Abstract
Epidemiological evidence indicates that physical activity between menarche and first pregnancy is associated with a lower risk of breast cancer among women with at least 20 years between these reproductive events. The mechanism by which physical activity during this interval confers protection is unknown. This study used a novel animal model to assess potentially protective effects of physical activity on tumor development in delayed parity. Thirty-six female Sprague Dawley rats received an i.p. injection of 50 mg/kg N-methyl-N-nitrosourea (MNU) at 5 weeks of age. Estrogen and progesterone pellets were implanted subcutaneously 1 week (early parity, EP, n = 8) or 4 weeks (delayed parity, DP, n = 11) following MNU injection. An additional group of DP rats were progressively exercise trained (Ex + DP, n = 9) on a treadmill following MNU injection for 7 weeks (up to 20 m/min at 15% incline for 30 min). We observed the greatest tumor latency and smallest tumor burden in Ex + DP animals. Ductal hyperplasia and inflammation of non-tumor bearing mammary glands were only found in DP, and we detected a significant increase in collagen for DP and Ex + DP compared to EP. Exercise induced differential gene expression of cyclin-dependent kinase-inhibitor 1C (Cdkn1c) and urokinase-plasminogen activator (Plau) in mammary tissue of Ex + DP animals compared to DP alone. While there are delayed parity-induced changes in mammary gland collagen and gene expression levels, Ex + DP animals had longer tumor latency, smaller tumor burden, and glandular tissue resistant to ductal hyperplasia. Exercise may induce protection through beneficial regulation of gene expression profiles.
Keywords: Neoplasm, Exercise, Delayed parity, Gene expression
1. Introduction
Breast cancer is the most common malignancy among women in the United States [1]. Reproductive factors such as earlier age at menarche, later age at first pregnancy, and a longer interval between these two reproductive events are consistently associated with higher risk of breast cancer [2–4]. Available global evidence indicates the menarche to first pregnancy interval has lengthened in recent years [5], supporting a need to identify lifestyle interventions to mitigate this accumulating excess risk. There is convincing epidemiological evidence that moderate-to-vigorous intensity physical activity is associated with a 10–25% lower risk of breast cancer, compared to inactivity [6]. Pregnancy- and exercise-induced protections against breast cancer are also well-established in female rats using carcinogen-induced breast cancer models [7–10]. Also consistent with human data, delayed parity in rats increases breast cancer risk compared to rats with pregnancy induced earlier in life [11].
We have recently shown in epidemiological data that the influence of leisure-time physical activity between menarche and first pregnancy on future breast cancer risk varies by the length of the interval between menarche and first pregnancy [12]. Physical activity was significantly associated with a 27% lower breast cancer risk for high risk women with the longest time between menarche and first pregnancy (≥20 years). This was not observed among women with a shorter time between menarche and first pregnancy. Here, a parallel pre-clinical rat model was devised to examine potential mechanisms concerning the role of physical activity in mammary gland tumorigenesis associated with delayed parity. We investigated the role of exercise not only on breast cancer incidence, but latency, mammary gland morphology, and pertinent gene expression levels.
Many cellular adaptations occur in response to exercise and there are marked differences between mammary tissue of animals with early parity and with delayed parity. We hypothesized several pathways would be modified by our intervention. Preliminary data indicated the gene Cdkn1c was differentially regulated by exercise. Cdkn1c encodes for the protein cyclin dependent kinase 1c, which is an inhibitor of several G1 cyclin/CDK complexes and a negative regulator of the cell cycle at the G1 checkpoint [13]. Decreased mRNA levels of CDKN1C are associated with multiple cancer types [14–18], and several observations implicate a role for CDKN1C in breast tumorigenesis. Decreased CDKN1C expression occurs during human mammary epithelial cell immortalization [19]. Additionally, decreased CDKN1C gene expression is seen in the large majority of human breast cancers and is associated with poor prognosis [20,21]. No study to-date has investigated potential mechanisms underlying the role of physical activity in preventing increased mammary gland tumorigenesis associated with delayed parity. Therefore, we seek to identify candidate genes associated with breast cancer which may be beneficially modulated by exercise during the menarche-to-first-pregnancy interval.
