Abstract
Chronic itch is one of the disturbing symptoms of inflammatory skin diseases. Kappa opioid receptor agonists are effective in suppressing scratching in mice against different pruritogens. Nalbuphine, a nonscheduled kappa opioid receptor agonist and mu opioid receptor antagonist, has been in clinical use for post-operative pain management since the 1980s and recently has been in clinical trials for chronic itch of prurigo nodularis (https://www.trevitherapeutics.com/nalbuphine). We studied whether nalbuphine is effective against chronic scratching induced by rostral neck application of 1-fluoro-2,4-dinitrobenzene (DNFB), an accepted mouse model of contact dermatitis to study pruritoceptive itch. Mice were treated once a week with either saline or nalbuphine 20 min before the third, fifth, seventh, and ninth sensitizations with DNFB and the number of scratching bouts was counted for 30 min. Skin samples from the neck of mice at week 4 were used to measure protein levels and mRNA expressions of chemokines and cytokines. Different sets of mice were used to study sedation and anhedonic-like behavior of nalbuphine. We found that: nalbuphine (a) antagonized scratching in a dose- and time-dependent manner without affecting locomotion, b) decreased IL-31, and increased anti-inflammatory IL-10, and c) induced more elevations in the levels of CCL2, CCL3, CCL12, CXCL1, CXCL2, CXCL9, CXCL10, IL-1β IL-16, TIMP-1, M-CSF, TREM-1 and M1-type macrophages compared to saline. Increases in chemokines and cytokines and M1 macrophages by nalbuphine suggest an inflammatory phase of healing in damaged skin due to scratching. Our data indicate that nalbuphine is an effective antipruritic in murine model of pruritoceptive itch.
Keywords: Nalbuphine, pruritus, contact dermatitis, DNFB, cytokines, chemokines
1. Introduction
Chronic itch is one of the most disturbing symptoms encountered not only in some inflammatory skin diseases but also in systemic, neuropathic and psychological disorders (Yosipovitch and Bernhard, 2013; Xu et al., 2016). Treatment of chronic pruritus requires a wide range of systemic medications including antihistamines, glucocorticoids, antidepressants, gabapentin, methotrexate, cyclosporine, capsaicin, and calcineurin inhibitors (Pereira and Stander, 2017; Giavina-Bianchi and Giavina-Bianchi, 2019). Clinical studies targeting neurokinin 1, opioid, histamine, leukotriene, interleukin and TRPV1 receptors, and inhibitors on janus kinase and bile acid transporters are underway (Erickson et al., 2018; McEwen et al., 2018; Patel and Dao, 2018; Fowler and Yosipovitch, 2019).
The therapeutic value of kappa opioid receptor agonists against chronic pruritus has increased. Previous studies have shown that several kappa opioid receptor antagonists induce compulsive scratching (Kamei and Nagase, 2001; Inan et al., 2011; Cowan et al., 2015) whereas kappa opioid receptor agonists suppress scratching in mouse models of acute (Togashi et al., 2002; Ko et al., 2003; Inan and Cowan, 2004; Inan et al., 2009; Sakakihara et al., 2016), and chronic itch (Umeuchi et al., 2005; Inan and Cowan 2006; Akiyama et al., 2015; Takahashi et al., 2017). Further, dynorphin, an endogenous kappa opioid receptor agonist, plays a neuromodulatory role in the conduction of itch stimuli along the spinal cord (Kardon et al., 2014). Recent preclinical studies have focused on the ability of kappa opioid receptor agonists to signal preferentially via G proteins rather than β-arrestin 2 (thus causing fewer side effects) in targeting pruritus and pain (Brust et al., 2016).
Nalfurafine, the kappa opioid receptor agonist, has been approved for the treatment of uremic pruritus and is in clinical trials for pruritus in chronic liver disease in Japan (Kamimura et al., 2017). Difelikefalin and asimadoline, peripherally acting kappa opioid receptor agonists, are in clinical trials for uremic pruritus and atopic dermatitis, respectively (Chalmers, 2011; McEwen et al., 2018). Promising results have been reported with nalbuphine, a mu opioid receptor antagonist and kappa opioid receptor agonist, against prurigo nodularis (https://www.trevitherapeutics.com/nalbuphine). Nalbuphine has been in clinical practice since the 1980s for postoperative pain management (Beaver and Feise, 1978; Beaver and Feise 1981), and since the 1990s for reversal of morphine-induced pruritus without affecting analgesia suggesting an action through kappa opioid receptor agonism (Alhashemi et al., 1997; Yeh et al., 2009; Moustafa et al., 2016).
The role of the immune system in itch in mice and humans with inflammatory skin diseases has been reported (Dillon et al., 2004; Bilsborough et al., 2006; Takamori et al., 2018; Hashimoto et al., 2019). The present study investigated nalbuphine’s effect on pruritus and chemokine and cytokine levels in a DNFB-induced contact dermatitis, a mouse model of pruritoceptive itch (Zhang et al., 2015; Vaia et al., 2016; Mu et al., 2017; Mack and Kim, 2018). The presence of regulatory T (Treg) cells, and M1 and M2 monocytes/macrophages were also examined. Additionally, sedation and anhedonic-like effects were studied as recognized side effects of nalbuphine.
2. Materials and methods
2.1. Animals
Male Swiss Webster mice (Taconic Biosciences, Germantown, NY) weighing 25-30 g were used. Mice were housed in a temperature- and humidity-controlled environment with a 12-hr light-dark cycle. They were supplied with food and water ad libitum. Before any procedure was applied, the mice were acclimated for 1 week in the animal facility. Behavioral testing was performed between 11:00 AM and 5:00 PM. All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of Temple University and conducted according to the NIH Guide for the Care and Use of Laboratory Animals. In all experiments, 6-8 mice/group were used.
2.2. Acute administration of nalbuphine against DNFB-induced scratching
We used a model of contact allergic dermatitis induced by repeated exposure to DNFB-acetone solution (Zhang et al., 2015, Mu et al., 2017). Two days before DNFB application, the rostral area of the neck and the abdomen of mice were shaved. Initial sensitization with DNFB-acetone solution (100 μl) was conducted on abdominal skin. One week later, a cutaneous reaction was evoked on a rostral area of the neck by repeated (2 times a week for 4 weeks) applications of 50 μl of DNFB-acetone solution. Nine applications were made, including initial sensitization. The timeline and sequence for these experiments are shown in Fig. 1. Mice were randomly divided into the following four groups: saline + DNFB, nalbuphine 1 mg/kg + DNFB, nalbuphine 3 mg/kg + DNFB, and nalbuphine 10 mg/kg + DNFB. Every week (for 4 weeks) before the second sensitization, mice were placed into observation boxes for at least for an hr for acclimation. Twenty min before application of the DNFB solution, mice were injected with either saline or nalbuphine (1, 3, or 10 mg/kg, s.c.). The DNFB-acetone solution was then applied to the rostral area of neck and 1 min later, the number of scratching bouts directed to the neck was counted for 30 min. At the end of observations on week 4, the mice were euthanized using CO2 followed by cervical dislocation. Neck skin tissue and abdominal skin tissue (as control) were obtained from mice treated with saline or nalbuphine (10 mg/kg) and kept at −80 °C to measure chemokine and cytokine levels.
Fig. 1. Timeline for DNFB sensitizations and treatments.
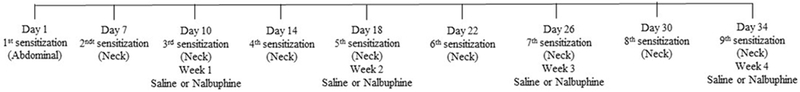
Mice were sensitized twice a week (one week after first sensitization on abdominal skin) by rostral neck application of DNFB (0.1% solution in acetone). Twenty min before the third, fifth, seventh and ninth applications mice were treated (s.c.) with either saline or nalbuphine (1, 3, or 10 mg/kg). Following application of DNFB, the number of scratching bouts to the neck was counted for 30 min.
