Overexpression of the HvCslF6 gene in hull-less barley grain perturbs sucrose uptake and allocation, and impairs transfer tissue and endosperm development
Keywords: Barley, carbohydrate partitioning, cavity, fructan, (1,3;1,4)-β-glucan, grain morphology, starch, sucrose
Abstract
In cereal grain, sucrose is converted into storage carbohydrates: mainly starch, fructan, and mixed-linkage (1,3;1,4)-β-glucan (MLG). Previously, endosperm-specific overexpression of the HvCslF6 gene in hull-less barley was shown to result in high MLG and low starch content in mature grains. Morphological changes included inwardly elongated aleurone cells, irregular cell shapes of peripheral endosperm, and smaller starch granules of starchy endosperm. Here we explored the physiological basis for these defects by investigating how changes in carbohydrate composition of developing grain impact mature grain morphology. Augmented MLG coincided with increased levels of soluble carbohydrates in the cavity and endosperm at the storage phase. Transcript levels of genes relating to cell wall, starch, sucrose, and fructan metabolism were perturbed in all tissues. The cell walls of endosperm transfer cells (ETCs) in transgenic grain were thinner and showed reduced mannan labelling relative to the wild type. At the early storage phase, ruptures of the non-uniformly developed ETCs and disorganization of adjacent endosperm cells were observed. Soluble sugars accumulated in the developing grain cavity, suggesting a disturbance of carbohydrate flow from the cavity towards the endosperm, resulting in a shrunken mature grain phenotype. Our findings demonstrate the importance of regulating carbohydrate partitioning in maintenance of grain cellularization and filling processes.
Introduction
The major storage carbohydrates in cereal grains include starch, fructan, and mixed linkage (1,3;1,4)-β-glucan (MLG). In Hordeum vulgare L. (barley) grain, starch represents the dominant storage polysaccharide contributing between 52% and 72% of the dry weight (Henry, 1988). In contrast, fructan constitutes 1–4% and MLG 4–10% of the total dry weight (Åman et al., 1985; Henry and Saini, 1989; Burton and Fincher, 2012; Nemeth et al., 2014). Changing carbohydrate metabolic pathways significantly influences grain development and final composition, and thus represents a key factor for future development of barley varieties with application-specific grain quality. In addition, the need to lower processing costs and reduce wastage of valuable nutrients, such as nutrients lost during pearling, have resulted in greater interest in hull-less barley varieties.
Allocation of carbohydrate occurs as soon as the grain begins to form and continues throughout development. At the pre-storage phase [0–6 days after pollination (DAP)], the maternal pericarp, besides its own chloroplast activities, receives additional sucrose from vascular tissue; this sucrose undergoes conversion to hexose sugars which are then used for transient starch reserves (Radchuk et al., 2009; Sreenivasulu et al., 2010). Concurrently, the maternal nucellus begins to disintegrate partly to support cell division and cellularization events in the early endosperm at 3 DAP (Sreenivasulu et al., 2010; Domínguez and Cejudo, 2014). MLG is first deposited in the earliest cell walls at 4–5 DAP (Kohorn et al., 2006), and oligofructans are detected in the vacuoles (Wagner and Wiemken, 1986). From 6 to 8 DAP, genes related to energy production and storage are expressed (Wobus et al., 2005; Sreenivasulu et al., 2010). Maintaining cytosolic sucrose homeostasis is indispensable for correct starch synthesis. Starch storage in the endosperm begins at 9 DAP (Olsen, 2001; Sreenivasulu et al., 2010), coinciding with a high sucrose to hexose ratio (Wobus et al., 2005).
Starch biosynthesis enzymes include granule-bound starch synthase (GBSS), starch synthase (SS), isoamylase (ISO), limit dextrinase (LD), starch branching enzyme (SBE), and ADP-glucose pyrophosphorylase (Radchuk et al., 2009; Jeon et al., 2010; Keeling and Myers, 2010). Fructans are synthesized by sucrose:sucrose-1-fructosyltransferase (1-SST), fructan:fructan-1-fructosyltransferase (1-FFT), sucrose:fructan-6-fructosyltransferase (6-SFT), and fructan:fructan-6G-fructosyltransferase (6G-FFT). Hydrolysis of fructans is mediated by fructan 1-exohydrolase (1-FEH) and fructan 6-exohydrolase (6-FEH) (Vijn and Smeekens, 1999). MLG accounts for 70% of cell wall material in barley endosperm (Fincher, 1975; Guillon et al., 2011; Burton and Fincher, 2014), and MLG synthesis requires cellulose synthase-like enzymes HvCslF6, HvCslH1, and HvCslJ (Doblin et al., 2009; Burton et al., 2011; Little et al., 2018) in balance with (1,3;1,4)-β-glucanase (Hrmova and Fincher, 2001).
Manipulation of genes associated with these pathways is a common strategy to produce crops with the desired grain composition. This manipulation can have a significant influence on grain development and composition. For example, the M292 mutation in the starch synthase IIa (SSIIa) gene produced a shrunken grain phenotype, with a reduction in starch content coinciding with significantly increased sucrose, fructan, and MLG content (Clarke et al., 2008). The same phenotype was reported for low-starch barley lines with RNAi suppression of all starch branching enzyme genes (Carciofi et al., 2012). It has been suggested that reduced metabolic uptake of sucrose in the endosperm transfer cell (ETC) region can cause an excessive accumulation of fluid that enlarges the grain cavity, resulting in shrunken grain after desiccation (Carciofi et al., 2012; Shaik et al., 2016). Larger grain cavities have previously been observed in barley lines overexpressing HvCslF6, and grain from these lines had higher MLG and reduced starch content. Morphological changes in these lines were detected in aleurone tissues that were on average three cell layers thick, with strongly defined walls and a pronounced cubic shape as well as deformed and, in places, completely missing transfer cells at the grain-filling phase (15 DAP). Mature transgenic grain had reduced size relative to control lines, a shrunken and darkened appearance, and increased brittleness (Lim et al., 2018). In this study, we used transgenic grains overexpressing the HvCslF6 gene as a model system to investigate the effects of increased MLG on carbohydrate metabolism, cavity formation, and cell identity during grain development.
Materials and methods
Plant materials
Barley plants were grown following Lim et al. (2018) under a day/night temperature regime of 23 °C/15 °C. Progeny of the T3 generation from four independent transformed lines (15-3, 18-6, 25-5, and 16-5) overexpressing the HvCslF6 gene were selected; these lines were referred to as lines F6-15, F6-18, F6-25, and F6-16 in Lim et al. (2018); in addition, Torrens [wild type (WT)] and WT plants regenerated from tissue culture [WT(tc)] were selected and analysed. Developing grains from individual plants were collected from the middle of the spike from 7 to 24 DAP at approximately mid-day, covering all phases of grain development. Mature grain was also collected. The ‘outer tissues', endosperm, and embryo samples were separated using a scalpel and fine forceps, snap-frozen in liquid nitrogen, and kept at –80 °C until required. Experiments were performed on three biological replicates, consisting of at least 10 grains per replicate, with two technical replicates each, except where noted in the figure legend.
Vector construction and Agrobacterium-mediated barley transformation of pAsGlo1:3xnlsYFP
DNA fragments were synthesized by Genscript (USA) and inserted into the pUC57 vector. The 2.6 kb 3xnlsYFP gene (MA044) was adapted from Ueda et al. (2011) and codon optimized for barley using the online tool at https://sg.idtdna.com/CodonOpt (accessed 1 October 2019). The sequence for the 994 bp oat globulin AsGlo1 promoter sequence (pAsGlo1; MA003) was obtained from Vickers et al. (2006) and modified to include 5'-HindIII and 3'-KpnI restriction sites. The HindIII/KpnI pAsGlo1 fragment from MA003 was excised and inserted into the Gateway-compatible pMDC32 vector (Curtis and Grossniklaus, 2003) in place of the double 35S promoter, creating MA009. The 3xnlsYFP sequence was amplified from pUC57 using pUC57-attL1_FWD (5'-ACCTCGCGAATGCATCTAGATCA-3') and SacI-attL2_REV (5'-CAAATAATGATTTTATTTTGACTGATAGTGACCTGTTCGTTGCAACAAATTGATGAGCAATTATTTTTTATAATGCCAACTTTGTACAAGAAAGCTGGGTTCATTATTTGGAGCTC-3') primers and HiFi Platinum Taq (Thermo Fisher Scientific), resulting in a PCR fragment flanked by attL1 and attL2 Gateway-compatible sites. The 3xnlsYFP insert was transferred into the MA009 (pAsGlo1:pMDC32) vector using LR clonase II (Thermo Fisher Scientific) according to the manufacturer's instructions. The resulting vector MA032 (pAsGlo1:3xnlsYFP) was transformed into Agrobacterium tumefaciens strain AGL1 and used for transformation of the Hordeum vulgare cv. WI4330 following the protocol outlined in Lim et al. (2018).
(1,3;1,4)-β-Glucan (MLG) assay
For the measurements of storage carbohydrates (MLG and starch) from developing and mature grain, the ‘outer tissues' and the endosperm were analysed as a single sample. The embryo, containing low amounts of storage carbohydrates, was removed and used for analysis of soluble sugars. Freeze-dried samples were ground and weighed (10 mg). To remove free sugars and chlorophyll, samples were pre-treated with 70% ethanol (1 ml) at 97 °C for 30 min, centrifuged at 5000 rpm for 5 min, and supernatants removed. Pellets were washed with 100% ethanol (1 ml) at 97 °C for 10 min, centrifuged at 5000 rpm for 5 min, and supernatants removed. MLG content was measured using the Megazyme Mixed-linkage Beta-glucan Assay Kit (K-BGLU) (McCleary and Codd, 1991) as outlined in Lim et al. (2018).
Starch assay
Starch was measured using the Megazyme Total Starch Assay (AA/AMG) (McCleary et al., 1994), with 10 mg of freeze-dried sample, pre-treated with 70% ethanol (30 min, 97 °C) and 100% ethanol (10 min, 97 °C).
Soluble sugar analysis
For the soluble sugar measurements, the developing and mature grains were separated into ‘outer tissues', endosperm, and embryo tissue samples. Grains were ground and weighed (10 mg). Soluble sugars were extracted in 80% ethanol at 85 °C for 30 min followed by Milli-Q water at 50 °C for 30 min in a final dilution of 1:40 (w/v, mg µl–1) and supernatants combined (Verspreet et al. (2012). For cavity sap, 10 barley grains were cut in half and fluids from the endosperm cavity were collected using a microsyringe (Hamilton). Cavity sap was immediately heated to 90 °C to inactivate endogenous enzymes and diluted in Milli-Q water to a final dilution of 1:100 (v/v). Extracts from grain tissues and cavity sap were treated or not with fructanase (Megazyme fructan assay kit, Deltagen), incubated at 40 °C for 2 h for complete hydrolysis, and heated to 90 °C for 5 min. Soluble sugars were analysed by high pH anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) on a Dionex ICS-5000 system using a DionexCarboPAC™PA-20 column (3×150 mm) with a guard column (3×50 mm) kept at 30 °C and operated at a flow rate of 0.5 ml min–1. The eluents used were (A) 0.1 M sodium hydroxide and (B) 0.1 M sodium hydroxide with 1 M sodium acetate. The gradient used was: 0% (B) from 0 min to 9 min, 10% (B) from 9 min to 10 min, 100% (B) from 10 min to 12 min, and 0% (B) from 12 min to 20 min. Detector temperature was maintained at 20 °C, data collection was at 2 Hz, and the Gold Standard PAD waveform (std. quad. potential) was used. Standards used were: glucose, fructose, sucrose, raffinose, 1-kestose, and nytose at concentrations of 4, 2, 1, 0.5, 0.25, and 0.125 mg l–1, respectively. Total fructan (µM) content was calculated according to Huynh et al. (2008) and equals the sum of glucose and fructose after hydrolysis minus the sum of free glucose, fructose, sucrose, and raffinose (before hydrolysis), taking into account the loss of one water molecule per monosaccharide.
Monosaccharide and arabinoxylan analysis
Monosaccharides were analysed following Hassan et al. (2017) using reversed phase HPLC separation coupled to diode array detection (Agilent Technologies). Total arabinoxylan content (% w/w) was calculated from the combined amount of % l-arabinose and % d-xylose, taking into account the loss of one water molecule per monosaccharide.
