Abstract
A 43-year-old Chinese man with a silicosis history was admitted to our hospital due to bilateral lower extremity edema for 1 year, exacerbating with hematuria for 2 months. He started working as a coal miner 30 years ago, and was diagnosed as silicosis 3 months ago. Lab tests revealed hematuria 3+, proteinuria 3+, and a serum creatinine value 2.47 mg/dl on routine check. He was diagnosed with focal proliferative IgA nephropathy (IgAN) and acute tubulo-interstitial nephritis by renal biopsy. He was treated with corticosteroids and got a remission 4 months later. Immunohistochemical staining showed the deposition of macrophage receptor with collagenous structure (MARCO), nod-like receptor pyrin domain-containing-3 (NLRP3), Caspase-1, apoptosis-associated speck (ASC), interleukin (IL)-1β, and IL-18 in both glomerular and tubulo-interstitial areas. We proposed that the silicon exposure could be related to his kidney disease in the patient and NLRP3 mediated inflammation might be involved in its pathogenesis which needs further explorations.
Keywords: Silicosis, silica nephropathy, IgA nephropathy, MARCO receptor, the NLRP3 inflammasome
Introduction
Silicosis is the most common one in pneumoconiosis, which was defined as a fibrotic lung disease caused by inhalation of free crystalline silicon dioxide or silica. Occupational exposure to repairable crystalline silica dust particles occurred in many industries, like sandblasters, miners, quarry workers, etc. [1] It was reported that silica exposure was associated with several disorders, including autoimmune diseases [2,3], renal diseases[4,5], etc.
Although the alveolar macrophages are believed to initiate the inflammatory responses of silicosis, its true mechanism remained to be elucidated. It was proposed that mechanical stimulation, chemical poisoning, oxygen radical, and immune reaction might be involved in the pathophysiological development of the disease [6,7].
More interestingly, recent studies suggested that nod-like receptor pyrin domain-containing-3 (NLRP3, also called cryopyrin or NALP3) inflammasome (a cytoplasmic multi-protein which was consisted of a sensor NLRP3, an apoptosis-associated speck (ASC) adaptor, and an effector caspase-1 and regulated the mutation and secretion of pro-inflammatory cytokines like interleukin (IL)-1β and IL-18 played a vital role in the inflammatory response and subsequent development of pulmonary fibrosis in silicosis [8–12]. NLRP3 inflammasome was also related to several crystal-associated renal diseases, like gout, oxalate nephropathy, etc. [11,13].
Herein, we described a patient with renal biopsied proven injury who had a history of silicosis, and the NLRP3 pathway related to the association of silicosis and the kidney disease was further explored.
Case presentation
Clinical history and laboratory data
A 43-year-old Chinese Han man was admitted to our hospital because of edema of lower limbs bilaterally for 1 year, exacerbating with gross hematuria in the last 2 months.
One year ago, he developed pitting edema of lower limbs and also found bubbles in urine at the same time. He visited the local hospital and the diagnosis of lower limbs varicose vein was made. Ten months later, his edema aggravated consciously and he presented with the whole course gross hematuria. The routine urinalysis showed proteinuria (3+) and hematuria (3+). Urinary protein excretion amount was 3.7 g/24h. The serum creatinine value was in the normal range and increased to 2.47 mg/dL one month later.
The past history revealed that he was a coal miner for 30 years and diagnosed as silicosis 3 months ago. He presented with hypertension for 4 years and it could be controlled at the range of 120–130/80–90 mmHg by regular medications. He did not abuse alcohol, cigarettes, or other drugs.
After admission, physical examination revealed that his temperature was 36.5 °C, respiratory rate was 20 breaths/min, pulse rate was 76 beats/min and blood pressure was 130/80 mmHg. There was no jaundice, rash and bleeding by skin examination and the superficial lymph nodes was not touched. Pitting edema of lower limbs was found bilaterally.
Table 1 summarized all the laboratory indices after his admission.
Table 1.
Laboratory indices of the patient.
| Urinalysis | Blood chemistry | Serology | |||
|---|---|---|---|---|---|
| Protein | 2+ | Sodium | 141.80 mmol/L | C-reactive protein | 2.05 mg/L |
| Glucose | – | Potassium | 3.52 mmol/l | Rheumatoid factor | <20 IU/ml |
| Sediments | Chloride | 105.70 mmol/L | Antistrptolysin | 33.10 IU/ml | |
| Red blood cell | 170–180/high-power field | Blood urea nitrogen | 13.30 mmol/L | Antinuclear antibodies | 1:100 |
| Hyaline cast | 0 | Creatinine | 2.51 mg/dl | Anti-ENA autoantibodies | – |
| Granular cast | 0–1/high-power field | Total protein | 63.10 g/L | MPO-ANCA | – |
| RBC cast | 0 | Albumin | 35.90 g/L | Anti-GBM | – |
| Total bilirubin | 13.00 μmol/L | PR3-ANCA | – | ||
| Aspartate aminotransferase (AST) | 17 IU/L | Immune globulin G (IgG) | 9.99 g/L | ||
| Pulmonary function test | Alanine transaminase (ALT) | 22 IU/L | IgA | 3.19 g/L | |
| Vital capacity (VC) | 3.93 L | Peripheral blood | IgM | 0.80 g/L | |
| VC%pred | 83.6% | Peripheral blood | 112 g/L | Complement 3 | 1.09 g/L |
| Forced expiratory volume in 1.0 s (FEV1.0) | 3.12 L | Platelet | 248 × 1012/L | Complement 4 | 0.22 g/L |
| FEV1.0% | 84.4% | White blood cell | 9.20 × 109/L | Anti-hepatitis B and C virus antibody | – |
| DLCO%pred | 69.7% | Erythrocyte sedimentation rate | 40 mm/h | Immunofixation electrophoresis | No monoclonal lane |
High-resolution computed tomography (HRCT) showed that there were multiple small nodular lesions on both lung fields and multiple calcifications were on the left upper lobe.
