Abstract
Glial fibrillary acidic protein (GFAP), ubiquitin carboxyl-terminal hydrolase-L1 (UCH-L1), and S100B have been shown to be predictive of patients with brain injury. Kinetics of these biomarkers in injured humans have not been extensively examined. This prospective multi-center study included patients with mild-to-moderate traumatic brain injury. Blood samples obtained at enrollment and every 6 h up to 24 h post-injury were assayed for GFAP, UCH-L1, and S100B. Random effects models examined changes in the biomarkers' level over time. A total of 167 patients were enrolled; mean age was 46.0 ± 17.8, 61.1% were male, 143 (85.6%) had a Glasgow Coma Scale score of 15, and 33 (19.8%) had a positive head computed tomography (CT) scan. Baseline median biomarker concentrations for all three were higher among CT-positive patients (p < 0.0001) but GFAP was the only biomarker that significantly increased over time among CT-positive patients relative to CT-negative patients (log transformed values 0.037; 95% confidence interval 0.02, 0.05; p < 0.001), indicating a 3.7% per hour rise in GFAP concentration. There was no significant increase in either UCH-L1 or S100B in CT-positive patients (p = 0.15 and p = 0.47, respectively). GFAP concentrations increased 3.7% per hour among CT-positive patients whereas neither UCH-L1 nor S100B increased, compared with CT-negative patients. The kinetics and temporal profile of GFAP suggest it may be a more robust biomarker to detect patients with positive CT findings, particularly at later post-injury times. Further study is needed to determine if GFAP is a useful test to follow throughout a patient's clinical course.
Keywords: : biomarkers, glia cell response to injury, neural injury, traumatic brain injury
Introduction
Over the past decade, the number of traumatic brain injury (TBI)–related emergency department (ED) visits, hospitalizations, and deaths have increased in the United States, making TBI a serious public health concern as well as a leading cause of morbidity and mortality.1 Despite the availability of clinical decision rules,2,3 increasing pressure to deliver cost-effective care, and the controversy regarding potentially hazardous ionizing radiation exposure,4,5 computed tomography (CT) remains a frequently used but often uninformative diagnostic test for patients presenting to the ED with mild-to-moderate TBI (mmTBI).6 In an attempt to reduce CT use in mmTBI, numerous studies have evaluated the diagnostic significance of serum biomarkers, which are released as a result of head trauma and may potentially predict the presence of brain injury.7,8
Among the most widely studied biomarkers for this purpose are S100B,9,10 a protein involved in intracellular regulation that is expressed in astroglia cells,11 and glial fibrillary acidic protein (GFAP),12 a cytoskeletal protein expressed in astroglia cells, as well.11 Although several studies have reported the diagnostic value of S100B13,14 and GFAP as biomarkers for mmTBI,15,16 to date there remains insufficient evidence to warrant reliance on either as a screening tool to determine the need for head CT in the U.S.10,17 However, recent work has suggested low serum levels of the less-studied biomarker ubiquitin carboxyl-terminal hydrolase-L1 (UCH-L1), a neuron-specific deubiquitinase, obtained within 6 h of injury have better early diagnostic utility in screening for and excluding acute intracranial lesions found on head CT scans in adult patients with mmTBI than either S100B or GFAP.18
While this suggests a singular, more sensitive threshold value of UCH-L1 may be sufficient to define a negative head CT scan early after injury, there is limited data regarding the temporal profiles of serum UCH-L1 and GFAP in patients with mmTBI. Prior work in an animal model of moderate TBI determined that the time window of GFAP to detect acute brain injury following TBI was wider than UCH-L1,19 suggesting GFAP may be the more robust biomarker for mmTBI beyond the acute post-injury phase. This finding is consistent with a recent study (2016) in humans, which demonstrated serum UCH-L1 levels rose early after mmTBI, peaked at 8 h, then rapidly declined, whereas GFAP peaked later (20 h), and declined gradually thereafter but remained elevated over a 7-day period. Further, serum GFAP concentrations obtained across 7 days were consistent in their ability to detect CT lesions and the need for neurosurgical intervention.20 Given that serum concentration of UCH-L1 may be useful in the evaluation of patients with suspected mmTBI in the acute post-injury phase and that GFAP levels rise later and remain elevated longer, we sought to 1) further characterize the kinetics of these biomarkers and 2) examine the potential use of determining both biomarker levels during the 24-h post-injury period when evaluating the need for CT use and hospital admission for patients with suspected mmTBI.
The goal of this study was to determine the association of CT findings with serum levels of GFAP, UCH-L1, and S100B measured repeatedly over 24 h among patients with suspected mmTBI. In particular, the main interest was to determine potential differences in the biomarkers' kinetics among CT-positive and CT-negative patients and to assess their potential clinical application during the initial 24 h after injury.
Methods
Patients and study procedures
This secondary analysis of data derived from a prospective multi-center observational study included patients 18–80 years of age who presented at one of seven study site hospital EDs with a blunt closed-head injury and were assessed for potential mmTBI.18 Study sites included both U.S. and European hospitals and were composed of Level 1 and 2 trauma centers and a non-trauma center (Table 1). Eligible patients were those with an initial Glasgow Coma Score (GCS) of 9-15 (defined as mmTBI ) for whom an emergency head CT scan was obtained for evaluation of head injury as deemed necessary by the attending ED physician. All sites were aware of and considered available clinical decision rules to guide the need for imaging; however, no formal rules for obtaining a CT were utilized so as to best reflect current practice in the U.S. and Europe.
Table 1.
Description of Study Site Hospitals
| Study site | Location | Annual ED volume | Designation |
|---|---|---|---|
| Washington University, Barnes-Jewish Hospital | St. Louis, MO | 95,000 | Level I |
| University of Florida, Shands Hospital | Gainesville, FL | 66,000 | Level I |
| Gwinnett Medical Center - Lawrenceville | Atlanta, GA | 100,000 | Level II |
| Dekalb Medical - North Decatur | Atlanta, GA | 92,000 | Non-trauma |
| Wayne State - Detroit Receiving Hospital | Detroit, MI | 90,000 | Level I |
| University of Pécs Medical Center | Pecs, Hungary | 25000Ω | Level 1* |
| Albert Szent-Györgyi Medical Center - University of Szeged | Szeged, Hungary | 90,000 | Level 1* |
The ED volume is >25,000/year, and the facility cares for more than 2000 neurotrauma cases/year.
This is equivalent to a Level I facility in the United States.
ED, emergency department.