2.3. Measurement of locomotion and anhedonia-like effect
In a different group of mice (n=8), we examined whether the anti-scratch effect of nalbuphine is a result of behavioral depression and/or anhedonic-like behavior since sedation and anhedonia are known side effects of kappa opioid agonists. For locomotor activity, mice were placed individually in a cage (27 cm x 48 cm x 20 cm) and acclimated for 1 hr. They were injected s.c. with either saline or nalbuphine (10 mg/kg) and monitored for total distance traveled over 1 hr using a Digiscan D Micro System (AccuScan, Columbus, OH). We chose the highest dose of nalbuphine since the sedation effect is dose-related.
The possible anhedonic-like effect was measured using the splash test which quantitates self-grooming (Yalcin et al., 2008; Butelman et al., 2019). Mice were acclimated individually to observation cages for at least 1 hr and were then injected with either saline or nalbuphine (3 or 10 mg/kg, s.c.). Twenty min later, using a spray bottle, approximately 0.7 ml of 10 % sucrose (w/v) solution in water was sprayed from about 10 cm distance on the flank areas of the mouse. The time spent grooming on both flank areas was recorded for 5 min and for 10 min (in different mice) using a stopwatch.
2.4. Measurement of chemokine and cytokine levels
Chemokine and cytokine levels (except IL-31) were measured in neck tissue lysates using a proteome profiler mouse cytokine array kit purchased from R&D Systems Inc. (Minneapolis, MN). The relative expression levels of 40 mouse cytokines were detected using nitrocellulose membranes that have antibodies spotted in duplicate. Neck tissue lysates were prepared as follows: tissues were homogenized in phosphate-buffered saline with protease inhibitors. After homogenization, Triton X-100 was added (final concentration was 1% in homogenate). Samples were frozen at −80 °C, thawed, and then centrifuged at 10,000 x g for 5 min to remove cellular debris. Homogenates were kept at −80 °C. Protein concentration of samples was measured using a Thermo Fisher Scientific NanoDrop 2000 spectrophotometer. The proteome profiler array kit had 4 nitrocellulose membranes loaded with the same antibodies. Each membrane was used for one group. For each group, cytokine and chemokine levels were measured in pooled samples (6-8 mice). 300 μg samples for each group were used. Sample/antibody/streptavidine-HRP complex was measured using chemiluminescent detection reagents. Light is produced at each spot in proportion to the amount of cytokine bound. Luminescence was quantitated by a Fuji Digital camera using imageGauge® software. Results were stated as Arbitrary Units and reported as fold change from control (abdominal skin results for saline + DNFB group).
IL-31 was measured using a Mouse IL-31 PicoKine ELISA kit (Boster Biological Technology Co., Pleasanton, CA) in duplicate homogenized neck skin tissue samples from mice treated with saline and nalbuphine (10 mg/kg) according to the manufacturer’s guidelines.
2.5. Measurement of mRNA expression
Homogenized neck tissue samples from mice treated with saline and nalbuphine (10 mg/kg) were used. Analyses of RNA expression were performed as previously described (Sanmiguel et al., 2009). Briefly, RNA was isolated using a RNeasy kit (Qiagen) and cDNA synthesized with AMV Reverse Transcriptase (Promega) and random hexamers. Real time PCR was carried out using a StepOnePlus Real-Time PCR System (Applied Biosystems) and the primers listed below. FoxP3 (Treg), iNOS (M1 monocyte/macrophage), CD80 and CD163 (M2 monocyte/macrophage) markers were used. We also measured mRNA expression for IL-10 since it is more sensitive than protein measurement. Relative expression of every gene was normalized to GAPDH by the 2ΔCT method. The following primers were used:
GAPDH (F) CTTGTGCAGTGCCAGCC (R) GCCCAATACGGCCAAATC;
FoxP3 (F) CTCCCTGCTCCTCCTATTC (R) CTCCTAATGCCTCCCAGAG;
IL-10 (F) GCCCTTTGCTATGGTGTC (R) TCCCTGGTTTCTCTTCCC;
iNOS (F) CAGCTACGCCTTCAACAC (R) TGGGACAGTCTCCATTCC;
CD80 (F) CTCGCTTCTCTTGGTTGG (R) TGGTTGCGAGTCGTATTG;
CD163 (F) ACTCCAGGAAGGGCATAC (R) CACAGCCCAACTGCTTAC
2.6. Statistical analysis
Data are expressed as mean ± standard error of the mean (S.E.M.) and P<0.05 was accepted as statistically significant. GraphPad Prism, version 7, was used for data analysis. To analyze results for the effects of nalbuphine on pruritus induced by DNFB, two-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test was used. IL-31 levels, IL-10, FoxP3, iNOS, CD80, CD163, and locomotion were analyzed using unpaired Student’s t-test. Splash test results were analyzed using one-way ANOVA followed by Dunnett’s multiple comparison test. Statistics on the results of the RT2 Profiler® Arrays were done by the Student’s t-test, using the online GeneGlobe Data Analysis Center (Qiagen).
2.7. Compounds
1-Fluoro-2,4-dinitrobenzene and nalbuphine hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO). DNFB 0.1% solution was prepared in acetone and nalbuphine was dissolved in saline.
3. Results
3.1. Acute systemic administration of nalbuphine attenuates DNFB-induced pruritus
DNFB sensitization induced scratching beginning during the first week and continuing until the end of observations on week 4 (Fig. 2). Nalbuphine at 3 and 10 mg/kg given 20 min before the fifth (week 2), seventh (week 3), and ninth (week 4) DNFB challenges significantly decreased scratching (*P<0.05, **P<0.01, ****P<0.0001). At the third sensitization (week 1), there was a decrease in scratching with nalbuphine 10 mg/kg pretreatment, however it did not reach a statistically significance level. Nalbuphine antagonized scratching in a time- and dose-dependent manner.
Fig. 2. Nalbuphine inhibits scratching in a time- and dose-dependent manner.
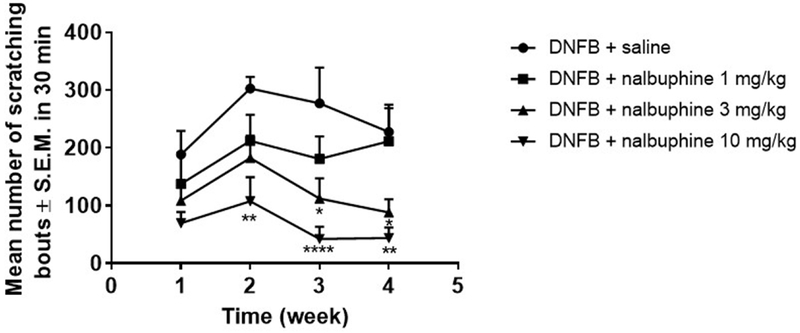
Nalbuphine at 3 and 10 mg/kg significantly reduced scratching compared to saline (Two-way ANOVA (time and treatment) followed by Dunnett’s multiple comparison test, *P<0.05, **P<0.01, ****P<0.0001; n=6-8).
3.2. The effect of nalbuphine on locomotion and anhedonia
To establish if the antagonizing effect of nalbuphine on pruritus is a consequence of sedation, we measured locomotion in mice treated with saline or nalbuphine 10 mg/kg. Ambulation was not significantly suppressed in mice treated with nalbuphine compared to saline administered mice (Fig. 3).
Fig. 3. Nalbuphine does not significantly affect locomotion.
