDS 1 loop will be compared. Statistical analysis will be performed with R [22].
3.8. Methods for minimising bias
Minimising selection bias: All patients will be screened consecutively and, in cases of eligibility they will be asked for informed consent. Number of screened, included and analysed patients will be reported and drop-outs explained. A precondition for randomization in step 3 is the applicability and feasibility of both techniques, as determined by the operating surgeon’s intraoperative assessment. When feasible, the patient will be randomized by a consecutively numbered and sealed opaque envelope containing a card marked “Monomax” or marked “PDS.” The randomization sequence will be computer-generated with a mixture of variable block sizes of 4, 6, 8 or 10.
Minimising performance and detection bias: The operating surgeon cannot be blinded to the suture technique. Patients are blinded to the suture material. The outcome assessors are aware of the suture material for short-term outcomes but not for the outcomes at 12 months. Statistical analysis will be performed after closure of the database.
Minimising attrition bias: Statistical measurements such as imputation will be taken to minimise risk of bias due to incomplete outcome data [23]. The study will be reported according to the CONSORT statement [24], where applicable.
Minimising reporting bias: To avoid risk of selective reporting, the study protocol (including full information about its outcomes and statistical analysis) is hereby published according to the SPIRIT statement [25]. The study was registered with www.germanctr.de (DRKS00013001) before inclusion of the first patient.
Minimising other bias: Any financial relationship or any conflict of interest that could inappropriately influence the work within this project will be stated explicitly.
4. Ethics, informed consent and data protection
The ReLap study will be conducted in accordance with the current version of the Declaration of Helsinki [26], and according to the professional code for physicians in Germany (§15 BOÄ). The study protocol was reviewed and approved by the Ethics Committee of the medical faculty of the University of Heidelberg (S-442/2017).
Before inclusion in the ReLap study, patients will be informed both orally and in writing about all relevant aspects of the study, e.g., the aims, methods, anticipated benefits, and potential risks of the study, as well as any discomfort it may entail. The patients’ decision to participate will be documented by signature on the informed consent form. All patient-related information is subject to medical confidentiality and to the Federal Data Protection Act. Data transfer will be pseudonymised. Third parties will not have any insight into the original data.
5. Discussion
Patients undergoing relaparotomies are underrepresented in clinical trials. Standard of care, relative outcomes compared to primary laparotomy, and ideal fascial closure technique are unknown. The ReLap study is a monocentric, prospective, mixed-methods, exploratory study with three steps (health care research, translational research and randomized controlled trial) aimed to detect differences in the standard of care between relaparotomies and primary laparotomies and the relation between biomarkers and adhesions, and to gain evidence for the comparative effectiveness on fascial closure techniques following relaparotomy. Thus, a significant knowledge gap concerning standard of care and outcomes of relaparotomies will be closed.
Conflicts of interest statement
All authors declare that they have no competing interests that could compromise the outcome of this study.
Funding statement
This research did not receive any specific funding from any agency in the public, commercial or not-for-profit sectors. After the conduct of the study, half of the open access publication fee was covered by Deutsche Forschungsgemeinschaft within the funding programme Open Access Publishing, by the Baden-Württemberg Ministry of Science, Research and the Arts and by Ruprecht-Karls-Universität Heidelberg.
Ethical approval
This study protocol has been reviewed and approved by the Ethics Committee of the medical faculty at the University of Heidelberg (S-442/2017).
Consent
Before inclusion in the ReLap study, patients will be informed both orally and in writing about all relevant aspects of the study, e.g., the aims, methods, anticipated benefits and potential risks of the study, and any discomfort it may entail. The patients’ free decision to participate will be documented by signature on the informed consent form. All patient-related information is subject to medical confidentiality and to the Federal Data Protection Act. Data transfer will be pseudonymised. Third parties will not have any insight into the original data.
Authors' contributions
PP, DTT, CDH, FJH, and JCH developed the study concept and wrote the manuscript. PK, MS, MWB, and MKD helped to develop the study concept and revised the man.uscript critically for important intellectual content. All authors approved the final version for publication agreed to be accountable for all aspects of the work, and ensure that any questions related to the accuracy or integrity of any part of the work will be appropriately investigated and resolved.
Registration of research studies
Deutsches Register Klinischer Studien DRKS00013001 (www.germanctr.de).
Guarantor
Pascal Probst.
Acknowledgements
This is an investigator-initiated study and the standard resources and facilities available to researchers at the University of Heidelberg were availed for conducting the study.
Contributor Information
Dinh Thien-An Tran, Email: ThienAn.Tran@med.uni-heidelberg.de.