Included patients presented within 4 h of injury, received a CT scan as part of routine care, and had the first blood sample obtained for analysis within 6 h of injury. For this study, patients having only one serum sample obtained for biomarker analysis were excluded. In some instances, the first sample quantity obtained was inadequate for analysis but these patients were still included if they had at least two subsequent adequate samples for analysis. Table 2 details the inclusion and exclusion criteria. Institutional Review Board approval was obtained at each study site and each site also obtained approval by the ethics board of the U.S. Army Medical Research and Materiel Command Office of Research Protections Human Research Protection Office Department of Defense.
Table 2.
Inclusion/Exclusion Criteria
| Inclusion criteria |
|---|
| • The subject was ≥18 years of age and ≤80 years of age. |
| • Acceleration or deceleration closed injury to the head that was either self-reported or witnessed. |
| • Presented to an ED within 4 h of injury. |
| • An initial GCS score of 9-15 in the ED performed by PI or trained study personnel. |
| • ED workup included a head CT scan (based on standard practice and/or decision rules). |
| • Informed consent was obtained from the subject or his/her legal representative; oral consent for the initial blood draw and/or deferred consent to 24 h was allowed for patients who were unable to consent at initial evaluation or exception from the informed consent requirement by use of “community consent” if approved by an Institutional Review Board. |
| • The PI deemed the subject to be an appropriate study candidate. |
| Exclusion criteria |
|---|
| • Participation in another potentially confounding clinical study. |
| • Inability to accurately determine time of injury. |
| • Head CT not completed as part of clinical emergency care. |
| • Primary diagnosis of ischemic or hemorrhagic infarct. |
| • Not available for the 35-day follow-up visit. |
| • Venipuncture not feasible. |
| • Blood donation within 1 week of screening. |
| • The subject was otherwise determined medically unsuitable for study participation |
ED, emergency department; GCS, Glasgow Coma Scale; PI, principal investigator; CT, computed tomography.
Baseline patient characteristics
Baseline patient data collected by trained research personnel included demographics, medical history, substance use history, GCS scores, loss of consciousness (LOC), and circumstances related to the mechanism of injury. This was used to describe the study patient's general characteristics and injury patterns.
Serum sample and handling
Blood samples were collected at time of study enrollment and every 6 h up to the time of discharge (either ED or hospital) or up to 24 h (maximum of five samples during index visit). Patients were evaluated at follow up (Day 35 ± 5 days) for neuropsychological testing and to obtain another blood sample. The neuropsychological testing was not part of this study's outcome assessment and blood samples were rarely obtained and therefore were not included in the analysis and are not reported here. Blood samples were processed and the resulting serum was stored at −80°C and then shipped on dry ice to a central repository for storage until time of testing as per a pre-defined specimen handling procedure.
Serum analysis for GFAP, UCH-L1, and S100B
Serum samples were analyzed for UCH-L1 and GFAP concentration by an enzyme-linked immunosorbent assay at a later time by technicians blinded to clinical data and CT results. Samples were run in triplicate and the mean used to determine the final concentration. If one replicate was more than 4 standard deviations different from the mean of the other two replicates, the mean concentration was determined using just the two replicates. Duplicate and high and low positive controls were included with each plate. Details of the assay procedure can be found elsewhere.18 UCH-L1 and GFAP tests were performed by Banyan Biomarker, Inc. S100B concentrations were determined using an electrochemiluminescence immunoassay designed for in vitro diagnostic testing (Roche, Cobas 6000). Results for S100B are reported in μg/L with the standard normal reference intervals of 0.00-0.09 μg/L. Although various cut-off values have been proposed, for the purposes of this study an S100B ≥ 0.10 μg/L (100 pg/mL) was considered to be abnormal and could indicate a traumatic abnormality on head CT.21,22 S100B samples were tested at Halland's Hospital Halmsead, Department of Clinical Chemistry, Halmstead, Sweden.
The lower limit of quantification (LLQ) for both UCH-L1 and GFAP was 30 pg/mL (none provided for S100B), and the lower limit of detection (LLD) for UCH-L1, GFAP, and S100B was 10 pg/mL, 20 pg/mL, and 5 pg/mL, respectively. Table 3 summarizes these values and the intra-assay and inter-assay coefficients of variation for each study serum biomarker. For this analysis, serum concentrations above the LLD but less than or equal to the LLQ were assigned the LLQ value (30 pg/mL for GFAP and UCH-L1; no LLQ provided for S100B). Serum concentrations at or below the LLD were assigned the value of the LLD (5 pg/mL for S100B, 10 pg/mL for UCH-L1, and 20 pg/mL for GFAP). There is no clear standard or ideal method used to analyze values below the LLD (left-truncated values). Some advocate eliminating those values, others suggest imputing values, using a fraction of the LLD, a reverse Kaplan-Meier Estimator, or other techniques to determine a final value.23–26 For this analysis, we chose not to eliminate the values, use a fraction of the value, or use an imputation method, but rather chose to “round-up” for a variety of reasons. The very low values at the LOD and LLQ would not impact our results given the larger variation and it was thought to be a more conservative approach when considering a change over time (less change from baseline if values rise since we were not interested in small non-clinically useful but statistically significant changes). Assay results were not available to the treating clinician and were not used to guide treatment. For this main analysis, we considered results for all samples collected within 24 h after injury.
Table 3.
Analytical Performances of Each Biomarker
| Biomarker | S100B | UCH-L1 | GFAP |
|---|---|---|---|
| Limit of detection, pg/mL | 5 | 10 | 20 |
| Lower limit of quantification, pg/mL | N/A | 30 | 30 |
| Intra-assay coefficient of variation, % | 2.1 | 4.5 | 3.7 |
| Inter-assay coefficient of variation, % | 2.8 | 4.6 | 5.8 |
UCH-L1, ubiquitin carboxyl-terminal hydrolase-L1; GFAP, glial fibrillary acidic protein; N/A, not available.
Head CT scans
Each subject's head CT images were reviewed by an independent committee consisting of three blinded board-certified neuro-radiologists. The neuro-radiologists determined whether a CT scan was positive, defined as the presence of an acute trauma-related intracranial lesion. A charter outlining the criteria and procedures to be followed for scoring the CT scans was developed prior to the reading and interpretation of any of the CT images. Two of the neuro-radiologists, who had no access to any other clinical or laboratory data except subject age and gender, reviewed all of the study subjects' CT scans. Any discrepancy with respect to CT-positive or CT-negative was adjudicated by a third blinded radiologist and in those instances, was the final interpretation. Inter-rater reliability between the two primary radiologists was determined using Cohen's kappa statistic.