2. Methods
2.1. Animals and experimental protocol
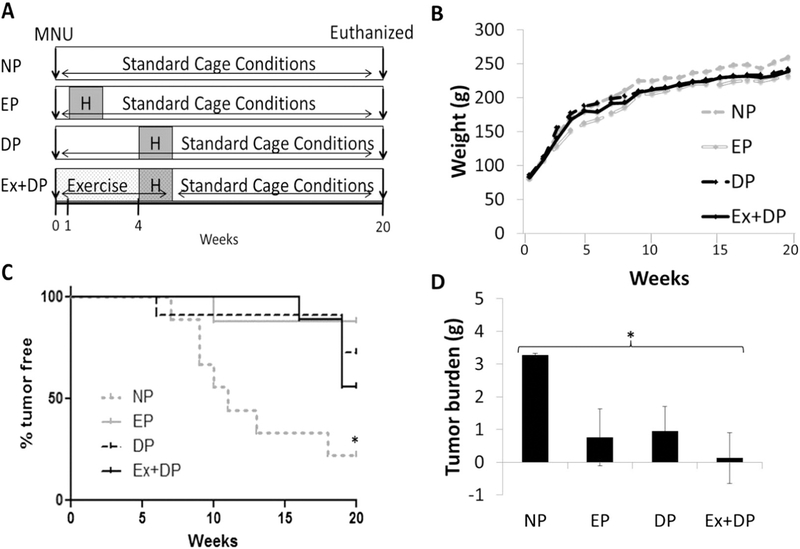
The paradigm of the study is presented in Fig. 1A. Five-week old female Sprague Dawley rats (Harlan Laboratories) were randomly divided into four cohorts as described below. Of note, thirty-five days of age is the approximate age of reproductive maturity for female Sprague Dawley rats [9]. All rats (n = 38) were injected (week 0) intraperitoneally with 50 mg/kg N-methyl-N-nitrosourea (MNU) in sterile 0.85% NaCl solution at pH 5.0 as an accepted model of mammary tumor induction [11]. Pregnancy was mimicked by estrogen (35 mg) and progesterone (35 mg) 21 day release hormone pellets (Innovative Research of America, FL, USA) implanted subcutaneously (anesthesia: (60 mg/kg) ketamine/(7.5 mg/kg) xylazine/(1.0 mg/kg) on alternate sides of the scruff of the neck [22]. Our early parity (EP, n = 9), group was implanted with hormone pellets 1 week following MNU injection as it has been shown that inducing pregnancy 1 week following the administration of MNU confers protection against mammary gland tumor development [8]. In contrast, the delayed parity (DP, n = 11) group was implanted with hormone pellets 4 weeks following MNU injection. Others have observed tumor development as early as 5 weeks following MNU administration in female Sprague Dawley rats administered MNU at 5 weeks of age [23]. Therefore, a 4 week time frame was chosen for delayed parity.
Fig. 1.
Experimental paradigm and physical characteristics. Thirty-four to thirty-five day old animals were injected with MNU at time 0 (weeks) and followed for 20 weeks. Animals were progressively exercise trained (Ex), or remained sedentary, prior to and during exposure to hormone (H) pellets (A). Exercise training did not alter body weight (B), but did increase tumor latency (C) leading to smaller tumors (D) in exercise trained animals. *p < 0.05. Nulliparous (NP), early parity (EP), delayed parity (DP).
Rats were acclimated to the treadmill for 3 days at 10 m/min for 10 min prior to starting the exercise training protocol. During week 4 animals did not run the day of surgery for subcutaneous hormone pellet implantation, and the following day animals were exercise at a reduced intensity of 20 m/min and 5% incline for 30 min. Duration indicated in the table does not include a 5 min warm up and 5 min cool down on the treadmill.
The exercise plus DP (Ex + DP, n = 9) group was progressively exercise trained (Table 1) during this menarche to first pregnancy interval and throughout the 21 day hormone release from the pellets (total 7 weeks exercise training). Ex + DP animals then remained under standard cage conditions for weeks 8–20. A positive control of nulliparous animals (NP, n = 9) was also included which received neither exercise nor hormone pellet implantation. Animals in the NP, EP, and DP groups remained in standard cage conditions throughout the study and all animals were euthanized by decapitation at either humane tumor endpoint criteria or the end of the study (week 20). Animals were housed under 12-hour light/12-hour dark cycles with access to food and water ad libitum. All animal experiments were approved by the Institutional Animal Care and Use Committee of The University of Pennsylvania, and all animals received humane care in compliance with National Institutes of Health standards.
Table 1.
Exercise training protocol.
| Week | Day | Speed (m/min) | Grade (%) | Duration (min) |
|---|---|---|---|---|
| 1 | 1 | 15 | 0 | 10 |
| 2 | 17.5 | 0 | 15 | |
| 3 | 20 | 0 | 20 | |
| 4 | 20 | 0 | 25 | |
| 5 | 20 | 0 | 30 | |
| 2 | 1 | 20 | 5 | 30 |
| 2 | 20 | 5 | 30 | |
| 3 | 20 | 10 | 30 | |
| 4 | 20 | 10 | 30 | |
| 5 | 20 | 15 | 30 | |
| 3–7 | 20 | 15 | 30 | |
2.2. Physical characteristics
Rats were palpated twice weekly for tumors. Animals were skinned at euthanasia and examined for any tumor not detected via palpation. If tumors were discovered, the tissue was visualized, measured, and weighed. Body weight was recorded once weekly throughout the experiment and body fat was assessed at study end in a sub-set of animals (n = 25) via underwater weighing to estimate body composition using established methods [24]. Briefly, animals were skinned, weighed, and a surgical suture was tied to the tip of the tail. The carcass was submerged in water, palpated to remove air from lungs, gastrointestinal tract, and from remaining hair. The silk suture was then attached to a spring scale mounted over a bucket of water (24 °C). Submerged water weight (g) was recorded. The weighing procedure was repeated 3 times and averaged. Specific gravity was calculated and a regression formula ((−394.498 * specific gravity) + 431.35) was used to determine body fat as previously published [24].