Protein content
The amount of nitrogen in mature grain samples was analysed using the Dumas combustion method following Jung et al. (2003). Protein was calculated from the nitrogen content of the material, using a nitrogen conversion factor of 6.25.
RNA isolation and cDNA synthesis
Total RNA was extracted from all tissue homogenates using an Invitrogen™ TRIzol™ (Thermo Fisher Scientific), DNA was removed using a DNA-free kit following the manufacturer's instructions (Ambion®, Thermo Fisher Scientific), and was used as the template for cDNA synthesis following Burton et al. (2008).
Real-time quantitative PCR (qPCR)
Gene-specific primers were designed from the 3'-untranslated regions of full-length cDNAs. Fragments amplified using the gene-specific primers were purified using HPLC following Burton et al. (2008) and sequenced at the Australian Genome Research Facility, Adelaide, South Australia. PCR primer sequences are provided in Supplementary Table S2 at JXB online. qPCR analysis was carried out following Burton et al. (2008). Heatmap representation was generated using Multiple Experiment Viewer (MeV) software (http://mev.tm4.org/ accessed 1 October 2019).
Protein extraction, SDS–PAGE, and immunoblotting
Microsomes from frozen barley grain tissues (10 grains each) were isolated according to Song et al. (2015). Protein concentration was measured using the BCA protein assay kit (Thermo Fisher Scientific). Protein gel electrophoresis was performed with 30 µg of protein using the NuPAGE Bis-Tris electrophoresis system (Invitrogen) on 4–12% gradient gels (Novex, Life Technologies) with the molecular mass marker Precision Plus Protein Kaleidoscope (BioRad). After electrophoresis, proteins were transferred onto nitrocellulose membrane (Nitrobind, 0.22 µm, Thermo Fisher Scientific) using the XCell II Blot Module (Invitrogen) according to the manufacturer's protocol and processed according to Doblin et al. (2009). The membrane was incubated with a polyclonal HvCslF6 primary antibody (Wilson et al., 2015) at 1:2000 dilution followed by anti-rabbit horseradish peroxidase (HRP) conjugate (BioRad) at a 1:10 000 dilution. Signal detection was performed with SuperSignal West Pico chemiluminescent substrate (Thermo Fisher Scientific) according to the manufacturer's instructions and using a ChemiDoc MP Imaging System (BioRad).
Tissue preparation for MALDI-MSI measurement
Sectioning of barley grains and matrix application was performed following Peukert et al. (2014). The MS imaging (MSI) measurements were performed using an ultrafleXtreme matrix-assisted laser desorption/ionization (MALDI) time-of-flight (TOF)/TOF device (Bruker Daltonics) following Peukert et al. (2016a).
Tissue fixation, embedding, and microscopy for barley grain
Developing grains were halved using a razor blade and fixed in 0.25% glutaraldehyde, 4% paraformaldehyde, and 4% sucrose in phosphate-buffered saline (PBS), pH 7.2, dehydrated, and embedded in LR White resin following Burton et al. (2011). Embedded tissue was sectioned (1 µm) on an ultramicrotome using a diamond knife and dried onto polylysine-coated microscope slides (Thermo Fisher Scientific). Immunochemical analysis of cell wall polysaccharides was performed according to Burton et al. (2011) using primary antibodies including (1,3)-β-glucan (Biosupplies), hetero-(1,4)-β-mannan (Pettolino et al., 2001), BG1 (Biosupplies), LM11 (McCartney et al., 2005), LM19 (PlantProbes), and a recombinant crystalline cellulose CBM3a protein (PlantProbes). For detection of crystalline cellulose, a monoclonal anti-polyhistidine antibody (Sigma-Aldrich) [1:100 dilution in 1× PBS with 1% (w/v) BSA] was applied after CBM3a (Blake et al., 2006). For detection of arabinoxylan, sections were incubated in α-l-arabinofuranosidase (Megazyme International, Deltagen) under moist conditions at 40 °C for 2 h for unmasking of antibody epitopes prior to LM11 antibody incubation (Wilson et al., 2012). For detection of homogalacturonan, sections were either not treated or treated with 0.1 M sodium carbonate (Na2CO3) for 1 h at room temperature for unmasking of antibody epitopes prior to LM19 antibody incubation (Verhertbruggen et al., 2009). Alexa Fluor® 555 goat anti-mouse IgG (H+L) secondary antibody (Thermo Fisher Scientific) was used against BG1, (1,3)-β-glucan, and anti-polyhistidine antibodies, while Alexa Fluor® 555 goat anti-rat IgG (H+L) secondary antibody (Thermo Fisher Scientific) was used against LM11 and LM19 antibodies. Images were taken using a fluorescence microscope (Axio Imager M2; Carl Zeiss). Tissue sections were stained with Epoxy Tissue Stain 14950 (Proscitech) for morphological examination and were photographed using a Nikon Ni-E optical microscope. For environmental SEM, mature grains were cut in half, mounted on SEM stubs, and examined using an FEI Quanta 450 FEG environmental scanning electron microscope at an accelerating voltage of 10 kV.
Sections were prepared from the equatorial part of the grain. Two biological replicates with two technical replicates of each line were examined.
Aleurone layer dimension and cell shape measurements
To observe the aleurone layer dimensions in mature samples, grains were hand-dissected (transversely) in the mid-region and analysed as described in Aubert et al. (2018). Measurements were taken in triplicate from 2–5 sections of three individual grains using a Zeiss M2 AxioImager with an attached AxioCam MrM camera (Zeiss, Germany). Images were analysed using ZEN 2012 software (Zeiss, Germany) at dorsal, left, and right positions. Aleurone layer width was measured as the distance from the edge of the endosperm to the innermost autofluorescent aleurone cell wall. Aleurone proportion was measured by calculating the aleurone area as a percentage of the total endosperm area.
Aleurone cell shape of WT and 18-6 grains was measured similarly from toluidine blue-stained grain sections of 24 DAP grains using ZEN 2012 software from two biological replicates (four sections each). Cell length was defined as the distance from the outward-directed cell wall to the innermost cell wall, while the cell width was measured as the distance from the left-neighbouring-directed cell wall to the right-neighbouring cell wall. In total, 127 and 231 cells were randomly analysed from each line, respectively.
Starch granule size measurements
Ground barley flour was mixed with 1 ml of 2% SDS (Sigma-Aldrich) and 5 µl of 1 M DTT (Sigma-Aldrich) for 10 min. After centrifugation (3000 g) for 10 min, the supernatant was decanted. The pellet was washed in 1 ml of Milli-Q water, centrifuged (3000 g) for 10 min, and the supernatant decanted. The pellet was suspended in 150 µl of Lugol's iodine solution containing 2% potassium iodine (E. Merck) and 1% iodine (Sigma-Aldrich), and diluted further with 300 µl of Milli-Q water. A drop of the mixed sample was placed on a Bright-Line Hemacytometer (Hausser Scientific); images were taken using a Nikon Ni-E optical microscope. Measurement of starch particles was performed using Image J software (http://imagej.net/Fiji (accessed 1 October 2019)). RGB images were converted to 32 bit black and white format and the default threshold ImageJ settings applied, followed by watershed analysis to separate granules in close physical proximity. Granules were then measured using the ‘Analyse particle' function, with size constraints of 1 µm infinity and of 0.8–1.00 circularity. Haemocytometer lines were used to establish scale.
Results
pAsGlo1:HvCslF6 is expressed in the peripheral endosperm and correlates with increased HvCSLF6 protein and MLG abundance
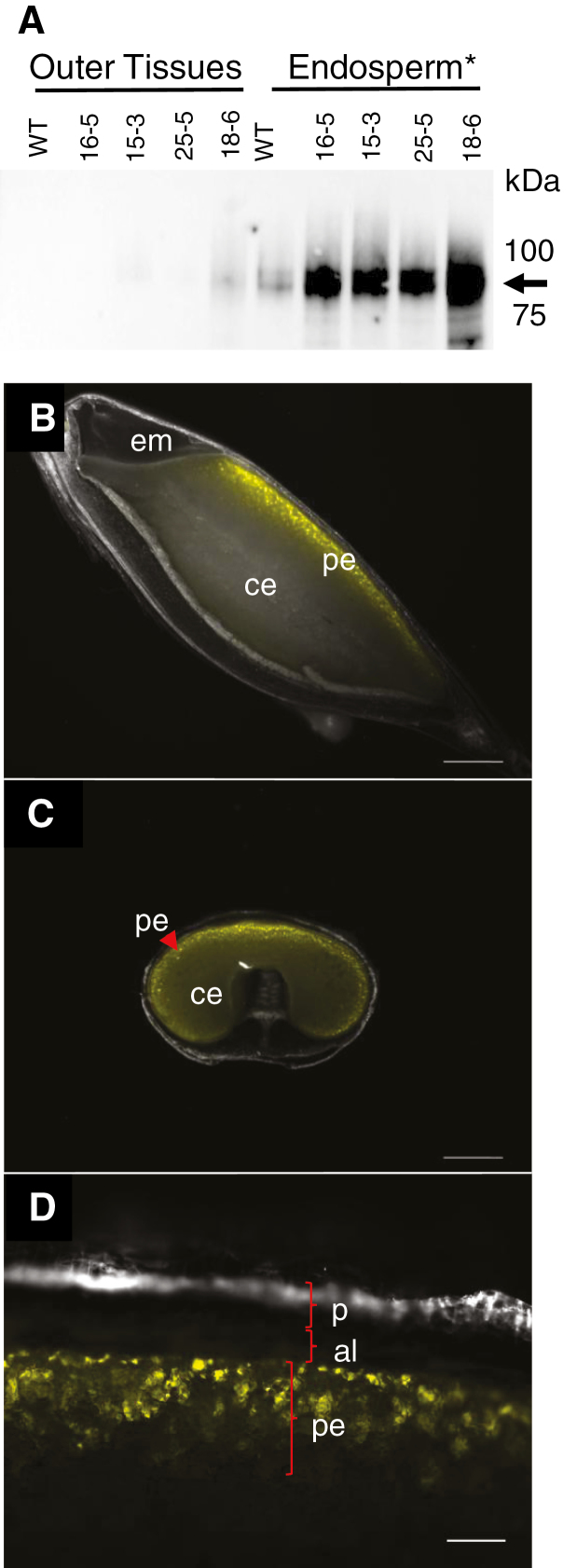
To determine HvCSLF6 protein abundance, a specific HvCSLF6 antibody was used (Fig. 1A). Consistent with Wilson et al. (2015), a strong doublet of HvCSLF6 protein between 89 kDa and 100 kDa was observed in endosperm extracts of transgenic and WT grain at 15 DAP, although the predicted size for HvCSLF6 is 105 kDa (Wilson et al., 2015). Levels of HvCSLF6 were greater in transgenic endosperm than in the WT; a weak HvCSLF6 signal was also observed in the 18-6 ‘outer tissues' sample (Fig. 1A). Out of the four transgenic lines tested, the highest accumulation of HvCSLF6 was observed in line 18-6, which also showed the strongest grain morphological phenotype.
Fig. 1.
Detection of HvCslF6 protein and YFP fluorescence in developing barley grain. (A) Total HvCslF6 protein levels in the transgenic grains are slightly higher in ‘outer tissues' and clearly higher in endosperm tissues relatively to the WT at 15 DAP. The arrow indicates HvCslF6 protein at 89–100 kDa. (B–D) Fluorescence from YFP is detected in the peripheral endosperm of barley grain at 20 DAP. Images (B) and (C) are under ×1 magnification with scale bars equivalent to 1000 µm. Image (D) is under ×10 magnification with a scale bar equivalent to 100 µm. Sections for image (C) were prepared in the equatorial part of the grain. *Endosperm sample includes embryo tissues. Abbreviations: aleurone (al), central endosperm (ce), embryo (em), pericarp (p), peripheral endosperm (pe).
Accumulation of HvCslF6 transcript was measured in WT and transgenic grain tissues at multiple DAP. From 7 to 19 DAP, transcript levels were higher in transgenic ‘outer tissues' and endosperm compared with the WT and WT(tc), and they were also slightly higher in 24 DAP transgenic ‘outer tissues' compared with the WT. Lowest expression of the HvCslF6 transcript was observed for line 15-3. In the embryo, only the 18-6 transgenic line had higher HvCslF6 transcript levels at 19 DAP relative to the WT (Supplementary Fig. S1A).