Outcomes
For this study, the primary clinical outcome was the result of the head CT scan (positive/negative) among patients with repeated blood samples drawn over 24 h post-injury. The main outcomes of interest, however, were the change in the three biomarkers' values over time among CT-positive and CT-negative patients.
Data analysis and reporting
A descriptive analysis for all subjects was performed. Patient groups (CT positive and CT negative) were compared using proportions and means or medians where appropriate. For selected binomial proportions, the exact (Clopper-Pearson) 95% confidence intervals (CIs) were provided. To compare biomarker values between CT-negative and CT-positive patients, the non-parametric Wilcoxon rank-sum test was used.
Individual graphical displays of the three biomarkers' serum concentration over time were constructed—one for the patient's individual trend overlaid with a locally weighted scatter plot smoothing (LOESS) plots (with 95% confidence limits) and another scatter plot that showed biomarker concentrations at various time-points obtained post-injury (also overlaid with LOESS plots). This was done to conceptualize the crude kinetics of each biomarker and provide a starting reference for model development.
For this study, the main interest was association of the CT results (positive or negative) with the change in each serum biomarker's concentrations measured up to 24 h post injury (CT*Time interaction) that was in addition to (independent of) other important clinical factors. Since it was likely that study subjects would have different initial biomarker concentrations (the baseline starting point or intercept), different biomarker kinetics over time (slope) and potentially correlated data due to the multiple samples obtained, mixed linear models with random effects (intercept and slope) were used for each of the three biomarkers. Given the distribution of biomarker values and model diagnostics, a natural log transformation of the biomarkers' values were utilized (lognormal distribution in SAS PROC GLIMMIX). Based on prior information and potentially theoretical importance, gender and presenting GCS score were mandatorily included in each model. Other factors considered for inclusion were age, race, LOC, and mechanism of injury. The Log Likelihood ratio and Bayesian information criterion tests were used to determine if non-mandatory variables should be included in the model and the optimal covariance matrix for the random effects.
Two sensitivity analyses were performed to examine results for consistency. Since there is no consensus on how to handle serum biomarker values below the LLQ and the LOD, the same models were fit using the actual reported values, even when below the detection limits. Some values were reported as 0 pg/mL and these were re-coded to 1 pg/mL so that those values could be log transformed for the models. Since it was likely that patients with a GCS <15 would be imaged regardless of the initial biomarker concentration (at least in the U.S.), we performed the main analysis again but included only patients with an initial GCS = 15. For all model results, we reported the parameter estimates for the transformed values with 95% CI. For the main results, we also back-transformed the parameter estimate values and provide the interpreted percent increase of each biomarker for the variable in the main models.
Finally, we calculated the sensitivity and specificity for each biomarker at various biomarker values using logistic regression and plotted the areas under the receiver operating characteristics curves (AUROCs) at 6-h intervals (0-6 h, 6-12 h, 12-18 h, and 18-24 h) to determine diagnostic characteristics at these various time intervals; however, one should note that this is only exploratory and the derived AUROC cannot be considered definitive. A p value of less than 0.05 was considered significant. All data were analyzed using SAS version 9.4 (Carey, NC) and published guidelines for statistical reporting were adhered to.27
Results
Patient characteristics
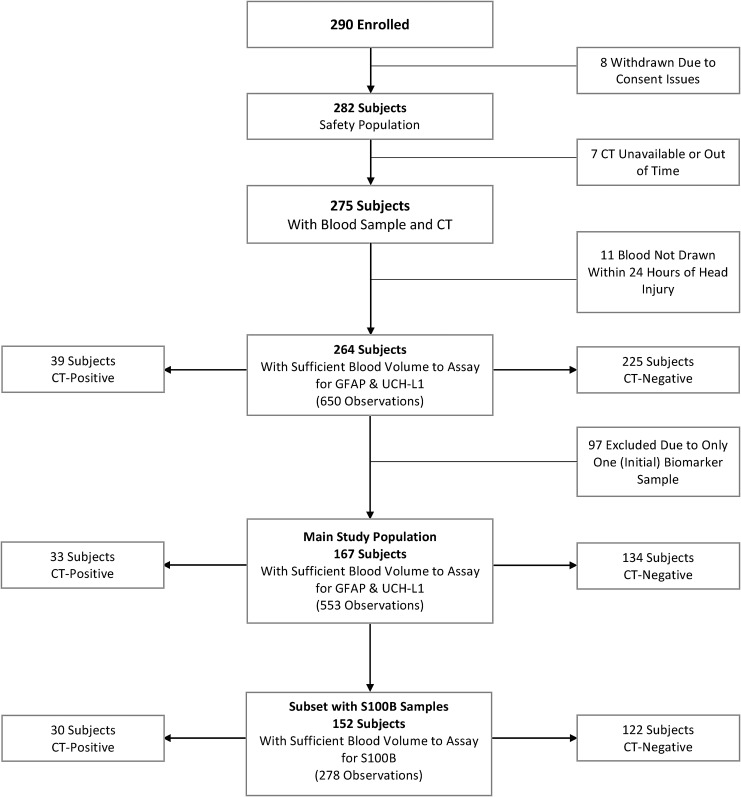
The study enrolled 290 patients: 26 were initially excluded for a variety of reasons; thus, leaving a total of 264 patients who had data for at least one blood sample and the required CT results. We excluded patients with only one blood sample (n = 97), thus leaving 167 patients included in this study. Fifteen of the 167 patients, however, were not tested for S100B since this part of the study procedure was added after study initiation and/or some subjects did not have enough blood obtained for analysis of all three markers. This resulted in 152 patients with S100B data. Figure 1 outlines the study patient selection process. Table 4 details the characteristics of included and excluded patients. Since exclusion of patients with only one serum biomarker determination was likely not a random factor, no formal statistical tests were used to define differences.
FIG. 1.
Flow diagram describing excluded and included patients with mild-to-moderate traumatic brain injury. CT, computed tomography; UCH-L1, ubiquitin carboxyl-terminal hydrolase-L1; GFAP, glial fibrillary acidic protein.
Table 4.