2.3. Histological analysis
Inguinal mammary glands were dissected post-mortem from the skin starting from the proximal area close to the nipple and working towards the distal end of the gland. The gland was immediately spread onto a glass slide and allowed to sit at room temperature for adherence. The slide was then placed in 10% formalin overnight and subsequently washed in 70% and 95% ethanol. Whole mounted glands were paraffin embedded, sectioned, and stained with H&E or Masson’s trichrome. Photographs of mammary gland cross sections were taken with a mounted digital camera (DP25, Olympus, Tokyo, Japan) and light microscope (BX53 Olympus, Tokyo, Japan). A blinded veterinary pathologist classified collagen, lobuloalveolar hyperplasia, ductal hyperplasia, and inflammation by grading: 0 = not present, 1 = minimal, 2 = mild, 3 = moderate, 4 = severe and 5 = marked.
2.4. Gene array and RT-PCR
As a hypothesis generating tool, we conducted a gene array associated with breast cancer to explore differences in gene expression of the mammary gland between groups. Total RNA was extracted from snap frozen abdominal mammary glands using the RNeasy kit (Qiagen, CA, USA). Sixty nanograms of RNA was equally pooled from 3 animals for reverse transcription into cDNA using RT2 PreAMP cDNA Synthesis Kit (Qiagen, CA, USA). RT-PCR was performed on the rat breast cancer array (PARN-131Z, Qiagen, CA, USA) using the RT2 SYBR Green qPCR mastermix (Qiagen, CA, USA) with an ABI 7300 instrument. Analysis of mRNA expression level was subsequently performed for collagen 1a (Col1a1) (PPR42922A, Qiagen, CA, USA), collagen 5a (Col5a1) (PPR42270A, Qiagen, CA, USA), (cyclin dependent kinase inhibitor 1C (Cdkn1c) (PPR06692C, Qiagen, CA, USA) and urokinase-plasminogen activator (Plau) (PPR43507E, Qiagen, CA, USA). Expression of the target genes were normalized to the level of the housekeeping gene Actin (PPR06570, Qiagen, CA, USA).
2.5. Statistical analysis
Tumor burden and histology values are reported as mean ± SEM, gene expression is reported as fold change (relative to EP) ± SD. Differences in physiological, histological, and PCR experiments were analyzed by a global F-test and subsequently assessed by Student’s t-test to examine two null hypotheses: 1) parity timing does not affect physiological, histological, or gene expression levels (EP vs. DP) and 2) exercise training does not affect physiological, histological, or gene expression levels (DP vs. Ex + DP). To minimize multiple hypothesis testing, genes from the gene array were only assessed if both DP and Ex + DP groups had standard deviations of fold change <4.0, had detectable levels for all 9 arrays, and were not housekeeping genes. This excluded 40 genes from analysis. A post-hoc Benjamini and Hochbert false discovery rate correction was applied to all genes analyzed. All statistical testing was carried out by STATA 12.1 (College Station, Texas). An alpha level of <0.05 was deemed significant.
3. Results
3.1. Exercise training increased tumor latency and decreased tumor burden
Exercise training did not alter body weight, (Fig. 1B), nor did exercise training result in a change in body fat (data not shown). The NP control group had significantly more tumor-bearing animals than any other group, with only 22% tumor-free throughout the duration of the experiment (Fig. 1C, p < 0.05). EP animals demonstrated 87% tumor-free survival. The Ex + DP group had the greatest tumor latency (+15 weeks post-MNU) compared to DP at 6 weeks and EP at 10 weeks post-MNU. Post-hoc tests revealed that NP animals had the largest tumors, and this group was significantly different from the Ex + DP group which had the smallest tumors (Fig. 1D, p < 0.05).
3.2. Delayed parity increased collagen levels independent of exercise training
Histological examination of tumor-free mammary glands revealed significantly lower collagen levels in EP animals compared to both DP and Ex + DP (Fig. 2A: global F-test p < 0.01). No differences were seen in lobuloalveolar hyperplasia between groups (p = 0.73) (Fig. 2B), and while not statistically significant, ductal hyperplasia (p = 0.21) and inflammation (p = 0.24) were only observed in DP animals (Fig. 2C and D, respectively). While not significant, Col1a1 was 37% elevated in DP animals, and Col5a1 was 35% and 36% elevated in DP and Ex + DP animals respectively compared to EP.
Fig. 2.