Ultrasound showed that the left and right kidneys were both in normal size. No stenosis or thrombus of renal artery and vein were found by Doppler ultrasound.
The patients presented with nephritic syndrome and acute kidney injury (AKI), which could not be excluded with silicosis associated renal disease. Thus, renal biopsy was crucial for the diagnosis and it was performed after his admission.
Diagnosis
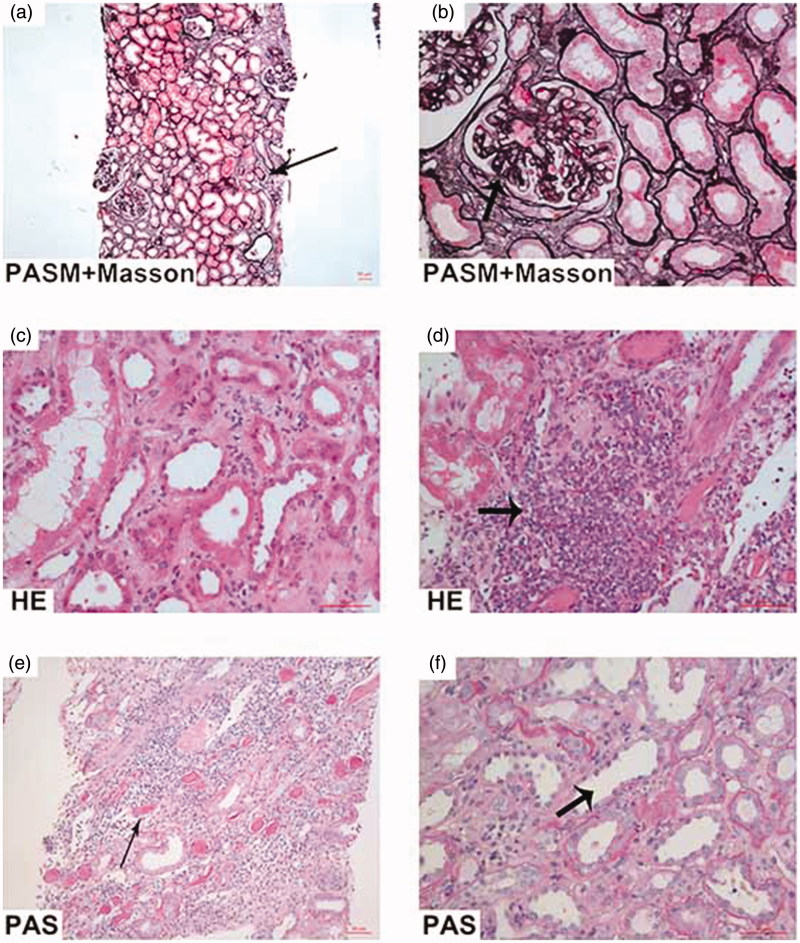
His renal biopsy specimen was examined by light microscopy, immunofluorescence, and electron microscopy. By light microscopy, 23 glomeruli were included in the specimen. One glomerulus was ischemic sclerosed and the remaining glomeruli manifested as mild mesangial cell and matrix proliferation with segmental endocapillary hypercellularity. Fuchsinophilic deposits were observed in mesangium. There were one cellular crescent and four fibro-cellular crescents. Tubular epithelial cells showed cytoplasmic vacuolization and focal loss of brush border with focal tubular atrophy. There was moderately interstitial infiltration of lymphocytes, mononuclear cells and a few eosinophils with focal interstitial fibrosis. Arterioles were thickened with hyalinosis. Immunofluorescence revealed lump and granular staining of IgA (3+) and C3 (3+) in mesangium and others including IgG, IgM, C1q, and fibrin were all negative (Figure 1(a–f)).
Figure 1.
The renal pathological findings. (a, b) PASM-Masson stain, (left ×100, right ×400) showed focal interstitial fibrosis accompanied by collections of interstitial inflammatory cells (left arrow). The renal capsules adhered (right arrow) with atrophic tubules and focal tubule dropout. (c, d) Hematoxylin-Eosin stain, (×400) showed inflammatory cell infiltrate (arrow), including lymphocytosis, mononuclear cells and a few eosinophils. (e, f) Periodic acid-Schiff stain, (left ×200, right ×400) showed a massive protein casts in the dilated tubules (left arrow) accompanied by brush border of proximal tubule dropout (right arrow).