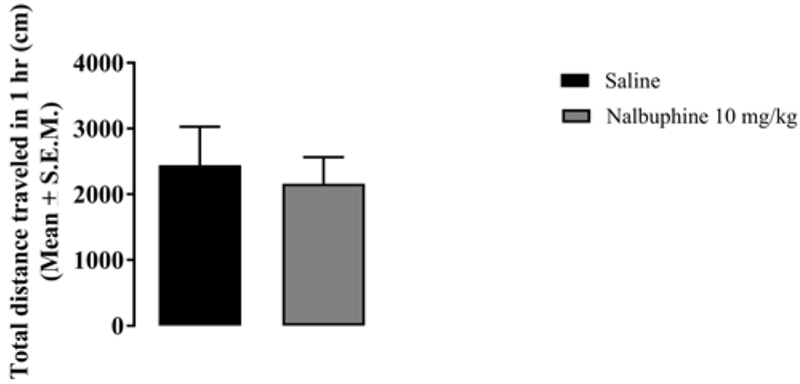
Mice (n=8) were injected with either saline or nalbuphine (10 mg/kg) and then total distance traveled was measured for 1 hr. Ambulatory activity was similar in mice treated with saline and nalbuphine.
Anhedonic-like behavior was tested using the splash test that measures self-grooming. We measured grooming for 5 min and 10 min durations in different groups of mice (Fig. 4). Nalbuphine (3 and 10 mg/kg) significantly reduced grooming time by 70-75% compared to saline during the 5 min observation period (Panel A, **P<0.01). In mice observed through 10 min, both 3 and 10 mg/kg of nalbuphine significantly decreased grooming by approximately 20% compared to saline (Panel B, *P<0.05).
Fig. 4. Measurement of anhedonic-like effect of nalbuphine.
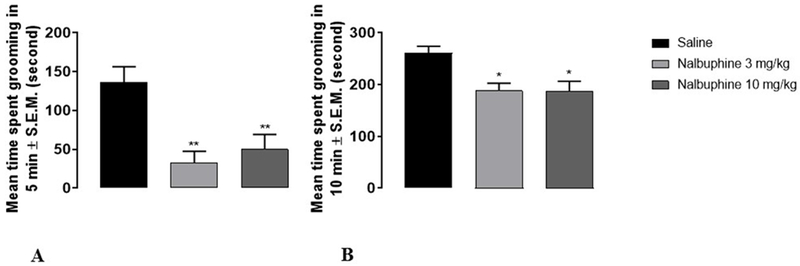
Mice were acclimated individually to observation boxes. They were injected (s.c.) with either saline or nalbuphine (3, or 10 mg/kg) 20 min before spraying 10% sucrose solution in water. Following spraying sucrose solution to the flank areas of mice, the time to spent grooming the flank areas was recorded during 5 min and 10 min (different group of mice). Both 3 and 10 mg/kg nalbuphine significantly reduced grooming time 70-75% compared to saline during the 5 min observation period (Panel A, **P<0.01,). In mice observed through 10 min, both 3 and 10 mg/kg nalbuphine significantly decreased grooming about 20% compared to saline (Panel B, *P<0.05). (One-way ANOVA followed by Dunnett’s test, n=6-8).
3.3. Changes in chemokine and cytokine levels
To examine if nalbuphine would influence inflammation in dermatitis, we used an array that measures a wide spectrum of chemokines and cytokines. Array images from abdominal and neck tissues of mice treated with saline or nalbuphine (10 mg/kg) are shown in Fig. 5. Abdominal skin of mice treated with nalbuphine did not show any detectable increase in chemokine expression from that of mice treated with saline. Increases were only noted on neck skin where DNFB was applied. This clearly shows that chemokine levels were increased as a consequence of dermatitis. Fold changes in chemokine (A) and cytokine (B) levels in neck tissue of saline + DNFB, abdominal tissue of nalbuphine + DNFB, and neck tissue of nalbuphine + DNFB compared to abdominal tissue of saline + DNFB are summarized in Table 1. CCL2, CCL3, CCL12, C5a, CXCL1, CXCL2, CXCL9, CXCL10 levels were increased 16-, 13-, 4-, 7-, 4-, 10-, 7-, and 4-fold, respectively, in mice with dermatitis treated with saline. However, the same chemokines were increased to a greater extent in nalbuphine-treated mice with dermatitis (60-, 57-, 10-, 13-, 8-, 80-, 14-, and 7-fold, respectively). While CCL1, CCL5, and CXCL11 did not show any change on neck tissue of mice treated with saline, they were slightly increased in nalbuphine-treated animals (4-, 4-, and 3-fold, respectively). CCL4, CXCL12, and CXCL13 levels did not change in either saline- or nalbuphine-treated mice with dermatitis. For cytokines, abdominal skin also did not show any increase except for IL-16 (6-fold). IL1-β, IL-13, IL-16, TIMP-1, M-CSF, TREM-1, SICAM, and IFN-γ levels were increased 4-, 5-, 10-, 6-, 4-, 4-, 5-, and 3-fold, respectively, in mice with dermatitis treated with saline. Similar to chemokines, these cytokines (except IL-13, SICAM, and IFN-δ) were increased more in mice with dermatitis treated with nalbuphine (16-, 3-, 16-, 12-, 10-, 25-, 4-, and 3-fold, respectively). While IL-1ra, IL-12p7c, IL-17, IL-23, and TNF-α did not show any changes in mice with dermatitis injected with saline, they were increased slightly in nalbuphine-injected mice (4-, 3-, 3-, 3-, and 4-fold, respectively). IL-1α, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-10, IL-27, G-CSF, and GM-CSF levels did not change in either saline- or nalbuphine-treated mice with dermatitis (Table 1.). Nalbuphine has an effect on the release of chemokines and cytokines in skin with dermatitis.
Fig. 5. Chemokine and cytokine levels were measured using proteome profiler array.
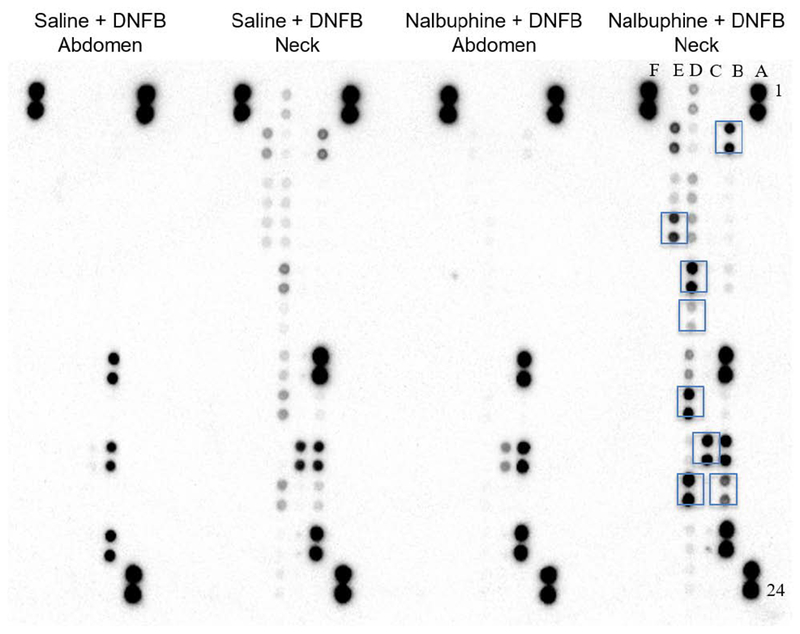
Nitrocellulose membranes are shown for abdominal and neck tissue for saline and nalbuphine (10 mg/kg) treatment. A1, 2, 23, 24, F1, 2 are reference spots, and F23, 24 are negative control. Boxed points on neck tissue sample treated with nalbuphine represent chemokines and cytokines that have increased to a greater extent compared to saline treatment: B3, 4 (CCL5); B19, 20 (IL-1β); C17, 18 (IL-16); D7, 8 (M-CSF); D9, 10 (CCL2); D15, 16 (CCL3); D19, 20 (CXCL2); E3, 4 (TIMP-1); E7, 8 (TREM-1).