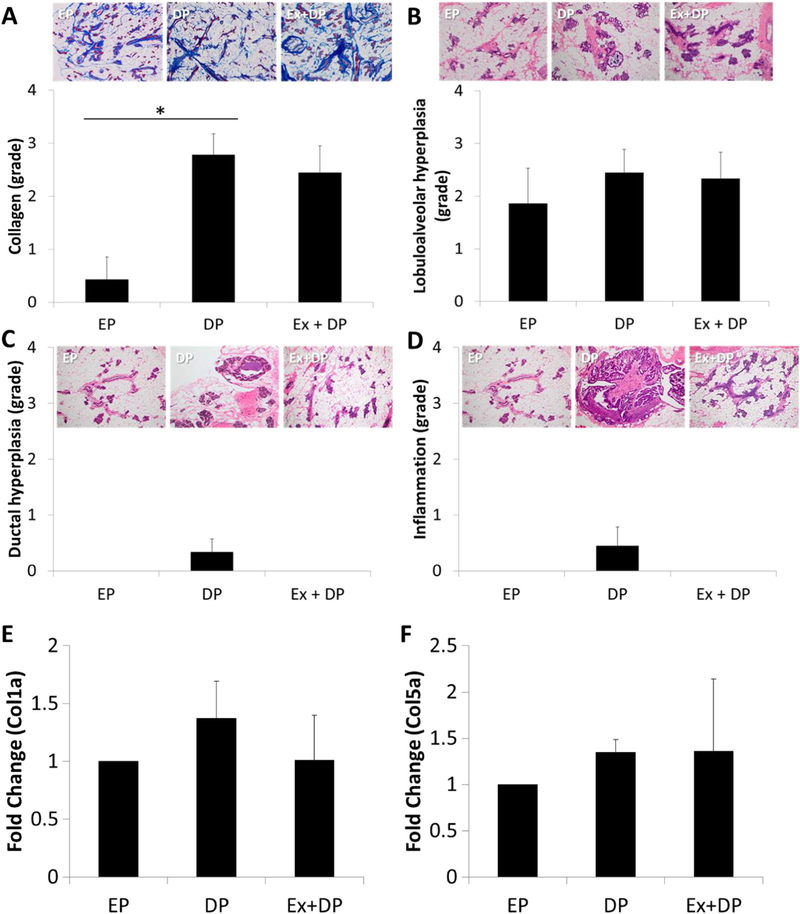
Histological assessment of non-tumor mammary glands. Collagen levels (A) (Masson’s trichrome stain - 4×) were significantly decreased in rats with early parity (EP) compared to delayed parity (DP), independent of exercise (Ex). There were no significant differences in lobuloalveolar hyperplasia (B), ductal hyperplasia (C), or inflammation (D). No differences were observed for gene expression levels of collagen 1a (Col1a1) (E) or 5a (Col5a1) (F). H&E stained images are shot at 10×. *p < 0.01.
3.3. Gene array and expression levels
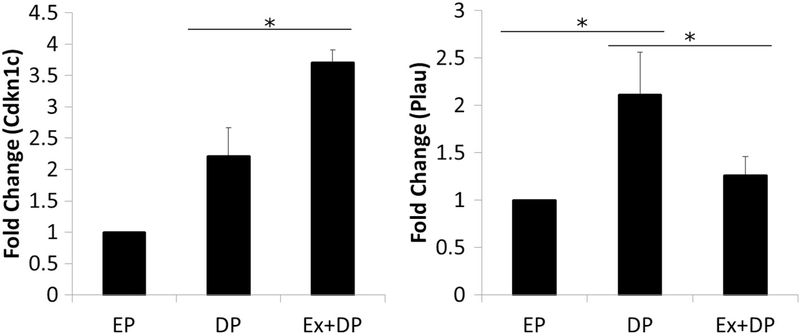
Table 2 depicts results from the gene array with standard deviations in both the DP and Ex + DP groups of <4.0, and demonstrated a significant difference between experimental groups following post-hoc correction, or were significant or near significant on the global F-test. We observed a pattern of delayed parity-induced increases in gene expression levels of genes associated with breast cancer (ataxia telangiectasia mutated (Atm), forkhead box A1 (Foxa1), keratin 18 (Krt18), keratin 8 (Krt8), and matrix metallopeptidase 2 (Mmp2)). The delayed parity-induced increase in these genes was not altered by exercise training. However, we did see exercise-induced changes as Cdkn1c expression was significantly enhanced with exercise training (global F-test, p < 0.05) and there was a trend for reduced expression of Plau (global F-test, p = 0.06). The exercise-induced changes in gene expression of Cdkn1c and Plau were confirmed via RT-PCR (Fig. 3). We observed significantly increased Cdkn1c expression levels in the Ex + DP group compared to DP (global F-test, p < 0.001). We also observed significantly higher levels of Plau gene expression in DP, compared to EP and Ex + DP (global F-test, p < 0.01).
Table 2.
