Abstract
Pursuing on our efforts toward searching for efficient hCA IX and hCA XII inhibitors, herein we report the design and synthesis of new sets of benzofuran-based sulphonamides (4a,b, 5a,b, 9a–c, and 10a–d), featuring the zinc anchoring benzenesulfonamide moiety linked to a benzofuran tail via a hydrazine or hydrazide linker. All the target benzofurans were examined for their inhibitory activities toward isoforms hCA I, II, IX, and XII. The target tumour-associated hCA IX and XII isoforms were efficiently inhibited with KIs spanning in ranges 10.0–97.5 and 10.1–71.8 nM, respectively. Interestingly, arylsulfonehydrazones 9 displayed the best selectivity toward hCA IX and XII over hCA I (SIs: 39.4–250.3 and 26.0–149.9, respectively), and over hCA II (SIs: 19.6–57.1 and 13.0–34.2, respectively). Furthermore, the target benzofurans were assessed for their anti-proliferative activity, according to US-NCI protocol, toward a panel of sixty cancer cell lines. Only benzofurans 5b and 10b possessed selective and moderate growth inhibitory activity toward certain cancer cell lines.
Keywords: Benzenesulfonamides, carbonic anhydrases, benzofurans, synthesis, anticancer
Introduction
Carbonic anhydrases (CAs, EC 4.2.1.1) are metalloenzymes, present in all kingdoms life, catalyse the reversible reaction of the hydration of carbon dioxide to bicarbonate and protons1. This simple reaction play a vital role in many physiological and pathological processes associated with pH control, ion transport, and fluid secretion2–4. The Zn(II) containing metalloenzyme α-CAs have been reported in vertebrates and, in humans, which is further distinguished by sixteen different hCA isoforms including cytosolic isoforms (hCA I, II, III, VII, and XIII), membrane bound isoforms (hCA IV, IX, XII, XIV, and XV), mitochondrial isoforms (hCA VA and VB) and secreted isoforms (hCA VI) depending upon their distribution in tissues, cellular localisation, and molecular features5–7. It is well established that these metalloenzymes possess a significant role in several pathological processes1,8–10. So, modulators of these enzymes could be used as diuretics11, anti-glaucoma agents12, anti-epileptics13, and more recently as antitumor agents13,14. In particular, the human (h) isoform CA IX is ectopically expressed in hypoxic tumours, thus acting as a key player in cancer cells survival, proliferation, and metastasis15, and its inhibition has been suggested as a promising strategy for treatment of human malignancies15–17.
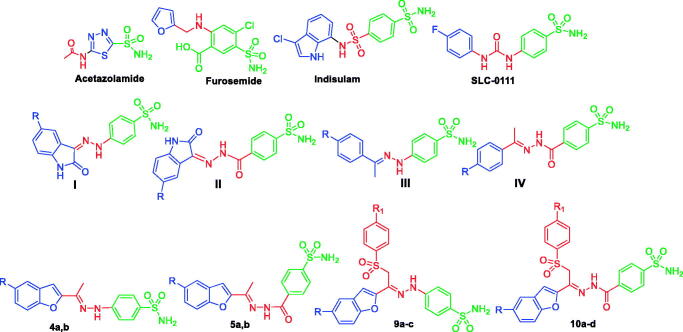
Amongst the various classes of CA inhibitors (CAIs), the primary sulphonamides and their bioisosteres represent the most important ones18, with many small molecules in clinical use, such as zacetazolamide (AAZ) and furosemide, or in clinical development, such as indisulam and SLC-0111 (Figure 1). Of special interest, SLC-0111 is an ureido-based benzenesulfonamide with selective hCA IX inhibitory activity that is currently being tested in Phase I/II clinical trials for the treatment of advanced hypoxic tumours13,19. Inhibition of CA with the zinc anchoring sulphonamide derivatives is mediated via coordination of SO2NH− (the deprotonated form) to the positively charged Zn(II) ion in the CA active site. In addition, the sulfamoyl functionality engages two H-bonds: the NH− group acts as donor, while the S = O as acceptor with T199 OG1 atom and backbone NH respectively.
Figure 1.
Structures of some CAIs, and the target benzofuran-based sulphonamides 4a, b, 5a, b, 9b–d and 10a–d.
The “tail approach” is considered to be the most successful approach that could be utilised to afford isoform selective CAIs. In details, the aromatic/heterocyclic ring incorporating the primary sulphonamide functionality, the zinc binding group (ZBG), is to be appended with tail moieties through diverse functionalised linkers. Recently our research team has utilised the tail approach to develop several small molecules as effective CAIs, like structures I–IV (Figure 1)20–24.
In continuation to our previous effort in the search for efficient hCA IX and hCA XII inhibitors25–27, herein we report the design and synthesis of new sets of benzofuran-based sulphonamides (4a,b, 5a,b, 9a–c, and 10a–d, Figure 1), featuring the zinc anchoring benzenesulfonamide moiety linked to a benzofuran tail via a hydrazine or hydrazide linker. In series 9 and 10, an arylsulfone moiety was incorporated to probably promote binding to the hydrophilic part of the active site.
The target benzofurans (4a,b, 5a,b, 9a–c, and 10a–d) were evaluated in vitro for their inhibitory activity towards the physiologically relevant hCA isoforms I, II, IX, and XII using stopped-flow CO2 hydrase assay. Additionally, they were screened for their anti-proliferative toward a panel of 60 cancer cell lines at dose of 10 mM following the US-NCI single dose assay protocol.
Materials and methods
Chemistry
All reaction solvents and reagents were purchased from commercial suppliers and used without further purification. Melting points were measured with a Stuart melting point apparatus and were uncorrected. The NMR spectra were obtained on Bruker Avance 400 (400 MHz 1H and 100 MHz 13C NMR). 1H NMR spectra were referenced to tetramethylsilane (δ = 0.00 ppm) as an internal standard and were reported as follows: chemical shift, multiplicity (b = broad, s = singlet, d = doublet, t = triplet, dd = doublet of doublet, m = multiplet). IR spectra were recorded with a Bruker FT-IR spectrophotometer. Reaction courses and product mixtures were routinely monitored by thin layer chromatography (TLC) that carried out using glass sheets pre-coated with silica gel 60 F254 purchased by Merk.