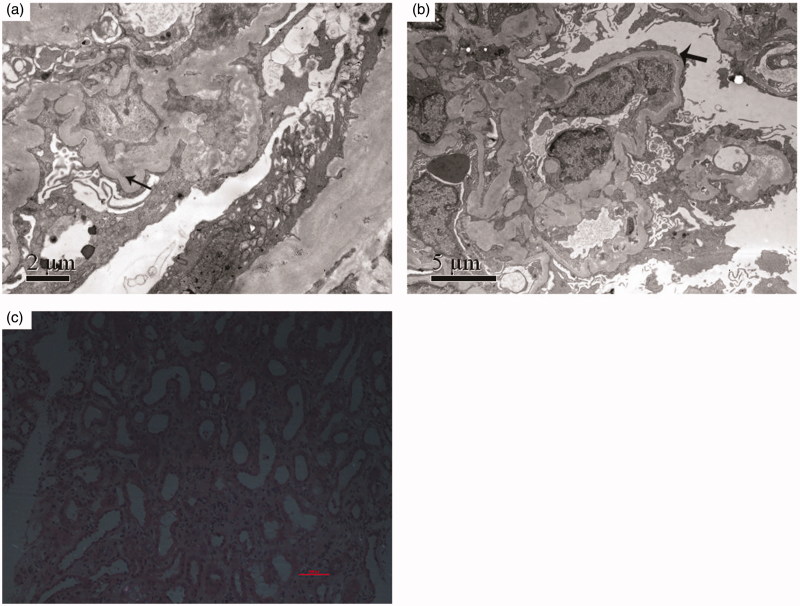
By electron microscopy, mild mesangial expansion with electron dense deposits in mesangial and para-mesangial matrix were observed. No remarkable changes were seen in glomerular basement membrane and the foot processes of podocytes were effaced diffusely (Figure 2(a,b)).
Figure 2.
The results of electron microscope and polarization microscope. (a) Electron microscopy showed mesangial electron dense deposits (arrow) with matrix increase. (b) Electron microscopy showed diffuse visceral epithelial cell foot process effacement (arrow). (c) Polarization microscopy showed no silica or silicon dioxide crystal deposition neither in glomerulus nor tubulo-interstitial areas.
The final pathological diagnosis was focal proliferative IgA nephropathy (Oxford classification: M1E1S0T1) and acute tubulo-interstitial nephritis.
Based on the renal pathological findings and his occupation, we proposed that the kidney disease might be associated with the silica exposure. Then, the kidney sections of the patients were further scanned using polarization microscopy for the quantity of silica or silicon dioxide crystal deposition. However, we did not find silica or silicon dioxide crystal deposition neither in glomerulus nor tubulo-interstitial areas (Figure 2(c)).
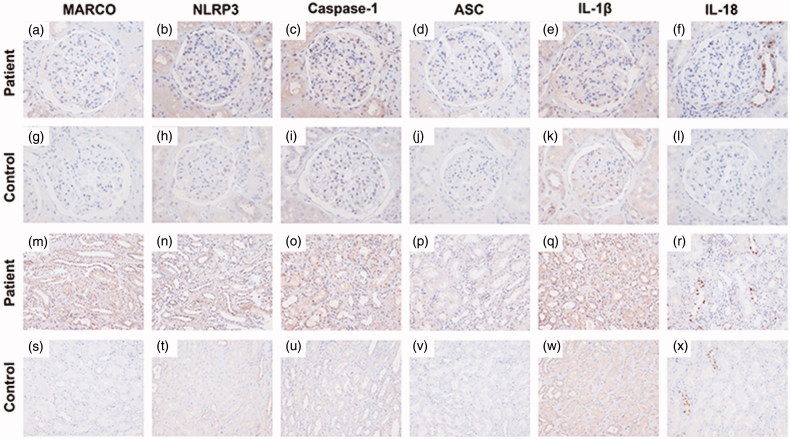
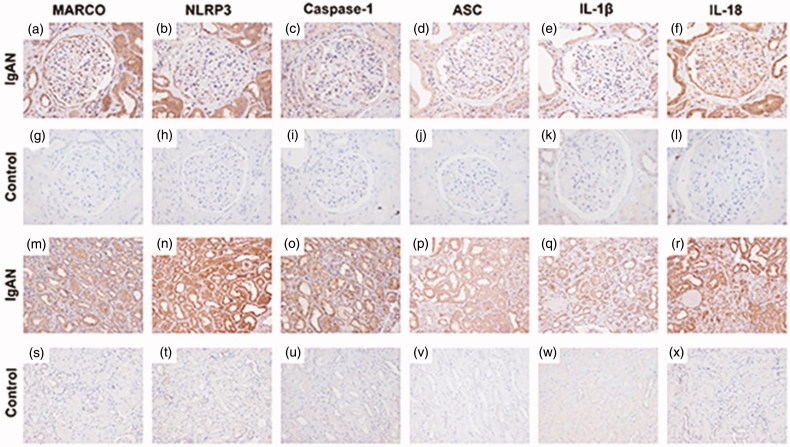
As the NLRP3-mediated inflammation might be involved in the oxalate nephropathy and silicosis, the MARCO, NLRP3, Caspase-1, ASC, IL-1β, and IL-18 were further stained by immunohistochemistry which were all positive in both glomerular and tubulo-interstitial areas in our patient and they were all virtually negative in the normal control (normal part of one nephrectomized kidney due to renal carcinoma) (Figure 3(a–x)). Furthermore, we selected one primary IgA nephropathy patient (Oxford classification: M1E1S0T1) as the disease control and we found that the expressions of MARCO, ASC, Caspase-1, and IL-1β were similar with our patient. However, the staining of NLRP3 was significantly higher in tubulo-interstitial areas than that in glomerular areas in the disease control, and the staining of IL-18 was specifically expressed in the distal convoluted tubules and some part of glomerular areas in our patient and it was dispersive around glomerular and tubulo-interstitial areas in the primary IgA nephropathy patient (Figure 4(a–x)).