Table. 1.
Fold changes in chemokine (A) and cytokine (B) levels in neck tissue of saline + DNFB, abdominal tissue of nalbuphine + DNFB, and neck tissue of nalbuphine + DNFB compared to abdominal tissue of saline + DNFB.
| A. | |||
|---|---|---|---|
| Saline + DNFB Neck | Nalbuphine + DNFB Abdomen | Nalbuphine + DNFB Neck | |
| CCL-1 | 1.53 | 1.24 | 4.34*** |
| CCL-2 | 16.48**** | 1.77 | 59.79**** |
| CCL-3 | 13.26**** | 2.20* | 57.46**** |
| CCL-4 | 1.97 | 1.52 | 1.52 |
| CCL-5 | 1.88 | 1.42 | 3.92** |
| CCL-12 | 3.73** | 1.45 | 10.75*** |
| C5a | 7.67*** | 1.45 | 13.46**** |
| CXCL1 | 4.14** | 1.12 | 9.01*** |
| CXCL2 | 11.22*** | 1.87 | 82.84**** |
| CXCL9 | 6.22*** | 1.43 | 13.14**** |
| CXCL10 | 4.26** | 1.03 | 6.76** |
| CXCL11 | 1.91 | 1.10 | 3.05* |
| CXCL12 | 1.68 | 1.01 | 2.03 |
| CXCL13 | 1.26 | 1.03 | 0.98 |
| B. | |||
| IL-1a | 1.03 | 2.31* | 1.74 |
| IL-1ß | 4.80** | 1.67 | 15.71*** |
| IL-1rd | 2.60** | 3.61** | 4.35** |
| IL-2 | 1.20 | 1.19 | 1.54 |
| IL-3 | 1.10 | 0.93 | 1.19 |
| IL-4 | 1.27 | 0.97 | 1.50 |
| IL-5 | 1.09 | 0.94 | 1.45 |
| IL-6 | 1.48 | 0.99 | 1.96 |
| IL-7 | 1.80 | 1.18 | 2.26* |
| IL-10 | 1.66 | 1.49 | 1.90 |
| IL-12p7c | 1.94 | 1.48 | 2.88* |
| IL-13 | 5.43** | 2.88** | 3.52** |
| IL-16 | 10.25*** | 5.80** | 16.75**** |
| IL-17 | 2.23* | 1.48 | 3.54* |
| IL-23 | 2.42* | 1.74 | 3.08* |
| IL-27 | 1.26 | 1.12 | 1.57 |
| TIMP-1 | 6.56*** | 1.48 | 12.60**** |
| G-CSF | 1.55 | 1.05 | 2.19* |
| GM-CSF | 1.46 | 1.10 | 2.39* |
| M-CSF | 3.93** | 1.79 | 10.03*** |
| TNF-α | 2.37* | 0.92 | 3.72* |
| TREM-1 | 4.93** | 1.43 | 25.01**** |
| SICAM | 5.20*** | 3.40** | 4.70*** |
| IFNγ | 3.40* | 1.91 | 3.05* |
P<0.05,
P<0.01,
P<0.001,
P<0.0001)
3.4. Nalbuphine decreases IL-31 level and increases IL-10 mRNA expression
Previous studies have reported that IL-31, a cytokine for type 2 immune responses, plays an important role for inducing pruritus and inflammation in atopic dermatitis and chronic contact dermatitis in mice and humans (Dillon et al., 2004; Bilsborough et al., 2006; Sonkoly et al., 2006; Cevikbas et al., 2014; Takamori et al., 2018). To study if nalbuphine has an effect on IL-31, we measured IL-31 levels in mice treated with saline or nalbuphine (10 mg/kg) using ELISA. The IL-31 level was significantly decreased in nalbuphine-administered mice compared to saline-injected mice (Fig. 6 Panel A, *P<0.05).
Fig. 6. Nalbuphine decreases IL-31 and increases IL-10.
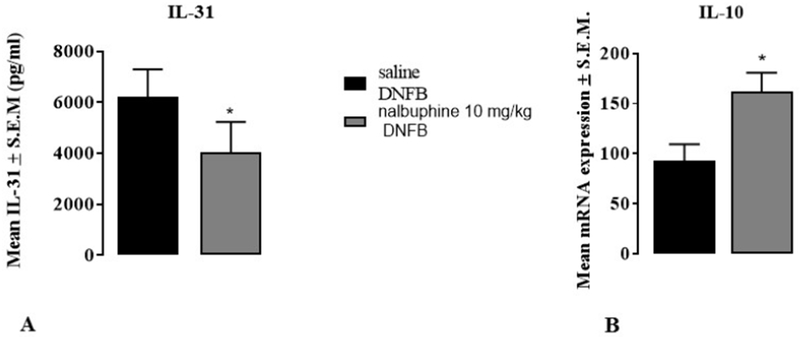
A. Nalbuphine (10 mg/kg) significantly reduces IL-31 levels compared to saline in neck tissue samples. The IL-31 value was measured using ELISA. B. Nalbuphine (10 mg/kg) significantly increases IL-10 mRNA expression compared to saline in neck tissue samples. (*P<0.05, unpaired Student’s t-test, n=6-8).
IL-10 is a Type 2 anti-inflammatory cytokine that downregulates expressions of Type 1 cytokines, IFN-γ, IL-2, IL-3, TNF-α, GM-CSF, and IL-31. When IL-10 was given to patients with psoriasis their lesions were decreased (Couper et. al., 2008; Mosser and Zhang, 2008; Shachar and Karin, 2013). We found IL-10 protein levels to be below the detection limit of the arrays (Fig. 5); therefore, we examined IL-10 mRNA expression, since it is more sensitive than protein measurements. IL-10 mRNA expression was significantly increased in mice treated with nalbuphine compared to mice treated with saline (Fig. 6 Panel B, *P<0.05). Nalbuphine decreased pruritic IL-31 and increased anti-inflammatory IL-10. Since IL-10 downregulates IL-31, it is suggested that nalbuphine decreases IL-31 by increasing IL-10.
3.5. Nalbuphine increases M1 monocyte/macrophage response
Given the chemokine and cytokine results with nalbuphine (Table 1.), we next measured mRNA expression of FoxP3 (Treg), iNOS (M1 monocyte/macrophage), and CD80 and CD163 (M2 monocyte/macrophage) to identify the type of immune cells present in skin with dermatitis. Expression for FoxP3 mRNA was similar in mice treated with saline or nalbuphine (Fig. 7 Panel A). Nalbuphine significantly increased iNOS mRNA expression compared to saline (Fig. 7 Panel B, *P<0.05). Both CD80 and CD163 mRNA expressions did not show any difference in mice injected with saline or nalbuphine (Fig. 7 Panel C and D, respectively). Nalbuphine increased Type 1 monocyte/macrophages in skin with dermatitis.
Fig. 7. mRNA expressions for markers for Treg, and M1 and M2 monocyte/macrophage.