General procedure for preparation of compounds 4a,b and 5a,b
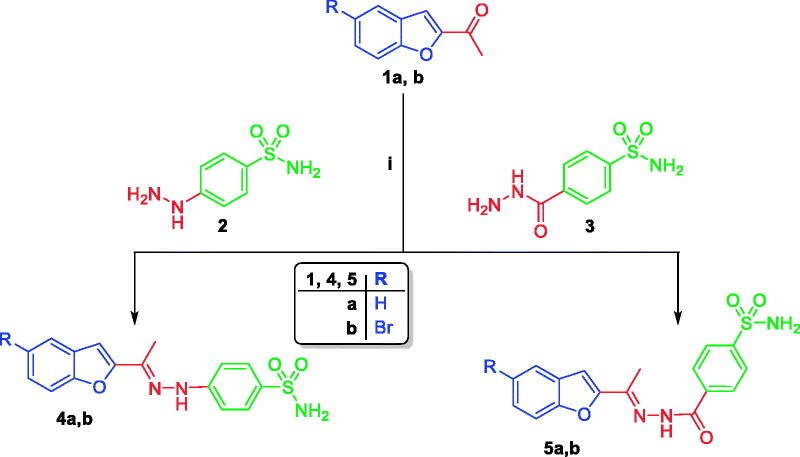
To a solution of 2-acetylbenzofuran derivative 1a or1b (1 mmol) in glacial acetic acid (5 mL), 4-hydrazinylbenzenesulfonamide 2 or 4-(hydrazinecarbonyl)benzenesulfonamide 3 (0.187 g, 1 mmol) was added. The reaction mixture was stirred under reflux temperature for 4 h. The precipitated solid was collected by filtration while hot, washed with cold ethanol, dried and recrystallised from dioxan to afford the target benzofuran-based sulphonamides 4a,b and 5a,b, respectively.
4–(2-(1-(Benzofuran-2-yl)ethylidene)hydrazineyl)benzenesulfonamide (4a)
White powder (yield 83%), m.p. 202–205 °C; IR (KBr, ν cm−1): 3447 (NH), 3326, 3214 (NH2) and 1322, 1147 (SO2); 1H NMR (DMSO-d6) δ ppm: 2.33 (s, 3H, CH3), 7.15 (s, 1H, Ar-H), 7.24–7.27 (m, 1H, Ar-H), 7.37, 7.39 (2s, 2H, NH2 D2O exchangeable of -SO2NH2), 7.52–7.56 (m, 1H, Ar-H), 7.61–7.66 (m, 2H, Ar-H), 7.70–7.74 (m, 2H, Ar-H), 7.83 (d, 1H, J = 8.0 Hz, Ar-H), 7.90 (s, 1H, Ar-H), 9.98 (s, 1H, NH D2O exchangeable); 13C NMR (DMSO-d6) δ ppm: 13.53, 105.23, 112.74, 114.79, 121.67, 123.64, 124.15, 124.50, 127.75, 128.89, 128.92, 134.70, 135.68, 148.35, 152.59, 155.45; MS m/z [%]: 329 [M+, 89.27], 89 [100]; Anal. calcd. for C16H15N3O3S (329.37): C, 58.35; H, 4.59; N, 12.76. Found C, 58.73; H, 4.53; N, 12.78.
4–(2-(1–(5-Bromobenzofuran-2-yl)ethylidene)hydrazineyl)benzenesulfonamide (4b)
White powder (yield 81%), m.p. >300 °C; IR (KBr, ν cm−1): 3434 (NH), 3227, 3316 (NH2) and 1343, 1162 (SO2); 1H NMR (DMSO-d6) δ ppm: 2.42 (s, 3H, CH3), 7.52–7.58 (m, 3H, Ar-H and NH2 D2O exchangeable of -SO2NH2), 7.65 (d, 1H, J = 8.0 Hz, Ar-H), 7.92 (s, 1H, Ar-H), 7.95–7.98 (m, 3H, Ar-H), 8.05–8.07 (m, 2H, Ar-H), 11.07 (s, 1H, NH D2O exchangeable); 13C NMR (DMSO-d6) δ ppm: 14.52, 108.00, 113.94, 116.13, 124.64, 126.03, 12811, 128.97, 129.30, 130.62, 137.18, 146.41, 147.11, 153.94, 155.30, 163.84; Anal. calcd. for C16H14BrN3O3S (408.27): C, 47.07; H, 3.46; N, 10.29. Found C, 47.27; H, 3.49; N, 10.28.
4–(2-(1-(Benzofuran-2-yl)ethylidene)hydrazine-1-carbonyl)benzenesulfonamide (5a)
White powder (yield 80%), m.p. >300 °C; IR (KBr, ν cm−1): 3434 (NH), 3316, 3227 (NH2), 1682 (C=O) and 1343, 1149 (SO2); 1H NMR (DMSO-d6) δ ppm: 2.44 (s, 3H, CH3), 7.28 (t, 1H, J = 8.0 Hz, Ar-H), 7.39 (t, 1H, J = 8.0 Hz, Ar-H), 7.49–7.58 (m, 3H, Ar-H and NH2 D2O exchangeable of -SO2NH2), 7.66 (d, 1H, J = 8.0 Hz, Ar-H), 7.71 (d, 1H, J = 8.0 Hz, Ar-H), 7.96 (d, 2H, J = 8.0 Hz, Ar-H), 8.06 (d, 2H, J = 8.0 Hz, Ar-H), 11.04 (s, 1H, NH D2O exchangeable); 13C NMR (DMSO-d6) δ ppm: 14.57, 108.91, 111.86, 122.33, 123.87, 126.05, 126.14, 128.12, 128.35, 129.26, 136.72, 137.28, 146.58, 147.05, 153.96, 155.16, 163.78; MS m/z [%]: 357 [M+, 52.05], 184 [100]; Anal. calcd. for C17H15N3O4S (357.38): C, 57.13; H, 4.23; N, 11.76. Found C, 57.27; H, 4.29; N, 11.78.
