Highlights
-
•
Traditionally, synchronous colorectal cancer and CRLM are resected separately.
-
•
Many institutions have begun performing these procedures simultaneously.
-
•
Minimal data support simultaneous resection including major liver resection.
-
•
Complications will be investigated following simultaneous resection.
-
•
This protocol will be implemented in 5 high-volume tertiary care centres worldwide.
Keywords: Synchronous colorectal cancer liver metastases, Postoperative complications, Comprehensive complication index, Mortality
Abstract
Introduction
The “traditional approach” to resect synchronous colorectal cancer with liver metastases (CRLM) is to perform staged resections. Many institutions perform simultaneous resection. Disadvantages to the simultaneous approach include longer operating room times, which may increase major postoperative complication rates. Data supporting simultaneous resection are limited to retrospective studies that are subject to selection bias. Therefore, we have proposed a single-arm prospective cohort pilot study to evaluate the postoperative complications following simultaneous resection of synchronous CRLM.
Methods and analysis
This single-arm study will be performed in five high-volume hepatobiliary centres to prospectively evaluate the following objectives: (1) To determine the 90-day postoperative complication rate of patients diagnosed with synchronous CRLM undergoing a simultaneous colorectal and liver resection, including major liver resections; (2) To determine the postoperative mortality rate at 90 days following index surgery; (3) To determine change in global health-related Quality of Life (QoL) following simultaneous resection at three months compared to baseline; and (4) To build a costing model for simultaneous resection, We will also evaluate the feasibility of performing combined resection in these patients by evaluating the number of eligible patients enrolled in the study and determining the reasons eligible patients were not enrolled. This protocol has been registered with ClinicalTrials.gov (NCT02954913).
Ethics and dissemination
This study has been provincially approved by the central research ethics board. Study results will inform the design a randomized controlled trial by providing information about the comprehensive complication index in this patient population used to calculate the sample size for the trial.
1. Background and rationale
Approximately 30% of patients with CRLM present with synchronous disease [1]. Resection of colorectal cancer metastases confined to the liver has been shown to offer long-term survival [2], [3], [4]. However, the optimal timing of surgical resection of synchronous liver metastases in relation to the primary tumour is not well defined. Prior retrospective cohorts and meta-analyses suggest that the simultaneous approach carries similar postoperative complication and perioperative mortality rates [5], [6], [7], [8], [9], [10], [11], [12]. However, most reports carry a significant selection bias, as surgeons tend to combine limited liver resections and “straightforward” colorectal resections as opposed to complex resections. Recent studies suggest that the postoperative complication risk is similar even in the case of complex liver resections as well as complex colon resections and rectal cancer resections [13], [14]. Rectal resections when compared to colon resections are thought to be more complex, due to: a higher risk of anastomotic leakage, [15] the use of specific surgical procedures, such as total mesorectal excision [16], [17] and laparoscopic surgery [18] and the involvement of a multidisciplinary team to determine the use and timing of neoadjuvant chemoradiotherapy [19], [20].
Improvements in anaesthesia, critical care and surgical resection techniques for both liver and colorectal surgery have enabled innovative surgeons and institutions to safely perform simultaneous resections in complex liver and colorectal cases, and the simultaneous approach has been adopted by many surgeons despite the lack of studies with rigorous methodology to provide good quality data. The decision to perform simultaneous resection varies greatly between surgeons and institutions and often depends on patients and tumour characteristics. Currently, there is no standard approach to this problem and it continues to be a topic of debate amongst surgeons, medical oncologists and radiation oncologists. A recent population-based study found that the majority of synchronous CRLM are performed in a staged manner [21]. A recent large database study found simultaneous resection led to reduced length of stay and reduced health care utilization in patients who underwent simultaneous resection including those who underwent complex colorectal resections and total hepatic lobectomies [22].