To examine tissue specificity of the AsGlo promoter (pAsGlo1), fresh barley grain sections from plants transformed with the pAsGlo1:3xnlsYFP transcriptional fusion construct were observed under a fluorescence microscope. Yellow fluorescent protein (YFP) fluorescence was first detected at ~7 DAP (data not shown) and by 20 DAP was seen specifically in the peripheral endosperm of the grain. No signal was detected in aleurone or pericarp tissues (Fig. 1B–D).
To establish whether contamination from the peripheral endosperm tissue had carried over into the manually prepared ‘outer tissues' samples, transcript levels of the aleurone-specific gene, lipid transfer protein 2 (HvLtp2) (Kalla et al., 1994) and the HvGBSS1a gene, which is not detectable in barley pericarp (Radchuk et al., 2009), were examined (Supplementary Fig. S1D, E). HvLtp2 transcript expression was detected at similar levels in both endosperm and ‘outer tissues' samples, while HvGBSS1a transcript expression was found at a higher level in endosperm preparations. These results indicate that a considerable proportion of peripheral endosperm was present in the manual preparations of ‘outer tissues' of all samples. These samples were named ‘outer tissues' throughout the manuscript, and they contained pericarp, testa, nucellar epidermis, and some aleurone cells.
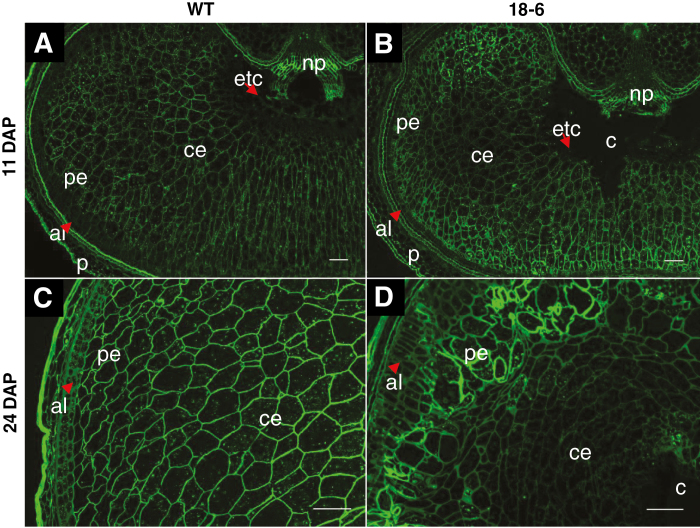
Distribution of MLG was examined by immunolabelling of grain sections (Fig. 2A–D). At 7 DAP, BG1 antibody labelling was evenly detected across both WT and 18-6 transgenic grain (data not shown). At 11 and 24 DAP, signals from BG1 antibody labelling were uniformly distributed across the tissue cell walls in WT grain (Fig. 2A, C) whereas for 18-6 transgenic grain, the fluorescence signals were stronger in the peripheral endosperm cell walls (cell walls appeared thicker; Fig. 2B, D), appearing similar to the pAsGlo1:3xnlsYFP expression pattern (Fig. 1C). BG1-associated fluorescence also indicated that peripheral and central endosperm cells in transgenic grain were irregularly arranged and the central endosperm cells appeared more ‘squashed' (Fig. 2D). Higher BG1 labelling in transgenic grain coincided with increased endosperm MLG content at 24 DAP (Supplementary Fig. S2A, B) and increased MLG content (% w/w) in the mature endosperm in all transgenic lines compared with the WT (Supplementary Table S1A).
Fig. 2.
Immunodetection of (1,3;1,4)-β-glucan in developing barley grain. Labelling with anti-(1,3;1,4)-β-glucan (BG1) monoclonal antibody shows stronger fluorescence signals in the peripheral endosperm of transgenic grain (B) and (D) relative to the WT (A) and (C) at 11 and 24 DAP. (A) and (B) are under ×5 magnification; (C) and (D) are under ×10 magnification. Scale bars are equivalent to 100 µm. Sections were prepared in the equatorial part of the grain for two biological replicates with two technical replicates each. Abbreviations: aleurone (al), cavity (c), central endosperm (ce), endosperm transfer cells (etc), pericarp (p), peripheral endosperm (pe), nucellar projection (np).
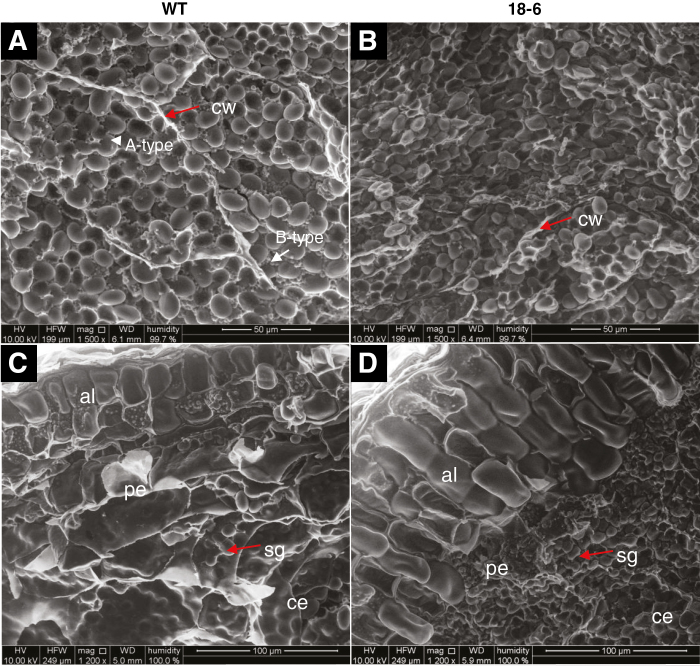
Starch granules are smaller in pAsGlo1:HvCslF6 transgenic grain
The ‘squashed' endosperm cells in 18-6 transgenic grain were packed with starch granules which appeared smaller than A-type (large) but much larger than B-type granules (small) in the WT (Fig. 3A, B). Small starch granules were also found in the peripheral endosperm layer of 18-6 transgenic grain, while the equivalent cells in the WT contained fewer, larger granules (Fig. 3C, D). The cell walls surrounding the starch granules were more obvious in WT endosperm compared with transgenic endosperm (Fig. 3A, B).
Fig. 3.
Environmental scanning electron micrographs of starch granules of mature barley grain. (A) WT endosperm contains A-type (large) and B-type (small) starch granules and the surrounding cell walls are apparent. (B) Starch granules in the 18-6 transgenic endosperm appear packed in an intermediate size between A-type and B-type in the WT grain and the cell walls are less apparent. (C) Aleurone cells in the WT appear cubic, while those of 18-6 transgenic grain appear rectangular cuboid (D). Starch granules are highly packed in the peripheral endosperm layer of 18-6 transgenic grain. (A) and (B) are taken in the distal endosperm regions of grains cross-sectioned in the middle part, viewed at ×1500 magnification with scale bars equivalent to 50 µm. (C) and (D) are taken next to the aleurone layer with ×1200 magnification and scale bars equivalent to 100 µm. Abbreviations: aleurone (al), cell wall (cw), central endosperm (ce), peripheral endosperm (pe), starch granule (sg).
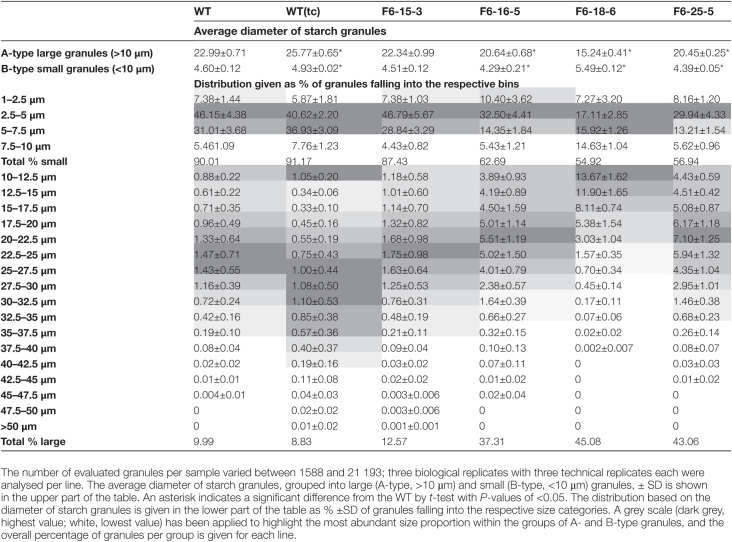
The size and distribution of starch granules in grain from the WT, WT(tc), and all transgenic lines were further examined (Table 1). Barley grain starch comprises large granules with diameters in the range of 10–40 µm and small granules ranging from 1 µm to 10 µm (Palmer, 1972; Chmelik et al., 2001). Therefore, granules with diameters <10 µm were grouped as small granules (B-type) while granules with diameters >10 µm were grouped as large granules (A-type). WT, WT(tc), 15-3, 16-5, and 25-5 had small granules with diameters ranging between 4.3 µm and 4.9 µm, whereas granules from 18-6 had diameters >5 µm. Line 18-6 had the smallest A-type granules, with average diameters of ~15 µm, whereas those of WT, WT(tc), and other transgenic lines ranged between 20 µm and 26 µm. In transgenic grain, the overall size distribution was shifted to a higher proportion of A-type granules while the median granule size in this group was reduced compared with the WT(tc) and WT grain. Lowest effects on starch granules size and distribution were observed for line 15-3 (Table 1).
Table 1.
Analysis of starch granules in mature barley grain
Abnormal structures of the cavity and ETCs in pAsGlo1:HvCslF6 transgenic grain
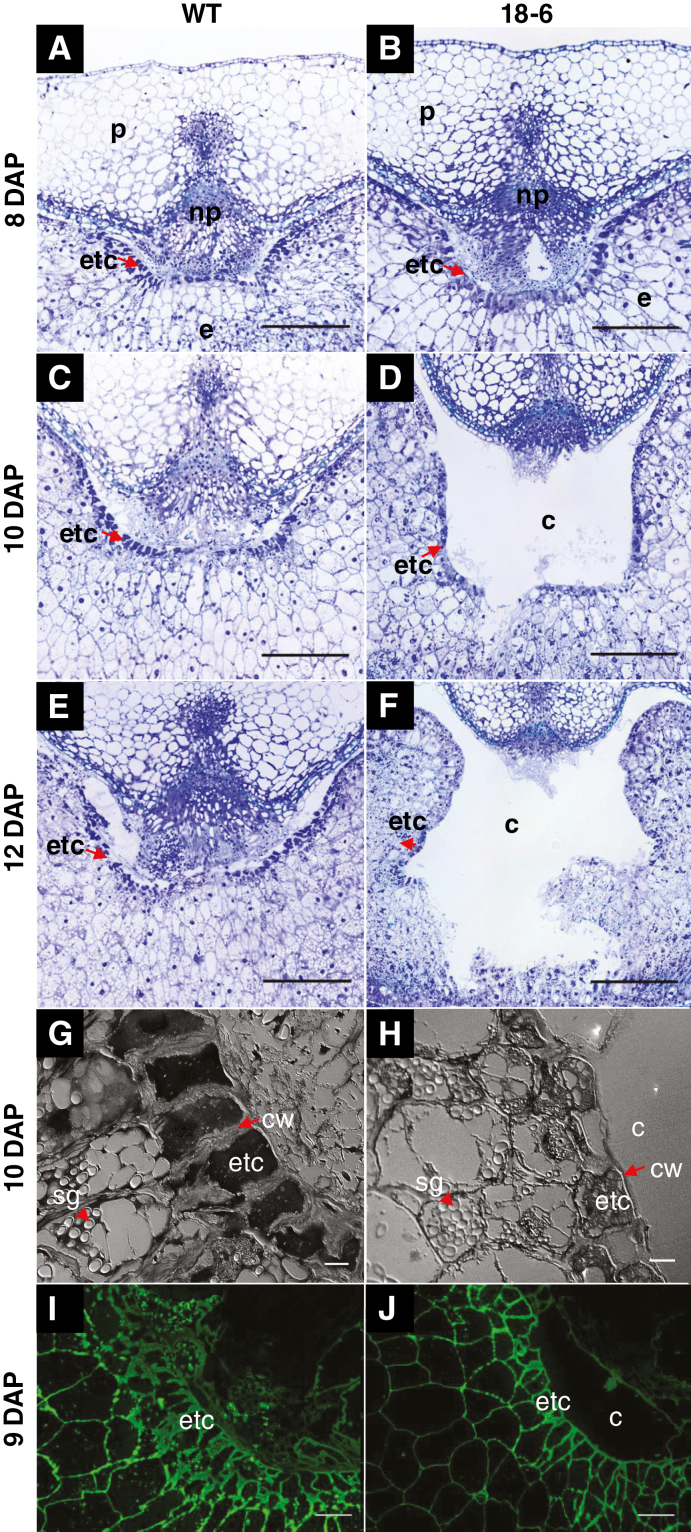
Similar to each of the other HvCslF6 transgenic lines, line 18-6 had a smaller endosperm area than the WT due to the presence of the large central cavity (Lim et al., 2018). The ETCs surrounding the central cavity were deformed or sporadically missing. Rupture of ETCs as an artefact of sample preparation can be excluded as this was observed in many different replicates and treatments as well as during cryo-sectioning such as performed for MSI experiments. This phenotype was absent in the WT and WT(tc) grain. We investigated histological changes during the transition phase of grain development (8–14 DAP), where cellularization of transfer tissues has been completed and storage product accumulation starts (Fig. 4A–F; Supplementary Fig. S3A–D). As early as 8 DAP, rupture of tissues adjacent to the nucellar projection (NP), the newly forming cavity, can be observed in transgenic grain while other transfer tissues appear similar to those in the WT (Fig. 4A, B). At 9 DAP, the cavity size in transgenic grain was larger compared with the WT, and the ETCs showed irregular cell types while the cavity surrounding the cell layer remained intact (Supplementary Fig. S3A, B). At 10 DAP, the ETCs in transgenic grain were ruptured (Fig. 4C, D), becoming increasingly pronounced during subsequent development, whereas the ETCs remained intact in the WT grain (Fig. 4E, F; Supplementary Fig. S3C, D). In WT grain, cell walls of the ETCs were denser and thicker (Fig. 4G) compared with those of transgenic grain which were thinner and surrounded by much less regularly shaped cells (Fig. 4H).