4–(2-(1–(5-Bromobenzofuran-2-yl)ethylidene)hydrazine-1-carbonyl)benzenesulfonamide (5b)
White powder (yield 79%), m.p. 270–272 °C; IR (KBr, ν cm−1): 3424 (NH), 3320, 3210 (NH2), 1597 (C=O) and 1316, 1150 (SO2); 1H NMR (DMSO-d6) δ ppm: 2.31 (s, 3H, CH3), 7.16–7.20 (m, 3H, Ar-H and NH2 D2O exchangeable of -SO2NH2), 7.37–7.40 (m, 2H, Ar-H),7.42–7.46 (m, 1H, Ar-H), 7.58–7.61 (m, 1H, Ar-H), 7.72–7.75 (m, 2H, Ar-H), 7.83–7.84 (m, 1H, Ar-H), 10.04 (s, 1H, NH D2O exchangeable); 13C NMR (DMSO-d6) δ ppm: 13.44, 104.36, 112.85, 113.57, 115.93, 123.93, 127.76, 127.81, 131.22, 134.91, 135.14, 148.19, 153.66, 156.47; Anal. calcd. for C17H14BrN3O4S (436.28): C, 46.80; H, 3.23; N, 9.63. Found C, 46.67; H, 3.19; N, 9.70.
General procedures for preparation of the target compounds 9a–c and 10a–d
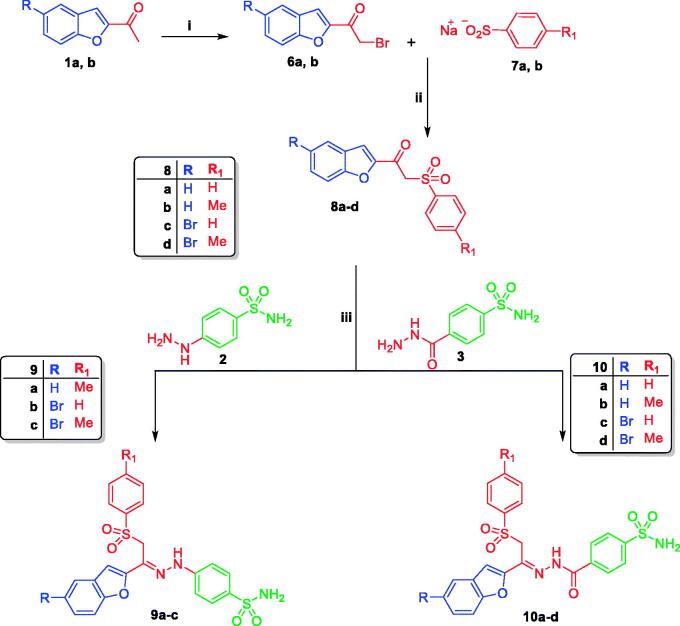
A mixture of 1-(benzofuran-2-yl)-2-(phenylsulfonyl)ethanone 8a–d (1 mmol), and 4-hydrazinylbenzenesulfonamide 2 (0.187 g, 1 mmol) or 4-(hydrazinecarbonyl)benzenesulfonamide 3 (0.215 g, 1 mmol) was refluxed in absolute ethanol in the presence of catalytic amount of glacial acetic acid. The solid formed was filtered, dried and recrystallised from ethanol/DMF to afford the target benzofuran-based sulphonamides 9a–c and 10a–d, respectively.
4–(2-(1-(Benzofuran-2-yl)-2-tosylethylidene)hydrazineyl)benzenesulfonamide (9a)
Yellow powder (yield 80%), m.p. 269–270 °C; IR (KBr, ν cm−1): 3430 (NH), 3309, 3280 (NH2) and 1343, 1309, 1265, 1159 (2SO2); 1H NMR (DMSO-d6) δ ppm: 2.13, 2.29 (2s, 3H, CH3), 4.75, 5.12 (2s, 2H, -SO2CH2-), 6.97 (d, 1H, J = 8.0 Hz, Ar-H), 7.08, 7.77 (s, 1H, Ar-H), 7.16, 7.17 (2s, 2H, NH2 D2O exchangeable of -SO2NH2), 7.23–7.29 (m, 2H, Ar- H), 7.31–7.37 (m, 2H, Ar- H), 7.44–7.52 (m, 2H, Ar- H), 7.57–7.73 (m, 3H, Ar-H), 7.74–7.76 (m, 2H, Ar-H), 10.29, 10.55 (2s, 1H, NH D2O exchangeable); 13C NMR (DMSO-d6) δ ppm: 21.33, 21.48, 53.15, 60.98, 105.77, 110.04, 111.44, 112.39, 112.98, 113.40, 121.56, 122.46, 123.61, 124.17, 124.25, 125.18, 125.35, 126.59, 127.22, 127.70, 128.73, 128.75, 128.82, 129.89, 129.93, 135.72, 136.00, 136.34, 136.41, 144.80, 145.37, 147.05, 147.38, 148.91, 153.82, 154.23, 154.56; MS m/z [%]: 483 [M+, 1.26], 143 [100]; Anal. calcd. for C23H21N3O5S2 (483.56): C, 57.13; H, 4.38; N, 8.69. Found C, 57.19; H, 4.39; N, 8.73.
4–(2-(1–(5-Bromobenzofuran-2-yl)-2-(phenylsulfonyl)ethylidene)hydrazineyl)benzenesulfonamide (9b)
Yellow powder (yield 78%), m.p. 281–283 °C; IR (KBr, ν cm−1): 3423 (NH), 3324, 3262 (NH2) and 1305, 1263, 1148, 1088 (2SO2); 1H NMR (DMSO-d6) δ ppm: 4.82, 5.18 (2s, 2H, -SO2CH2-), 6.97 (d, 1H, J = 8.0 Hz, Ar-H), 7.08, 7.99 (2s, 1H, Ar-H), 7.16, 7.19 (2s, 2H, NH2 D2O exchangeable of -SO2NH2), 7.27 (d, 1H, J = 8.0 Hz, Ar-H), 7.43–7.56 (m, 2H, Ar-H), 7.58–7.65 (m, 3H, Ar-H), 7.66–7.78 (m, 3H, Ar-H), 7.86 (d, 1H, J = 8.0 Hz, Ar-H), 7.91 (d, 1H, J = 8.0 Hz, Ar-H), 10.47, 10.62 (2s, 1H, NH D2O exchangeable); 13C NMR (DMSO-d6) δ ppm: 52.87, 60.71, 104.84, 109.23, 113.17, 113.46, 113.51, 114.48, 115.95, 116.51, 123.84, 124.31, 124.77, 127.29, 127.77, 128.76, 129.15, 129.55, 129.90, 131.01, 134.16, 134.62, 136.07, 136.21, 139.22, 146.94, 147.26, 150.20, 153.11, 153.35, 155.21; Anal. calcd. for C22H18BrN3O5S2 (548.43): C, 48.18; H, 3.31; N, 7.66. Found C, 48.39; H, 3.29; N, 7.67.