Colette Doerr-Harim, Email: colette.doerr-harim@med.uni-heidelberg.de.
Felix J. Hüttner, Email: felix.huettner@med.uni-heidelberg.de.
Julian C. Harnoss, Email: Julian-Camill.Harnoss@med.uni-heidelberg.de.
Phillip Knebel, Email: phillip.knebel@med.uni-heidelberg.de.
Martin Schneider, Email: martin.schneider@med.uni-heidelberg.de.
Markus W. Büchler, Email: markus.buechler@med.uni-heidelberg.de.
Markus K. Diener, Email: markus.diener@med.uni-heidelberg.de.
Pascal Probst, Email: pascal.probst@med.uni-heidelberg.de.
References
- 1.Hackert T., Büchler W.A. The broad view of reoperative surgery. Reoperat. Abdom. Surg. 2014:10–16. [Google Scholar]
- 2.Ellis H. The causes and prevention of intestinal adhesions. Br. J. Surg. 1982;69:241–243. doi: 10.1002/bjs.1800690502. [DOI] [PubMed] [Google Scholar]
- 3.Menzies D., Ellis H. Intestinal obstruction from adhesions – how big is the problem? Ann. R Coll. Surg. Engl. 1990;72:60–63. [PMC free article] [PubMed] [Google Scholar]
- 4.Weibel M.A., Majno G. Peritoneal adhesions and their relation to abdominal surgery A postmortem study. Am. J. Surg. 1973;3:345–353. doi: 10.1016/s0002-9610(73)80123-0. [DOI] [PubMed] [Google Scholar]
- 5.Lalountas M., Ballas K.D., Michalakis A. Postoperativeadhesion prevention using a statin-containing cellulose filmin an experimental model. Br. J. Surg. 2012;99:423–429. doi: 10.1002/bjs.7817. [DOI] [PubMed] [Google Scholar]
- 6.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 7.Coccolini F, World J Peritoneal adhesion index (PAI): proposal of a score for the “ignored iceberg” of medicine and surgery. Emerg. Surg. 2013;8(1):6. doi: 10.1186/1749-7922-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strowitzki M.J., Ritter A.S. Pharmacological HIF-inhibition attenuates postoperative adhesion formation. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-13638-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabin R., Gudex C., Selai C., Herdman M. Value Health. 2014;17(1):70–76. doi: 10.1016/j.jval.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Dindo D. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slankamenac K. The comprehensive complication index: a novel and more sensitive endpoint for assessing outcome and reducing sample size in randomized controlled trials. Ann. Surg. 2014;260(5):757–762. doi: 10.1097/SLA.0000000000000948. [DOI] [PubMed] [Google Scholar]
- 12.Rahbari Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147(3):339–351. doi: 10.1016/j.surg.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention, Surgical Site Infection https://www.cdc.gov/hai/ssi/ssi.html, 2018 (accessed 17 January 2018)
- 14.Horan T.C. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am. J. Infect. Control. 1992;20(5):271–274. doi: 10.1016/s0196-6553(05)80201-9. [DOI] [PubMed] [Google Scholar]
- 15.Rahbari N.N. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149(5):713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Koch M. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149(5):680–688. doi: 10.1016/j.surg.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Wente M.N., Veit J.A. Postpancreatectomyhemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142(1):20–25. doi: 10.1016/j.surg.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Wente M.N. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142(5):761–768. doi: 10.1016/j.surg.2007.05.005. PMID: 17981197. [DOI] [PubMed] [Google Scholar]
- 19.Bassi C. International Study Group on Pancreatic Fistula Definition: Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138(1):8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Besselink Definition and classification of chyle leak after pancreatic operation: a consensus statement by the International Study Group on Pancreatic Surgery. Surgery. 2017;161(2):365–372. doi: 10.1016/j.surg.2016.06.058. [DOI] [PubMed] [Google Scholar]
- 21.Dellinger R.P. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Core Team R. R Foundation for Statistical Computing; Vienna, Austria: 2014. R: A language and environment for statistical computing. http://www.R-project.org/. (accessed February 10th 2018) [Google Scholar]
- 23.Cochrane Methods Bias, Assessing Risk of Bias in Included Studies, http://methods.cochrane.org/bias/assessing-risk-bias-included-studies (accessed February 10th 2018)
- 24.Schulz K.F. 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan A.-W., SPIRIT Statement: Defining standard protocol items for clinical trials. Ann. Intern. Med. 2013;158(2013):200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Association W.M. World medical association declaration of helsinki. Ethical principles for medical research involving human subjects. Bull. World Health Organ. 2001;79(4):373. [PMC free article] [PubMed] [Google Scholar]