Fig. 4.
Histological images of the ventral crease region in WT and transgenic (18-6) barley grain. The endosperm transfer cells (ETCs) in the WT appear intact (A, C, and E) while the ETCs in transgenic grain are poorly formed in the region adjacent to the emerging cavity and start to be disrupted during the early storage phase (B, D, and F). ETCs in WT grain are denser with thicker cell walls (G) compared with loosely organized ETCs with thinner cell walls in transgenic grain (H) at 10 DAP. (I) and (J) Immunodetection of mannan [(1–4)-β-d-mannan and galacto-(1–4)-β-d-mannan antibody] revealed stronger fluorescence in the cell walls of WT ETCs relative to transgenic grain at 9 DAP. (A–F) Toluidine blue-stained grain sections under ×4 magnification; scale bars=100 µm. (G) and (H) ×60 magnification; scale bars=10 µm. (I) and (J) ×20 magnification; scale bars=50 µm. Abbreviations: cavity (c), cell wall (cw), endosperm (e), endosperm transfer cell (etc), nucellar projection (np), pericarp (p), starch granule (sg).
Altered cell wall composition in the ETCs and endosperm of pAsGlo1:HvCslF6 transgenic grain
To determine whether ETC rupture might be associated with changes in cell wall composition, sections of WT and 18-6 transgenic grain were examined. From 9 to 11 DAP, the labelling patterns for the mannan antibody were similar between WT and transgenic grain, although the intensity of mannan labelling was higher in the WT (Fig. 4I, J). Labelling of MLG (Fig. 2A, B), callose (Supplementary Fig. S4A, B), crystalline cellulose (Supplementary Fig. S4C, D), and arabinoxylan (Supplementary Fig. S5A, B) was not detected in the ETC walls of either WT or transgenic grain. Arabinoxylan labelling was greater in the nucellar epidermis of transgenic grain when compared with the WT (Supplementary Fig. S5A, B). Labelling of pectin was detected in the cell walls of the NP, and notably only in the walls of ETCs adjacent to the cavity in both transgenic and WT grain (Supplementary Fig. S6A–D).
Clear differences were observed in the abundance of arabinoxylan, crystalline cellulose, and callose in the endosperm walls of WT and 18-6 grain (Supplementary Fig. S5A–H) where transgenic grain showed stronger labelling for arabinoxylan and callose relative to the WT (Supplementary Fig. S5A–D). More intense labelling for callose and crystalline cellulose was detected in the peripheral endosperm of transgenic grains relative to the WT, similar to MLG (Fig. 2A–D; Supplementary Fig. S5C–F). Labelling of crystalline cellulose was completely absent in the nucellar epidermis and adjacent cell rows of transgenic grain (Supplementary Fig. S5E, F), as noted for ETCs (Supplementary Fig. S4C, D). The labelling intensity of mannan (Supplementary Fig. S5G, H) was similar between WT and transgenic endosperm, as was that of LM19, which was detected in maternal grain tissues following pre-treatment with 0.1 M sodium carbonate (Supplementary Fig. S6E–H).
Modified aleurone cell shape in pAsGlo1:HvCslF6 transgenic grain
At 9 DAP, peripheral endosperm cells in 18-6 and WT grain appeared similarly developed (Supplementary Fig. S7A, B), while at 12–14 DAP, aleurone cells in 18-6 appeared larger relative to the WT (Supplementary Fig. S7C–F). By 24 DAP, they were notably elongated in 18-6 while the equivalent WT cells remained cube shaped (Supplementary Fig. S7G, H). From 14 to 24 DAP, increasing thickness of 18-6 aleurone and peripheral endosperm cell walls was observed relative to the WT (Supplementary Fig. S7E–H) coinciding with increased MLG, arabinoxylan, callose, and cellulose deposition in these cell walls (Fig. 2C, D; Supplementary Fig. S5A–F).
Measuring aleurone cell shape revealed that at 24 DAP, the average length in 18-6 was 44.8±0.2 µm (SE) and the width was 16.1±0.3 µm (SE). For the WT, the average length was 18.7±0.5 µm (SE) with a width of 17.8±0.2 µm (SE), confirming that WT aleurone cells were cuboid and transgenic cells were over twice as long as, but similar in width to, those of the WT. Transgenic aleurone cells remained relatively elongated inwardly compared with the WT at each stage through to grain maturity (Fig. 3D), and the aleurone layer was thicker at maturity in all transgenic lines relative to the WT (Supplementary Fig. S8).
pAsGlo1:HvCslF6 transgenic barley grain accumulated more (1,3;1,4)-β-glucan, sucrose, and total fructan, and less starch in the endosperm compared with the WT
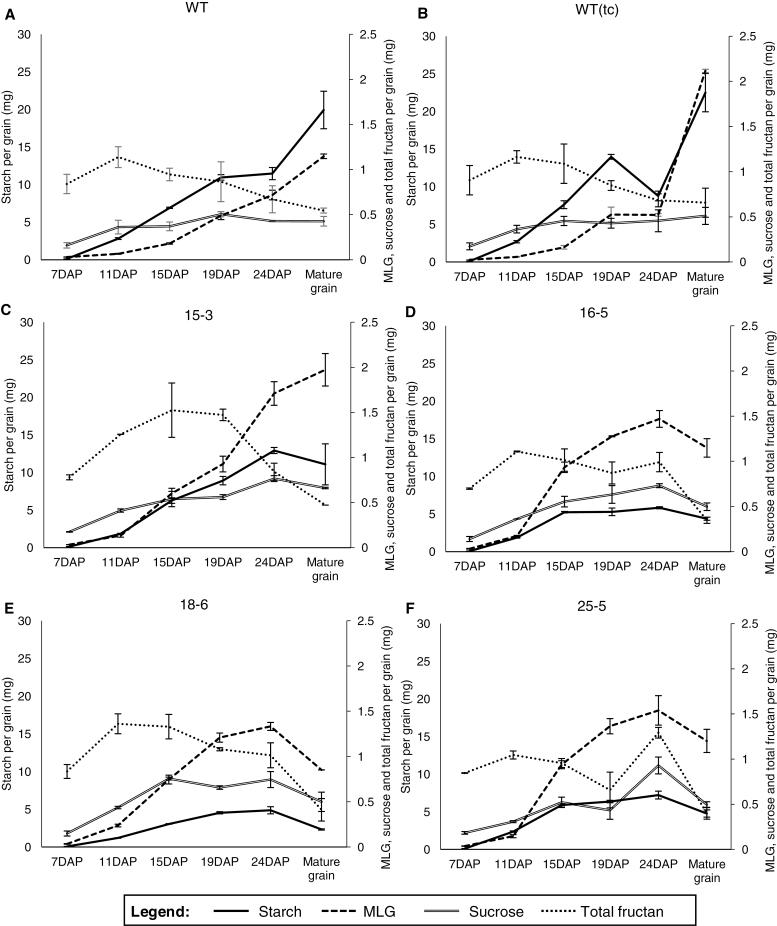
Storage carbohydrate deposition was examined in WT, WT(tc), and transgenic lines from 7 DAP until maturity (Fig. 5A–F). Differences were observed in the MLG content of the WT(tc) lines relative to the WT, whereas the levels of starch, sucrose, and total fructan were similar (Fig. 5A, B; Supplementary Table S1A). Likewise, with the transgenic lines, the greatest variations were observed in 18-6 and the smallest in 15-3, which had similar levels of MLG to WT(tc) per grain but significantly lower levels of starch. WT and WT(tc) grain accumulated starch and MLG throughout grain development (Fig. 5A, B), whilst starch and MLG declined slightly after 24 DAP in transgenic grain (Fig. 5C–F). WT and WT(tc) grain reached maximum total fructan levels at 11 DAP at ~1.2 mg, while transgenic lines ranged between 1.2 mg and 1.5 mg from 11 to 24 DAP (Fig. 5C–F). Sucrose levels in WT and WT(tc) grain were constant from 11 DAP to maturity at 0.5 mg, and increased in transgenic grain from 11 to 19 DAP, from 0.7 mg to 1 mg (Fig. 5C–F). MLG levels were increased in transgenic developing grain from 11 DAP onwards relative to the WT. At maturity on a per grain basis, transgenic grain (except line 16-5 with similar and line 18-6 with lower MLG levels) contained more MLG but less starch than WT grain (Fig. 5; Supplementary Table S1A). While glucose and fructose levels were lower in all transgenic grain samples, sucrose and total fructan levels were comparable with those of the WT and WT(tc) (Supplementary Table S1A). On a weight per weight basis, all transgenic grain contained more MLG, sucrose, and total fructan (except line 15-3 with similar total fructan levels) but less starch than the WT in developing and mature endosperm (Supplementary Figs S2A, C, S9A, C; Supplementary Table S1A). Protein levels were reduced in grain of all transgenic lines on a per grain basis, which is mainly attributed to the reduced endosperm weight relative to WT and WT(tc) grain (Supplementary Table S1). The fraction of other grain constituents, including lipids, cellulose, lignin, and other secondary metabolites (e.g. tocopherol and phytosterols), minerals, and vitamins was higher in mature ‘outer tissues' and endosperm of transgenic grain (Supplementary Table S1A). Cellulose is likely to be the major component of the grain cell wall, as shown in Supplementary Fig. S5E and F.
Fig. 5.
The carbohydrate content: MLG, starch, sucrose, and total fructan during barley grain development on a per grain basis (mg). (A) WT, (B) WT(tc), and pAsGlo1:HvCslF6 transgenic lines (C) 15-3, (D) 16-5, (E) 18-6, and (F) 25-5. For measurements of storage carbohydrates (MLG and starch) from developing and mature grains, ‘outer tissues' and endosperm were analysed as a single sample. The embryo, containing low amounts of storage carbohydrates, was removed and used for analysis of soluble sugars. For soluble sugar measurements, developing and mature grains were separated into ‘outer tissues', endosperm, and embryo. The total sum of values is shown. Additional carbohydrate content and statistics for mature grain are presented in Supplementary Table S1 for ‘outer tissues' plus endosperm as well as for embryo tissues and in Supplementary Figs S2, S9, and S10 for developing grain tissues.