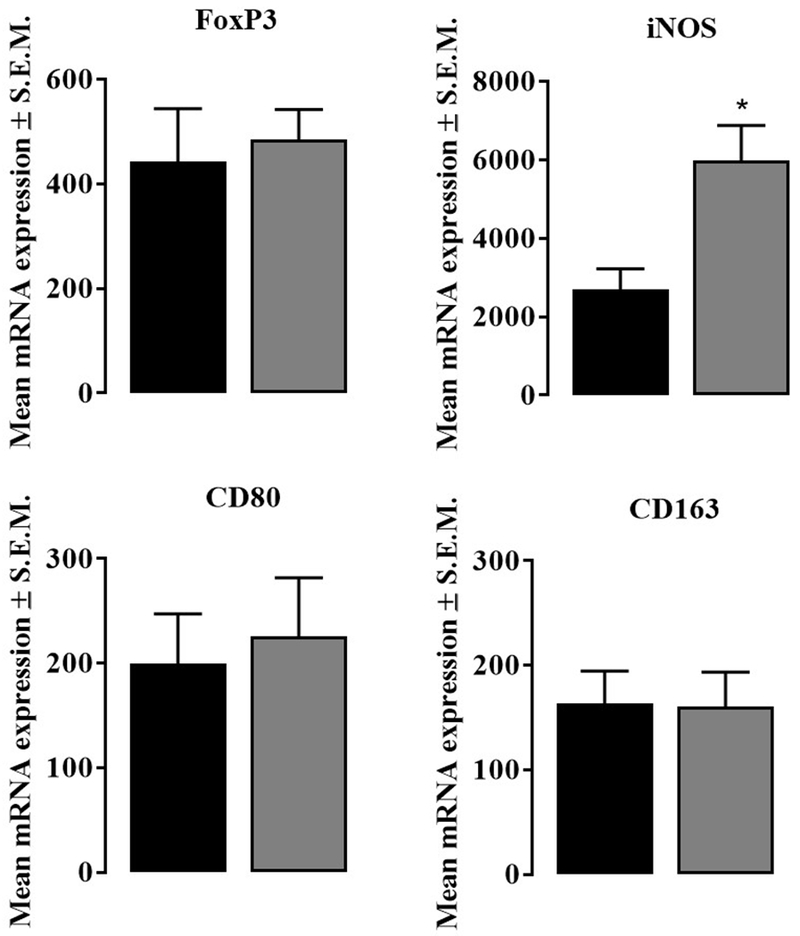
FoxP3 (marker for T regular cells), iNOS (marker for M1 type monocyte/macrophage), CD80, and CD163 (markers for M2 type monocyte/macrophage). FoxP3, CD80, and CD163 mRNA expressions were similar in neck tissue samples from mice treated with saline and nalbuphine. iNOS mRNA expression was significantly increased in neck tissue samples from mice treated with nalbuphine (*P<0.05, unpaired Student’s t-test, n=6).
4. Discussion
We investigated if nalbuphine would be effective against pruritoceptive pruritus in chronic and inflammatory skin diseases like contact dermatitis. We used the DNFB-induced dermatitis mouse model to study the effect of nalbuphine on scratching as well as on chemokine and cytokine levels since it is an inflammatory situation. Further, we studied sedation and anhedonia-like behavioral side effects of nalbuphine. Major findings of this study are: 1) Nalbuphine, a mixed kappa opioid receptor agonist and mu opioid receptor antagonist, suppresses pruritus in the DNFB-induced mouse model of chronic contact dermatitis 2) Nalbuphine increases anti-inflammatory cytokine IL-10, and reduces pro-inflammatory IL-31 levels in the skin with dermatitis, and 3) Nalbuphine increases M1-type monocyte/macrophage as well as some chemokines that are possibly involved in the inflammatory phase of the healing process. These data suggest that nalbuphine promotes skin healing. Our study reports for the first time that there are changes in the release of a wide range of chemokines and cytokines due to nalbuphine treatment in pruritus.
Previously, anti-scratch activity of nalbuphine (10-30 mg/kg) was reported against Substance P-induced scratching in mice (Hawi et al., 2013), however, to our knowledge, the present study is the first to report that nalbuphine (3 and 10 mg/kg) is antipruritic in a mouse model of chronic contact dermatitis (a pruritoceptive pruritus) without inducing sedation with a high dose of nalbuphine. Recently, we have also reported that nalbuphine suppresses TAT-HIV-1 (trans activator of transcription)-, chloroquine- (Inan et al., 2019), and deoxycholic acid (a secondary bile acid)-induced (Cowan et al., 2019) scratching in mice. Itch is a common symptom in patients with HIV. Chronic itch can be due to skin diseases that develop in almost 90% of HIV-positive patients or idiopathic (6%) (Gelfand and Rudikoff, 2001; Zancanaro et al., 2006; Kaushik et al., 2014; Parker, 2014). Prevalence of chronic pruritus in HIV-positive patients has been reported as 31% from a study conducted in Spain and 45% from a study conducted in southeastern United States (Blanes et al., 2012; Kaushik et al., 2014). Quality of life has been found negatively affected in HIV-patients with chronic itch in these studies. Cholestatic itch is an ongoing challenge in medicine that is often refractory to available therapeutics. Bile acids are believed to cause the repetitive scratching behavior by activating MRGPRX4 receptors in mice (Meixiong et al., 2019). Together all these results indicate that nalbuphine is a widely effective suppressor of scratching (in mice).
Also, nalbuphine at 3 and 10 mg/kg decreased only 20-25% grooming during a 10 min observation. Nalbuphine has advantages of over other candidate kappa opioid receptor agonists. First, it has been in clinical practice since the 1980s; second, it is not a scheduled compound in the USA, for example nalfurafine is scheduled as class II; and third, in clinical studies it has been shown to induce fewer psychotomimetic episodes compared to other kappa opioid agonists such as pentazocine and butorphanol (Schmidt et al., 1985).
Takamori et al. (2018) reported that IL-31-deficient mice develop contact dermatitis with less scratching to DNFB-induced sensitization. They suggested that IL-31 is required for the induction of pruritus. In our study, nalbuphine significantly reduced IL-31 levels (Fig. 6A) and scratching (Fig.2) also suggesting that IL-31 plays an important role in the development of pruritus. Further, nalbuphine significantly increased IL-10, an anti-inflammatory cytokine (Fig. 6B). IL-10 downregulates expression of Th1 cytokines like IL-2 and interferon-γ (IFN-γ), limits pro-inflammatory cytokine release (Tumor Necrosis Factor-α (TNF-α), IL-1, IL-6, IL-12), and controls differentiation of macrophages, T and B cells (Couper et al., 2008; Shachar and Karin, 2013). The anti-inflammatory effect of kappa opioid agonists has been reported in inflammatory pain models in rats (Walker et al., 1995; Binder et al., 2001; Walker 2003; Bileviciute-Ljungar et al. 2006) and in mice (Paton et al., 2017). Also, Bastos et al. (2011) showed that U-50,488 (a kappa opioid receptor agonist) reduced bone loss and IL-6 levels and increased IL-10 in ligature-induced periodontal disease in rats.
In our study, mice treated with saline had major increased levels of CCL2, CCL3, CXCL2, IL-1β, IL-13, and IL-16 (Table 1.). There was no increase in IL-4, IL-5 and IL-9 that are involved in the type 2 immune response. Only IL-13 was increased in terms of type 2 response. Pro-inflammatory chemokines CCL2, CCL3, CXCL2 and cytokines IL-β, and IL-16 were increased. Nalbuphine induced further increases in CCL2, CCL3, CXCL2, IL-1β, and IL-16 in addition to increases in CXCL9, CXCL10, Macrophage Colony-Stimulating Factor (M-CSF), Metalloproteinase Inhibitor 1 (TIMP-1) and Triggering Receptor Expressed on Myeloid cells-1 (TREM-1) (Table 1.). Previous studies have shown that chemokines are involved in wound healing. It was shown that CCL3 (DiPietro et. al., 1998) and CCL2 (Wood et. al., 2014) are required for wound healing as chemoattractants for macrophages. During the inflammation phase of wound healing polymorphonuclear neutrophils and monocytes (differentiate to macrophages) come to the site. As the wound heals, Ml pro-inflammatory macrophages are replaced by M2 anti-inflammatory macrophages (Rees et al., 2015; Krzyszczyk et al., 2018). M1 macrophages secrete TNF-α, IL-1β and IL-6 for a strong inflammatory response. CXCL2, CXCL10 and CXCL11 are important chemokines for wound healing, especially, CXCL10 and 11 initiating the remodeling phase (Behm et al., 2012; Brubaker et al., 2013; Rees et al., 2015). Nalbuphine treatment increased CCL2, CCL3, CXCL2, IL-β, CXCL9 and CXCL10 (Table 1.) as well as M1 type macrophages (Fig. 7) compared to saline administration. Further, M-CSF was also increased by nalbuphine (Table 1.). The positive role of M-CSF on wound healing has been reported previously (Ikeda et al., 2008; Sugiyama et al., 2008; Li et al., 2016). TIMP-1, also increased by nalbuphine (Table 1.), is a member of matrix metalloproteinases inhibitors that degrade the extracellular matrix, as well as being involved in cell growth and differentiation, cell migration, anti-angiogenesis, anti- and pro-apoptosis, and synaptic plasticity (Brew and Nagase, 2010). Positive effects of TIMP-1 in wound healing have also been reported (Arndt et al., 2018; Trestrup et al., 2018). It has been shown that TREM-1 plays a critical role in the development of inflammation in atherosclerosis and colitis (Boufenzer et al., 2015; Che et al., 2018). However, a recent study has reported that an increase in TREM-1 correlates with faster wound healing in cutaneous leishmaniasis (Nunes et al., 2018). Our results with nalbuphine suggest that skin with dermatitis collected on week 4 might reveal the beginning phase of wound healing. Further studies are required to answer the following questions: 1) What would the results be at weeks 5 or 6 in terms of chemokines and cytokines?, 2) What would the results be if nalbuphine was given chronically?, and 3) What would the histologic evidence be following chronic administration of nalbuphine?