Simultaneous resection has the potential advantage to decrease the number of complications following surgery, avoiding a second operation thereby improving patient’s QoL, decreasing overall health care costs and avoiding delays in the administration of postoperative chemotherapy. Although the total number of complications can be reduced, the operating room time is higher which could lead to a higher proportion of major postoperative complications due to hypothermia, prolonged hypovolemia and higher blood loss, which could lead to a higher proportion of postoperative mortality at 90 days following surgery. Therefore, we propose to undertake a prospective single arm pilot study of patients with synchronous CRLM undergoing simultaneous resection requiring major liver resection to provide us with important information to prepare a large randomized controlled study of simultaneous vs. staged resection. This pilot study will provide valuable data on the number of eligible patients enrolled and the number of patients who complete both resections in the same anaesthetic setting. It will also provide information on the type and proportion of postoperative complications at 90 days following surgery as measured by the comprehensive complication index [8] which will help us better understand the postoperative complication rate of the simultaneous approach and also calculate a sample size for a randomized controlled trial based on this primary outcome. Criteria for success of this pilot study will be clearly set in order to determine if it is possible to move forward with a larger trial. The results of this study could lead to changes in surgical practice by introducing an innovative approach to treat this disease, in a way that could improve patient’s QoL by decreasing postoperative complications and the number of surgical procedures and at the same time has the potential for cost savings to the health care system (i.e., release resources for other uses).
2. Methods and analysis
2.1. Study design
A single-arm, multi-centre prospective pilot study for patients undergoing simultaneous resection of synchronous colorectal cancer liver metastases (CRLM).
2.2. Study setting
This pilot study that will be performed at five academic tertiary care centres.
2.3. Study intervention
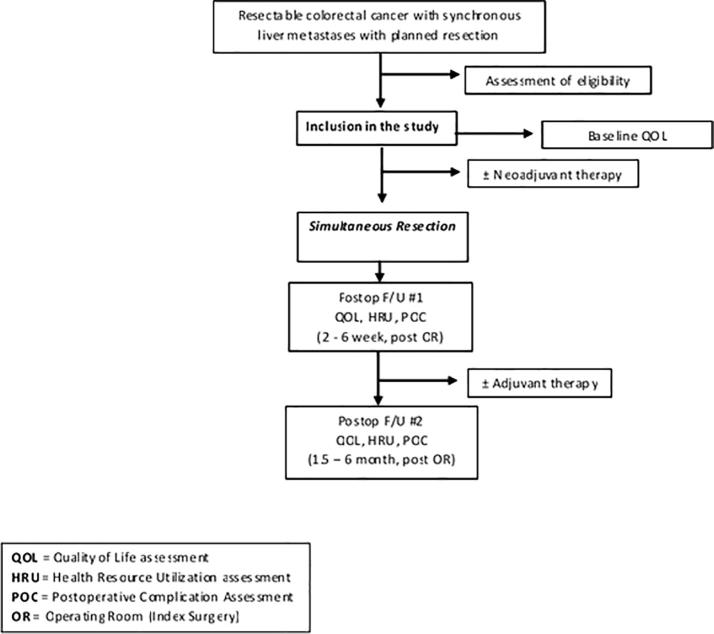
Patients will undergo resection of the colon or rectum and liver in the same anaesthetic setting. The operation should happen within 8 weeks from inclusion of the study. If patients need to undergo neoadjuvant chemotherapy or radiation therapy, they will be assessed for eligibility once the decision for surgery has been made. The type of colorectal and liver resection will be decided by the treating physician. The type of liver resection will be described according to the Couinaud classification and the Brisbane terminology of liver anatomy [23], [24]. The anaesthetic technique and the order of liver resection or rectal resection will be determined by each surgeon’s standards. It is recommended that a low central venous pressure be maintained in order to decrease intraoperative blood loss [25], [26] and that liver resection be performed prior to colorectal resection in order to keep a low central venous pressure during that part of the case. Any deviation from the intended intervention (i.e. colon or liver resection not performed at the same time of the index operation) will be noted with a reason. For a summary of the study procedures refer to Appendix 2.
2.4. Patient population
2.4.1. Inclusion criteria
Inclusion criteria are adult men and women who are medically fit for resection with an ECOG performance status ≤2, presenting with resectable, histological confirmation of colorectal cancer primary, and confirmed synchronous CRLM. Patients who only require resection of another pelvic organ or another abdominal organ are eligible. Resectable oligometastatic disease to the lung (up to 3 metastatic deposits) are eligible. Resectability status will be defined at a multidisciplinary gastrointestinal cancer conference according to the standard of each institution.