Sucrose and fructans accumulate in the developing pAsGlo1:HvCslF6 grain
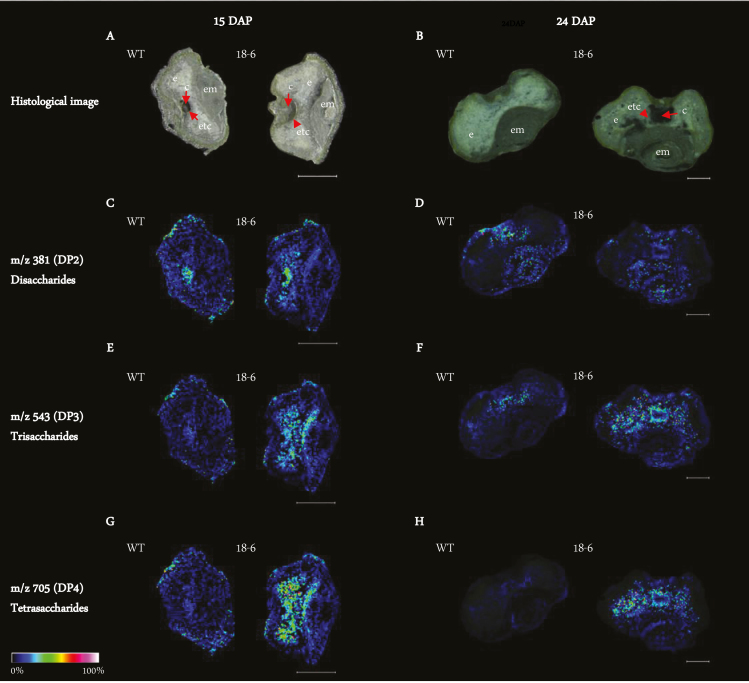
Drastic morphological changes in transgenic ETCs coincided with the early phase of storage product formation (9–14 DAP) (Fig. 4A–F; Supplementary Fig. S3A–D), and were followed by pronounced differences in endosperm storage product accumulation from 15 DAP onwards (Fig. 5A–F; Supplementary Figs S2A–D, S9A–D). MALDI-MSI was used to visualize the spatio-temporal distribution of oligosaccharides in WT and 18-6 transgenic grain at both 15 and 24 DAP. Sections were prepared from basal grain parts to also observe the embryo region (Fig. 6A–H). At 15 DAP, accumulation of disaccharides [degree of polymerization 2 (DP2)] in transgenic and WT cavity regions was similar (Fig. 6C), while signals for trisaccharides (DP3) and tetrasaccharides (DP4) were higher in transgenic endosperm and between the endosperm and embryo (Fig. 6E, G). At 24 DAP, DP2 levels were comparable in the cavity and embryo of both transgenic and WT grain (Fig. 6D), whilst DP3 and DP4 signals remained higher in the transgenic grain, mainly adjacent to the cavity and endosperm (Fig. 6F, H).
Fig. 6.
Visualization of oligosaccharides in WT and 18-6 transgenic grain at 15 and 24 DAP using a MALDI-MSI approach. (A) and (B) are histological images of WT and transgenic grain at 15 and 24 DAP, respectively. (C) At 15 DAP, disaccharides (DP2) are mostly accumulated in and around the endosperm cavity of WT and transgenic grain. (D) At 24 DAP, DP2 signal intensity is highest in the endosperm cavity and embryo of WT and transgenic grain. (E) and (G) show stronger signal intensities of trisaccharides (DP3) and tetrasaccharides (DP4) in the endosperm and tissues adjacent to the embryo of transgenic grain relative to the WT at 15 DAP. (F) and (H) show stronger signal intensities of DP3 and DP4 in the endosperm and endosperm cavity of transgenic grain relative to the WT at 24 DAP. Scale bars in (A), (C), (E), and (G) are equivalent to 500 µm. Scale bars in (B), (D), (F), and (H) are equivalent to 200 µm. Sections were prepared in the basal part of the grain. Colours used in the single ion intensity maps correspond to signal intensity scaling displayed at the bottom right. Abbreviations: cavity (c), embryo (em), endosperm (e), endosperm transfer cell (etc).
Quantitative measurement of soluble sugars in cavity fluids revealed that glucose, fructose, sucrose, and total fructan levels, as a proportion of the total sugars from one whole grain, were similar between WT and transgenic grain at 11 DAP (Table 2). At 15 DAP, glucose, sucrose, and total fructan levels increased significantly in the transgenic cavity compared with the WT (Table 2), with transgenic sucrose and total fructan levels still higher at 24 DAP (Table 2). At 15 DAP, twice as much volume of cavity fluid was obtained from transgenic lines compared with WT grain (Supplementary Fig. S11).
Table 2.
Percentage of soluble sugar content (glucose, fructose, sucrose, and total fructan) in the endosperm cavity per total corresponding sugar from a single grain at 11, 15, and 24 DAP on a w/w basis
| Glucose (%w/w) ±SD | Fructose(%w/w) ±SD | Sucrose(%w/w) ±SD | Total fructan(%w/w) ±SD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DAP | DAP | DAP | DAP | |||||||||
| 11 | 15 | 19 | 11 | 15 | 19 | 11 | 15 | 19 | 11 | 15 | 19 | |
| WT | 0.33±0.01 | 0.40±0.32 | 0.48±0.27 | 0.26±0.02 | 0.17±0.13 | 0.24±0.18 | 0.26±0.02 | 0.19±0.09 | 0.02±0.03 | 0.32±0.02 | 0.22±0.14 | 0.06±0.06 |
| WT (tc) |
0.23±0.18 | 0.37±0.41 | 0.66±0.79 | 0.17±0.14 | 0.28±0.32 | 0.35±0.44 | 0.16±0.13 | 0.03±0.03* | 0.02±0.01 | 0.20±0.16 | 0.07±0.02* | 0.10±0.02 |
| 15-3 | 0.50±0.34 | 0.75±0.00* | 0.94±0.54 | 0.24±0.18 | 0.17±0.00 | 0.33±0.24 | 0.42±0.29 | 0.47±0.05* | 0.31±0.20* | 0.51±0.39 | 0.52±0.09* | 0.60±0.34* |
| 16-5 | 0.31±0.25 | 0.84±0.09* | 0.85±0.62 | 0.22±0.18 | 0.28±0.03 | 0.28±0.19 | 0.30±0.16 | 0.82±0.07* | 0.15±0.03* | 0.39±0.27 | 0.81±0.11* | 0.39±0.13* |
| 18-6 | 0.27±0.20 | 1.04±0.72* | 0.19±0.02* | 0.13±0.06* | 0.72±0.66 | 0.18±0.08 | 0.25±0.17 | 0.52±0.14* | 0.08±0.00* | 0.28±0.16 | 0.75±0.29* | 0.14±0.03* |
| 25-5 | 0.10±0.07* | 0.82±0.08* | 0.83±0.23 | 0.07±0.06* | 0.27±0.07 | 0.25±0.07 | 0.13±0.13* | 0.57±0.06* | 0.24±0.05* | 0.13±0.12* | 0.63±0.06* | 0.58±0.17* |
An asterisk indicates a significant difference from the WT by t-test with P-values of <0.05.
Carbohydrate metabolic pathways are altered in pAsGlo1:HvCslF6 grain
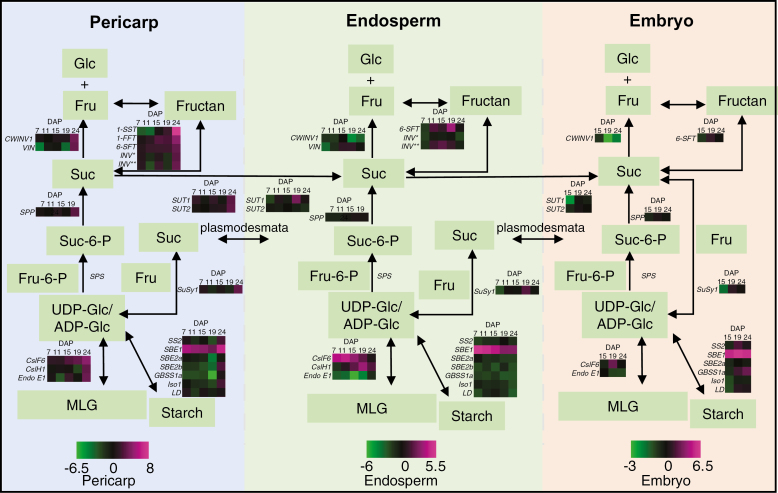
Transcript levels of genes encoding key enzymes associated with carbohydrate synthesis and encoding proteins with carbohydrate transport roles were measured in ‘outer tissues', endosperm, and embryo across development. The genes were cell wall-related [HvCslF6, HvCslH1, endo-β-(1,3;1,4)-glucanase E1 (Endo E1), cellulose synthase 2 (CesA2), and cellulose synthase-like F9 (CslF9)], starch-related [GBSS1a, starch synthase 2 (SS 2), SBE2a, SBE2b, Iso1, LD, and sucrose synthase1 (SuSy1)], sucrose metabolism-related [cell wall invertase 1 (CWINV1), vacuolar invertase (VIN), sucrose phosphate synthase (SPS), sucrose phosphate phosphatase (SPP), sucrose transporter 1 (SUT1), and sucrose transporter 2 (SUT2)], and fructan biosynthetic genes (1-SST, 1-FFT, and 6-SFT). HORVU6Hr1G011260 (INV*) and HORVU2Hr1G109120 (INV**) were selected based on their putative role in hydrolysing glycosidic bonds between two carbohydrate molecules, including fructans (Fig. 7; Supplementary Table S2).
Fig. 7.
Schematic diagram of the carbohydrate metabolic pathway in ‘outer tissues', endosperm, and embryo of a barley grain and the differential transcript expression of involved genes in 18-6 transgenic grain from 7 to 24 DAP. Expression of HvCslF6 transcripts includes the transgene and wild-type (WT) gene expression. For each tissue, the transcript expression values are normalized against the WT, calculated as log2, and represented in a heat map. Genes where the maximum transcript value was <2000 arbitrary units were not included in the heat map. Vertical dashed lines separate the three grain tissues. UDP-glucose (UDP-Glc) and ADP-glucose (ADP-Glc) are substrates for the biosynthesis of (1,3;1,4)-β-glucan and starch, respectively. INV* indicates the HORVU6Hr1G011260 gene. INV** indicates the HORVU2Hr1G109120 gene, which are both involved in the hydrolysis of glycosidic bonds between the carbohydrates, including fructans. Abbreviations: fructose (Fru), fructose-6-phosphate (Fru-6-P), glucose (Glc), sucrose (Suc), sucrose-6-phosphate (Suc-6-P). Expanded gene names and accession information is included in Supplementary Table S2. Magenta indicates increased expression, while green indicates decreased expression.
Consistent with increased expression from the pAsGlo1:HvCslF6 construct, HvCslF6 transcript was up-regulated in transgenic endosperm at 7 and 11 DAP, declining until 24 DAP, whilst HvCslH1 was up-regulated in transgenic endosperm from 15 to 24 DAP (Fig. 7). In embryo tissues, HvCslF6 was up-regulated at 19 DAP. Endo E1, encoding a protein that hydrolyses MLG, was down-regulated in transgenic endosperm and embryo across development (Fig. 7). HvCslF9, encoding another MLG synthase with the highest expression at ~8 DAP in developing grain (Burton et al., 2008), was down-regulated in transgenic endosperm from 7 to 15 DAP relative to the WT (Supplementary Fig. S1B) while HvCesA2, encoding a cellulose synthase (Burton et al., 2004; Desprez et al., 2007), showed similar transcript levels in both WT and transgenic endosperm from 7 to 15 DAP and was down-regulated from 19 to 24 DAP (Supplementary Fig. S1C).
Starch metabolic gene transcripts, SS2, SBE2a, SBE2b, GBSS1a, Iso1, and LD, were down-regulated in transgenic endosperm across development, with the lowest levels at 24 DAP. In contrast, SBE1 was highly up-regulated compared with the WT in all transgenic tissues during development (Fig. 7). Sucrose is hydrolysed by cell wall invertase (CWINV) and vacuolar invertase (VIN). CWINV1 was down-regulated in transgenic endosperm and embryo during late grain filling from 19 to 24 DAP. In the fructan metabolic pathway, 6-SFT was up-regulated in the transgenic endosperm from 11 to 19 DAP and in the embryo slightly at 19 DAP, while 6-SFT, 1-SST, and 1-FFT were all increased during late grain filling from 19 to 24 DAP relative to the WT (Fig. 7). The HORVU2Hr1G109120 gene was up-regulated concurrently with the 6-SFT gene in transgenic endosperm, while HORVU6Hr1G011260 transcript levels changed oppositely to 6-SFT (Fig. 7).