Patient Characteristics
| CT negative | CT positive | All subjects | |
|---|---|---|---|
| 80.2% (total n = 134) | 19.8% (total n = 33) | (total n = 167) | |
| Age (mean ± SD) | 43.8 ± 17.3 | 55.2 ± 16.8 | 46.0 ± 17.8 |
| Sex | |||
| Female | 41.8% (56) | 27.3% (9) | 38.9% (65) |
| Male | 58.2% (78) | 72.7% (24) | 61.1% (102) |
| Race | |||
| White | 74.6% (100) | 93.9% (31) | 78.4% (131) |
| Black | 20.9% (28) | 0.0% (0) | 16.8% (28) |
| Other | 4.5% (6) | 6.1% (2) | 4.8% (8) |
| Employment status | |||
| Employed | 49.2% (66) | 39.4% (13) | 47.3% (79) |
| Unemployed | 20.1% (27) | 15.1% (5) | 15.2% (32) |
| Student | 8.2% (11) | 0.0% (0) | 6.6% (11) |
| Homemaker | 1.5% (2) | 0.0% (0) | 1.2% (2) |
| Retired | 16.4% (22) | 39.4% (13) | 21.0% (35) |
| Alcohol | |||
| Yes | 29.8% (40) | 36.4% (12) | 31.1% (52) |
| No | 70.1% (94) | 63.6% (21) | 68.9% (115) |
| Drugs | |||
| Yes | 13.4% (18) | 3.0% (1) | 11.4% (19) |
| No | 86.6% (116) | 97.0% (32) | 88.6% (148) |
| Smoke | |||
| Yes | 34.3% (46) | 36.4% (12) | 34.7% (58) |
| No | 65.7% (88) | 63.6% (21) | 65.3% (109) |
| Mechanism of injury | |||
| MVC | 41.8% (56) | 12.1% (4) | 35.9% (60) |
| Assault | 8.2% (11) | 12.1% (4) | 9.0% (15) |
| Fall (n = 166) | 55.6% (74) | 78.8% (26) | 60.2% (100) |
| Sports | 4.5% (6) | 0.0% (0) | 3.6% (6) |
| LOC | |||
| Yes | 73.1% (98) | 72.7% (24) | 73.0% (122) |
| No | 26.9% (36) | 27.3% (9) | 26.9% (45) |
| LOC Information | |||
| Self-reported | 38.1% (51) | 27.3% (9) | 35.9% (60) |
| Unknown | 35.1% (47) | 39.4% (13) | 35.9 % (60) |
| Witnessed | 26.9% (36) | 33.3% (11) | 28.1% (47) |
| GCS | |||
| 15 | 90.3% (121) | 66.7% (22) | 85.6% (143) |
| 14 | 7.5% (10) | 18.2% (6) | 9.6% (16) |
| 13 | 0.75% (1) | 9.1% (3) | 2.4% (4) |
| 9 to 12 | 1.5% (2) | 6.1% (2) | 2.4% (4) |
| Initial biomarker concentration (median; 25th, 75th; pg/mL) | |||
| GFAP | 10 (4, 31) | 122 (20, 437) | 14 (5, 74) |
| UCH-L1 | 63 (27, 110) | 132 (98, 235) | 75 (32, 142) |
| S100B (n = 152) | 110 (70, 240) | 225 (180, 410) | 120 (80, 270) |
| Number of biomarker determinations per patient | |||
| 2 | 43.3% (58) | 12.1% (4) | 37.1% (62) |
| 3 | 21.0% (28) | 21.2% (7) | 21.0% (35) |
| 4 | 12.7% (17) | 27.3% (9) | 15.6% (26) |
| 5 | 23.1% (31) | 39.4% (13) | 26.3% (44) |
| Number of patients with values below LOD | |||
| GFAP | 66.4% (89) | 21.2% (7) | 57.5% (96) |
| UCH-L1 | 4.5% (6) | 0.0% (0) | 3.6% (6) |
| S100B (n = 145) | N/A | N/A | N/A |
| Number of patients with values below LLQ | |||
| GFAP | 73.1% (98) | 27.3% (9) | 64.1% (107) |
| UCH-L1 | 28.4% (38) | 3.0% (1) | 23.3% (39) |
| S100B (n = 145) | N/A | N/A | N/A |
| Number of biomarker samples during time intervals (n = 553) | |||
| 0–6 h | 48.9% (207) | 39.2% (51) | 46.6% (258) |
| > 6–12 h | 23.2% (98) | 18.5% (24) | 22.1% (122) |
| >12–18 h | 13.9% (59) | 20.8% (27) | 15.5% (86) |
| >18–24 h | 13.9% (59) | 21.5% (28) | 15.7% (87) |
CT, computed tomography; SD, standard deviation; MVC, motor vehicle collision; LOC, loss of consciousness; GCS, Glasgow Coma scale; GFAP, glial fibrillary acidic protein; UCH-L1, ubiquitin carboxyl-terminal hydrolase-L1; LOD, level of detection; N/A, not available; LLQ, lower limit of quantification.
The mean age of all 167 patients was 46.0 ± 17.8 years and 61.1% (95% CI 53.2% to 68.5%) were male. The main mechanisms of injury were motor vehicle crashes and/or falls. There were 143 of the total 167 patients (85.6%; 95% CI 79.4% to 90.6%) who had an initial GCS of 15 of which 22 (15.4%; 95% CI 9.9% to 22.4%) had a positive CT scan. Among patients with a GCS <15 (n = 24), 11 (45.8%; 95% CI 25.5% to 67.2%) had a positive CT scan. The initial serum concentration for each biomarker was higher among CT-positive patients (p < 0.0001 for UCH-L1, GFAP, and S100B). Table 5 details these and other patient characteristics.
Table 5.
Excluded Subjects
| Subjects (total n = 97) | |
|---|---|
| Age (mean ± SD) | 45.1 ± 19.1 |
| Sex | |
| Female | 39.2% (38) |
| Male | 60.8% (59) |
| Race | |
| White | 54.6% (53) |
| Black | 34.0% (33) |
| Other | 11.3% (11) |
| Alcohol | |
| Yes | 16.5% (16) |
| No | 83.5% (81) |
| Drugs | |
| Yes | 16.5% (16) |
| No | 83.5% (81) |
| Smoke | |
| Yes | 25.8% (25) |
| No | 74.2% (72) |
| Loss of consciousness | |
| Yes | 51.5% (50) |
| No | 48.5% (47) |
| GCS | |
| 15 | 94.8% (92) |
| 14 | 3.1% (3) |
| 13 | 2.1% (2) |
| 9 to 12 | 0% (0) |
| Initial biomarker values (median; 25th, 75th; pg/mL) | |
| GFAP | 5 (0, 19) |
| UCH-L1 | 51 (21, 100) |
| S100B (n = 93) | 110 (6, 170) |
| CT reading | |
| Positive | 6.2% (6) |
| Negative | 93.8% (91) |
SD, standard deviation; GCS, Glasgow Coma Scale; GFAP, glial fibrillary acidic protein; UCH-L1, ubiquitin carboxyl-terminal hydrolase-L1; CT, computed tomography.