4–(2-(1–(5-Bromobenzofuran-2-yl)-2-tosylethylidene)hydrazineyl)benzenesulfonamide (9c)
Yellow powder (yield 76%), m.p. 200–202 °C; IR (KBr, ν cm−1): 3401 (NH), 3334, 3288 (NH2) and 1307, 1265, 1149, 1086 (2SO2); 1H NMR (DMSO-d6) δ ppm: 2.13, 2.29 (2s, 3H, CH3), 4.74, 5.23 (2s, 2H, -SO2CH2-), 7.00 (d, 1H, J = 8.0 Hz, Ar-H), 7.09, 7.96 (2s, 1H, Ar-H), 7.20 (s, 2H, NH2 D2O exchangeable of -SO2NH2), 7.24–7.33 (m, 3H, Ar- H), 7.48 (s, 1H, Ar- H), 7.57 (d, 1H, J = 8.0 Hz, Ar-H), 7.63 (d, 2H, J = 8.0 Hz, Ar-H), 7.67–7.71 (m, 3H, Ar-H), 10.29, 10.55 (2s, 1H, NH D2O exchangeable); 13C NMR (DMSO-d6) δ ppm: 21.31, 21.46, 60.99, 109.22, 113.12, 113.51, 114.47, 115.89, 116.45, 123.58, 123.81, 124.70, 27.23, 127.58, 128.82, 129.08, 129.83, 129.92, 129.94, 131.10, 135.83, 136.21, 136.25, 136.47, 144.82, 145.28, 147.04, 147.28, 150.06, 153.06, 153.35, 155.36; Anal. calcd. for C23H20BrN3O5S2 (562.45): C, 49.12; H, 3.58; N, 7.47. Found C, 49.29; H, 3.59; N, 7.57.
4–(2-(1-(Benzofuran-2-yl)-2-(phenylsulfonyl)ethylidene)hydrazine-1-carbonyl)benzenesulfonamide (10a)
White powder (yield 80%), m.p. 281–283 °C; IR (KBr, ν cm−1): 3429 (NH), 3372, 3310 (NH2), 1679 (C=O) and 1343, 1309, 1266, 1159 (2SO2); 1H NMR (DMSO-d6) δ ppm: 4.89, 5.43 (2s, 2H, -SO2CH2-), 7.26 (t, 1H, J = 8.0 Hz, Ar-H), 7.37–7.41 (m, 4H, NH2 D2O exchangeable of -SO2NH2and Ar-H), 7.54–7.60 (m, 4H, Ar-H), 7.76–7.88 (m, 2H, Ar-H), 7.98–8.06 (m, 5H, Ar-H), 11.32, 11.76 (2s, 1H, NH D2O exchangeable); 13C NMR (DMSO-d6) δ ppm: 53.42, 110.06, 111.77, 122.29, 123.94, 126.20, 126.69, 128.15, 128.63, 128.78, 129.23, 129.59, 129.77, 134.51, 134.89, 136.63, 139.02, 147.48, 152.72, 155.03, 163.37; MS m/z [%]: 497 [M+, 7.32], 77 [100]; Anal. calcd. for C23H19N3O6S2 (497.54): C, 55.52; H, 3.85; N, 8.45. Found C, 55.23; H, 3.90; N, 8.47.
4–(2-(1-(Benzofuran-2-yl)-2-tosylethylidene)hydrazine-1-carbonyl)benzenesulfonamide (10b)
White powder (yield 78%), m.p. >300 °C; IR (KBr, ν cm−1): 3423 (NH), 3370, 3307 (NH2), 1680 (C=O) and 1343, 1306, 1267, 1159 (2SO2); 1H NMR (DMSO-d6) δ ppm: 2.20, 2.26 (2s, 3H, CH3), 4.82, 5.37 (2s, 2H, -SO2CH2-), 7.27 (d, 2H, J = 8.0 Hz, Ar-H), 7.34 (t, 1H, J = 8.0 Hz, Ar-H), 7.47, 7.49 (2s, 2H, NH2 D2O exchangeable of -SO2NH2), 7.56–7.60 (m, 3H, Ar-H), 7.69–7.71 (m, 2H, Ar-H), 7.73–7.82 (m, 3H, Ar-H), 7.96–8.00 (m, 2H, Ar-H), 11.24, 11.27 (s, 1H, NH D2O exchangeable); 13C NMR (DMSO-d6) δ ppm: 19.03, 21.04, 53.57, 110.08, 111.77, 122.27, 123.90, 126.19, 126.67, 128.16, 128.62, 128.80, 129.20, 130.02, 130.18, 134.80, 136.09, 136.54, 145.67, 147.48, 152.69, 154.98, 163.23; MS m/z [%]: 511 [M+, 5.06], 184 [100]; Anal. calcd. for C24H21N3O6S2 (511.57): C, 56.35; H, 4.14; N, 8.21. Found C, 56.48; H, 4.18; N, 8.27.
4–(2-(1–(5-Bromobenzofuran-2-yl)-2-(phenylsulfonyl)ethylidene)hydrazine-1-carbonyl)benzenesulfonamide (10c)
White powder (yield 77%), m.p. >300 °C; IR (KBr, ν cm−1): 3401 (NH), 3324, 3288 (NH2), 1682 (C=O) and 1341, 1307, 1157, 1085 (2SO2); 1H NMR (DMSO-d6) δ ppm: 5.26, 5.42 (2s, 2H, -SO2CH2-), 7.32 (2s, 1H, Ar-H), 7.50–7.55 (m, 3H, NH2 D2O exchangeable of -SO2NH2 and Ar-H), 7.57–7.61 (m, 6H, Ar-H),7.87 (s, 1H, Ar-H), 7.95–8.00 (m, 4H, Ar-H), 10.98, 11.36 (2s, 1H, NH D2O exchangeable); 13C NMR (DMSO-d6) δ ppm: 53.30, 56.50, 109.03, 113.85, 116.21, 124.60, 126.19, 128.78, 129.31, 129.80, 130.36, 134.95, 136.50, 138.90, 153.76, 153.98, 163.36; Anal. calcd. for C23H18BrN3O6S2 (576.44): C, 47.92; H, 3.15; N, 7.27. Found C, 47.79; H, 3.19; N, 7.33.