Figure 3.
Immunohistochemistry staining for MARCO, NLRP3, Caspase-1, ASC, IL-1β, IL-18 in the patient and the normal control (an adult kidney biopsy obtained from nephrectomy away from cancer). (a–f) Immunohistochemical staining of MARCO, NLRP3, Caspase-1, ASC, IL-1β, and IL-18 in glomerulus of the patient, respectively, (×400). (g–l) Immunohistochemical staining of MARCO, NLRP3, Caspase-1, ASC, IL-1β, and IL-18 in glomerulus of the normal control, respectively, (×400). (m–r) Immunohistochemical staining of MARCO, NLRP3, Caspase-1, ASC, IL-1β, and IL-18 in tubulo-interstitial areas of the patient, respectively, (×200). (s–x) Immunohistochemical staining of MARCO, NLRP3, Caspase-1, ASC, IL-1β, and IL-18 in tubulo-interstitial areas of the normal control, respectively, (×200).
Figure 4.
Immunohistochemistry staining for MARCO, NLRP3, Caspase-1, ASC, IL-1β, IL-18 in a primary IgA nephropathy (IgAN) patient and the normal control (an adult kidney biopsy obtained from nephrectomy away from cancer). (a–f) Immunohistochemical staining of MARCO, NLRP3, Caspase-1, ASC, IL-1β, and IL-18 in glomerulus of the primary IgAN patient, respectively, (×400). (g–l) Immunohistochemical staining of MARCO, NLRP3, Caspase-1, ASC, IL-1β, and IL-18 in glomerulus of the normal control, respectively, (×400). (m–r) Immunohistochemical staining of MARCO, NLRP3, Caspase-1, ASC, IL-1β, and IL-18 in tubulo-interstitial areas of the primary IgAN patient, respectively, (×200). (s–x) Immunohistochemical staining of MARCO, NLRP3, Caspase-1, ASC, IL-1β, and IL-18 in tubulo-interstitial areas of the normal control, respectively, (×200).
Follow-up
As the patient was diagnosed as IgA nephropathy combined with acute tubulo-interstitial nephritis, the prednisone (30 mg/d) was then initiated with tapering regularly in combination with ACEI treatment. The patient got a significant improvement both for renal function and proteinuria after 2 months, which kept stable still now. He also changed his job to remove the occupational factor. His laboratory data at follow-up were showed in Table 2.
Table 2.
Clinical data of the patient at follow-up.
| Initial | 18 February 2014 | 8 April 2016 | 28 July 2018 | 21 September 2018 | 11 November 2018 | 27 January 2019 | 17 March 2019 | 10 May 2019 | 22 June 2019 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Prednisone | / | 30 | ||||||||
| Qd | withdrawal | / | / | / | / | / | / | / | ||
| Urine | ||||||||||
| Proteinuria | ++ | ++ | ± | – | – | – | + | + | ± | ++ |
| Proteinuria amount (g/24h) | 3.41 | 2.84 | 0.21 | / | / | / | / | / | / | 0.37 |
| Hematuria | +++ | +++ | ± | – | – | – | + | + | – | – |
| Blood chemistry | ||||||||||
| Creatinine (mg/dl) | 2.51 | 1.79 | 1.53 | 1.26 | 1.27 | 1.27 | 1.29 | 1.07 | 1.20 | 1.19 |
| Albumin(g/L) | 35.9 | 37.4 | 42.5 | 41.5 | 42.3 | 41.6 | 41.2 | 43 | 41.9 | 42.5 |
Discussion
Occupational exposure to silica or silicon dioxide dust has been examined as a possible risk factor with respect to several diseases, like tuberculosis, lung cancer [14], systemic vasculitis [15], rheumatoid arthritis [16], systemic sclerosis [17], systemic lupus erythematosus [18], renal involvement [19], etc.
Early in 1951, Saita G et al. [20] firstly reported that the renal functions were decreased in some silicosis patients. Subsequently, several epidemiological evidences suggested that the silica exposure was associated with an increased risk of end-stage renal disease (ESRD), chronic kidney disease (CKD), or specifically glomerulonephritis [21–24]. Our patient had a history of silicosis and was diagnosed with focal proliferative IgA nephropathy and acute tubulo-interstitial nephritis by renal biopsy. We proposed that his renal disease might be associated with the silicosis history.