Head CT results
Of the 167 CT scans obtained, 126 were classified as negative by both primary radiologists, 29 were classified as positive by both primary radiologists, and 11 received discrepant positive and negative classifications from the two primary radiologists (one radiologist did not read one case; Kappa = 0.80; 95% CI 0.69 to 0.91). After the final adjudicated by a third independent radiologist, 33 patients (19.8%; 95% CI 14.0% to 26.6%) had a scan that was positive for an acute traumatic intracranial lesion. Table 6 lists the findings considered positive and the number of patients with each specific finding. Since some patients had more than one abnormality, the total adds up to more than 33.
Table 6.
Definition of Acute Intracranial Lesion
| Acute intracranial lesion is defined as any trauma induced or related finding. Acute lesions may include the following and the number of each finding: | |
| N | |
| Extra-axial lesions | |
| Acute epidural hematoma | 3 |
| Acute subdural hematoma | 21 |
| Cortical contusion | 11 |
| Ventricular compression | 3 |
| Ventricular trapping | 0 |
| Brain herniation | 0 |
| Intraventricular hemorrhage | 1 |
| Hydrocephalus | 1 |
| Subarachnoid hemorrhage | 27 |
| Petechail hemorrhagic or bland sheer injury | 0 |
| Brain edema | 0 |
| Post-traumatic ischemia | 0 |
| Intracerebral hematoma | 0 |
| Dural venous sinus injury and/or thrombosis | 0 |
Biomarker samples
For the 167 study subjects, 553 samples were collected for both UCH-LI and GFAP. Sixty-two patients had two samples obtained prior to study termination and 105 patients had three or more samples obtained during the 24-h study period. There were a total of 278 serum samples available for S100B analysis from the 152 patients in whom S100B values were obtained (median of three samples per subject).
Graphical displays
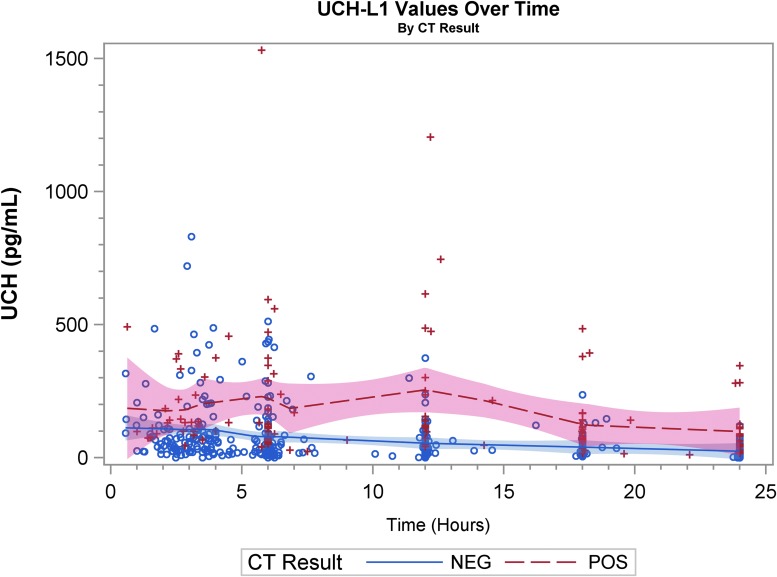
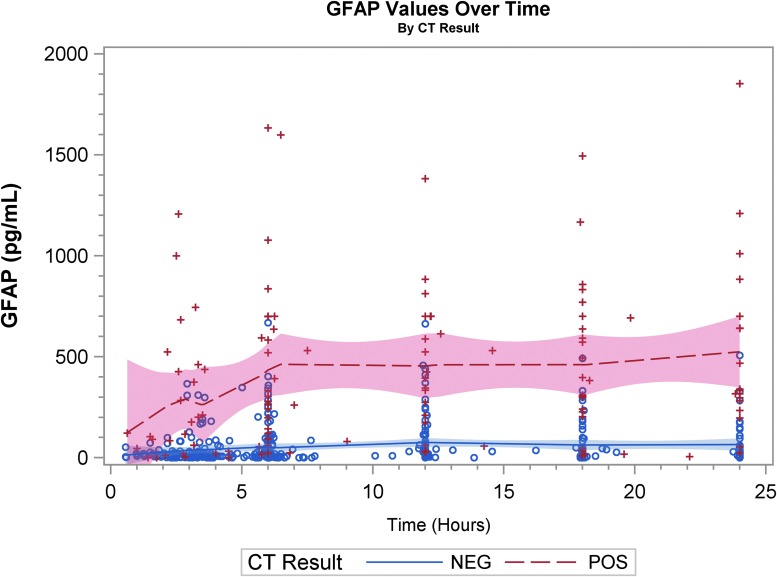
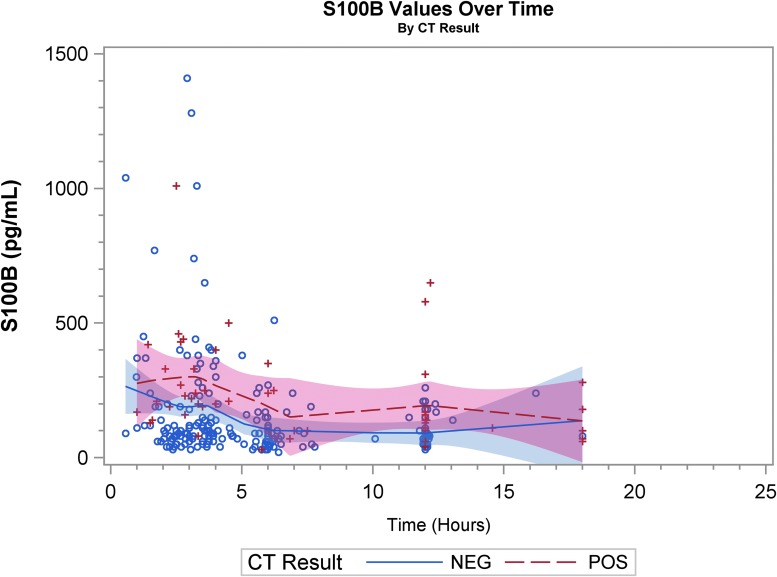
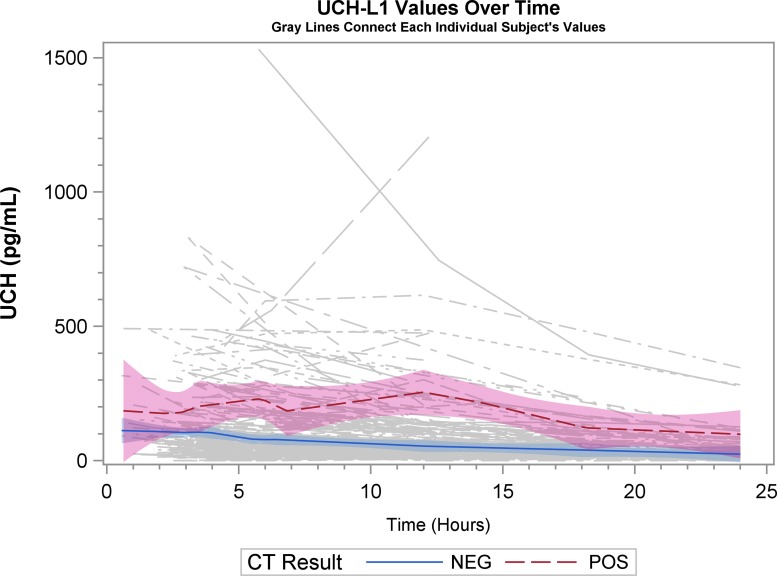
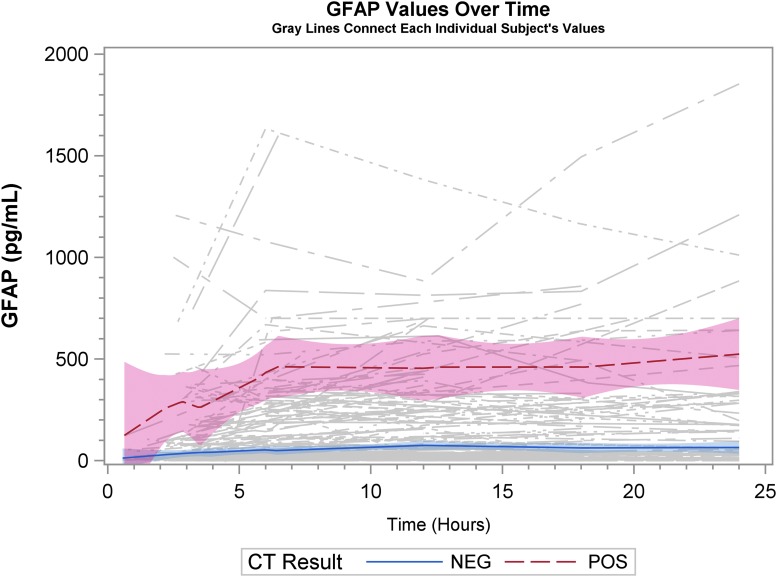
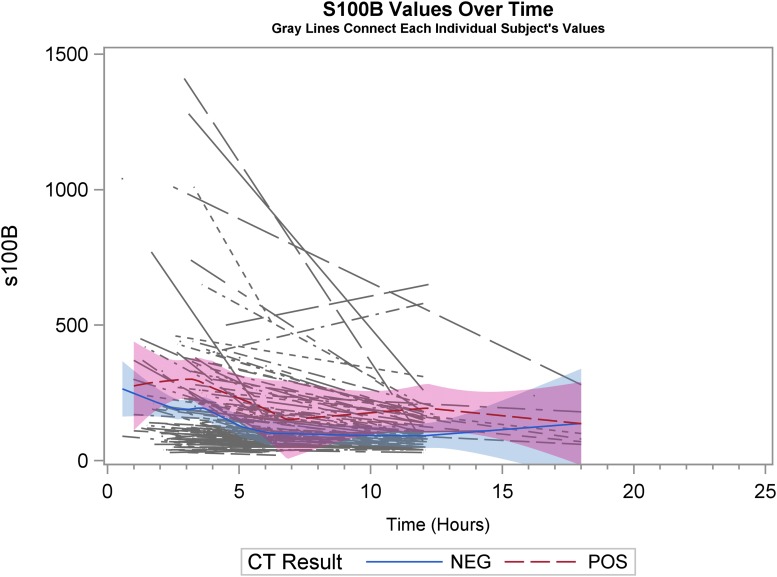