4–(2-(1–(5-Bromobenzofuran-2-yl)-2-tosylethylidene)hydrazine-1-carbonyl)benzenesulfonamide (10d)
White powder (yield 74%), m.p. 283–285 °C; IR (KBr, ν cm−1): 3405 (NH), 3323, 3280 (NH2), 1673 (C=O) and 1343, 1305,1159, 1086 (2SO2); 1H NMR (DMSO-d6) δ ppm: 2.20, 2.25 (2s, 3H, CH3), 4.83, 5.37 (2s, 2H, -SO2CH2-), 7.25–7.31 (m, 3H, Ar-H), 7.49–7.60 (m, 3H, NH2 D2O exchangeable of -SO2NH2 and Ar-H), 7.62–7.72 (m, 2H, Ar-H), 7.78 (d, 1H, J = 8.0 Hz, Ar-H), 7.86 (s, 1H, Ar-H), 7.96–8.10 (m, 4H, Ar-H), 11.30, 11.74 (2s, 1H, NH D2O exchangeable); 13C NMR (DMSO-d6) δ ppm: 21.38, 53.52, 109.04, 113.84, 116.17, 124.58, 126.17, 126.38, 128.69, 128.80, 129.11, 130.19, 130.40, 134.34, 136.06, 145.67, 147.52, 153.73, 153.99, 163.34; Anal. calcd. for C23H20BrN3O6S2 (590.46): C, 48.82; H, 3.41; N, 7.12. Found C, 48.92; H, 3.34; N, 7.29.
CA inhibitory assay
An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity28. Phenol red (at a concentration of 0.2 mM) has been used as indicator, working at the absorbance maximum of 557 nm, with 20 mM Hepes (pH 7.5) as buffer, and 20 mM Na2SO4 (for maintaining constant the ionic strength), following the initial rates of the CA-catalysed CO2 hydration reaction for a period of 10–100 s. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constants. For each inhibitor at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The uncatalyzed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitor (0.1 mM) were prepared in distilled-deionised water and dilutions up to 0.01 nM were done thereafter with the assay buffer. Inhibitor and enzyme solutions were preincubated together for 15 min at room temperature prior to assay, in order to allow for the formation of the E-I complex. The inhibition constants were obtained by non-linear least-squares methods using PRISM 3 and the Cheng–Prusoff equation, as reported earlier22,23,29–31, and represent the mean from at least three different determinations.
Anti-proliferative activity against sixty NCI-cancer cell lines panel
The anti-proliferative assay was carried out in accordance with the protocol of the Drug Evaluation Branch, NCI, Bethesda32,33, as described previously34,35.
Results and discussion
Chemistry
The preparation of benzofuran-based sulphonamides 4a,b, 5a,b 9b–d, and 10a–d in this study is illustrated in Schemes 1 and 2. The synthesis was initiated by condensation of 2-acetylbenzofuran 1a and 5-bromo-2-acetylbenzofuran 1b with 4-hydrazinylbenzenesulfonamide 2 or 4-(hydrazinecarbonyl)benzenesulfonamide 3 in refluxing glacial acetic acid to furnish the target benzofuran-based sulphonamides 4a,b and 5a,b in 79–83% yield (Scheme 1).
Scheme 1.
Reagent and conditions: (i) Glacial Acetic acid, reflux 4 h.
Scheme 2.
Reagent and conditions: (i) Br2/Acetic Acid, Stirring at r.t 4 h; (ii) Abs.Ethanol, reflux 4 h; (iii) Ethanol/Acetic acid, reflux 4 h.
In Scheme 2, 2-acetylbenzofurans 1a and 1b were brominated by the use of bromine in glacial acetic acid to afford 1-(benzofuran-2-yl)-2-bromoethan-1-ones 6a and 6b, respectively. Thereafter, the brominated intermediates 6a and 6b were refluxed with sodium benzene sulfinates 7a and 7b in ethanol to obtain key intermediates 8a and 8b. Consequently, these key intermediates were condensed with 4-hydrazinylbenzenesulfonamide 2 or 4-(hydrazinecarbonyl)benzenesulfonamide 3 in refluxing ethanol containing catalytic amount of glacial acetic acid to furnish the target benzofurans 9a–c and 10a–d, respectively (Scheme 2).
Postulated structure of the newly synthesised benzofuran-based sulphonamides 4a,b, 5a,b 9b–d, and 10a–d were in full agreement with their spectral and elemental analyses data.
Biological evaluation
Carbonic anhydrase inhibition
The newly prepared benzofuran-based sulphonamides 4a,b, 5a,b 9b–d, and 10a–d were evaluated for their ability to inhibit the physiologically relevant hCA isoforms, hCA I, II (cytosolic), and hCA IX and XII (trans membrane, tumour associated isoforms) using acetazolamide (AAZ) as standard inhibitor by a stopped flow CO2 hydras assay . The inhibition data of the prepared benzensulfonamides and AAZ against the examined isoforms are summarised in Table 1.
Table 1.
Inhibition data of human CA isoforms hCA I, II, IX and XII for the target sulphonamides (4a,b, 5a,b, 9a–c, and 10a–d), using (AAZ) as a standard drug.
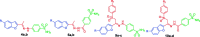 | ||||||
|---|---|---|---|---|---|---|
| Compound | R | R1 |
KI (nM)* |
|||
| hCA I | hCA II | hCA IX | hCA XII | |||
| 4a | H | – | 162.8 | 12.3 | 33.3 | 26.9 |
| 4b | Br | – | 92.7 | 73.5 | 48.4 | 38.8 |
| 5a | H | – | 37.4 | 33.6 | 60.4 | 10.1 |
| 5b | Br | – | 63.9 | 44.2 | 27.7 | 32.5 |
| 9a | H | CH3 | 1292 | 643.7 | 32.8 | 49.7 |
| 9b | Br | H | 2159 | 888.2 | 44.6 | 33.4 |
| 9c | Br | CH3 | 2503 | 571.1 | 10.0 | 16.7 |
| 10a | H | H | 4625 | 353.9 | 76.6 | 71.8 |
| 10b | H | CH3 | 3921 | 228.5 | 51.1 | 38.9 |
| 10c | Br | H | 1822 | 438.8 | 85.4 | 66.8 |
| 10d | Br | CH3 | 827.6 | 727.1 | 97.5 | 27.5 |
| AAZ | – | – | 250.0 | 12.0 | 25.0 | 5.7 |
Mean from three different assays, by a stopped flow technique (errors were in the range of ±5–10% of the reported values).