Silica nephropathy referred to the floorboard of kidney diseases after exposure to silica or silicon dioxide, including tubulo-interstitial disease, immune-mediated disease, chronic kidney disease, and end-stage renal disease [25–29]. In literatures, the renal histopathology of silica nephropathy was varied, including focal glomerulonephritis, necrotizing glomerulonephritis, crescentic glomerulonephritis, etc. [25–46] (Details in Table 3).
Table 3.
Cases describing silicosis combined with renal pathological changes in literatures.
| Year | Author | Case descriptions | Renal pathologic features | References |
|---|---|---|---|---|
| 1975 | Saldanha LF et al. | A 44-year-old man with history of significant industrial silica exposure who presented with hypertension and proteinuria. | Focal glomerulonephritis, intraluminal sloughing of the proximal convoluted tubule, cytoplasmic vacuolization and granularity. | [25] |
| 1977 | Suratt PM et al. | Four men developed silicosis after sandblasting tombstones for an average of 35 months and two of them developed lupus erythematosus and focal glomerulonephritis respectively. | Mild proliferative glomerulonephritis. | [30] |
| 1978 | Giles RD et al. | A 23-year-old sandblaster who developed acute onset massive proteinuria and fatal renal failure. | Mild proliferative glomerulonephritis with loss of colloidal iron staining for sialoprotein, and electron microscopy disclosed an increased density of epithelial cytoplasm, altered lysosomes and endothelial cell microtubular structures. | [26] |
| 1980 | Hauglustaine D et al. | A 37-year-old white male, working in a tile factory, presented proteinuria with no obvious tubular dysfunctional. | Mild focal segmental proliferative glomerulonephritis. Distinct alterations were found by electron microscopy, especially in the proximal tubular cells. | [31] |
| 1981 | Bolton WK et al. | Rapidly progressive renal failure developed in 4 patients with silica exposure. 3 presented with manifestations of a connective tissue disorders. All had abnormal proteinuria, hypoalbuminemia and active urinary sediments. | Glomerular hypercellularity and sclerosis, crescents, interstitial cellular infiltrates and tubular necrosis with red cell casts as seen on light microscopy. On electron microscopy there was foot process obliteration, characteristic cytoplasmic dense lysosomes, microtubules and dense deposits. | [32] |
| 1983 | Cledes J et al. | A 59-year old sand-blaster, histologically proven silicosis was complicated by systemic lupus erythematosus (SLE) and nephritis. | Focal glomerulonephritis with IgG, IgA and C1q deposits. | [33] |
| 1983 | Banks DE et al. | A coal miner presented with pulmonary changes consistent with acute silico lipoproteinosis who developed proteinuria and hematuria. | Diffusely thickened glomerular basement membrane, foot process effacement, and dense lamellar inclusions in swollen glomerular epithelial cells, similar to those seen in Fabry’s disease. | [34] |
| 1987 | Osorio AM et al. | A 54-year-old foundry worker with extensive silica exposure, developed the nephrotic syndrome and renal failure over a 3-month period. | Proliferative glomerulonephritis. | [27] |
| 1987 | Bonnin A et al. | Three men, (50, 67 and 69 years old) with the history of pulmonary silicosis, and two of them presented with microscopic hematuria, mild renal failure, and high blood pressure, and all had glomerular type proteinuria. | Crescentic IgA mesangial nephropathy. | [28] |
| 1989 | Arnalich F et al. | A 55-year-old white male, with silicosis diagnosed 10 years earlier, presented massive proteinuria with microscopic hematuria, moderate renal failure and distal polyneuropathy. | Focal segmental necrotizing glomerulonephritis and arteriolitis. | [35] |
| 1989 | Sherson D et al. | A 56-year-old man worked as a sandblaster for 30 years, and had rapidly progressive crescentic glomerulonephritis. | Severe crescentic glomerulonephritis with significant edema and cellular infiltration in the interstitium. | [36] |
| 1990 | Dracon M et al. | A series of 11 coal miners demonstrating a progressive renal failure with a syndrome of rapidly progressive glomerulonephritis and 3 of them had IgA deposition. | Crescentic glomerulonephritis. | [37] |
| 1991 | Pouthier D et al. | A 43-year-old stone cutter with 13 years of exposure to silica developed a pulmonary silicosis and a glomerulonephritis with moderate renal failure. | Segmental and focal mesangial proliferation and on electron microscopy, distinct alterations of the proximal tubular cells. | [38] |
| 1994 | Neyer U et al. | A 55-year-old male had Wegener’s granulomatosis after silica exposure. | Severe active glomerulonephritis with intra- and extra-capillary proliferation and crescents in more than 50% of the glomerulus. | [39] |
| 1996 | Wilke RA et al. | A 58-year-old coal miner as an employee of the chemical industry suffered from end-stage renal disease. | Glomerulosclerosis and chronic interstitial nephritis | [40] |
| 2001 | Nakajima H et al. | A 63-year-old man had the history of silicosisand had been diagnosed as glomerulonephritis. | Pauci-immune necrotizing crescentic glomerulonephritis | [41] |
| 2001 | Fujii Y et al. | A 51-year-old male who had been working as a building wrecker for 20 years suffered from IgA nephropathy. | Mild mesangial matrix expansion and mesangial cell proliferation with IgA deposition. | [42] |
| 2003 | Mulloy KB et al. | A 63-year-old male who worked in Department of Energy facilities and was diagnosed as microscopic polyangiitis, systemic necrotizing vasculitis, vasculitis, and glomerulonephritis. | Proliferative (crescentic) and necrotizing glomerulonephritis. | [43] |
| 2010 | Dahlgren J et al. | A 38-year-old male was diagnosed as Goodpasture’s syndrome after high level of exposure to crystalline silica | Global glomerulonephritis | [44] |
| 2016 | Riccò M et al. | A 68-year-old male (smoker) was diagnosed as IgA nephropathy after exposure to magnanimous silica dusts. | Glomerular sclerosis with IgA deposition, signs of diffuse vasculitis and tubular atrophy | [45] |
| 2016 | Lee JW et al. | A 56-year-old male presented with silicosis and had an occupational history of precious metal processing for 30 years and a 30 pack-year smoking history, and diagnostic analysis revealed perinuclear ANCA-associated microscopic polyangiitis | Chronic sclerosing glomerulonephritis suggesting ANCA-associated crescentic glomerulonephritis | [46] |
The mechanisms underlying silica nephropathy have not yet been fully elucidated. Most evidences were consistent with the interplay of at least the following two ways: the direct toxic effect of the deposited crystalline material in the renal parenchyma or an autoimmune process involving interaction of silica particles with the immune system, mainly by activation of macrophages through which the kidneys were affected [22,29,47].