2.4.2. Exclusion criteria
Patients will be excluded based on the following criteria: extrahepatic disease other specified above, tumours treated with local transanal excision, two stage liver resection and prior liver resection, pregnant or lactating female, complex multi-organ pelvic or abdominal resection.
2.5. Sample size justification and feasibility
We plan to enroll 46 patients. At all centres there are 240 liver resections performed annually for colorectal cancer. There are also approximately 60 liver resections performed within 12 months of a colorectal resection (synchronous disease), therefore, we believe 46 patients would be eligible for this study over an 18-month period. With a 3-month follow-up, we expect the study to finalize within 24 months of initiation.
2.6. Study objectives
2.6.1. General objective
To improve the management of patients presenting with synchronous CRLM.
2.6.2. Primary objectives
To determine the 90-day postoperative complication rate of patients diagnosed with synchronous CRLM, undergoing a simultaneous colorectal and liver resection (including major liver resections).
2.6.3. Secondary objectives
(1) To determine the postoperative mortality rate at 90 days following index surgery, (2) to determine change in global health-related QoL following simultaneous resection at three months compared to baseline, and (3) to build a costing model for patients with synchronous CRLM undergoing simultaneous resection.
2.7. Study data collection
A research assistant will gather postoperative complication data from patients’ charts, or electronic medical records and transcribe it onto case report forms daily while the patient is in the hospital. An adjudication committee, consisting of two independent adjudicators who are medical professionals will review patient complications using de-identified source documents. The adjudicators will confirm that all reported complications are accurate, not duplicated, and appropriately classified. Each complication must be supported by source documents. The outcome assessment will follow strict criteria set by Clavien-Dindo and the Comprehensive Complication Index. The completed de-identified forms will be given to the responsible clinical trials group for data entry into a secure electronic database. Survival information will be obtained from electronic medical records, paper charts, death registries, and/or any physician participating in the patient’s care (i.e. family physician, oncologist, and/or palliative care physician).
2.8. Participant Follow-up
After enrollment, patients will undergo a baseline QoL assessment during their clinic visit. Patients will then be assessed at their first post-operative clinic visit, 4 weeks (±1 week) following the index operation. Patients will be classified according to the Clinical Risk Score as defined by Fong [27]. The second postoperative follow-up will happen at 12 weeks (±2 weeks) following surgery. At each assessment, complete history and ECOG performance status will be recorded. Patients will complete the QoL questionnaire in the surgical clinics. Questionnaires will be mailed prior to their visit to facilitate compliance. Health Resource Utilization will also be collected at each assessment. We will ensure complete follow-up for all patients; phone calls and home visits will be performed, if necessary.
2.9. Outcomes
2.9.1. Primary outcome measure
We will determine the proportion and 95% confidence intervals (CI) of postoperative complications, including both minor and major complications, for patients undergoing simultaneous colorectal and major liver resections. Postoperative complications will be recorded during the patient’s hospital stay and up to 90 days from the index operation. Each post-operative complication will be classified according to Clavien-Dindo and this will be used to calculate the Comprehensive Complication Index [28], [29], [30].
2.9.2. Secondary outcome measures
(1) The proportion with 95% CI of overall postoperative complications and the comprehensive complication index will be determined. Postoperative complications will be recorded during and following up of each patient’s hospital stay up to 90 days from the index operation and classified according to Clavien-Dindo [28], [29], [31]. (2) The proportion with its 95% CI of patients who die at 90 days or during the hospital stay for the index operation (perioperative mortality) will be calculated. Mortality will also be determined via linkage with population databases. (3) The change in global health related QoL from baseline to 3 months following index surgery will be assessed at one and three months following index surgery. QoL will be measured using the EORTC-QLQ-C30 [32], [33] instrument administered at baseline, at 1 and 3 months following surgery. (4) Cost analysis of patients undergoing simultaneous resection up to 3 months following index surgery will be performed. Health Resource Utilization forms will be used at each patient assessment following surgery (at one and three months from index surgery) to determine the number of health-related visits and imaging performed. A costing model will be performed by including all factors that drive cost in this patient population.