Graphical representation of biomarker levels during the first 24 h are shown. Figures 2A, 2B, and 2C are scatter plots and Figures 3A, 3B, and 3C are spaghetti plots all with the LOESS regression lines demonstrating biomarker values (please note the different concentrations scales for each biomarker). The figures suggest that UCH-L1 levels were elevated early but began to slowly decline after 12 h, whereas GFAP levels rose a bit later and remained elevated over the 24-h study time period and S100B levels seem to decline early. These same figures suggest that there was variability among patients in the starting point (intercept) and rate of change (slope) for all three biomarkers. When comparing the rate of change in serum biomarker concentration between CT-positive and CT-negative patients, there was little obvious difference for UCH-L1 and S100B, but GFAP concentration rose more rapidly in the CT-positive population. There were significant correlations in biomarker values measured over 24 h for UCH-L1 and S100B, UCH-L1 and GFAP, and GFAP and S100B (Spearman correlation coefficient 0.77, 0.50, and 0.37, respectively; p < 0.0001 for all three comparisons).
FIG. 2A.
Scatter plot with the locally weighted scatter plot smoothing regression lines demonstrating ubiquitin carboxyl-terminal hydrolase-L1 (UCH-L1) values. “+” and “o” represent the biomarker value (Y-axis) concentration at the given time-point (X-axis). CT, computed tomography.
FIG. 2B.
Scatter plot with the locally weighted scatter plot smoothing regression lines demonstrating glial fibrillary acidic protein (GFAP) values. “+” and “o” represent the biomarker value (Y-axis) concentration at the given time-point (X-axis). CT, computed tomography.
FIG. 2C.
Scatter plot with the locally weighted scatter plot smoothing regression lines demonstrating S100B values. “+” and “o” represent the biomarker value (Y-axis) concentration at the given time-point (X-axis). CT, computed tomography.
FIG. 3A.
Spaghetti plot with the locally weighted scatter plot smoothing regression lines demonstrating ubiquitin carboxyl-terminal hydrolase-L1 (UCH-L1) values. Each thin line of the spaghetti plot represents one patient. CT, computed tomography.
FIG. 3B.
Spaghetti plots with the locally weighted scatter plot smoothing regression lines demonstrating glial fibrillary acidic protein (GFAP) values. Each thin line of the spaghetti plot represents one patient. CT, computed tomography.