More importantly, scavenger receptor, especially the macrophage receptor with collagenous structure (MARCO) expressed in alveolar macrophages [48–52] and the associated NLRP3 inflammasome were thought to be the key regulators for binding and uptake of crystalline silica particle, recognition and clearance of silica and the subsequent development of pulmonary fibrosis in recent studies [3,8,9,11]. NLRP3 inflammasome could be activated after uptake of silica by scavenger receptors, lysosomal rupture and release of cathepsin B [53]. Then, it could cleave several proinflammatory cytokines leading to the secretion of IL-1, IL-18, etc.
Positive depositions of MARCO, NLRP3, Caspase-1, ASC, IL-1β, and IL-18 in the glomerular and tubulo-interstitial areas of our patients further highlight their roles in the pathogenesis of silica nephropathy. Interestingly, the immunohistochemistry results from the disease control of one primary IgA nephropathy patient showed that the distributions of MARCO, Caspase-1, ASC, and IL-1β were not different between our patient and disease control and the expression of IL-18 was mainly expressed in the distal convoluted tubules and some parts of glomerular areas in our patient although it was dispersive around glomerular and tubulo-interstitial areas in the primary IgA nephropathy patient. More importantly, we found that the staining of NLRP3 was significantly higher in tubulo-interstitial areas than that in glomerular areas in the disease control, which was consistent with the previous work by Chun J in primary IgA nephropathy [54]. They found that in primary IgA nephropathy, NLRP3 principally expressed in tubular areas with lesser glomerular localization. Firstly, they detected NLRP3 localization in normal human renal tissues and biopsies from primary IgA nephropathy patients by immunochemistry and immunofluorescence (IF). The results suggested that NLRP3 predominately localized on tubular epithelium with almost no expression in glomerular areas in normal human kidney by IF. Then, they concentrated on tubular expression of NLRP3 and discovered the high expression of NLRP3 (protein and mRNA) in human primary renal tubular cells (HPTC) through immunostaining and mRNA analyzing. Also, when HPTC lost their epithelial phenotype and obtained the characteristic of fibroblastic phenotype by persistent stimulation of TGF-β1 which led to chronic tubular epithelial cell and fibrosis, NLRP3 expression (protein and mRNA) was decreased obviously. Moreover, NLRP3 mRNA expression of renal biopsies was detected by real-time PCR in fifty-four primary IgA nephropathy patients. They found that NLRP3 mRNA expression levels in renal biopsies from IgA nephropathy patients were two to several hundred-folds higher than normal controls. IgA nephropathy patients with high levels of NLRP3 expression had less risk of the composite endpoint compared with low expression of NLRP3 patients. Furthermore, in progressive IgA nephropathy, NLRP3 expression of renal tissues was decreased during tubular atrophy/interstitial fibrosis (the best predictor of outcome in IgA nephropathy upon established on the Oxford classification) which was connected with a worse clinical outcome. Therefore, they pointed that NLRP3 was a predominant tubular protein in primary IgA nephropathy. A recent study by Mascarenhas S et al. [55] found that silica had nephrotoxicity on human-kidney proximal-tubular cells (the toxin’s prime targets) in vitro, which exhibited by induction of prolonged tubular-cell apoptosis and inflammation, through NLRP3-mediated pathway. Thus, we proposed that the kidney disease of our patient might be a secondary IgA nephropathy which could be associated with silicosis based on the different expressions of NLRP3 and its related cytokines/chemokines. This highlight the further explorations of NLRP3 inflammasome on the patho-mechanism of silica nephropathy.
Conclusion
Silicon exposure might be related to the kidney disease in our patient and the NLRP3 mediated inflammation might be involved in its pathogenesis which needs further explorations.