The ubiquitous cytosolic hCA I isoform was inhibited by the herein reported benzofuran-based sulphonamides in a variable degree. The benzofuran hydrazones 4a and 4b displayed moderate potency with inhibition constant (KI) values of 162.8 and 92.7 nM, respectively, whereas the benzofuran hydrazides 5a and 5b potently inhibited hCA I isoform with KI values of 37.4 and 63.9 nM, respectively. Contrariwise, hCA I was weakly inhibited by both arylsulfonehydrazones 9a–c and arylsulfonehydrazides 10a–d with KIs ranging in the micromolar range, in detail, between 1.292 and 4.625 μM, except for the Br-substituted tolylsulfonehydrazide 10d which displayed lower KI value (827.6 nM).
-
The in vitro kinetic data listed in Table 1 revealed that the physiologically dominant cytosolic hCA II isoform was inhibited in a similar fashion to hCA I inhibition profile. While, benzofuran hydrazones/hydrazides 4a,b/5a,b effectively inhibited hCA II (KIs: 12.3–73.5 nM), arylsulfonehydrazones 9a–c and arylsulfonehydrazides 10a–d displayed weak inhibitory activity with KIs spanning in the high nanomolar range: 228.5–888.2 nM.
In particular, benzofuran hydrazone 4a (KI=12.3 nM) emerged as the most potent hCA II inhibitor in this study with comparable activity to the standard drug AAZ (KI=12 nM). It is noteworthy that grafting 5-Br substituent to the benzofuran moiety elicited a worsening of effectiveness toward hCA II, except for compound 9c which exhibited a reduced KI (571.1 nM) than its un-substituted analogue 9a (KI=1643.7 nM).
-
The tumour-associated hCA IX isoform was efficiently inhibited by the herein reported benzofuran-based sulphonamides (4a,b, 5a,b, 9a–c and 10a–d) with KI values in the nanomolar range, 10.0–97.5 nM, Table 1. Superiorly, sulphonamide 9c displayed the best hCA IX inhibitory activity in this study (KI=10.0 nM) which is 2.5-times more potent than the standard drug AAZ (KI=25 nM). Also, compounds 4a, 5b and 9a displayed potent inhibitory activity toward hCA IX isoform with KI values equal 33.3, 27.7 and 32.8 nM, respectively.
It is worth emphasising that replacement of the hydrazine linker in arylsulfonehydrazones 9a–c (KIs = 32.8, 44.6 and 10.0 nM, respectively) with the hydrazide one furnished arylsulfonehydrazides 10a–d with decreased hCA IX inhibitory activity (KIs = 76.6, 51.1, 85.4 and 97.5 nM, respectively).
The data listed in Table 1 ascribed to the newly synthesised benzofuran-based sulphonamides (4a,b, 5a,b, 9a–c and 10a–d) potent efficiency in inhibiting the transmembrane tumour-associated hCA XII isoform. The target sulphonamides possessed activity with KI values spanning in the nanomolar range: 10.1–71.8 nM, Table 1. In particular, compound 5a was the most potent hCA XII inhibitor in this study with KI value of 10.1 nM. It is worth highlighting that the benzofuran hydrazides 5a and 5b showed an improved inhibitory profile (KIs = 10.1 and 32.5 nM, respectively) against hCA IIX in comparison to their benzofuran hydrazone analogues 4a and 4b (KIs = 26.9 and 38.8 nM, respectively).
The calculated selectivity indexes (SIs) displayed in Table 2 undeniably ascribed to the arylsulfonehydrazones 9 excellent selectivity towards hCA IX and XII over hCA I (SIs ranges: 39.4–250.3 and 26.0–149.9, respectively) and over hCA II (SIs ranges: 19.6–57.1 and 13.0–34.2, respectively). Besides, arylsulfonehydrazides 10 displayed good selectivity towards hCA IX and XII over hCA I (SIs ranges: 8.5–76.7 and 27.3–100.8, respectively) and over hCA II (SIs ranges: 5.1–7.5 and 4.9–26.4, respectively). Conversely, both hydrazones 4 and hydrazides 5 failed to display a satisfied selectivity towards hCA IX and XII. The distinctive selectivity of series 9 and 10 could be attributed to incorporation of arylsulfone moieties which elicited a dramatic worsening of effectiveness against hCA I and II.
Table 2.
Selectivity ratios for the inhibition of hCA IX and XII over hCA I and II for targeted compounds 4a, b, 5a, b, 9a–c and 10a–d.
| Compound | I/IX | II/IX | I/XII | II/XII |
|---|---|---|---|---|
| 4a | 4.9 | 0.4 | 6.1 | 0.5 |
| 4b | 1.9 | 1.5 | 2.4 | 1.9 |
| 5a | 0.6 | 0.6 | 3.7 | 3.3 |
| 5b | 2.3 | 1.6 | 2.0 | 1.4 |
| 9a | 39.4 | 19.6 | 26.0 | 13.0 |
| 9b | 48.4 | 19.9 | 64.6 | 26.6 |
| 9c | 250.3 | 57.1 | 149.9 | 34.2 |
| 10a | 60.4 | 4.6 | 64.4 | 4.9 |
| 10b | 76.7 | 4.5 | 100.8 | 5.9 |
| 10c | 21.3 | 5.1 | 27.3 | 6.6 |
| 10d | 8.5 | 7.5 | 30.1 | 26.4 |
| AAZ | 10.0 | 0.5 | 43.9 | 2.2 |
In vitro antitumor activity towards 60 cancer cell lines (NCI, USA)
The newly prepared benzofuran-based sulphonamides 4a,b, 5a,b 9a-c, and 10a–d were selected to be evaluated for their antitumor activity at the NCI-Developmental Therapeutic Programme (www.dtp.nci.nih.gov). They were evaluated at one dose primary anticancer screening assay, at 10 μM, toward the full panel of sixty cancer cell lines, in accordance with the protocol of the Drug Evaluation Branch, NCI, Bethesda34. The cell growth was evaluated using the sulforhodamine B (SRB) colorimetric assay35–37. The obtained data were reported as mean-graph of the percentage growth of the different treated tumour cells (Supplementary Materials).