Candidate gene array.
| Gene name | EP | DP | ap-Value | Ex + DP | bp-Value | cF-test |
|---|---|---|---|---|---|---|
| Atm | 1.0 ± 0.0 | 2.2 ± 0.2 | <0.01 | 2.3 ± 1.0 | 0.81 | 0.06 |
| Cdkn1c | 1.0 ± 0.0 | 1.8 ± 0.6 | 0.11 | 4.0 ± 1.7 | 0.09 | 0.03 |
| Foxa1 | 1.0 ± 0.0 | 10.5 ± 3.9 | 0.02 | 9.1 ± 0.9 | 0.66 | 0.01 |
| Krt18 | 1.0 ± 0.0 | 1.4 ± 0.1 | <0.01 | 1.4 ± 0.7 | 0.93 | 0.46 |
| Krt8 | 1.0 ± 0.0 | 1.8 ± 0.1 | <0.01 | 1.7 ± 0.6 | 0.82 | 0.05 |
| Mmp2 | 1.0 ± 0.0 | 1.6 ± 0.1 | 0.01 | 1.7 ± 1.5 | 0.92 | 0.62 |
| Plau | 1.0 ± 0.0 | 0.9 ± 0.5 | 0.87 | 0.3 ± 0.2 | 0.11 | 0.06 |
A hypothesis generating targeted gene array was run on RNA pooled from 3 animals per reaction (n = 3) mean ± SD.
EP vs. DP comparison.
DP vs. Ex + DP comparison.
Global F-test.
Fig. 3.
Cdkn1c and Plau gene expression levels. Cdkn1c and Plau demonstrate exercise-induced regulation which was confirmed via follow-up using single primers. *p < 0.05.
4. Discussion
Overall, this study provides evidence for exercise-induced protection against breast cancer in the context of a delayed parity model in the rat. We observed significantly elevated mammary collagen levels with delayed parity that was independent of exercise as well as both exercise independent and dependent changes in gene expression levels for genes known to be associated with breast cancer. Cdkn1c and Plau expression in mammary glands was significantly enhanced or decreased with exercise, respectively; suggesting that exercise training may decrease tumorigenesis via multiple points of regulation. These findings are novel in that for the first time they implicate exercise training in the beneficial improvement of these genes in breast cancer risk.
Previous studies of breast cancer in animal models have utilized intraperitoneal injection of MNU to reliably induce mammary tumors similar in hormone responsiveness and histology to those of human mammary carcinomas [9]. Further, previous studies indicate that pregnancy, or pregnancy mimicking hormone pellets, 1 week following administration of MNU confers protection against mammary gland tumor development [8,25]. Models of delayed parity in rats also demonstrate increased incidence of breast cancer [11]. In concurrence, we observed earlier onset of tumor incidence and greater tumor number in DP animals compared to EP animals.
Exercise-induced protection against breast cancer has been observed previously. In an adolescent rodent model, Westerlind et al. utilized an eight week treadmill based exercise training intervention of 30 min per day, with a moderate intensity of 20 m/min and 15% grade [26]. This protocol has also demonstrated prevention of breast cancers among older animals [10]. Further, this exercise level in rats is equivalent to exercise levels associated with positive health benefits in humans [27,28]. Using the same protocol, we saw protection against breast cancer with exercise in a delayed parity model. Animals in the Ex + DP group had the greatest tumor latency and smallest tumor burden.
Histological examination of mammary tissue revealed lower collagen levels in EP animals compared to both DP and Ex + DP. Collagen is the primary stromal protein of the mammary gland and animal models have causally linked increased stromal collagen to mammary tumor formation [29]. In humans, denser breasts are associated with an increased risk for breast cancer and El-Bastawissi et al. observed greater breast density with later age at first birth in humans [30]. With regard to exercise and breast density, previous research has been inconclusive. Recently, Azam et al. found no association between physical activity and breast density after adjusting for other risk factors [31]. While collagen levels may not be altered by exercise, inflammation and ductal hyperplasia may be modulated by lifestyle choices such as early parity or exercise. We observed the presence of inflammation and ductal hyperplasia only in the mammary tissue of DP animals.
Type I collagen is the major structural component of extracellular matrix. We observed that Col1a1, a component of type I collagen was elevated only in DP animals. Type V collagen only comprises 2–5% of collagen in healthy tissue. However in breast cancer, collagen V levels are elevated [32]. While Col5a1gene expression levels were increased over 30% in DP and Ex + DP animals compared to EP, there was no significant difference. It is unclear how gene expression levels of collagen in adult animals may reflect collagen deposition and ultimately breast density of the mammary gland.
Just as we observed exercise independent and dependent changes to the morphology of mammary tissue from rats with delayed parity, we also observed similar patterns in gene expression levels. Atm, Krt18, Krt8, and Mmp2 were significantly upregulated in DP compared to EP, and there was no difference between DP and Ex + DP. Additionally, Foxa1 demonstrated increased gene expression levels in DP and Ex + DP.
The above genes, which have increased gene expression with DP and Ex + DP, may point towards an enhanced state of, or, response to, stress in the mammary tissue compared to EP. Atm is well known to be activated by cellular stress such as double stranded DNA breaks [33]. The observed increase in gene expression levels seen with the experimental treatment of DP may be a byproduct of carcinogenesis as increased levels of Krt8 and Foxa1 are associated with luminal type breast cancers [34,35].
Two genes were differentially regulated by exercise compared to sedentary DP animals. Cdkn1c was significantly elevated and there was a trend for decreased levels of Plau. Previous studies have shown that these two genes appear to be important in tumorigenesis, and we observed that exercise training resulted in beneficial regulation [19,36].