2.10. Statistical analyses
Following the grading of each postoperative complication according to Clavien-Dindo, the mean and standard deviation of the Comprehensive Complication Index at 90 days from index operation will be determined [28], [29], [30], [31]. The proportion with 95% CI of overall and major postoperative complications and the mortality at 90 days will be calculated using the Wilson-Score method. Changes in the continuous QoL outcomes will be defined as a 10% change, which corresponds to a 10-point difference in the EORTC-QLQ LMC21 or in the EORTC-QLQ C30 global health scale. To detect minimal clinically important difference in the continuous QOL outcomes, repeated-measure ANOVA models were constructed, with the within-subject correlation expected to follow an autoregressive structure. P values at or below .05 were considered statistically significant. Costs will be grouped by baseline costs, cost of hospitalization for surgery and follow up costs (i.e. postoperative complications leading to re-hospitalization, adjuvant chemotherapy and emergency room visits). Mean costs per patient with its associated 95% credible intervals will be calculated using Bayesian approach.
2.11. Criteria for success of the pilot study
Approximately 20% of patient experience complications followed staged resection [34]. The steering committee agreed that a major complication rate of 30% will be the highest proportion accepted for patients undergoing simultaneous resection, a relative risk increase of 50%, which translates to a mean 12.5-point increase in the Comprehensive Complication Index [30]. For patients not included in the trial and undergo staged surgery, data on postoperative complications will be collected retrospectively and compared to our cohort.
3. Trial organization and quality assurance
Study Steering Committee: The PI and three co-investigators will form the steering committee for this study and will meet prior to the study start-up, and every 4 months. They will be responsible for monitoring patient safety throughout the study. Members are experts in the fields of clinical trials methodology, oncology and surgery and will receive study data pertinent to patient safety at the midpoint of patient accrual. The committee will meet once 15 patients have been accrued to the study and followed for at least 90 days.
Central Adjudication Committee: Two professionals that are experts in the medical field independent of the research team will be responsible for assessing the postoperative complications for each patient using de-identified source documents.
Methods Centre: A clinical trials group will perform data management, statistical analyses, and provide methodological and administrative support to all research personnel. Study coordination will be performed by the Principal Investigator and research team.
Ethical approval
The study will be performed in accordance with the recommendations adopted by the 18th World Medical Assembly, Helsinki, Finland, 1964. The centralized REB (HiREB) has approved the study protocol and documents prior to commencement. Written informed consent will be obtained from all patients prior to enrollment.
Significance
Currently, many patients with synchronous disease undergo staged resection mostly due to fear of the impact of a major operation on patient’s QoL and postoperative complications. However, as the safety and techniques of liver and colorectal resection continue to improve (i.e. laparoscopic liver surgery, enhanced recovery after surgery pathways), the feasibility of performing a combined colorectal and liver surgery is higher. Despite many surgeons embracing staged as opposed to simultaneous resection as the preferred management strategy for the treatment of synchronous colorectal cancer with liver resections, we feel there is sufficient clinical equipoise to support a well-designed, randomized controlled trial addressing this question. This study will provide the background data necessary to prepare such trial.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contribution
All authors contributed substantially to the conception and design, acquisition of data, or analysis and interpretation of data; drafted the article or revised it critically for important intellectual content; and gave final approval of the version to be published. Dr. Simunovic, Dr. Gallinger, Dr. Wei, Dr. Ruo and Dr. Karanicolas are experts in clinical trials research. The 5 aforementioned co-investigators have worked with the principal investigator, Dr. Pablo Serrano, to design and revise this protocol. Dr. Meyers, Dr. Devaud, and Dr. Reiter are experts in medical, surgical, and radiation oncology and have also helped with the logistical aspect of designing this study (i.e. appropriate time points for patient enrolment). Dr. Hallet has been responsible for assessing the study design to ensure appropriate health research methodologies have been incorporated in this protocol. Dr. Gafni has been responsible for designing the cost-analysis portion of this protocol. Dr. Parpia has been responsible for the preparation of the statistical analysis plan mentioned in this protocol. Dr. Levine has been overseeing the preparation of this protocol since the initial phases and has provided feedback to ensure the study meets the rigorous clinical trials requirements as set by the Ontario Clinical Oncology Group.
Conflict of interest
All authors report no conflict of interest.
Guarantor
The principal investigator of the study, Pablo Serrano will be the guarantor.