The sucrose transporter gene, SUT1, was up-regulated in transgenic endosperm at 19 DAP and down-regulated in transgenic embryo at 15 DAP, while SUT1 and SUT2 were both slightly up-regulated in transgenic ‘outer tissues' at 24 DAP relative to the WT. SuSy1, SPS, and SPP, all involved in sucrose metabolism, showed only small transcript changes compared with the WT (Fig. 7). During the storage phase overall (15–24 DAP), fructan biosynthetic and sucrose transporter genes were up-regulated, whilst invertase and starch metabolic genes were down-regulated in the transgenic endosperm, coinciding with increased sucrose and fructans and decreased starch contents, as shown in the sugar quantitative data (Supplementary Figs S2C, D, S9A, C, D).
Discussion
Specificity of pAsGlo1 promoter activity in barley grain and HvCslF6 regulation
Increased endosperm MLG content in pAsGlo1:HvCslF6 lines is consistent with the early activity of the pAsGlo1 promoter shortly after the completion of endosperm cellularization (Wilson et al., 2006), specifically in the peripheral endosperm and matching the up-regulation of HvCslF6 transcripts at 7 DAP (Fig 1A–D; Supplementary Fig. S1A). The pAsGlo1-driven YFP was not detected in transgenic ‘outer tissues' or embryo, so higher HvCslF6 transcript levels here may be due to contamination from the peripheral endosperm layer. Similar contamination arising from hand dissection of grain tissues has been reported for endosperm transcriptome data sets (Schon and Nodine, 2017). Based on these observations, we assume that HvCslF6 transgene overexpression occurs exclusively in the endosperm, and not in the ‘outer tissues' or embryo.
Previous studies have indicated that regulation of MLG synthesis, and HvCSlF6 transcript levels can change in response to varying environmental conditions (Dickin et al., 2011; and reviewed in Houston et al., 2016). Consistent with these previous observations, here we observed that wild type lines that had been through tissue culture [WT(tc)] were similar to the WT in relation to their grain starch, sucrose, and total fructan, but they differed in the MLG content, indicating that there may be feedback from the environmental cues that can influence the regulation of HvCslF6 in grain. Differences in HvCslF6 expression (Supplementary Fig. S1A) are also likely to be the reason for the variation between transgenic lines in their grain MLG, starch, sucrose, and total fructan levels (Fig. 5; Supplementary Table S1).
Altered aleurone development in pAsGlo1:HvCslF6 transgenic barley
The aleurone cells in 18-6 transgenic grain appeared permanently elongated inwardly by 24 DAP (Fig. 3C, D; Supplementary Fig. S7A–H). Differentiation of aleurone cells is initiated at ~8 DAP (Bosnes et al., 1992) driven by signals released from the peripheral starchy endosperm (Becraft and Asuncion-Crabb, 2000; Gruis et al., 2006). A number of genes are involved in regulating aleurone cell fate and tissue differentiation in cereal grains, including defective kernel1 (Dek1), crinkly4 (Cr4), and supernumerary aleurone1 (Sal1) (Lid et al., 2002; Olsen et al., 2008). Aleurone development is also associated with hormonal regulation (Bethke et al., 1999; Gómez-Cadenas et al., 2001; Geisler-Lee and Gallie, 2005) whilst aleurone cell number has been linked to endosperm and embryo development as well as to differential hydrolytic enzyme activity (Aubert et al., 2018). For example, disorgal maize mutants have more aleurone cells, a shrunken endosperm with reduced starch granule density, and a smaller embryo (Lid et al., 2004); defective seed5 (des5) barley mutants have fewer aleurone cells, a shrunken endosperm, and altered starch granule types, and embryos prematurely abort (Olsen et al., 2008).
Peripheral endosperm cells containing more HvCslF6 transcript and HvCslF6 protein appear to be structurally different from the WT from 9 DAP onwards (Figs 2A–D, 3A–D). The thicker cell walls in transgenic aleurone (Fig. 4E–H) relative to the WT probably result from an extended period of MLG deposition in the peripheral endosperm cells, as also shown for Brachypodium distachyon (Trafford et al., 2013). Due to the specific grain size, cell expansion is only possible towards the middle of the grain, resulting in inwardly elongated aleurone cells and ‘squeezed' adjacent starchy endosperm.
Morphology and cell wall composition changes in pAsGlo1:HvCslF6 ETCs
A larger cavity and unevenly formed ETC layer were observed in transgenic grain relative to the WT from 9 DAP onwards, and the ETCs began to rupture at 10 DAP (Fig. 4C, D; Supplementary Fig. S3A, B). At this stage, the ETC walls in transgenic grain showed less intense mannan labelling than the WT (Fig. 4I, J). In the ETC walls, no MLG, arabinoxylan, callose, or crystalline cellulose was detected (Supplementary Fig. S5A–D), while the labelling of pectin probably relates to the remnants from the nucellus (Supplementary Fig. S6–H) (Greenwood et al., 2005).
These results do not match the transcriptome analysis by Thiel et al. (2012), who demonstrated up-regulation of genes related to callose and cellulose metabolism at the same time point (10 DAP). The failure of antibodies to detect cell wall polysaccharides other than mannan could be due to epitope masking, making them unavailable for binding, as has been reported by Xue et al. (2013). Closer examination of the cell wall polysaccharides in ETCs may be achieved using immunogold labelling and TEM, with appropriate unmasking techniques. Alternatively, laser microdissection of the ETCs and surrounding endosperm would allow examination of gene expression and link it directly to cell wall composition.
Altered ETC morphology may trigger abnormal accumulation of sucrose and fructans in the grain cavity and endosperm
Correct formation of ETCs is important to ensure nutrient transfer from the cavity to the endosperm for development and storage of carbohydrates. A study by Felker et al. (1985) showed that some barley mutants with abnormal endosperm had missing ETCs, such as seg2, seg4, seg5, and seg8, and starch filling in the endosperm was also perturbed. Here, in HvCslF6 transgenic grain during the early storage phase, a significant increase in glucose, sucrose, and fructan content in the cavity and endosperm compared with the WT was observed, whilst starch levels were reduced (Figs 5C–F, 6C–H; Table 2; Supplementary Figs S2C, S9A–D, S11A–D). We hypothesize that the high sucrose and fructan pools in the cavity at 15 DAP may be due to earlier perturbations in ETC differentiation, which may have subsequently perturbed the active flow of sucrose towards the endosperm. A link between changed carbohydrate partitioning and altered development of the transfer region has been suggested previously (Vijn and Smeekens, 1999; Weichert et al., 2010; Ruan, 2014). However, a detailed histological examination of ETC development in pAsGlo1:HvCslF6 grain is required to confirm changes in ETC identity and function. In contrast, the lys5 mutant Risø13, harbouring a mutation in the plastidial ADP-glucose transporter resulting in reduced starch biosynthesis, did not show such morphological changes or spatial alterations of the sucrose allocation, but decreased velocity of the sucrose stream through the cavity, as compared with the WT (Melkus et al., 2011). Thus, the wrinkled phenotype of mature Risø13 grains is possibly associated with decreased starch deposition in the endosperm and the strongly altered starch granule morphology (Patron et al., 2004).
Fructans in the endosperm cavity are involved in detoxification of hydroxyl radicals (Peukert et al., 2014; Matros et al., 2015) and maintaining the sucrose gradient between the vascular bundle and sink tissues (Pollock and Cairns, 1991; Peukert et al., 2016b). In 18-6 transgenic grain, ETCs were irregularly formed and the sucrose uptake pathway was likely to have been compromised. At the same time, the increased demand for glucose during MLG synthesis in the endosperm would be likely to have stimulated sucrose import. Fructan synthesis can be induced by sucrose (Vijn and Smeekens, 1999; Ruan, 2014), towards keeping the levels of free fructose temporarily low. In the transgenic endosperm, the fructosyltransferase gene, 6-SFT, is up-regulated, encoding an enzyme catalysing the formation of branched and levan-type fructans containing β(2,6) linkages (Vijn and Smeekens, 1999). Down-regulation of CWINV1 and VIN in the transgenic endosperm may also contribute to the increased amounts of sucrose and fructan, and small amounts of free glucose.
Starch metabolism is perturbed in pAsGlo1:HvCslF6 endosperm
pAsGlo1:HvCslF6 grain showed up-regulation of the glucan synthase HvCslH1 and down-regulation of the endo-glucanase E1 in the developing endosperm (Fig. 7), probably resulting from increased abundance of metabolic precursors and constitutively induced MLG synthesis, respectively. High MLG content in barley is likely to be balanced by lower starch levels as previously reported (Munck et al., 2004; Seefeldt et al., 2009; Burton et al., 2011) and, as seen here, linked to the down-regulation of some starch biosynthetic genes (Fig. 7). This coincides with results from B. distachyon where MLG accumulated as the main storage carbohydrate in cell walls during grain development at the expense of starch in mature grain (Guillon et al., 2011). The low capacity for starch synthesis in Brachypodium is reflected by reduced transcription of SS1 and SBE1 as well as reduced activity of starch synthase and ADP-glucose pyrophosphorylase (Trafford et al., 2013). In our study, SBE1 was up-regulated in transgenic endosperm. Previously, SBE1 was found to be involved in producing long-chain amylopectin in rice (Satoh et al., 2003) and in maize (reviewed in Tetlow and Emes, 2014). However, alterations in amylopectin chain length have not been investigated in our study and it is unclear whether up-regulation of SBE1 contributes to overall starch granule size differences seen in barley (Fig. 3A, B; Table 1). Recently, RNAi suppression of all three HvSBE isoforms was shown to result in a severe amylose-only phenotype with irregular granules, while hyperphosphorylation caused WT-like structures (Shaik et al., 2016). Further investigations need to include the amylose/amylopectin ratio and starch phosphorylation status in pAsGlo1:HvCslF6 grain.
Conclusions
Increased MLG levels are desirable for human health benefits, but high levels in barley grain from early stages perturb carbohydrate metabolism, leading to impairment in grain development (Fig. 8). Major changes affect the cell wall composition and morphology of the peripheral endosperm regions (aleurone and adjacent endosperm cells), and, notably, of the ETC layer. The increased MLG synthesis in transgenic grain is compromised by reduced starch and increased fructan synthesis. The high demand for maternally derived sucrose together with the disturbed active import to the endosperm via the ETC border probably result in excessive accumulation of sucrose and fructans in the cavity. The shrunken phenotype of the HvCslF6 transgenic grain is likely to be due to the increased amount of cavity fluids and higher osmotic potential, forcing water uptake and causing wrinkling due to greater water loss during desiccation. Our investigations of the metabolic, transcriptional, and morphological changes associated with increasing MLG early in grain development (this report) or systemically (Burton et al., 2011) have shown the importance of regulation of carbohydrate metabolism homeostasis in controlling grain phenotypes, suggesting that these transgenic approaches may not be an ideal avenue in pursuit of this outcome. Engineering of barley lines with MLG synthesis inducible at the grain-filling stage as well as selection for high MLG lines from available barley mutant collections may overcome these limitations in the near future.
Fig. 8.