Decreased gene expression levels of CDKN1C are associated with cancer [20,21]. We observed an increase in Cdkn1c gene expression levels due to exercise. Other wellness interventions have also shown increased Cdkn1c gene expression levels. Chisholm et al. observed that Cdkn1c gene expression levels were increased by the green tea polyphenol, epigallocatechin-3-gallate (EGCG), which has anti-oxidant effects associated with cancer suppression and in particular, breast cancer suppression [37,38].
Higher levels of uPA, the protein encoded by the gene Plau, are associated with breast cancer [39,40], and we observed exercise-induced decreases in Plau. In animal models, Almholdt et al. utilized the MMTV-PyMT transgenic breast cancer model crossed with uPA deficient mice [36] and observed marked reductions in metastasis compared to controls. Further, with regard to tumorigenesis, primary tumors of uPA-deficient mice were palpable for the first time approximately 4 days later than in wild-type mice. There was a trend (p = 0.07) for delayed tumor latency in uPA deficient mice. No other studies have reported on the possible role of uPA in tumorigenesis, but it appears lower levels of Plau are beneficial.
5. Conclusion
Given the particular susceptibility of breast tissue to exposures and the association between the length of time between menarche and first birth with cancer risk, our model focused on exercise training during the interval between menarche and first pregnancy. This animal model was developed to support epidemiological evidence linking physical activity from menarche to first pregnancy with risk of breast cancer among women with delayed parity (i.e., >20 years between menarche and first pregnancy) [12]. No previous animal studies have investigated the role of exercise in delayed parity. A limitation of our study was the observational nature. As exercise training affects multiple systems and many signaling pathways, there is much to discovery regarding the molecular pathways involved. Thus, there is no single pharmacological treatment or knock-out model available to succinctly examine mechanistic processes. Yet, we demonstrated a phenotype in rats that corresponds to epidemiological data from human studies. Also, our study did not assess the effects of exercise training on mammary tissue from animals with early parity. Nor did we assess the time course of differences in mammary tissue gene expression. Therefore, our future work will examine the mechanism of exercise induced protection against breast cancer during the reproductive interval of menarche to first birth and its relationship with timing of parity through the inclusion of an Ex + EP group in the experimental design.
Collectively, our data demonstrate patterns of delayed parity induced changes to the breast tissue which are both dependent and independent of exercise training completed between menarche and first pregnancy. While exercise training was beneficial for tumor latency and size, it did not mitigate enhanced collagen levels found in mammary tissue of delayed parity animals. Similarly, exercise training did not mitigate enhanced expression levels of several genes associated with breast cancer. However, there were exercise-dependent changes in the mammary gland. Exercise training prevented the development of inflammation and ductal hyperplasia. Exercise training also led to improved directional regulation of gene expression levels for Cdkn1c and Plau. Differential gene expression levels of mammary tissue Cdkn1c and Plau in animals physically active between menarche and first pregnancy suggest that these genes may play a role in exercise-induced protection against breast cancer later in life.
HIGHLIGHTS.
Patterns of delayed parity induced changes to the breast tissue which are both dependent and independent of exercise training completed between menarche and first pregnancy.
While exercise training was beneficial for tumor latency and size, it did not mitigate enhanced collagen levels found in mammary tissue of delayed parity animals.
Similarly, exercise training did not mitigate enhanced expression levels of several genes associated with breast cancer.
However, there were exercise-dependent changes in the mammary gland.
Exercise training prevented the development of inflammation and ductal hyperplasia.
Exercise training also led to improved directional regulation of gene expression levels for Cdkn1c and Plau.
Differential gene expression levels of mammary tissue Cdkn1c and Plau in animals physically active between menarche and first pregnancy suggest that these genes may play a role in exercise-induced protection against breast cancer later in life.
Acknowledgements
We gratefully acknowledge Elizabeth Durham, DVM, DACVP of the Comparative Pathology Core at the University of Pennsylvania, School of Veterinary Medicine for her expertise in histological grading of mammary gland slides. We also thank the Laboratory of Innovative and Translational Nursing Research at the University of Pennsylvania, School of Nursing, for reagents and expertise (Geetha Muthukumaran, PhD).
Financial support
This work was funded by sub awards from U54CA155626 to Deirdre Tobias, U54CA155850 to Kathleen Sturgeon, UM1CA176726 to Ying Liu, and IRG-78-002-36 pilot funds from the American Cancer Society to Bart De Jonghe. Ying Liu is also supported by the Breast Cancer Research Foundation and the Foundation for Barnes Jewish Hospital, St Louis, Missouri. Deirdre Tobias is funded by a K01 from the National Institute of Diabetes and Digestive and Kidney Diseases (DK103720).
Footnotes
Conflict of interests
None.