Research registration number
This study has been registered under ClinicalTrials.gov, registration number NCT02954913.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.isjp.2018.01.001.
Appendix 1.
Study schema.
Appendix 2.
Schedule of study procedures.
| Study procedure | Screening | Baseline | Day of surgery | Follow-up 1 | Follow-up 2 | End of study |
|---|---|---|---|---|---|---|
| Day 1 | 4 weeks (±1w) | 12 weeks (±2) | ||||
| Review of HPB referrals | X | |||||
| Eligibility Form | X | |||||
| Surgeon’s Confirmation of Eligibility | X | |||||
| Enrollment Log | X | |||||
| Informed Consent Process | X | |||||
| Baseline Case Report Form | X | |||||
| Quality of Life (EORTC-QLQ-C30 and EORTC-QLQ-LMC21) | X | X | X | |||
| Surgery Worksheet (must be provided to surgeon prior to surgery) | X | |||||
| Surgery Case Report Form | X | |||||
| Pathology Case Report Form | X | |||||
| Post-operative follow-up form (includes ECOG) | X | X | ||||
| Minor Complication Form (only if applicable) | X | X | ||||
| Major Complication Form (only if applicable) | X | X | ||||
| Health Resource Utilization Form | X | X | ||||
| Deidentify Data and send all study documents (enrollment log, case report forms and source) | X | |||||
| Survival Information (if applicable) | X | |||||
Appendix 3. Supplementary data
References
- 1.Manfredi S., Lepage C., Hatem C., Coatmeur O., Faivre J., Bouvier A.M. Epidemiology and management of liver metastases from colorectal cancer. Ann. Surg. 2006;244(2):254–259. doi: 10.1097/01.sla.0000217629.94941.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordlinger B., Sorbye H., Glimelius B., Poston G.J., Schlag P.M., Rougier P., Bechstein W.O., Primrose J.N., Walpole E.T., Finch-Jones M., Jaeck D., Mirza D., Parks R.W., Mauer M., Tanis E., Van Cutsem E., Scheithauer W., Gruenberger T., E.G.-I.T.C. Group, U.K. Cancer Research, O. Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft, G. Australasian Gastro-Intestinal Trials, D. Federation Francophone de Cancerologie Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14(12):1208–1215. doi: 10.1016/S1470-2045(13)70447-9. [DOI] [PubMed] [Google Scholar]
- 3.Robertson D.J., Stukel T.A., Gottlieb D.J., Sutherland J.M., Fisher E.S. Survival after hepatic resection of colorectal cancer metastases: a national experience. Cancer. 2009;115(4):752–759. doi: 10.1002/cncr.24081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordlinger B., Sorbye H., Glimelius B., Poston G.J., Schlag P.M., Rougier P., Bechstein W.O., Primrose J.N., Walpole E.T., Finch-Jones M., Jaeck D., Mirza D., Parks R.W., Collette L., Praet M., Bethe U., Van Cutsem E., Scheithauer W., Gruenberger T., E.G.-I.T.C. Group, U.K. Cancer Research, O. Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft, G. Australasian Gastro-Intestinal Trials, D. Federation Francophone de Cancerologie Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371(9617):1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reddy S.K., Pawlik T.M., Zorzi D., Gleisner A.L., Ribero D., Assumpcao L., Barbas A.S., Abdalla E.K., Choti M.A., Vauthey J.N., Ludwig K.A., Mantyh C.R., Morse M.A., Clary B.M. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann. Surg. Oncol. 2007;14(12):3481–3491. doi: 10.1245/s10434-007-9522-5. [DOI] [PubMed] [Google Scholar]
- 6.Slesser A.A., Simillis C., Goldin R., Brown G., Mudan S., Tekkis P.P. A meta-analysis comparing simultaneous versus delayed resections in patients with synchronous colorectal liver metastases. Surg. Oncol. 2013;22(1):36–47. doi: 10.1016/j.suronc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Yin Z., Liu C., Chen Y., Bai Y., Shang C., Yin R., Yin D., Wang J. Timing of hepatectomy in resectable synchronous colorectal liver metastases (SCRLM): simultaneous or delayed? Hepatology. 2013;57(6):2346–2357. doi: 10.1002/hep.26283. [DOI] [PubMed] [Google Scholar]