In conclusion, this study demonstrates that nalbuphine is effective against pruritoceptive pruritus in dermatitis in mice. It decreases IL-31 and increases anti-inflammatory IL-10. Increase in some chemokines and cytokines as well as M1-type monocyte/macrophages by nalbuphine may suggest that nalbuphine promotes healing of inflamed skin. Collectively, in addition to suppression of scratching, this outcome would be beneficial.
Acknowledgments:
This study was funded by National Institute of Health/National Institute on Drug Abuse [P30 Grant DA 013429]. Mr. Joseph J Meissler (Center for Substance Abuse Research, Neuroimmunology Core, Lewis Katz School of Medicine at Temple University, Philadelphia) is thanked for his assistance in reading Arbitrary Units.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
Authors declare no conflict of interest.
Author agreement
All authors are agree to submit European Journal of Pharmacology.
References
- Akiyama T, Carstens MI, Piecha D, Steppan S, Carstens E, 2015. Nalfurafine suppresses pruritogen- and touch-evoked scratching behavior in models of acute and chronic itch in mice. Acta. Derm. Venereol 95, 147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhashemi JA, Crosby ET, Grodecki W, Duffy PJ, Hull KA, Gallant C, 1997. Treatment of intrathecal morphine-induced pruritus following cesarean section. Can. J. Anaesth 44, 1060–1065. [DOI] [PubMed] [Google Scholar]
- Arndt S, Unger P, Berneburg M, Bosserhoff AJ, Karrer S, 2018. Cold atmospheric plasma (CAP) activates angiogenesis-related molecules in skin keratinocytes, fibroblasts and endothelial cells and improves wound angiogenesis in an autocrine and paracrine mode. J. Dermatol. Sci 89, 181–190. [DOI] [PubMed] [Google Scholar]
- Bastos JV, Queiroz-Junior CM, Caliari MV, Francischi JN, Pacheco CM, Maltos KL, 2011. Peripheral kappa opioid receptors activation reduces alveolar bone loss in rats by Modulating interleukin-6 and -10. Arch. Oral Biol 56, 540–548. [DOI] [PubMed] [Google Scholar]
- Beaver WT, Feise GA, 1978. A comparison of the analgesic effect of intramuscular nalbuphine and morphine in patients with postoperative pain. J. Pharmacol. Exp. Ther 204, 487–496. [PubMed] [Google Scholar]
- Beaver WT, Feise GA, 1981. Analgesic effect of intramuscular and oral nalbuphine in postoperative pain. Clin. Pharmacol. Ther 29, 174–180. [DOI] [PubMed] [Google Scholar]
- Behm B, Babilas P, Landthaler M, Schreml S, 2012. Cytokines, chemokines, and growth factors in wound healing. J. Eur. Acad. Dermatol. Venereol 26, 812–820. [DOI] [PubMed] [Google Scholar]
- Bileviciute-Ljungar I, Saxne T, Spetea M 2006. Anti-inflammatory effects of contralateral administration of the j-opioid agonist U-50,488H in rats with unilaterally induced adjuvant arthritis. Rheumatology 45, 295–302. [DOI] [PubMed] [Google Scholar]
- Bilsborough J, Leung DY, Maurer M, Howell M, Boguniewicz M, Yao L, Storey H, LeCiel C, Harder B, Gross JA, 2006. IL-31 is associated with cutaneous lymphocyte antigen-positive skin homing T cells in patients with atopic dermatitis. J. Allergy. Clin. Immunol 117, 418–425. [DOI] [PubMed] [Google Scholar]
- Binder W, Machelska H, Mousa S, Schmitt T, 2001. Analgesic and antiinflammatory effects of two novel k-opioid peptides. Anesthesiology 94, 1034–1044. [DOI] [PubMed] [Google Scholar]
- Blanes M, Belinchón I, Portilla J, Betlloch I, Reus S, Sánchez-Payá J, 2012. Pruritus in HIV-infected patients in the era of combination antiretroviral therapy: a study of its prevalence and causes. Int. J. STD AIDS 4, 255–257. [DOI] [PubMed] [Google Scholar]
- Boufenzer A, Lemarié J, Simon T, Derive M, Bouazza Y, Tran N, Maskali F, Groubatch F, Bonnin P, Bastien C, Bruneval P, Marie PY, Cohen R, Danchin N, Silvestre JS, Ait-Oufella H, Gibot S, 2015. TREM-1 mediates inflammatory injury and cardiac remodeling following myocardial infarction. Circ. Res 116, 1772–1782. [DOI] [PubMed] [Google Scholar]
- Brew K, Nagase H, 2010. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta 1803, 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker AL, Rendon JL, Ramirez L, Mashkoor A, Kovacs C, Kovacs E, 2013. Reduced neutrophil chemotaxis and infiltration contributes to delayed resolution of cutaneous wound infection with advanced age. J. Immunol 190, 1746–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust TF, Morgenweck J, Kim SA, Rose JH, Locke JL, Schmid CL, Zhou L, Stahl EL, Cameron MD, Scarry SM, Aubé J, Jones SR, Martin TJ, Bohn LM, 2016. Biased agonists of the kappa opioid receptor suppress pain and itch without causing sedation or dysphoria. Sci. Signal 9: ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelman ER, McElroy BD, Prisinzano TE, Kreek MJJ, 2019. Impact of pharmacological manipulation of the k-opioid receptor system on self-grooming and anhedonic-like behaviors in mice. J. Pharmacol. Exp. Ther 10.1124/jpet.119.256354 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, Kukova G, Buhl T, Ikoma A, Buddenkotte J, Soumelis V, Feld M, Alenius H, Dillon SR, Carstens E, Homey B, Basbaum A, Steinhoff M, 2014. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J Allergy Clin. Immunol 133, 448–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers D, 2011. Peripheral kappa agonists In: Sinatra RS, Jahr JS, Watkins-Pitchford (eds). The Essence of Analgesia and Analgesics, New York, NY, Cambridge University Press, 490–491. [Google Scholar]
- Che X, Park KC, Park SJ, Kang YH, Jin HA, Kim JW, Seo DH, Kim DK, Kim TI, Kim WH, Kim SW, Cheon JH, 2018. Protective effects of guggulsterone against colitis are associated with the suppression of TREM-1 and modulation of macrophages. Am. J. Physiol. Gastrointest. Liver Physiol 315, G128–G139. [DOI] [PubMed] [Google Scholar]
- Couper KN, Blount DG, Riley EM, 2008. The master regulator of immunity to infection. J. Immunol 180, 5771–5777. [DOI] [PubMed] [Google Scholar]
- Cowan A, Kehner GB, Inan S, 2015. Targeting itch with ligands selective for κ opioid receptors. Handb. Exp. Pharmacol 226, 291–314. [DOI] [PubMed] [Google Scholar]
- Cowan A, Dun NJ, Inan S, 2019 Nalbuphine suppresses deoxycholic acid-induced scratching in male, Swiss Webster mice. International Narcotics Research Conference, New York, NW, July 7-11, 2019, poster # W21. [Google Scholar]
- Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, Haugen HS, Maurer M, Harder B, Johnston J, Bort S, Mudri S, Kuijper JL, Bukowski T, Shea P, Dong DL, Dasovich M, Grant FJ, Lockwood L, Levin SD, LeCiel C, Waggie K, Day H, Topouzis S, Kramer J, Kuestner R, Chen Z, Foster D, Parrish-Novak J, Gross JA, 2004. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nature Immunol. 5, 752–760. [DOI] [PubMed] [Google Scholar]
- DiPietro LA, Burdick M, Low QE, Kunkel SL, Strieter RM, 1998. MIP-1 alpha as a critical macrophage chemoattractant in murine wound repair. J. Clin. Invest 101, 1693–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson S, Nahmias Z, Rosman I, Kim BS, 2018. Immunomodulating agents as antipruritics. Dermatol. Clin 36, 325–334. [DOI] [PubMed] [Google Scholar]
- Fowler E, Yosipovitch G, 2019. Chronic itch management: therapies beyond those targeting the immune system. Ann Allergy Asthma Immunol. pii: S1081-1206(19)30085-7 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Gelfand JM, Rudikoff D, 2001. Evaluation and treatment of itching in HIV-infected patients. Mt. Sinai J. Med 68, 298–308. [PubMed] [Google Scholar]
- Giavina-Bianchi M, Giavina-Bianchi P, 2019. Systemic treatment for severe atopic dermatitis. Arch. Immunol. Ther. Exp 67:69–78 . [DOI] [PubMed] [Google Scholar]
- Hawi A, Hunter R, Morford L, Sciascia T, 2013. Nalbuphine attenuates itch in the substance-P-induced mouse model. Acta. Derm. Venereol 93: 634. [Google Scholar]
- Ikeda O, Sekine Y, Muromoto R, Ohbayashi N, Yoshimura A, Matsuda T, 2008. Enhanced c-Fms/M-CSF receptor signaling and wound-healing process in bone marrow-derived macrophages of signal-transducing adaptor protein-2 (STAP-2) deficient mice. Biol. Pharm. Bull 31: 1790–1793. [DOI] [PubMed] [Google Scholar]
- Inan S, Cowan A, 2004. Kappa opioid agonists suppress chloroquine-induced scratching in mice. Eur. J. Pharmacol 502: 233–237. [DOI] [PubMed] [Google Scholar]
- Inan S, Cowan A, 2006. Nalfurafine, a kappa opioid receptor agonist, inhibits scratching behavior secondary to cholestasis induced by chronic ethynylestradiol injections in rats. Pharmacol. Biochem. Behav 85: 39–43. [DOI] [PubMed] [Google Scholar]
- Inan S, Dun NJ, Cowan A, 2009. Nalfurafine prevents 5’-guanidinonaltrindole- and compound 48/80-induced spinal c-fos expression and attenuates 5’-guanidinonaltrindole-elicited scratching behavior in mice. Neuroscience 163: 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan S, Dun NJ, Cowan A, 2011. Investigation of gastrin releasing peptide as a mediator for 5’-guanidinonaltrindole-induced compulsive scratching in mice. Peptides 32: 286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan S, Dun NJ, Cowan A, 2019. Nalbuphine, a kappa opioid receptor agonist and mu opioid receptor antagonist, suppresses scratching in mice induced by HIV-1 TAT and also by chloroquine, an anti-malarial agent. International Narcotics Research Conference, New York, NW, July 7-11, 2019, poster # W20. [Google Scholar]
- Kamei J, Nagase H, 2001. Norbinaltorphimine, a selective kappa opioid receptor antagonist, induces an itch-associated response in mice. Eur. J. Pharmacol 418: 141–145. [DOI] [PubMed] [Google Scholar]
- Kamimura K, Yokoo T, Kamimura H, Sakamaki A, Abe S, Tsuchiya A, Takamura M, Kawai H, Yamagiwa S, Terai S, 2017. Long-term efficacy and safety of nalfurafine hydrochloride on pruritus in chronic liver disease patients: Patient-reported outcome based analyses. PLoS One 12: e0178991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardon AP, Polgár E, Hachisuka J, Snyder LM, Cameron D, Savage S, Cai X, Karnup S, Fan CR, Hemenway GM, Bernard CS, Schwartz ES, Nagase H, Schwarzer C, Watanebe M, Furuta T, Kaneko T, Koerber HR, Todd AJ, Ross SE, 2017. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron 82, 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik SB, Cerci FB, Miracle J, Pokharel A, Chen SC, Chan YH, Wilkin A, Yosipovitch G, 2014. Chronic pruritus in HIV-positive patients in the southeastern United States: Its prevalence and effect on quality of life. J. Am. Acad. Dermatol 70, 659–664. [DOI] [PubMed] [Google Scholar]
- Ko MC, Lee H, Song MS, Sobczyk-Kojiro K, Mosberg HI, Kishioka S, Woods JH, Naughton NN, 2003. Activation of kappa opioid receptors inhibits pruritus evoked by subcutaneous or intrathecal administration of morphine in monkeys. J. Pharmacol. Exp. Ther 305: 173–179. [DOI] [PubMed] [Google Scholar]
- Krzyszczyk P, Schloss R, Palmer A, Berthiaume F, 2018. The role of macrophages in acute an chronic wound healing and interventions to promote pro-wound healing phenotypes. Front. Physiol 9: 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Jalili RB, Ghahary A, 2016. Accelerating skin wound healing by M-CSF through generating SSEA-1 and -3 stem cells in the injured sites. Sci. Rep 6: 28979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HS, Ha H, Lee H, Lee JK, Lee MY, Shin HK, 2014. Morus alba L suppresses the development of atopic dermatitis induced by the house dust mite in NC/Nga mice. B.M.C. Complement. Altern. Med 14: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack MR, Kim BS, 2018. The itch-scratch cycle: A neuroimmune perspective. Trends in Immunol. 39, 980–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur VS, Kumar J, Crawford PW, Hait H, Sciascia T, 2017. A multicenter, randomized, double-blind, placebo-controlled trial of nalbuphine ER tablets for uremic pruritus. Am. J. Nephrol 46: 450–458. [DOI] [PubMed] [Google Scholar]
- McEwen MW, Fite EM, Yosipovitch G, Patel T, 2018. Drugs on the horizon for chronic pruritus. Dermatol. Clin 36: 335–344. [DOI] [PubMed] [Google Scholar]
- Meixiong J, Vasavda C, Snyder SH, Dong X, 2019. MRGPRX4 is a G protein-coupled receptor activated by bile acids that may contribute to cholestatic pruritus. Proc. Natl. Acad. Sci 116, 10525–10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, Zhang X, 2008. Interleukin-10: new perspectives on an old cytokine. Immunol. Rev 226: 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa AAM, Baaror AS, Abdelazim IA, 2016. Comparative study between Nalbuphine and ondensatron in prevention of intrathecal morphine-induced pruritus in women undergoing cesarean section. Anesth. Essays Res 10: 238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D, Deng J, Liu KF, Wu ZY, Shi YF, Guo WM, Mao QQ, Liu HJ, Li H, Sun YG, 2017. A central neural circuit for itch sensation. Science 357: 695–699. [DOI] [PubMed] [Google Scholar]
