Abstract
Comatose patients after cardiac arrest should receive active targeted temperature management (TTM), with a goal core temperature of 32–36°C for at least 24 hours. Small variations in brain temperature may confer or mitigate a substantial degree of neuroprotection, which may be lost at temperatures near 37°C. The purpose of this study was to define the relationship between brain and core temperature after cardiac arrest through direct, simultaneous measurement of both. We placed intracranial monitors in a series of consecutive patients hospitalized for cardiac arrest at a single tertiary care facility within 12 hours of return of spontaneous circulation to guide postcardiac arrest care. We compared the absolute difference between brain and core (esophageal or rectal) temperature measurements every hour for the duration of intracranial monitoring and tested for a lag between brain and core temperature using the average square difference method. Overall, 11 patients underwent simultaneous brain and core temperature monitoring for a total of 906 hours of data (Median 95; IQR: 15–118 hours per subject). On average, brain temperature was 0.34C° (95% confidence interval [CI] 0.31–0.37) higher than core temperature. In 7% of observations, brain temperature exceeded the measured core temperature ≥1°C. Brain temperature lagged behind core temperature by 0.45 hours (95% CI = −0.27–1.27 hours). Brain temperature averages 0.34°C higher than core temperature after cardiac arrest, and is more than 1°C higher than core temperature 7% of the time. This phenomenon must be considered when carrying out TTM to a goal core temperature of <36°C.
Keywords: : monitoring, hypothermia, cardiac arrest, brain injury, neurocritical care, intracranial monitoring
Introduction
Cardiac arrest is the most common cause of death in the United States and the majority of patients who succumb after return of spontaneous circulation (ROSC) die from sequelae of brain injury (Coppler et al., 2015; Mozaffarian et al., 2015; Elmer et al., 2016). To minimize secondary brain injury, guidelines recommend active targeted temperature management (TTM) of comatose postcardiac arrest patients to a core temperature of 32–36°C for at least 24 hours after ROSC (Callaway et al., 2015). Since the publication of the large TTM trial (Nielsen et al., 2013), targeting a core temperature of 36°C has become increasingly common (Leary et al., 2015; Rittenberger et al., 2015; Deye et al., 2016). Unfortunately, although the brain is the target end organ for TTM-induced protection, only core temperature is usually measured and the relationship between brain and core temperature after cardiac arrest is poorly described. In other populations with severe acquired brain injury, core temperature is systematically lower than brain temperature (Childs and Lunn, 2013; Karaszewski et al., 2013).
Preclinical evidence suggests that reductions of brain temperature by 1°C from 37°C to 36°C may be neuroprotective (Jackson et al., 2015), and that these benefits are not achieved with active normothermia (37°C) (Logue et al., 2007). These data raise concern that the neuroprotective benefits of TTM may be reduced when targeting a core temperature of 36°C, if the unmeasured brain temperature were significantly higher. The purpose of this study, therefore, was to quantify the relationship between brain and core temperature in comatose postcardiac arrest patients through direct, simultaneous measurement of both. We hypothesized that core temperature would substantially underestimate brain temperature across a range of core temperatures. Secondarily, we hypothesized that brain temperature would lag behind core temperature during periods of dynamic temperature change.
Materials and Methods
The University of Pittsburgh Institutional Review Board approved this study. In brief, we identified a series of patients treated after cardiac arrest at a single tertiary care center between 2013 and 2015 who had intracranial monitors placed within 12 hours of ROSC to guide clinical care. We measured baseline severity of postcardiac arrest illness using a previously validated illness severity scale (Coppler et al., 2015). We excluded subjects who had cardiac arrest or had brain monitors placed because of severe trauma or a primary neurological process such as catastrophic cerebrovascular accident. Our neuromonitoring package included placement of a right frontal intracranial access bolt (Licox® IMC Bolt Fitting, Integra LifeSciences; or QFlow 500™ Titanium Bolt, Hemedex, Inc.), through which intracranial pressure, temperature, and tissue oxygen probes were placed in the subcortical white matter. We measure intracranial temperature with either a Licox (Integra LifeSciences) or Raumedic® Neurovent PTO (Raumedic) catheter. Core temperature was measured by either a rectal or esophageal temperature probe. We performed TTM using a CoolGard 3000© endovascular catheter (Alsius Corporation) or Arctic Sun© (Mediance, Bard Medical) surface cooling pads, and targeted a core temperature of 33°C for 24 hours followed by rewarming at 0.25°C/h to a goal of 37°C, which we maintained for at least 5 days after ROSC.