References
- [1].Group, U. S. C. S. W. United States Cancer Statistics: 1999–2012 Incidence and Mortality Web-based Report, Department of Health and Human Services: Centers for Disease Control and Prevention, and National Cancer Institute, Atlanta (GA), 2015. [Google Scholar]
- [2].Colditz GA, Rosner B, Cumulative risk of breast cancer to age 70 years according to risk factor status: data from the Nurses’ Health Study, Am. J. Epidemiol. 152 (2000) 950–964. [DOI] [PubMed] [Google Scholar]
- [3].Rosner B, Colditz GA, Nurses’ health study: log-incidence mathematical model of breast cancer incidence, J. Natl. Cancer Inst. 88 (1996) 359–364. [DOI] [PubMed] [Google Scholar]
- [4].Li CI, Malone KE, Daling JR, Potter JD, Bernstein L, Marchbanks PA, et al. , Timing of menarche and first full-term birth in relation to breast cancer risk, Am. J. Epidemiol. 167 (2008) 230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Biro FM, Greenspan LC, Galvez MP, Puberty in girls of the 21st century, J. Pediatr. Adolesc. Gynecol. 25 (2012) 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wu Y, Zhang D, Kang S, Physical activity and risk of breast cancer: a meta-analysis of prospective studies, Breast Cancer Res. Treat. 137 (2013) 869–882. [DOI] [PubMed] [Google Scholar]
- [7].Thompson HJ, Effects of physical activity and exercise on experimentally-induced mammary carcinogenesis, Breast Cancer Res. Treat. 46 (1997) 135–141. [DOI] [PubMed] [Google Scholar]
- [8].Tsubura A, Uehara N, Matsuoka Y, Yoshizawa K, Yuri T, Estrogen and progesterone treatment mimicking pregnancy for protection from breast cancer, In Vivo 22 (2008) 191–201. [PubMed] [Google Scholar]
- [9].Russo J, Russo IH, Experimentally induced mammary tumors in rats, Breast Cancer Res. Treat. 39 (1996) 7–20. [DOI] [PubMed] [Google Scholar]
- [10].Thompson HJ, Westerlind KC, Snedden JR, Briggs S, Singh M, Inhibition of mammary carcinogenesis by treadmill exercise, J. Natl. Cancer Inst. 87 (1995) 453–455. [DOI] [PubMed] [Google Scholar]
- [11].Tsukamoto R, Mikami T, Miki K, Uehara N, Yuri T, Matsuoka Y, et al. , N-methyl-N-nitrosourea-induced mammary carcinogenesis is promoted by short-term treatment with estrogen and progesterone mimicking pregnancy in aged female Lewis rats, Oncol. Rep. 18 (2007) 337–342. [PubMed] [Google Scholar]
- [12].Liu Y, Tobias DK, Sturgeon KM, Rosner B, Malik V, Cespedes E, et al. , Physical activity from menarche to first pregnancy and risk of breast cancer, Int. J. Cancer (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].De Clercq A, Inze D, Cyclin-dependent kinase inhibitors in yeast, animals, and plants: a functional comparison, Crit. Rev. Biochem. Mol. Biol. 41 (2006) 293–313. [DOI] [PubMed] [Google Scholar]
- [14].Liu J, Kahri AI, Heikkila P, Voutilainen R, Ribonucleic acid expression of the clustered imprinted genes, p57KIP2, insulin-like growth factor II, and H19, in adrenal tumors and cultured adrenal cells, J. Clin. Endocrinol. Metab. 82 (1997) 1766–1771. [DOI] [PubMed] [Google Scholar]
- [15].Fan GK, Chen J, Ping F, Geng Y, Immunohistochemical analysis of P57(kip2), p53 and hsp60 expressions in premalignant and malignant oral tissues, Oral Oncol. 42 (2006) 147–153. [DOI] [PubMed] [Google Scholar]
- [16].Bonilla F, Orlow I, Cordon-Cardo C, Mutational study of p16CDKN2/MTS1/INK4A and p57KIP2 genes in hepatocellular carcinoma, Int. J. Oncol. 12 (1998) 583–588. [DOI] [PubMed] [Google Scholar]
- [17].Oya M, Schulz WA, Decreased expression of p57(KIP2)mRNA in human bladder cancer, Br. J. Cancer 83 (2000) 626–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kobatake T, Yano M, Toyooka S, Tsukuda K, Dote H, Kikuchi T, et al. , Aberrant methylation of p57KIP2 gene in lung and breast cancers and malignant mesotheliomas, Oncol. Rep. 12 (2004) 1087–1092. [PubMed] [Google Scholar]
- [19].Nijjar T, Wigington D, Garbe JC, Waha A, Stampfer MR, Yaswen P, p57KIP2 expression and loss of heterozygosity during immortal conversion of cultured human mammary epithelial cells, Cancer Res. 59 (1999) 5112–5118. [PubMed] [Google Scholar]