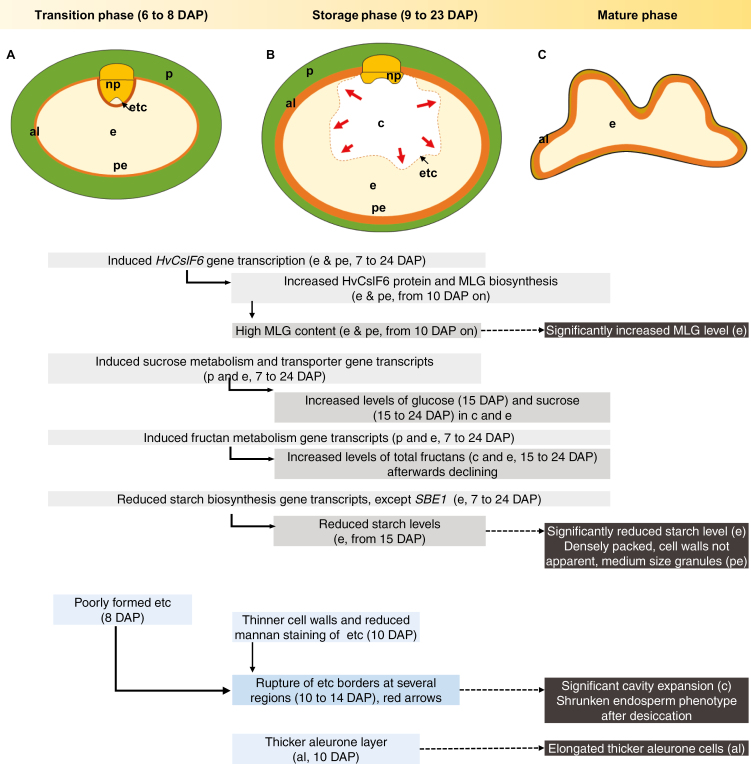
Schematic diagram of grain development of the pAsGlo1:HvCslF6 transgenic line (A–C) relative to the wild type. (A) In transgenic endosperm, the early up-regulation of HvCslF6 gene expression relative to the WT from 7 DAP results in an increased amount of (1,3;1,4)-β-glucan (MLG) during the grain storage phase, which is followed by a decreased amount of starch and increased levels of glucose, sucrose, and total fructans. Early changes in carbohydrate biosynthesis allocation appear to influence endosperm transfer cell (ETC) differentiation and subsequently perturb sucrose uptake in the endosperm. (B) Consequently, high amounts of sucrose and fructans are accumulated in the grain cavity, causing the cavity to aberrantly expand and subsequent rupture (as indicated by the dotted line). Thinner cell walls in the non-uniformly developed ETCs of transgenic grain and variations in mannan labelling may contribute to ETC rupture in transgenic grain. (C) The transgenic grain has an elongated aleurone layer compared with the WT grain which persisted to maturity. As the grain matures, fluid in the transgenic cavity dries out, causing the grain to become shrunken. High levels of MLG and low levels of starch persist in the mature endosperm. Abbreviations: aleurone (al), cavity (c), endosperm (e), endosperm transfer cells (etc), nucellar projection (np), pericarp (p), peripheral endosperm (pe). Red arrows indicate probable osmotic pressure exerted from the cavity towards the endosperm. Grey boxes indicate findings at the genetic and metabolic level, while blue boxes indicate findings at the morphological level. Dark grey boxes highlight the mature phenotype. Arrangement of boxes roughly reflects observation time during the course of grain development.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Normalized transcript levels of HvCslF6 transgene, HvCslF9, HvCesA2, HvLtp2, and HvGBSS1a in developing grain tissues.
Fig. S2. MLG and starch contents in developing grain.
Fig. S3. Morphology of the endosperm cavity in WT and 18-6 transgenic grain at 9 and 14 DAP.
Fig. S4. Immunohistochemical analysis of callose and crystalline cellulose in ETC walls at 10 DAP.
Fig. S5. Immunohistochemical analysis of arabinoxylan, callose, crystalline cellulose, and mannan in endosperm cell walls at 10 DAP.
Fig. S6. Immunohistochemical analysis of pectins with LM19 antibody on barley grain sections at 10 DAP.
Fig. S7. Morphology of aleurone cells in developing WT and 18-6 transgenic grain.
Fig. S8. Morphology of aleurone cells in WT and 18-6 transgenic grain at grain maturation and during grain development.
Fig. S9. Sucrose and total fructan contents in developing rain.
Fig. S10. Percentage of soluble glucose and fructose contents in developing grain on a w/w and per grain basis (mg).
Fig. S11. Total volume of fluids in the endosperm cavity.
Table S1. Carbohydrate content in mature grain (embryo removed) and embryo.
Table S2. List of primers used for real-time PCR.
Acknowledgments
The authors wish to acknowledge Dr Wei Zeng (University of Melbourne), Dr Gwenda Mayo, Dr Lisa O'Donovan, and Ken Neubauer (Adelaide Microscopy, University of Adelaide), Dr Rohan Singh, Katherine Allder, and Kylie Neumann (University of Adelaide) for their technical assistance, and Dr Hans-Peter Mock (Leibniz Institute of Plant Genetics and Crop Plant Research, for stimulating discussions. This work was supported by an Adelaide Graduate Research Scholarship (AGRS), the Australian Research Council (ARC) Centre of Excellence in Plant Cell Walls (CE110001007), ARC Fellowship funding (DE150100837 and FT140100780), a German Academic Exchange Service (DAAD) G08 Australia Germany Exchange Program, and a grant by the German Research Foundation (DFG, MA 4814/3-1) to AM. All authors state that they have no conflict of interest concerning this manuscript.
Glossary
Abbreviations:
- DAP
days after pollination
- DP
degree of polymerization
- ETC
endosperm transfer cell
- 1-FEH
fructan 1-exohydrolase
- 6-FEH
fructan 6-exohydrolase
- 1-FFT
fructan:fructan-1-fructosyltransferase
- 6G-FFT
fructan:fructan-6G-fructosyltransferase
- GBSS
granule-bound starch synthase
- HPAEC–PAD
high pH anion exchange chromatography with pulsed amperometric detection
- HvCslF6
cellulose synthase-like gene F6
- HvCslH1
cellulose synthase-like gene H1
- HvCslJ
cellulose synthase-like gene J
- ISO
isoamylase
- LD
limit dextrinase
- MALDI-MSI
matrix-assisted laser desorption/ionization mass spectrometry imaging
- MLG
mixed-linkage (1,3;1,4)-β-glucan
- NP
nucellar projection
- pAsGlo1
oat globulin AsGlo1 promoter sequence
- SBE
starch branching enzyme
- 6-SFT
sucrose:fructan-6-fructosyltransferase
- SS
starch synthase
- 1-SST
sucrose:sucrose-1-fructosyltransferase
- WT
wild type
- WT(tc)
wild type regenerated from tissue culture
References
- Åman P, Hesselman K, Tilly AC. 1985. The variation in chemical composition of Swedish barleys. Journal of Cereal Science 3, 73–77. [Google Scholar]
- Aubert MK, Coventry S, Shirley NJ, Betts NS, Würschum T, Burton RA, Tucker MR. 2018. Differences in hydrolytic enzyme activity accompany natural variation in mature aleurone morphology in barley (Hordeum vulgare L.). Scientific Reports 8, 11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becraft PW, Asuncion-Crabb Y. 2000. Positional cues specify and maintain aleurone cell fate in maize endosperm development. Development 127, 4039–4048. [DOI] [PubMed] [Google Scholar]
- Bethke PC, Lonsdale JE, Fath A, Jones RL. 1999. Hormonally regulated programmed cell death in barley aleurone cells. The Plant Cell 11, 1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake AW, McCartney L, Flint JE, Bolam DN, Boraston AB, Gilbert HJ, Knox JP. 2006. Understanding the biological rationale for the diversity of cellulose-directed carbohydrate-binding modules in prokaryotic enzymes. Journal of Biological Chemistry 281, 29321–29329. [DOI] [PubMed] [Google Scholar]
- Bosnes M, Weideman F, Olsen OA. 1992. Endosperm differentiation in barley wild-type and sex mutants. The Plant Journal 2, 661–674. [Google Scholar]
- Burton RA, Collins HM, Kibble NA, et al. 2011. Over-expression of specific HvCslF cellulose synthase-like genes in transgenic barley increases the levels of cell wall (1,3;1,4)-β-d-glucans and alters their fine structure. Plant Biotechnology Journal 9, 117–135. [DOI] [PubMed] [Google Scholar]
- Burton RA, Fincher GB. 2012. Current challenges in cell wall biology in the cereals and grasses. Frontiers in Plant Science 3, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton RA, Fincher GB. 2014. Evolution and development of cell walls in cereal grains. Frontiers in Plant Science 5, 456, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton RA, Jobling SA, Harvey AJ, Shirley NJ, Mather DE, Bacic A, Fincher GB. 2008. The genetics and transcriptional profiles of the cellulose synthase-like HvCslF gene family in barley. Plant Physiology 146, 1821–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton RA, Shirley NJ, King BJ, Harvey AJ, Fincher GB. 2004. The CesA gene family of barley. Quantitative analysis of transcripts reveals two groups of co-expressed genes. Plant Physiology 134, 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carciofi M, Blennow A, Jensen SL, Shaik SS, Henriksen A, Buléon A, Holm PB, Hebelstrup KH. 2012. Concerted suppression of all starch branching enzyme genes in barley produces amylose-only starch granules. BMC Plant Biology 12, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmelik J, Krumlová A, Budinská M, Kruml T, Psota V, Bohacenko I, Mazal P, Vydrová H. 2001. Comparison of size characterization of barley starch granules determined by electron and optical microscopy, low angle laser light scattering and gravitational field‐flow fractionation. Journal of the Institute of Brewing 107, 11–17. [Google Scholar]
- Clarke B, Liang R, Morell MK, Bird AR, Jenkins CL, Li Z. 2008. Gene expression in a starch synthase IIa mutant of barley: changes in the level of gene transcription and grain composition. Functional & Integrative Genomics 8, 211–221. [DOI] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U. 2003. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiology 133, 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desprez T, Juraniec M, Crowell EF, Jouy H, Pochylova Z, Parcy F, Höfte H, Gonneau M, Vernhettes S. 2007. Organization of cellulose synthase complexes involved in primary cell wall synthesis in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA 104, 15572–15577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickin E, Steele K, Frost G, Edwards-Jones G, Wright D. 2011. Effect of genotype, environment and agronomic management on β-glucan concentration of naked barley grain intended for health food use. Journal of Cereal Science 54, 44–52. [Google Scholar]
- Doblin MS, Pettolino FA, Wilson SM, Campbell R, Burton RA, Fincher GB, Newbigin E, Bacic A. 2009. A barley cellulose synthase-like CSLH gene mediates (1,3;1,4)-beta-d-glucan synthesis in transgenic Arabidopsis. Proceedings of the National Academy of Sciences, USA 106, 5996–6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez F, Cejudo FJ. 2014. Programmed cell death (PCD): an essential process of cereal seed development and germination. Frontiers in Plant Science 5, 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felker FC, Peterson DM, Nelson OE. 1985. Anatomy of immature grains of eight maternal effect shrunken endosperm barley mutants. American Journal of Botany 72, 248–256. [Google Scholar]
- Fincher GB. 1975. Morphology and chemical composition of barley endosperm cell walls. Journal of the Institute of Brewing 81, 116–122. [Google Scholar]
- Geisler-Lee J, Gallie DR. 2005. Aleurone cell identity is suppressed following connation in maize kernels. Plant Physiology 139, 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Cadenas A, Zentella R, Walker-Simmons MK, Ho T-HD. 2001. Gibberellin/abscisic acid antagonism in barley aleurone cells: site of action of the protein kinase PKABA1 in relation to gibberellin signaling molecules. The Plant Cell 13, 667–679. [PMC free article] [PubMed] [Google Scholar]
- Greenwood JS, Helm M, Gietl C. 2005. Ricinosomes and endosperm transfer cell structure in programmed cell death of the nucellus during Ricinus seed development. Proceedings of the National Academy of Sciences, USA 102, 2238–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruis DF, Guo H, Selinger D, Tian Q, Olsen OA. 2006. Surface position, not signaling from surrounding maternal tissues, specifies aleurone epidermal cell fate in maize. Plant Physiology 141, 898–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillon F, Bouchet B, Jamme F, Robert P, Quéméner B, Barron C, Larré C, Dumas P, Saulnier L. 2011. Brachypodium distachyon grain: characterization of endosperm cell walls. Journal of Experimental Botany 62, 1001–1015. [DOI] [PubMed] [Google Scholar]
- Hassan AS, Houston K, Lahnstein J, Shirley N, Schwerdt JG, Gidley MJ, Waugh R, Little A, Burton RA. 2017. A genome wide association study of arabinoxylan content in 2-row spring barley grain. PLoS One 12, e0182537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry R, Saini H. 1989. Characterization of cereal sugars and oligosaccharides. Cereal Chemistry 66, 362–365. [Google Scholar]
- Henry RJ. 1988. The carbohydrates of barley grains—a review. Journal of the Institute of Brewing 94, 71–78. [Google Scholar]
- Hrmova M, Fincher GB. 2001. Structure–function relationships of beta-d-glucan endo- and exohydrolases from higher plants. Plant Molecular Biology 47, 73–91. [PubMed] [Google Scholar]