- 8.Martin R., Paty P., Fong Y., Grace A., Cohen A., DeMatteo R., Jarnagin W., Blumgart L. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J. Am. College Surg. 2003;197(2):233–241. doi: 10.1016/S1072-7515(03)00390-9. discussion 241–2. [DOI] [PubMed] [Google Scholar]
- 9.Chua H.K., Sondenaa K., Tsiotos G.G., Larson D.R., Wolff B.G., Nagorney D.M. Concurrent vs. staged colectomy and hepatectomy for primary colorectal cancer with synchronous hepatic metastases. Dis. Colon Rectum. 2004;47(8):1310–1316. doi: 10.1007/s10350-004-0586-z. [DOI] [PubMed] [Google Scholar]
- 10.Feng Q., Wei Y., Zhu D., Ye L., Lin Q., Li W., Qin X., Lyu M., Xu J. Timing of hepatectomy for resectable synchronous colorectal liver metastases: for whom simultaneous resection is more suitable–a meta-analysis. PLoS One. 2014;9(8):e104348. doi: 10.1371/journal.pone.0104348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarnagin W.R., Gonen M., Fong Y., DeMatteo R.P., Ben-Porat L., Little S., Corvera C., Weber S., Blumgart L.H. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann. Surg. 2002;236(4):397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. discussion 406–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capussotti L., Ferrero A., Vigano L., Ribero D., Lo Tesoriere R., Polastri R. Major liver resections synchronous with colorectal surgery. Ann. Surg. Oncol. 2007;14(1):195–201. doi: 10.1245/s10434-006-9055-3. [DOI] [PubMed] [Google Scholar]
- 13.Silberhumer G.R., Paty P.B., Temple L.K., Araujo R.L., Denton B., Gonen M., Nash G.M., Allen P.J., DeMatteo R.P., Guillem J., Weiser M.R., D'Angelica M.I., Jarnagin W.R., Wong D.W., Fong Y. Simultaneous resection for rectal cancer with synchronous liver metastasis is a safe procedure. Am. J. Surg. 2015;209(6):935–942. doi: 10.1016/j.amjsurg.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vigano L., Karoui M., Ferrero A., Tayar C., Cherqui D., Capussotti L. Locally advanced mid/low rectal cancer with synchronous liver metastases. World J. Surg. 2011;35(12):2788–2795. doi: 10.1007/s00268-011-1272-7. [DOI] [PubMed] [Google Scholar]
- 15.Rullier E., Laurent C., Garrelon J.L., Michel P., Saric J., Parneix M. Risk factors for anastomotic leakage after resection of rectal cancer. Brit. J. Surg. 1998;85(3):355–358. doi: 10.1046/j.1365-2168.1998.00615.x. [DOI] [PubMed] [Google Scholar]
- 16.Heald R.J., Ryall R.D. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1(8496):1479–1482. doi: 10.1016/s0140-6736(86)91510-2. [DOI] [PubMed] [Google Scholar]
- 17.MacFarlane J.K., Ryall R.D., Heald R.J. Mesorectal excision for rectal cancer. Lancet. 1993;341(8843):457–460. doi: 10.1016/0140-6736(93)90207-w. [DOI] [PubMed] [Google Scholar]
- 18.Bonjer H.J., Deijen C.L., Haglind E., C.I.S. Group A randomized trial of laparoscopic versus open surgery for rectal cancer. New Engl. J. Med. 2015;373(2):194. doi: 10.1056/NEJMc1505367. [DOI] [PubMed] [Google Scholar]
- 19.Jeong S.Y., Park J.W., Nam B.H., Kim S., Kang S.B., Lim S.B., Choi H.S., Kim D.W., Chang H.J., Kim D.Y., Jung K.H., Kim T.Y., Kang G.H., Chie E.K., Kim S.Y., Sohn D.K., Kim D.H., Kim J.S., Lee H.S., Kim J.H., Oh J.H. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. 2014;15(7):767–774. doi: 10.1016/S1470-2045(14)70205-0. [DOI] [PubMed] [Google Scholar]