Funding Statement
This work was supported by the grants of National Natural Science Foundation of China [No. 81670640 and No. 81870479]).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Leung CC, Yu ITS, Chen W. Silicosis. Lancet. 2012; 379:2008–2018. [DOI] [PubMed] [Google Scholar]
- 2.Parks CG, Conrad K, Cooper GS. Occupational exposure to crystalline silica and autoimmune disease. Environ Health Perspect. 1999;107:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee S, Hayashi H, Mastuzaki H, et al. Silicosis and autoimmunity. Curr Opin Allergy Clin Immunol. 2017;17:78–84. [DOI] [PubMed] [Google Scholar]
- 4.Mohner M, Pohrt A, Gellissen J. Occupational exposure to respirable crystalline silica and chronic non-malignant renal disease: systematic review and meta-analysis. Int Arch Occup Environ Health. 2017;90:555–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maciejewska A. Health effects of occupational exposure to crystalline silica in the light of current research results. Med Pr. 2014;65:799–818. [PubMed] [Google Scholar]
- 6.Huaux F. New developments in the understanding of immunology in silicosis. Curr Opin Allergy Clin Immunol. 2007;7:168–173. [DOI] [PubMed] [Google Scholar]
- 7.Yao SQ, Rojanasakul LW, Chen ZY, et al. Fas/FasL pathway-mediated alveolar macrophage apoptosis involved in human silicosis. Apoptosis. 2011;16:1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassel SL, Eisenbarth SC, Iyer SS, et al. The Nalp3 inflammasome is essential for the development of silicosis. PNAS. 2008;105:9035–9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hornung V, Bauernfeind F, Halle A, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beamer CA, Migliaccio CT, Jessop F, et al. Innate immune processes are sufficient for driving silicosis in mice. J Leukoc Biol. 2010;88:547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dostert C, Petrilli V, Van Bruggen R, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Yan X, Wang Y, et al. NLRP3 inflammasome inhibition attenuates silica-induced epithelial to mesenchymal transition (EMT) in human bronchial epithelial cells. Exp Cell Res. 2018; 362:489–497. [DOI] [PubMed] [Google Scholar]
- 13.Knauf F, Asplin JR, Granja I, et al. NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy. Kidney Int. 2013; 84:895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Occupational Safety and Health Administration (OSHA), Department of Labor Occupational Exposure to Respirable Crystalline Silica. Final rule. Fed Regist. 2016;81:16285–16890. [PubMed] [Google Scholar]
- 15.Gomez-Puerta JA, Gedmintas L, Costenbader KH. The association between silica exposure and development of ANCA-associated vasculitis: systematic review and meta-analysis. Autoimmun Rev. 2013;12:1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sluis-Cremer GK, Hessel PA, Hnizdo E, et al. Relationship between silicosis and rheumatoid arthritis. Thorax. 1986;41:596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCormic ZD, Khuder SS, Aryal BK, et al. Occupational silica exposure as a risk factor for scleroderma: a meta-analysis. Int Arch Occup Environ Health. 2010;83:763–769. [DOI] [PubMed] [Google Scholar]
- 18.Ozoran K, Uçan H, Tutkak H, et al. Systemic lupus erythematosus arising in a patient with chronic silicosis. Rheumatol Int. 1997;16:217–218. [DOI] [PubMed] [Google Scholar]
- 19.Boujemaa W, Lauwerys R, Bernard A. Early indicators of renal dysfunction in silicotic workers. Scand J Work Environ Health. 1994;20:180–183. [DOI] [PubMed] [Google Scholar]
- 20.Saita G, Zavaglia O. [Renal function in silicotics]. Med Lav. 1951;42:41–48. [PubMed] [Google Scholar]
- 21.Vupputuri S, Parks CG, Nylander-French LA, et al. Occupational silica exposure and chronic kidney disease. Ren Fail. 2012;34:40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenman KD, Moore-Fuller M, Reilly MJ. Kidney disease and silicosis. Nephron. 2000;85:14–19. [DOI] [PubMed] [Google Scholar]
- 23.National Institute for Occupational Safety and Health Health effects of occupational exposure to respirabale crystalline silica. Cincinnati (OH): Department of Health and Human Service; 2002. [Google Scholar]
- 24.Steenland NK, Thun MJ, Ferguson CW, et al. Occupational and other exposures associated with male end-stage renal disease: a case/control study. Am J Public Health. 1990;80:153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saldanha LF, Rosen VJ, Gonick HC. Silicon nephropathy. Am J Med. 1975;59:95–103. [DOI] [PubMed] [Google Scholar]
- 26.Giles RD, Sturgill BC, Suratt PM, et al. Massive proteinuria and acute renal failure in a patient with acute silicoproteinosis. Am J Med. 1978;64:336–342. [DOI] [PubMed] [Google Scholar]
- 27.Osorio AM, Thun MJ, Novak RF, et al. Silica and glomerulonephritis: case report and review of the literature. Am J Kidney Dis. 1987;9:224–230. [DOI] [PubMed] [Google Scholar]