Statistics
We used descriptive statistics to summarize population characteristics, then compared brain and core temperature measurements every hour for the duration of intracranial monitoring within each patient and for the overall population. We tested for a lag between brain and core temperature using the average square difference method. The sum of the squared difference in temperature between each hourly measurement divided by the number of measurements was computed while varying the lag between the two temperature signals between −3 and +3 hours. The minimum of the seven resulting values corresponded to the optimal lag between the two signals for each patient. Computations were programmed in Matlab R2014a (The Mathworks). We calculated the mean lag for the 11 included patients and bootstrapped its 95% confidence interval using the BCa method with 10,000 bootstrap samples in SPSS 23 (IBM).
Results
Overall, 11 patients underwent simultaneous brain and core temperature monitoring for a total of 906 hours (Median 95; IQR: 15–118 hours per subject). The majority of subjects had an asystolic initial rhythm and presented with severe brain injury (Table 1). Esophageal core temperature was monitored in 64% of patients and most subjects (73%) were cooled using an endovascular device. Median time from arrival to placement of intracranial monitoring was 7 hours (IQR: 5.5–8.5 hours).
Table 1.
Subject Demographics
| Subject characteristics | N = 11 |
|---|---|
| Age, years | 47 ± 10 |
| Male sex | 6 (55%) |
| Out-of-hospital cardiac arrest | 11 (100%) |
| Initial rhythm (%) | |
| VT/VF | 2 (18) |
| PEA | 3 (27) |
| Asystole | 5 (46) |
| Unknown | 1 (9) |
| Pittsburgh cardiac arrest category (%) | |
| I | 0 (0) |
| II | 1 (9) |
| III | 0 (0) |
| IV | 10 (91) |
| Core temperature monitoring site (%) | |
| Rectal | 4 (36) |
| Esophageal | 7 (64) |
| Cooling device | |
| CoolGard 3000© | 9 (73%) |
| Artic Sun© | 2 (27%) |
| Arrival to brain monitoring interval, hours | 7 ± 3 |
| Survival to hospital discharge | 1 (9%) |
PEA, pulseless electrical activity; VT/VF, ventricular tachycardia/ventricular fibrillation.