FIG. 3C.
Spaghetti plots with the locally weighted scatter plot smoothing regression lines demonstrating S100B values. Each thin line of the spaghetti plot represents one patient. CT, computed tomography.
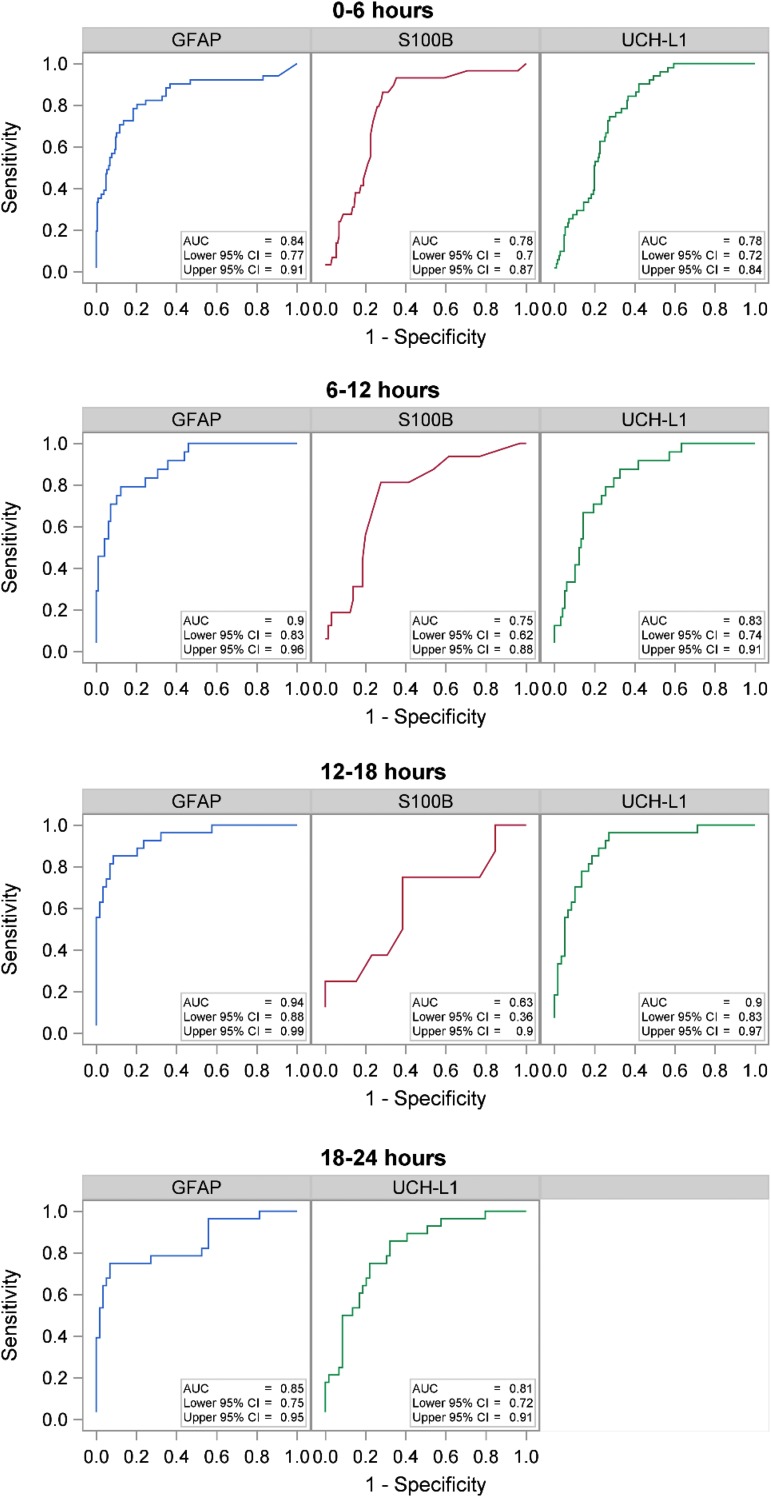
Graphics for each biomarkers' AUROC curves, paneled by time intervals, are shown with the AUROC (95% CI) inset within each (Fig. 4). No formal statistical testing was done but it appears that GFAP and UCH-L1 perform best in the 6-12 h and 12-18 h post-injury windows.
FIG. 4.
Area under the receiver operator characteristic curve (AUC) for each biomarker stratified by 6-h time intervals. CI, confidence interval; GFAP, glial fibrillary acidic protein; UCH-L1, ubiquitin carboxyl-terminal hydrolase-L1.
Biomarker kinetics model results
The final models included sex, GCS (< 15 vs. 15), time, CT results, and the CT*Time interaction. Other considered variables were removed since they exhibited collinearity (such as LOC) or did not add more information to the models (such as age). Consistent with the graphical display, all three biomarkers were, on average, higher among CT-positive versus CT-negative patients. This was true even after accounting for the initial GCS score. Only GFAP, however, significantly rose over time among CT-positive patients, compared with CT-negative patients (estimate = 0.036; 95% CI 0.02 to 0.05; p < 0.001). When back transforming the estimate of the log of GFAP (e0.03601 = 1.0367), we found that GFAP concentrations rose by 3.7% (95% CI 2.2% to 5.1%) per hour among CT-positive patients, compared with CT-negative patients. Table 7 shows the complete results for the fixed effects for all three biomarkers. All three biomarker concentrations were, on average, higher for patients who had a positive head CT, and both GFAP and UCH-L1 (but not S100B) were higher among patients who presented with a GSC <15. The random effects component analysis for all biomarkers found there was variation in the intercept and slope among study patients.
Table 7.