- [20].Larson PS, Schlechter BL, King CL, Yang Q, Glass CN, Mack C, et al. , CDKN1C/p57kip2 is a candidate tumor suppressor gene in human breast cancer, BMC Cancer 8 (2008) 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yang C, Nan H, Ma J, Jiang L, Guo Q, Han L, et al. , High Skp2/low p57(Kip2) expression is associated with poor prognosis in human breast carcinoma, Breast Cancer (Auckl.) 9 (2015) 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Blakely CM, Stoddard AJ, Belka GK, Dugan KD, Notarfrancesco KL, Moody SE, et al. , Hormone-induced protection against mammary tumorigenesis is conserved in multiple rat strains and identifies a core gene expression signature induced by pregnancy, Cancer Res. 66 (2006) 6421–6431. [DOI] [PubMed] [Google Scholar]
- [23].Thompson HJ, Adlakha H, Singh M, Effect of carcinogen dose and age at administration on induction of mammary carcinogenesis by 1-methyl-1-nitrosourea, Carcinogenesis 13 (1992) 1535–1539. [DOI] [PubMed] [Google Scholar]
- [24].Dahms WT, Glass AR, Correlation of percent body fat with body specific gravity in rats, J. Nutr. 112 (1982) 398–400. [DOI] [PubMed] [Google Scholar]
- [25].Zhu Z, Jiang W, Zacher JH, Neil ES, McGinley JN, Thompson HJ, Effects of energy restriction and wheel running on mammary carcinogenesis and host systemic factors in a rat model, Cancer Prev. Res. (Phila.) 5 (2012) 414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Westerlind KC, McCarty HL, Schultheiss PC, Story R, Reed AH, Baier ML, et al. , Moderate exercise training slows mammary tumour growth in adolescent rats, Eur. J. Cancer Prev. 12 (2003) 281–287. [DOI] [PubMed] [Google Scholar]
- [27].Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. , Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association, Med. Sci. Sports Exerc. 39 (2007) 1423–1434. [DOI] [PubMed] [Google Scholar]
- [28].Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, et al. , Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine, JAMA 273 (1995) 402–407. [DOI] [PubMed] [Google Scholar]
- [29].Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, et al. , Collagen density promotes mammary tumor initiation and progression, BMC Med. 6 (2008) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].El-Bastawissi AY, Aiello EJ, Buist DS, Taplin SH, Previous pregnancy outcome and breast density (United States), Cancer Causes Control 16 (2005) 407–417. [DOI] [PubMed] [Google Scholar]
- [31].Azam S, Physical activity does not influence breast density: protective effect against breast cancer is due to other mechanisms, ECCO-Eur. CanCer Organ.: ScienceDaily; (2016). [Google Scholar]
- [32].Barsky SH, Rao CN, Grotendorst GR, Liotta LA, Increased content of Type V Collagen in desmoplasia of human breast carcinoma, Am. J. Pathol. 108 (1982) 276–283. [PMC free article] [PubMed] [Google Scholar]
- [33].Rotman G, Shiloh Y, ATM: from gene to function, Hum. Mol. Genet. 7 (1998) 1555–1563. [DOI] [PubMed] [Google Scholar]
- [34].Mehta RJ, Jain RK, Leung S, Choo J, Nielsen T, Huntsman D, et al. , FOXA1 is an independent prognostic marker for ER-positive breast cancer, Breast Cancer Res. Treat. 131 (2012) 881–890. [DOI] [PubMed] [Google Scholar]
- [35].Page MJ, Amess B, Townsend RR, Parekh R, Herath A, Brusten L, et al. , Proteomic definition of normal human luminal and myoepithelial breast cells purified from reduction mammoplasties, Proc. Natl. Acad. Sci. U. S. A. 96 (1999) 12589–12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Almholt K, Lund LR, Rygaard J, Nielsen BS, Dano K, Romer J, et al. , Reduced metastasis of transgenic mammary cancer in urokinase-deficient mice, Int. J. Cancer 113 (2005) 525–532. [DOI] [PubMed] [Google Scholar]
- [37].Chisholm K, Bray BJ, Rosengren RJ, Tamoxifen and epigallocatechin gallate are synergistically cytotoxic to MDA-MB-231 human breast cancer cells, Anti-Cancer Drugs 15 (2004) 889–897. [DOI] [PubMed] [Google Scholar]
- [38].Hsu S, Lewis JB, Borke JL, Singh B, Dickinson DP, Caughman GB, et al. , Chemopreventive effects of green tea polyphenols correlate with reversible induction of p57 expression, Anticancer Res. 21 (2001) 3743–3748. [PubMed] [Google Scholar]
- [39].Duffy MJ, O’Grady P, Devaney D, O’Siorain L, Fennelly JJ, Lijnen HJ, Urokinase-plasminogen activator, a marker for aggressive breast carcinomas. Preliminary report, Cancer 62 (1988) 531–533. [DOI] [PubMed] [Google Scholar]
- [40].Grondahl-Hansen J, Hilsenbeck SG, Christensen IJ, Clark GM, Osborne CK, Brunner N, Prognostic significance of PAI-1 and uPA in cytosolic extracts obtained from node-positive breast cancer patients, Breast Cancer Res. Treat. 43 (1997) 153–163. [DOI] [PubMed] [Google Scholar]