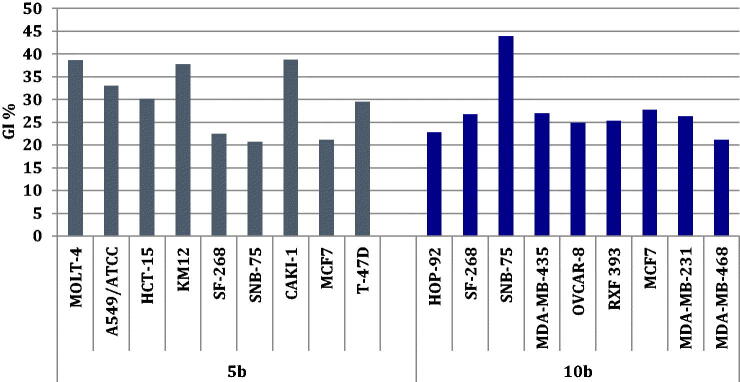
Investigation of the obtained results for this assay unveiled that only sulphonamides 5b and 10b possessed selective and moderate growth inhibitory activity toward certain cell lines, as displayed in Figure 2. Unfortunately, all the remaining target sulphonamides displayed non-significant anti-proliferative activity toward most NCI cancer cell lines.
Figure 2.
The most susceptible NCI cancer cell lines towards the impact of target sulphonamides 5b and 10b according to the GI%.
Conclusion
In summary, we successfully designed and synthesised novel benzofuran-based sulphonamides (4a,b, 5a,b, 9a–c, and 10a–d) as a potent and selective CAIs. All the examined hCA isoforms were inhibited by the prepared benzofurans in variable degrees with the following KIs ranges: 37.4–4625 nM for hCA I, 12.3–888.2 nM for hCA II, 10.0–97.5 nM for hCA XI, and 10.1–71.8 nM for hCA XII. Regarding the selectivity of the target compounds, arylsulfonehydrazones 9 showed excellent selectivity towards hCA IX and XII over hCA I (SIs ranges: 39.4–250.3 and 26.0–149.9, respectively) and over hCA II (SIs ranges: 19.6–57.1 and 13.0–34.2, respectively). Besides, arylsulfonehydrazides 10 displayed good selectivity towards hCA IX and XII over hCA I (SIs: 8.5–76.7 and 27.3–100.8, respectively) and over hCA II (SIs: 5.1–7.5 and 4.9–26.4, respectively). The distinctive selectivity of series 9 and 10 could be attributed to incorporation of arylsulfone moieties which elicited a dramatic worsening of effectiveness against hCA I and II. The prepared benzofuran-based sulphonamides were further evaluated for their antitumor activity at the NCI-Developmental Therapeutic Programme. The obtained results unveiled that only sulphonamides 5b and 10b possessed selective and moderate growth inhibitory effect against certain cell lines, whereas, the remaining compounds displayed non-significant anti-proliferative activity toward most NCI cancer cell lines.
Supplementary Material
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Supuran CT. Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32. [DOI] [PubMed] [Google Scholar]
- 2.Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168. [DOI] [PubMed] [Google Scholar]
- 3.Supuran CT. Carbonic anhydrase inhibitors as emerging agents for the treatment and imaging of hypoxic tumors. Expert Opin Investig Drugs 2018;27:963–70. [DOI] [PubMed] [Google Scholar]
- 4.Supuran CT. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov 2017;12:61–88. [DOI] [PubMed] [Google Scholar]
- 5.Innocenti A, Sarıkaya SB, Gülçin I, Supuran CT.. Carbonic anhydrase inhibitors. Inhibition of mammalian isoforms I–XIV with a series of natural product polyphenols and phenolic acids. Bioorg Med Chem 2010;18:2159–64. [DOI] [PubMed] [Google Scholar]
- 6.Innocenti A, Gülçin I, Scozzafava A, Supuran CT.. Carbonic anhydrase inhibitors. Antioxidant polyphenols effectively inhibit mammalian isoforms I–XV. Bioorg Med Chem Lett 2010;20:5050–3. [DOI] [PubMed] [Google Scholar]
- 7.Sentürk M, Gülçin I, Beydemir S, et al. . In vitro inhibition of human carbonic anhydrase I and II isozymes with natural phenolic compounds. Chem Biol Drug Des 2011;77:494–9. [DOI] [PubMed] [Google Scholar]
- 8.Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60. [DOI] [PubMed] [Google Scholar]
- 9.Capasso C, Supuran CT.. An overview of the alpha-, beta-and gamma-carbonic anhydrases from Bacteria: can bacterial carbonic anhydrases shed new light on evolution of bacteria? J Enzyme Inhib Med Chem 2015;30:325–32. [DOI] [PubMed] [Google Scholar]
- 10.Supuran CT. Carbonic anhydrases-an overview. Curr Pharm Des 2008;14:603–14. [DOI] [PubMed] [Google Scholar]
- 11.Carta F, Supuran CT.. Diuretics with carbonic anhydrase inhibitory action: a patent and literature review (2005–2013). Expert Opin Ther Pat 2013;23:681–91. [DOI] [PubMed] [Google Scholar]
- 12.Scozzafava A, Supuran CT, Glaucoma and the applications of carbonic anhydrase inhibitors. In: Frost SC, McKenna R, eds. Carbonic anhydrase: mechanism, regulation, links to disease, and industrial applications. Dordrecht: Springer. 2014:349–359. [DOI] [PubMed] [Google Scholar]
- 13.Mishra CB, Kumari S, Angeli A, et al. . Discovery of benzenesulfonamides with potent human carbonic anhydrase inhibitory and effective anticonvulsant action: design, synthesis, and pharmacological assessment. J Med Chem 2017;60:2456–69. [DOI] [PubMed] [Google Scholar]
- 14.Zha GF, Wang SM, Rakesh KP, et al. . Discovery of novel arylethenesulfonyl fluorides as potential candidates against methicillin-resistant of Staphylococcus aureus (MRSA) for overcoming multidrug resistance of bacterial infections. Eur J Med Chem 2019;162:364–77. [DOI] [PubMed] [Google Scholar]
- 15.Supuran CT, Alterio V, Di Fiore A, et al. . Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: three for the price of one. Med Res Rev 2018;38:1799–836. [DOI] [PubMed] [Google Scholar]
- 16.Supuran CT. Carbonic anhydrase inhibition and the management of hypoxic tumors. Metabolites 2017;7:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Prete S, Vullo D, Fisher GM, et al. . Discovery of a new family of carbonic anhydrases in the malaria pathogen Plasmodium falciparum—The η-carbonic anhydrases. Bioorg Med Chem Lett 2014;24:4389–96. [DOI] [PubMed] [Google Scholar]