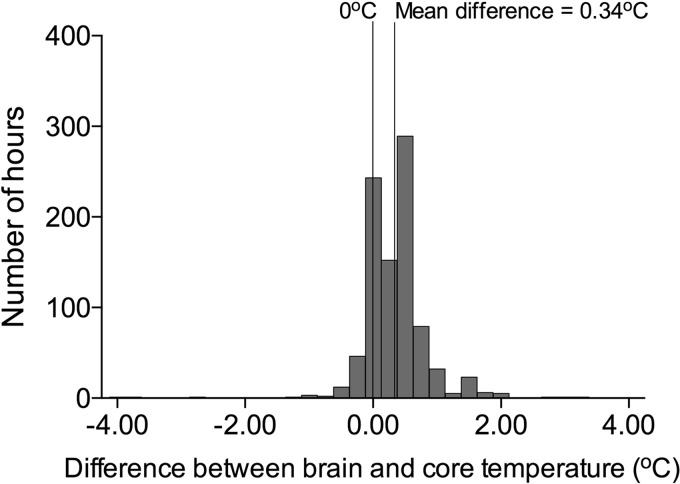
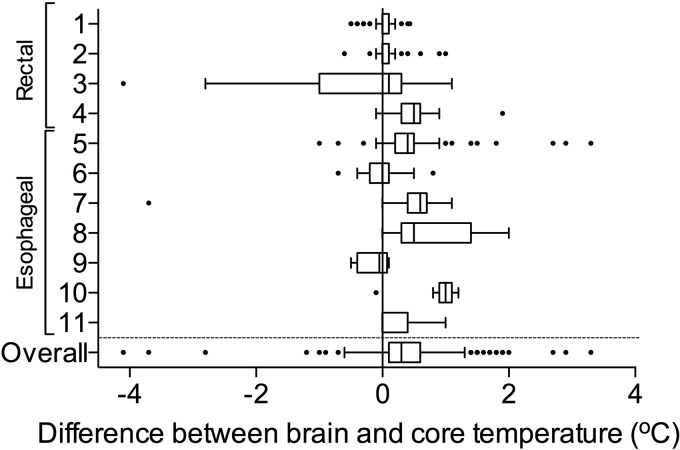
On average, brain temperature exceeded core temperature by 0.34°C (95% confidence interval [CI]: 0.31–0.37°C) (brain mean temperature −34.81°C; 95% CI: 34.68–34.95; core mean temperature −34.46°C; 95% CI: 34.33–34.59; Figs. 1 and 2). Individual patient data did not reveal any differences in brain–core gradient between patients with rectal and esophageal temperature measurements, nor between patients with endovascular or surface cooling (Fig. 2). In 7% of observations, brain temperature exceeded core temperature by at least 1°C. The bias for measurements of brain temperature to be higher than core temperature was nonsignificantly higher at higher temperatures based on the Bland–Altman plot (Fig. 3). Brain temperature lagged behind core temperature by 0.45 hours (95% CI: −0.27–1.27 hours).
FIG. 1.
Histogram of total hours of observation and differences between brain and core temperature.
FIG. 2.
Box and whisker plot of individual patient data and differences between brain and core temperature.
FIG. 3.
Bland–Altman plot of absolute difference in core and brain temperature and relative core temperature.
Discussion
To our knowledge, this is the first report to compare brain and core temperature in human cardiac arrest patients. We found that during active temperature management, core temperature consistently underestimated brain temperature, often by a substantial degree. This phenomenon has also been observed after brain trauma and stroke (Karaszewski et al., 2009; Childs and Lunn, 2013). Since the landmark TTM trial showing equivalent effects of treatment at 33°C versus 36°C on patient outcomes (Nielsen et al., 2013), clinical practice has shifted toward targeting 36°C in comatose survivors of cardiac arrest (Callaway et al., 2015; Leary et al., 2015; Deye et al., 2016). The TTM trial demonstrated equivalent patient outcomes at a population level, yet even within the context of this trial the 95% error bars in the 36°C cohort crossed 37°C (Nielsen et al., 2013). Clinical practice outside of the context of a robust trial may be less precise, and patients treated with a target core temperature of 36°C are at greater risk of exceeding 37°C than patients treated with a lower target core temperature (Rittenberger et al., 2015). Given the discrepancy we demonstrate between brain and core temperature, our results highlight the importance of vigilant prevention of even small rises in core temperature above 36°C to prevent unintended cerebral normothermia or fever during TTM.
Our work has several limitations. We present single center observational data including some of the most severe brain-injured cardiac arrest patients. The generalizability of our findings to less severe brain-injured postcardiac arrest patients is unknown. It may be that in a more mildly injured cohort, brain metabolism is less severely impaired and there is actually more cerebral thermogenesis, or that homeostasis is better preserved and there is less of a difference. In addition, the site of core temperature measurement and the device used for TTM were not consistent across patients. This variety likely reflects current clinical practice among cardiac arrest centers (Coppler et al. unpublished data 2016). Finally, with regard to detection of a lag between brain and core temperature, hourly measurements may be too infrequent and relatively insensitive to identify a lag that may exist on the order of minutes. However, we feel that such a brief lag, if it exists, is unlikely to be clinically relevant.