- 28.Bonnin A, Mousson C, Justrabo E, et al. Silicosis associated with crescentic IgA mesangial nephropathy. Nephron. 1987;47:229–230. [DOI] [PubMed] [Google Scholar]
- 29.Ghahramani N. Silica nephropathy. Int J Occup Environ Med. 2010;1:108–115. [PubMed] [Google Scholar]
- 30.Suratt PM, Winn WC, Brody AR, et al. Acute silicosis in tombstone sandblasters. Am Rev Respir Dis. 1977;115:521–529. [DOI] [PubMed] [Google Scholar]
- 31.Hauglustaine D, Van Damme B, Daenens P, et al. Silicon nephropathy: a possible occupational hazard. Nephron. 1980;26:219–224. [DOI] [PubMed] [Google Scholar]
- 32.Bolton WK, Suratt PM, Strugill BC. Rapidly progressive silicon nephropathy. Am J Med. 1981;71:823–828. [DOI] [PubMed] [Google Scholar]
- 33.Cledes J, Hervé JP, Clavier J, et al. [Pulmonary silicosis and disseminated lupus erythematosus]. Poumon Coeur. 1983;39:205–207. [PubMed] [Google Scholar]
- 34.Banks DE, Milutinovic J, Desnick RJ, et al. Silicon nephropathy mimicking Fabry’s disease. Am J Nephrol. 1983;3:279–284. [DOI] [PubMed] [Google Scholar]
- 35.Arnalich F, Lahoz C, Picazo ML, et al. Polyarteritis nodosa and necrotizing glomerulonephritis associated with long-standing silicosis. Nephron. 1989;51:544–547. [DOI] [PubMed] [Google Scholar]
- 36.Sherson D, Jorgensen F. Rapidly progressive crescenteric glomerulonephritis in a sandblaster with silicosis. Br J Ind Med. 1989;46:675–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dracon M, Noёl C, Wallaert B, et al. [Rapidly progressive glomerulonephritis in pneumoconiotic coal miners]. Nephrologie. 1990;11:61–65. [PubMed] [Google Scholar]
- 38.Pouthier D, Duhoux P, Van Damme B. [Pulmonary silicosis and glomerular nephropathy. Apropos of 1 case]. Nephrologie. 1991;12:8–11. [PubMed] [Google Scholar]
- 39.Neyer U, Woss E, Neuweiler J. Wegener’s granulomatosis associated with silicosis. Nephrol Dial Transplant. 1994;9:559–561. [DOI] [PubMed] [Google Scholar]
- 40.Wilke RA, Salisbury S, Abdel-Rahman E, et al. Lupus-like autoimmune disease associated with silicosis. Nephrol Dial Transplant. 1996;11:1835–1838. [PubMed] [Google Scholar]
- 41.Nakajima H, Miyazaki M, lmai N, et al. [A case of silicosis with MPO-ANCA-associated glomerulonephritis and alveolar hemorrhage]. Nihon Jinzo Gakkai Shi. 2001;43:351–356. [PubMed] [Google Scholar]
- 42.Fujii Y, Arimura Y, Waku M, et al. [A case of IgA nephropathy associated with silicosis]. Nihon Jinzo Gakkai Shi. 2001;43:613–618. [PubMed] [Google Scholar]
- 43.Mulloy KB. Silica exposure and systemic vasculitis. Environ Health Perspect. 2003;111:1933–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dahlgren J, Wardenburg M, Peckham T. Goodpasture’s syndrome and silica: a case report and literature review. Case Rep Med. 2010;2010:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ricco M, Thai E, Cella S. Silicosis and renal disease: insights from a case of IgA nephropathy. Ind Health. 2016;54:74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JW, Myong JP, Choi YJ, et al. Diagnosis of perinuclear anti-neutrophil cytoplasmic antibody-associated microscopic polyangiitis in silicotics: case report. Ann Occup Environ Med. 2016;28:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rapiti E, Sperati A, Miceli M, et al. End stage renal disease among ceramic workers exposed to silica. Occup Environ Med. 1999;56:559–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thakur SA, Beamer CA, Migliaccio CT, et al. Critical role of MARCO in crystalline silica-induced pulmonary inflammation. Toxicol Sci. 2009;108:462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Migliaccio CT, Hamilton RF Jr, Holian A. Increase in a distinct pulmonary macrophage subset possessing an antigen-presenting cell phenotype and in vitro APC activity following silica exposure. Toxicol Appl Pharmacol. 2005;205:168–176. [DOI] [PubMed] [Google Scholar]
- 50.Thakur SA, Hamilton R, Pikkarainen T, et al. Differential binding of inorganic particles to MARCO. Toxicol Sci. 2009;107:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamilton RF Jr, Thakur SA, Holian A. Silica binding and toxicity in alveolar macrophages. Free Radic Biol Med. 2008;44:1246–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamilton RF, Thakur SA, Mayfair JK Jr, et al. MARCO mediates silica uptake and toxicity in alveolar macrophages from C57BL/6 mice. J Biol Chem. 2006;281:34218–34226. [DOI] [PubMed] [Google Scholar]
- 53.Pollard KM. Silica, silicosis, and autoimmunity. Front Immunol. 2016;7:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chun J, Chung H, Wang X, et al. NLRP3 localizes to the tubular epithelium in human kidney and correlates with outcome in IgA nephropathy. Sci Rep. 2016;6:24667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mascarenhas S, Mutnuri S, Ganguly A. Silica – a trace geogenic element with emerging nephrotoxic potential. Sci Total Environ. 2018;645:297–317. [DOI] [PubMed] [Google Scholar]