- 18.Alterio V, Di Fiore A, D’Ambrosio K, et al. . Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev 2012;112:4421–68. [DOI] [PubMed] [Google Scholar]
- 19.Carta F, Vullo D, Osman SM, et al. . Synthesis and carbonic anhydrase inhibition of a series of SLC-0111 analogs. Bioorg Med Chem 2017;25:2569–76. [DOI] [PubMed] [Google Scholar]
- 20.Ibrahim HS, Allam HA, Mahmoud WR, et al. . Dual-tail arylsulfone-based benzenesulfonamides differently match the hydrophobic and hydrophilic halves of human carbonic anhydrases active sites: Selective inhibitors for the tumor-associated hCA IX isoform. Eur J Med Chem 2018;152:1–9. [DOI] [PubMed] [Google Scholar]
- 21.Abo-Ashour MF, Eldehna WM, Nocentini A, et al. . 3-Hydrazinoisatin-based benzenesulfonamides as novel carbonic anhydrase inhibitors endowed with anticancer activity: Synthesis, in vitro biological evaluation and in silico insights. Eur J Med Chem 2019;184:111768. [DOI] [PubMed] [Google Scholar]
- 22.Abo-Ashour MF, Eldehna WM, Nocentini A, et al. . Novel hydrazido benzenesulfonamides-isatin conjugates: Synthesis, carbonic anhydrase inhibitory activity and molecular modeling studies. Eur J Med Chem 2018;157:28–36. [DOI] [PubMed] [Google Scholar]
- 23.Eldehna WM, Abo-Ashour MF, Nocentini A, et al. . Enhancement of the tail hydrophobic interactions within the carbonic anhydrase IX active site via structural extension: Design and synthesis of novel N-substituted isatins-SLC-0111 hybrids as carbonic anhydrase inhibitors and antitumor agents. Eur J Med Chem 2019;162:147–60. [DOI] [PubMed] [Google Scholar]
- 24.Allam HA, Fahim SH, Abo-Ashour MF, Nocentini A, et al. . Application of hydrazino and hydrazido linkers to connect benzenesulfonamides with hydrophilic/phobic tails for targeting the middle region of human carbonic anhydrases active site: Selective inhibitors of hCA IX. Eur J Med Chem 2019;179:547–56. [DOI] [PubMed] [Google Scholar]
- 25.Al-Sanea MM, Elkamhawy A, Paik S, et al. . Synthesis and biological evaluation of novel 3-(quinolin-4-ylamino) benzenesulfonamides as carbonic anhydrase isoforms I and II inhibitors. J Enzyme Inhib Med Chem 2019;34:1457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eldehna WM, Abdelrahman MA, Nocentini A, et al. . Synthesis, biological evaluation and in silico studies with 4-benzylidene-2-phenyl-5(4H)-imidazolone-based benzenesulfonamides as novel selective carbonic anhydrase IX inhibitors endowed with anticancer activity. Bioorg Chem 2019;90:103102. [DOI] [PubMed] [Google Scholar]
- 27.Abdelrahman MA, Eldehna WM, Nocentini A, et al. . Novel diamide-based benzenesulfonamides as selective carbonic anhydrase IX inhibitors endowed with antitumor activity: synthesis, biological evaluation and in silico insights. Int J Mol Sci 2019;20:2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73. [PubMed] [Google Scholar]
- 29.Eldehna WM, Abo-Ashour MF, Berrino E, et al. . SLC-0111 enaminone analogs, 3/4-(3-aryl-3-oxopropenyl) aminobenzenesulfonamides, as novel selective subnanomolar inhibitors of the tumor-associated carbonic anhydrase isoform IX. Bioorg Chem 2019;83:549–58. [DOI] [PubMed] [Google Scholar]
- 30.Eldehna WM, Nocentini A, Al-Rashood ST, et al. . Tumor-associated carbonic anhydrase isoform IX and XII inhibitory properties of certain isatin-bearing sulfonamides endowed with in vitro antitumor activity towards colon cancer. Bioorg Chem 2018;81:425–32. [DOI] [PubMed] [Google Scholar]
- 31.Fares M, Eladwy RA, Nocentini A, et al. . Synthesis of bulky-tailed sulfonamides incorporating pyrido [2, 3-d][1, 2, 4] triazolo [4, 3-a] pyrimidin-1 (5H)-yl) moieties and evaluation of their carbonic anhydrases I, II, IV and IX inhibitory effects. Bioorg Med Chem 2017;25:2210–7. [DOI] [PubMed] [Google Scholar]
- 32.Boyd MR, Paull KD.. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev Res 1995;34:91–109. [Google Scholar]
- 33.(a) Eldehna WM, Hassan GS, Al-Rashood ST, et al. . Synthesis and in vitro anticancer activity of certain novel 1-(2-methyl-6-arylpyridin-3-yl)-3-phenylureas as apoptosis-inducing agents. J Enzyme Inhib Med Chem 2019;34:322.(b) Eldehna WM, Abo-Ashour MF, Ibrahim HS, Al-Ansary G, et al. . Novel [(3-indolylmethylene)hydrazono]indolin-2-ones as apoptotic anti-proliferative agents: design, synthesis and in vitro biological evaluation. J Enzym Inhib Med Chem 2018;33: 686–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monks A, Scudiero D, Skehan P, et al. . Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst 1991;83:757–66. [DOI] [PubMed] [Google Scholar]
- 35.Skehan P, Storeng R, Scudiero D, et al. . New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 1990;82:1107–12. [DOI] [PubMed] [Google Scholar]
- 36.Eldehna WM, El Kerdawy AM, Al-Ansary GH, et al. . Type IIA-Type IIB protein tyrosine kinase inhibitors hybridization as an efficient approach for potent multikinase inhibitor development: design, synthesis, anti-proliferative activity, multikinase inhibitory activity and molecular modeling of novel indolinone-based ureides and amides. Eur J Med Chem 2019;163:37–53. [DOI] [PubMed] [Google Scholar]
- 37.Eldehna W, Fares M, Ibrahim H, et al. . Synthesis and cytotoxic activity of biphenylurea derivatives containing indolin-2-one moieties. Molecules 2016;21:762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.