- 20.Kapiteijn E., Marijnen C.A., Nagtegaal I.D., Putter H., Steup W.H., Wiggers T., Rutten H.J., Pahlman L., Glimelius B., van Krieken J.H., Leer J.W., van de Velde C.J., G. Dutch Colorectal Cancer Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. New Engl. J. Med. 2001;345(9):638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 21.Nanji S., Mackillop W.J., Wei X., Booth C.M. Simultaneous resection of primary colorectal cancer and synchronous liver metastases: a population-based study. Can. J. Surg. 2017;60(2):122–128. doi: 10.1503/cjs.008516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abelson J.S., Michelassi F., Sun T., Mao J., Milsom J., Samstein B., Sedrakyan A., Yeo H.L. Simultaneous resection for synchronous colorectal liver metastasis: the new standard of care? J. Gastrointest. Surg. 2017;21(6):975–982. doi: 10.1007/s11605-017-3422-1. [DOI] [PubMed] [Google Scholar]
- 23.Bismuth H. Revisiting liver anatomy and terminology of hepatectomies. Ann. Surg. 2013;257(3):383–386. doi: 10.1097/SLA.0b013e31827f171f. [DOI] [PubMed] [Google Scholar]
- 24.Strasberg S.M. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J. Hepatobiliary Pancreat. Surg. 2005;12(5):351–355. doi: 10.1007/s00534-005-0999-7. [DOI] [PubMed] [Google Scholar]
- 25.Chen H., Merchant N.B., Didolkar M.S. Hepatic resection using intermittent vascular inflow occlusion and low central venous pressure anesthesia improves morbidity and mortality. J. Gastrointest. Surg. 2000;4(2):162–167. doi: 10.1016/s1091-255x(00)80052-9. [DOI] [PubMed] [Google Scholar]
- 26.Hughes M.J., Ventham N.T., Harrison E.M., Wigmore S.J. Central venous pressure and liver resection: a systematic review and meta-analysis. HPB. 2015;17(10):863–871. doi: 10.1111/hpb.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fong Y., Fortner J., Sun R.L., Brennan M.F., Blumgart L.H. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann. Surg. 1999;230(3):309–318. doi: 10.1097/00000658-199909000-00004. discussion 318–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slankamenac K., Graf R., Barkun J., Puhan M.A., Clavien P.-A. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann. Surg. 2013;258(1):1–7. doi: 10.1097/SLA.0b013e318296c732. [DOI] [PubMed] [Google Scholar]
- 29.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nederlof N., Slaman A.E., van Hagen P., van der Gaast A., Slankamenac K., Gisbertz S.S., van Lanschot J.J., Wijnhoven B.P., van Berge Henegouwen M.I., C.R.-S. Group Using the comprehensive complication index to assess the impact of neoadjuvant chemoradiotherapy on complication severity after esophagectomy for cancer. Ann. Surg. Oncol. 2016;23(12):3964–3971. doi: 10.1245/s10434-016-5291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slankamenac K., Nederlof N., Pessaux P., de Jonge J., Wijnhoven B.P., Breitenstein S., Oberkofler C.E., Graf R., Puhan M.A., Clavien P.A. The comprehensive complication index: a novel and more sensitive endpoint for assessing outcome and reducing sample size in randomized controlled trials. Ann. Surg. 2014;260(5):757–762. doi: 10.1097/SLA.0000000000000948. discussion 762–3. [DOI] [PubMed] [Google Scholar]
- 32.Aaronson N.K., Ahmedzai S., Bergman B., Bullinger M., Cull A., Duez N.J., Filiberti A., Flechtner H., Fleishman S.B., de Haes J.C. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J. Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 33.Groenvold M., Klee M.C., Sprangers M.A., Aaronson N.K. Validation of the EORTC QLQ-C30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreement. J. Clin. Epidemiol. 1997;50(4):441–450. doi: 10.1016/s0895-4356(96)00428-3. [DOI] [PubMed] [Google Scholar]
- 34.Serrano P.E., Gafni A., Gu C., Gulenchyn K.Y., Julian J.A., Law C., Hendler A.L., Moulton C., Gallinger S., Levine M. Positron emission tomography-computed tomography (PET-CT) versus no PET-CT in the management of potentially resectable colorectal cancer liver metastases: cost implications of a randomized controlled trial. J. Oncol. Pract. 2016;12:e765–e774. doi: 10.1200/JOP.2016.011676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.