Conclusion
Brain temperature exceeds core temperature in comatose patients after cardiac arrest, sometimes by more than 1°C. Small elevations in core temperature above 36°C may risk brain normothermia and reduce the neuroprotective effect of TTM.
Contributor Information
Collaborators: on behalf of the Pittsburgh Post-Cardiac Arrest Service
Acknowledgments
The Pittsburgh Post-Cardiac Arrest Service researchers are Jon C. Rittenberger, MD, MS; Clifton W. Callaway, MD, PhD; Francis X. Guyette, MD, MPH; Ankur A. Doshi, MD; Cameron Dezfulian, MD; Jonathan Elmer, MD, MS; Bradley J. Molyneaux, MD, PhD; and Lillian Emlet, MD, MS. Source of funding: Dr. Elmer's research time is supported by the NHLBI 5K12HL109068; Dr. Dezfulian's research time is supported by the NINDS KO8NS069817; and Dr. Elmer's research time is supported by the NHLBI 5K12HL109068.
Author Disclosure Statement
No competing financial interests exist.
References
- Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, Leary M, Meurer WJ, Peberdy MA, Thompson TM, et al. Part 8: post-cardiac arrest care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015;132:S465–S482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs C, Lunn KW. Clinical review: brain-body temperature differences in adults with severe traumatic brain injury. Crit Care 2013;17:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppler PJ, Elmer J, Calderon L, Sabedra A, Doshi AA, Callaway CW, Rittenberger JC, Dezfulian C. Validation of the Pittsburgh Cardiac Arrest Category Illness severity score. Resuscitation 2015;89:86–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deye N, Vincent F, Michel P, Ehrmann S, da Silva D, Piagnerelli M, Kimmoun A, Hamzaoui O, Lacherade JC, de Jonghe B, et al. Changes in cardiac arrest patients’ temperature management after the 2013 “TTM” trial: results from an international survey. Ann Intensive Care 2016;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer J, Torres C, Aufderheide TP, Austin MA, Callaway CW, Golan E, Herren H, Jasti J, Kudenchuk PJ, Scales DC, et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation 2016;102:127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson TC, Manole MD, Kotermanski SE, Jackson EK, Clark RS, Kochanek PM. Cold stress protein RBM3 responds to temperature change in an ultra-sensitive manner in young neurons. Neuroscience 2015;305:268–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaszewski B, Carpenter TK, Thomas RG, Armitage PA, Lymer GK, Marshall I, Dennis MS, Wardlaw JM. Relationships between brain and body temperature, clinical and imaging outcomes after ischemic stroke. J Cereb Blood Flow Metab 2013;33:1083–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaszewski B, Wardlaw JM, Marshall I, Cvoro V, Wartolowska K, Haga K, Armitage PA, Bastin ME, Dennis MS. Early brain temperature elevation and anaerobic metabolism in human acute ischaemic stroke. Brain 2009;132:955–964 [DOI] [PubMed] [Google Scholar]
- Leary M, Blewer AL, Delfin G, Abella BS. Variability in postarrest targeted temperature management practice: implications of the 2015 guidelines. Ther Hypothermia Temp Manag 2015;5:184–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue ES, McMichael MJ, Callaway CW. Comparison of the effects of hypothermia at 33 degrees C or 35 degrees C after cardiac arrest in rats. Acad Emerg Med 2007;14:293–300 [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015;131:e29–e322 [DOI] [PubMed] [Google Scholar]
- Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med 2013;369:2197–2206 [DOI] [PubMed] [Google Scholar]
- Rittenberger JC, Coppler P, Guyette FX, Callaway CW, Elmer J, Doshi AA, Slowey D. Abstract 17617: post-cardiac arrest mortality rates with different levels of targeted temperature management within patient Illness Severity Categories. Circulation 2015;132:A17617 [Google Scholar]