- Nunes S, Silva IB, Ampuero MR, de Noronha ALL, de Souza LCL, Correia TC, Khouri R, Boaventura VS, Barral A, Ramos PIP, Brodskyn C, Oliveira PRS, Tavares NM, 2018. Integrated analysis reveals that miR-193b, miR-671, and TREM-1 correlate with a good response to treatment of human localized cutaneous leishmaniasis caused by Leishmania braziliensis. Front. Immunol 9: 640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker SRS, 2014. The skin and HIV: No superficial matter. Top. Antivir. Med 22: 680–684. [PMC free article] [PubMed] [Google Scholar]
- Patel JM, Dao H, 2018. Chronic pruritus: a review of neurophysiology and associated immune neuromodulatory treatments. Skin Therapy Lett. 23: 5–9. [PubMed] [Google Scholar]
- Paton KF, Kumar N, Crowley RS Harper JL, Prisinzano TE, Kivell BM, 2017. The analgesic and anti-inflammatory effects of Salvinorin A analogue β-tetrahydropyran Salvinorin B in mice. Eur. J. Pain 21, 1039–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira MP, Stander S, 2017. Chronic pruritus: current and emerging treatment options. Drugs 77: 999–1007. [DOI] [PubMed] [Google Scholar]
- Rees PA, Greaves NS, Baguneid M, Bayat A, 2015. Chemokines in wound healing and as potential therapeutic targets for reducing cutaneous scarring. Adv. Wound Care 4: 687–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakihara M, Imamachi N, Saito Y, 2016. Effects of intrathecal κ-opioid receptor agonist on morphine-induced itch and antinociception in mice. Reg. Anesth. Pain Med 41: 69–74. [DOI] [PubMed] [Google Scholar]
- Sanmiguel JC, Olaru F, Li J, Mohr E, Jensen LE, 2009. Interleukin-1 regulates keratinocyte expression of T cell targeting chemokines through interleukin-1 receptor associated kinase-1 (IRAK1) dependent and independent pathways. Cell Signal 21: 685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt WK, Tam SW, Shotzberger GS, Dewey HS, Clark R, Vernier VG, 1985. Nalbuphine. Drug Alcohol Depend. 14: 339–362. [DOI] [PubMed] [Google Scholar]
- Shachar I, Karin N, 2013. The dual roles of inflammatory cytokines and chemokines in the regulation of autoimmune diseases and their clinical implication. J. Leukoc. Biol 93: 51–61. [DOI] [PubMed] [Google Scholar]
- Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, Alenius H, Dieu-Nosjean MC, Meller S, Rieker J, Steinhoff M, Hoffmann TK, Ruzicka T, Zlotnik A, Homey B, 2006. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J. Allergy Clin. Immunol 117: 411–417. [DOI] [PubMed] [Google Scholar]
- Sugiyama K, Ishii G, Ochiai A, Esumi H, 2008. Improvement of the breaking strength of wound by combined treatment with recombinant human G-CSF, recombinant human M-CSF, and a TGF-beta1 receptor kinase inhibitor in rat skin. Cancer Sci. 99: 1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto H, Matsuda H, Mitsuishi K, Hira K, Uchida T, Unno T, Ogawa H, Ra C, 1999. NC/Nga mice: a mouse model for atopic dermatitis. Int. Arch. Allergy. Immunol 120: 70–75. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Tominaga M, Kosaka R, Kamata Y, Umehara Y, Matsuda H, Sakaguchi A, Ogawa H, Takamori K, 2017. Involvement of μ-opioid receptors and κ-opioid receptors in itch-related scratching behavior of imiquimod-induced psoriasis-like dermatitis in mice. Acta. Derm. Venereol 97: 928–933. [DOI] [PubMed] [Google Scholar]
- Takamori A, Nambu A, Sato K, Yamaguchi S, Matsuda K, Numata T, Sugawara T, Yoshizaki T, Arae K, Morita H, Matsumoto K, Sudo K, Okumura K, Kitaura J, Matsuda H, Nakae S, 2018. IL-31 is crucial for induction of pruritus, but not inflammation, in contact hypersensitivity. Sci. Rep 8 :6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi Y, Umeuchi H, Okano K, Ando N, Yozhizawa Y, Honda T, Kawamura K, Endoh T, Utsumi J, Kamei J, Tanaka T, Nagase H, 2002. Antipruritic activity of the kappa opioid receptor agonist, TRK-820. Eur. J. Pharmacol 435: 259–264. [DOI] [PubMed] [Google Scholar]
- Trᴓstrup H, Holstein P, Kalsmark T, Moser C, Âgren MS, 2018. Uncontrolled gelatin degradation in non-healing chronic wounds. J. Wound Care 27: 724–734. [DOI] [PubMed] [Google Scholar]
- Umeuchi H, Kawashima Y, Aoki CA, Kurokawa T, Nakao K, Itoh M, Kikuchi K, Kato T, Okano K, Gershwin ME, Miyakawa H, 2005. Spontaneous scratching behavior in MRL/lpr mice, a possible model for pruritus in autoimmune diseases, and antipruritic activity of a novel kappa-opioid receptor agonist nalfurafine hydrochloride. Eur. J. Pharmacol 518: 133–139. [DOI] [PubMed] [Google Scholar]
- Vaia M, Petrosino S, De Filippis D, Negro L, Guarino A, Carnuccio R, Di Marzo V, Iuvone T, 2016. Palmitoylethanolamide reduces inflammation and itch in a mouse model of contact allergic dermatitis. Eur. J. Pharmacol 791: 669–674. [DOI] [PubMed] [Google Scholar]
- Walker JS, Howlett CR, Nayanar V, 1995. Anti-inflammatory effects of kappa-opioids in adjuvant arthritis. Life Sci. 57, 371–378. [DOI] [PubMed] [Google Scholar]
- Walker JS, 2003. Anti-inflammatory effects of opioids. Adv. Exp. Med. Biol 521, 148–160. [PubMed] [Google Scholar]
- Wood S, Jayaraman V, Huelsmann EJ, Bonish B, Burgad D, Sivaramakrishnan G, Qin S, DiPietro LA, Zloza A, Zhang C, Shafikhani SH, 2014. Pro-inflammatory chemokine CCL2 (MCP-1) promotes healing in diabetic wounds by restoring the macrophage response. PLoS One 9: e91574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu AZ, Tripathi SV, Kau AL, Schaffer A, Kim BS, 2016. Immune dysregulation underlies a subset of patients with chronic idiopathic pruritus. J. Am. Acad. Dermatol 74: 1017–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin I, Belzung C, Surget A, 2008. Mouse strain differences in the unpredictable chronic mild stress: a four-antidepressant survey. Behav. Brain Res 193: 140–143. [DOI] [PubMed] [Google Scholar]
- Yeh YC, Lin TF, Chang HC, Chan WS, Wang YP, Lin CJ, Sun WC, 2009. Combination of low-dose nalbuphine and morphine in patient-controlled analgesia decreases incidence of opioid-related side effects. J. Formos. Med. Assoc 108: 548–553. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Bernhard JD, 2013. Clinical practice. Chronic pruritus. N. Eng. J. Med 368: 1625–1634. [DOI] [PubMed] [Google Scholar]
- Zancanaro PCQ, McGirt LY, Mamelak AJ, Nguyen RHN, Martins CR, 2006. Cutaneous manifestations of HIV in the era of highly active antiretroviral therapy: An institutional urban clinic experience. J. Am. Acad. Dermatol 54, 581–588. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yan J, Hu R, Sun Y, Ma Y, Chen Z, Jiang H, 2015. Microglia are involved in pruritus induced by DNFB via the CX3CR1/p38 MAPK pathway. Cell. Physiol. Biochem 35: 1023–1033. [DOI] [PubMed] [Google Scholar]