- Houston K, Tucker MR, Chowdhury J, Shirley N, Little A. 2016. The plant cell wall: a complex and dynamic structure as revealed by the responses of genes under stress conditions. Frontiers in Plant Science 7, 984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh BL, Palmer L, Mather DE, Wallwork H, Graham RD, Welch RM, Stangoulis JCR. 2008. Genotypic variation in wheat grain fructan content revealed by a simplified HPLC method. Journal of Cereal Science 48, 369–378. [Google Scholar]
- Jeon JS, Ryoo N, Hahn TR, Walia H, Nakamura Y. 2010. Starch biosynthesis in cereal endosperm. Plant Physiology and Biochemistry 48, 383–392. [DOI] [PubMed] [Google Scholar]
- Jung S, Rickert D, Deak N, Aldin E, Recknor J, Johnson L, Murphy P. 2003. Comparison of Kjeldahl and Dumas methods for determining protein contents of soybean products. Journal of the American Oil Chemists' Society 80, 1169–1173. [Google Scholar]
- Kalla R, Shimamoto K, Potter R, Nielsen PS, Linnestad C, Olsen OA. 1994. The promoter of the barley aleurone-specific gene encoding a putative 7 kDa lipid transfer protein confers aleurone cell-specific expression in transgenic rice. The Plant Journal 6, 849–860. [DOI] [PubMed] [Google Scholar]
- Keeling PL, Myers AM. 2010. Biochemistry and genetics of starch synthesis. Annual Review of Food Science and Technology 1, 271–303. [DOI] [PubMed] [Google Scholar]
- Kohorn BD, Kobayashi M, Johansen S, Riese J, Huang LF, Koch K, Fu S, Dotson A, Byers N. 2006. An Arabidopsis cell wall-associated kinase required for invertase activity and cell growth. The Plant Journal 46, 307–316. [DOI] [PubMed] [Google Scholar]
- Lid SE, Al RH, Krekling T, Meeley RB, Ranch J, Opsahl-Ferstad HG, Olsen OA. 2004. The maize disorganized aleurone layer 1 and 2 (dil1, dil2) mutants lack control of the mitotic division plane in the aleurone layer of developing endosperm. Planta 218, 370–378. [DOI] [PubMed] [Google Scholar]
- Lid SE, Gruis D, Jung R, Lorentzen JA, Ananiev E, Chamberlin M, Niu X, Meeley R, Nichols S, Olsen OA. 2002. The defective kernel 1 (dek1) gene required for aleurone cell development in the endosperm of maize grains encodes a membrane protein of the calpain gene superfamily. Proceedings of the National Academy of Sciences, USA 99, 5460–5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim WL, Collins HM, Singh RR, Kibble NAJ, Yap K, Taylor J, Fincher GB, Burton RA. 2018. Method for hull-less barley transformation and manipulation of grain mixed-linkage beta-glucan. Journal of Integrative Plant Biology 60, 382–396. [DOI] [PubMed] [Google Scholar]
- Little A, Schwerdt JG, Shirley NJ, et al. 2018. Revised phylogeny of the cellulose synthase gene superfamily: insights into cell wall evolution. Plant Physiology 177, 1124–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matros A, Peshev D, Peukert M, Mock HP, Van den Ende W. 2015. Sugars as hydroxyl radical scavengers: proof-of-concept by studying the fate of sucralose in Arabidopsis. The Plant Journal 82, 822–839. [DOI] [PubMed] [Google Scholar]
- McCartney L, Marcus SE, Knox JP. 2005. Monoclonal antibodies to plant cell wall xylans and arabinoxylans. Journal of Histochemistry and Cytochemistry 53, 543–546. [DOI] [PubMed] [Google Scholar]
- McCleary B, Solah V, Gibson T. 1994. Quantitative measurement of total starch in cereal flours and products. Journal of Cereal Science 20, 51–58. [Google Scholar]
- McCleary BV, Codd R. 1991. Measurement of (1→3),(1→4)-β-d-glucan in barley and oats: a streamlined enzymic procedure. Journal of the Science of Food and Agriculture 55, 303–312. [Google Scholar]
- Melkus G, Rolletschek H, Fuchs J, et al. 2011. Dynamic 13C/1H NMR imaging uncovers sugar allocation in the living seed. Plant Biotechnology Journal 9, 1022–1037. [DOI] [PubMed] [Google Scholar]
- Munck L, Møller B, Jacobsen S, Søndergaard I. 2004. Near infrared spectra indicate specific mutant endosperm genes and reveal a new mechanism for substituting starch with (1→3,1→4)-β-glucan in barley. Journal of Cereal Science 40, 213–222. [Google Scholar]
- Nemeth C, Andersson AA, Andersson R, Mangelsen E, Sun C, Åman P. 2014. Relationship of grain fructan content to degree of polymerisation in different barleys. Food and Nutrition Sciences 5, 581–598. [Google Scholar]
- Olsen LT, Divon HH, Al R, Fosnes K, Lid SE, Opsahl-Sorteberg HG. 2008. The defective seed5 (des5) mutant: effects on barley seed development and HvDek1, HvCr4, and HvSal1 gene regulation. Journal of Experimental Botany 59, 3753–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen OA. 2001. Endosperm development: cellularization and cell fate specification. Annual Review of Plant Biology 52, 233–267. [DOI] [PubMed] [Google Scholar]
- Palmer GH. 1972. Morphology of starch granules in cereal grains and malts. Journal of the Institute of Brewing, 78, 326–332. [Google Scholar]
- Patron NJ, Greber B, Fahy BF, Laurie DA, Parker ML, Denyer K. 2004. The lys5 mutations of barley reveal the nature and importance of plastidial ADP-Glc transporters for starch synthesis in cereal endosperm. Plant Physiology 135, 2088–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettolino FA, Hoogenraad NJ, Ferguson C, Bacic A, Johnson E, Stone BA. 2001. A (1→4)-beta-mannan-specific monoclonal antibody and its use in the immunocytochemical location of galactomannans. Planta 214, 235–242. [DOI] [PubMed] [Google Scholar]
- Peukert M, Lim WL, Seiffert U, Matros A. 2016a Mass spectrometry imaging of metabolites in barley grain tissues. Current Protocols in Plant Biology 1, 574–591. [DOI] [PubMed] [Google Scholar]
- Peukert M, Thiel J, Mock HP, Marko D, Weschke W, Matros A. 2016b Spatiotemporal dynamics of oligofructan metabolism and suggested functions in developing cereal grains. Frontiers in Plant Science 6, 1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peukert M, Thiel J, Peshev D, Weschke W, Van den Ende W, Mock HP, Matros A. 2014. Spatio-temporal dynamics of fructan metabolism in developing barley grains. The Plant Cell 26, 3728–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock CJ, Cairns AJ. 1991. Fructan metabolism in grasses and cereals. Annual Review of Plant Physiology and Plant Molecular Biology 42, 77–101. [Google Scholar]
- Radchuk VV, Borisjuk L, Sreenivasulu N, Merx K, Mock HP, Rolletschek H, Wobus U, Weschke W. 2009. Spatiotemporal profiling of starch biosynthesis and degradation in the developing barley grain. Plant Physiology 150, 190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan YL. 2014. Sucrose metabolism: gateway to diverse carbon use and sugar signaling. Annual Review of Plant Biology 65, 33–67. [DOI] [PubMed] [Google Scholar]
- Satoh H, Nishi A, Yamashita K, Takemoto Y, Tanaka Y, Hosaka Y, Sakurai A, Fujita N, Nakamura Y. 2003. Starch-branching enzyme I-deficient mutation specifically affects the structure and properties of starch in rice endosperm. Plant Physiology 133, 1111–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon MA, Nodine MD. 2017. Widespread contamination of arabidopsis embryo and endosperm transcriptome data sets. The Plant Cell 29, 608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seefeldt HF, Blennow A, Jespersen BM, Wollenweber B, Engelsen SB. 2009. Accumulation of mixed linkage (1→3)(1→4)-β-d-glucan during grain filling in barley: a vibrational spectroscopy study. Journal of Cereal Science 49, 24–31. [Google Scholar]
- Shaik SS, Obata T, Hebelstrup KH, Schwahn K, Fernie AR, Mateiu RV, Blennow A. 2016. Starch granule re-structuring by starch branching enzyme and glucan water dikinase modulation affects caryopsis physiology and metabolism. PLoS One 11, e0149613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Zeng W, Wu A, et al. 2015. Asparagus spears as a model to study heteroxylan biosynthesis during secondary wall development. PLoS One 10, e0123878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivasulu N, Borisjuk L, Junker BH, Mock HP, Rolletschek H, Seiffert U, Weschke W, Wobus U. 2010. Barley grain development toward an integrative view. International Review of Cell and Molecular Biology 281, 49–89. [DOI] [PubMed] [Google Scholar]
- Tetlow IJ, Emes MJ. 2014. A review of starch-branching enzymes and their role in amylopectin biosynthesis. IUBMB Life 66, 546–558. [DOI] [PubMed] [Google Scholar]
- Thiel J, Riewe D, Rutten T, Melzer M, Friedel S, Bollenbeck F, Weschke W, Weber H. 2012. Differentiation of endosperm transfer cells of barley: a comprehensive analysis at the micro-scale. The Plant Journal 71, 639–655. [DOI] [PubMed] [Google Scholar]
- Trafford K, Haleux P, Henderson M, Parker M, Shirley NJ, Tucker MR, Fincher GB, Burton RA. 2013. Grain development in Brachypodium and other grasses: possible interactions between cell expansion, starch deposition, and cell-wall synthesis. Journal of Experimental Botany 64, 5033–5047. [DOI] [PubMed] [Google Scholar]
- Ueda M, Zhang Z, Laux T. 2011. Transcriptional activation of Arabidopsis axis patterning genes WOX8/9 links zygote polarity to embryo development. Developmental Cell 20, 264–270. [DOI] [PubMed] [Google Scholar]
- Verhertbruggen Y, Marcus SE, Haeger A, Ordaz-Ortiz JJ, Knox JP. 2009. An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohydrate Research 344, 1858–1862. [DOI] [PubMed] [Google Scholar]
- Verspreet J, Pollet A, Cuyvers S, Vergauwen R, Van den Ende W, Delcour JA, Courtin CM. 2012. A simple and accurate method for determining wheat grain fructan content and average degree of polymerization. Journal of Agricultural and Food Chemistry 60, 2102–2107. [DOI] [PubMed] [Google Scholar]
- Vickers CE, Xue G, Gresshoff PM. 2006. A novel cis-acting element, ESP, contributes to high-level endosperm-specific expression in an oat globulin promoter. Plant Molecular Biology 62, 195–214. [DOI] [PubMed] [Google Scholar]
- Vijn I, Smeekens S. 1999. Fructan: more than a reserve carbohydrate? Plant Physiology 120, 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W, Wiemken A. 1986. Properties and subcellular localization of fructan hydrolase in the leaves of barley (Hordeum vulgare L. cv Gerbel). Journal of Plant Physiology 123, 429–439. [Google Scholar]
- Weichert N, Saalbach I, Weichert H, et al. 2010. Increasing sucrose uptake capacity of wheat grains stimulates storage protein synthesis. Plant Physiology 152, 698–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Burton RA, Collins HM, Doblin MS, Pettolino FA, Shirley N, Fincher GB, Bacic A. 2012. Pattern of deposition of cell wall polysaccharides and transcript abundance of related cell wall synthesis genes during differentiation in barley endosperm. Plant Physiology 159, 655–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Burton RA, Doblin MS, Stone BA, Newbigin EJ, Fincher GB, Bacic A. 2006. Temporal and spatial appearance of wall polysaccharides during cellularization of barley (Hordeum vulgare) endosperm. Planta 224, 655–667. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Ho YY, Lampugnani ER, Van de Meene AM, Bain MP, Bacic A, Doblin MS. 2015. Determining the subcellular location of synthesis and assembly of the cell wall polysaccharide (1,3;1,4)-β-d-glucan in grasses. The Plant Cell 27, 754–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobus U, Sreenivasulu N, Borisjuk L, Rolletschek H, Panitz R, Gubatz S, Weschke W. 2005. Molecular physiology and genomics of developing barley grains. Recent Research Developments in Plant Molecular Biology 2, 1–29. [Google Scholar]
- Xue J, Bosch M, Knox JP. 2013. Heterogeneity and glycan masking of cell wall microstructures in the stems of Miscanthus × giganteus, and its parents M. sinensis and M. sacchariflorus. PLoS One 8, e82114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.