Main Models—Natural Log Transformation
| UCH-L1 (n = 167) | Estimate | SE | 95% CI | p Value | % Change |
|---|---|---|---|---|---|
| Intercept | 4.223 | 0.099 | 4.027, 4.418 | <0.0001 | |
| Female | −0.016 | 0.117 | −0.247, 0.214 | 0.89 | −1.6 |
| GCS <15 | 0.635 | 0.169 | 0.301, 0.968 | 0.0002 | 88.7 |
| Time | −0.043 | 0.004 | −0.051, −0.036 | <0.0001 | −4.3 |
| Positive CT | 0.710 | 0.195 | 0.326, 1.094 | 0.0003 | 103.4 |
| Positive CT*Time | 0.010 | 0.007 | −0.004, 0.024 | 0.15 | 1.0 |
| GFAP (n = 167) | Estimate | SE | 95% CI | p Value | % Change |
|---|---|---|---|---|---|
| Intercept | 3.420 | 0.105 | 3.212, 3.628 | <0.0001 | |
| Female | 0.037 | 0.146 | −0.251, 0.324 | 0.8 | 3.8 |
| GCS <15 | 0.355 | 0.209 | −0.057, 0.767 | 0.091 | 42.6 |
| Time | 0.004 | 0.004 | −0.004, 0.011 | 0.37 | 0.4 |
| Positive CT | 1.412 | 0.193 | 1.031, 1.793 | <0.0001 | 310.5 |
| Positive CT*Time | 0.036 | 0.007 | 0.022, 0.050 | <0.0001 | 3.7 |
| S100B (n = 152) | Estimate | SE | 95% CI | p Value | % Change |
|---|---|---|---|---|---|
| Intercept | 5.019 | 0.106 | 4.810, 5.227 | <0.0001 | |
| Female | 0.089 | 0.117 | −0.144, 0.321 | 0.45 | 9.3 |
| GCS <15 | 0.171 | 0.170 | −0.166, 0.507 | 0.32 | 18.6 |
| Time | −0.070 | 0.007 | −0.084, −0.057 | <0.0001 | −6.8 |
| Positive CT | 0.600 | 0.202 | 0.202, 0.998 | 0.003 | 82.2 |
| Positive CT*Time | 0.008 | 0.012 | −0.015, 0.032 | 0.475 | 0.9 |
% Change is for listed features relative to other (i.e., having a GCS <15, compared with a GCS = 15, results in a 95.0% change in serum UCH-L1 concentration) except time is for each 1-h increase. Calculated by (Estimate − 1) × 100 using unrounded estimates (rounded estimates presented in Table).
UCH-L1, ubiquitin carboxyl-terminal hydrolase-L1; SE, standard error; CI, confidence interval; GCS, Glasgow Coma Scale; CT, computed tomography; GFAP, glial fibrillary acidic protein.
The sensitivity analysis looking at only patients with GCS = 15 demonstrated similar findings. The sensitivity analysis that used reported values even when below the LOD and LLQ also were similar, with the exception that UCH-L1 did show an increase over time in CT-positive patients relative to CT-negative patients (a CT*Time interaction, estimate 0.028, 95% CI 0.007 to 0.048; p = 0.01), likely due to the lowering of some values below the LOD (Table 8). Since the lower 95% confidence interval is close to 0, this result must be interpreted with caution, does not represent our main result, and is exploratory in nature.
Table 8.
Sensitivity Analysis
| Only GCS = 15 | Actual reported values* | |||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | 95% CI | p Value | Estimate | SE | 95% CI | p Value | |
| UCH-L1 | (n = 143) | (n = 167) | ||||||
| Intercept | 4.320 | 0.102 | 4.119, 4.521 | <0.0001 | 4.155 | 0.125 | 3.908, 4.402 | <0.0001 |
| Female | −0.164 | 0.118 | −0.396, 0.069 | 0.17 | −0.016 | 0.153 | −0.318, 0.286 | 0.92 |
| GCS <15 | 0.687 | 0.221 | 0.250, 1.123 | 0.002 | ||||
| Time | −0.043 | 0.004 | −0.051, −0.036 | <0.0001 | −0.063 | 0.005 | −0.074, −0.052 | <0.0001 |
| Positive CT | 0.505 | 0.228 | 0.056, 0.955 | 0.028 | 0.746 | 0.242 | 0.270, 1.223 | 0.002 |
| Positive CT*Time | 0.009 | 0.009 | −0.008, 0.026 | 0.30 | 0.028 | 0.011 | 0.007, 0.048 | 0.01 |
| GFAP | ||||||||
| Intercept | 3.494 | 0.107 | 3.282, 3.707 | <0.0001 | 2.540 | 0.176 | 2.193, 2.887 | <0.0001 |
| Female | −0.019 | 0.155 | −0.324, 0.286 | 0.90 | −0.078 | 0.237 | −0.546, 0.389 | 0.74 |
| GCS <15 | 0.591 | 0.341 | −0.081, 1.262 | 0.084 | ||||
| Time | 0.002 | 0.003 | −0.005, 0.008 | 0.56 | 0.008 | 0.006 | −0.004, 0.0207 | 0.19 |
| Positive CT | 1.134 | 0.218 | 0.704, 1.564 | <0.0001 | 1.959 | 0.326 | 1.317, 2.601 | <0.0001 |
| Positive CT*Time | 0.036 | 0.007 | 0.023, 0.050 | <0.0001 | 0.038 | 0.012 | 0.016, 0.061 | 0.001 |
| Estimate | SE | 95% CI | p Value | Estimate | SE | 95% CI | p Value | |
|---|---|---|---|---|---|---|---|---|
| S100B | (n = 130) | (n = 152) | ||||||
| Intercept | 5.099 | 0.111 | 4.880, 5.318 | <0.0001 | 5.019 | 0.106 | 4.810, 5.227 | <0.0001 |
| Female | −0.005 | 0.122 | −0.247, 0.238 | 0.97 | 0.089 | 0.120 | −0.144, 0.321 | 0.45 |
| GCS <15 | 0.171 | 0.170 | −0.166, 0.507 | 0.32 | ||||
| Time | −0.072 | 0.007 | −0.085, −0.058 | <0.0001 | −0.070 | 0.007 | −0.084, −0.057 | <0.0001 |
| Positive CT | 0.429 | 0.238 | −0.044, 0.901 | 0.075 | 0.600 | 0.202 | 0.202, 0.998 | 0.003 |
| Positive CT*Time | 0.009 | 0.013 | −0.020, 0.038 | 0.49 | 0.008 | 0.012 | −0.015, 0.032 | 0.47 |
This uses reported values even if below the limit of detection; values of zero were recorded to 1 due to need of log transformation.
GCS, Glasgow Coma Scale; UCH-L1, ubiquitin carboxyl-terminal hydrolase-L1; SE, standard error; CI, confidence interval; CT, computed tomography; GFAP, glial fibrillary acidic protein.
Discussion
This prospective multi-center observational study evaluated the usefulness of serial serum concentrations of GFAP, UCH-L1, and S100B during the initial 24 h after mild-to-moderate traumatic brain injury to differentiate between patients with an acute injury on CT and those without. Our results demonstrated that all three serum biomarker concentrations were, on average, higher in CT-positive versus CT-negative patients with suspected mmTBI. When considering serum concentration changes over time, only GFAP showed a significant increase in CT-positive patients, compared with CT-negative patients. Among patients with a positive head CT, GFAP levels rise on average by 3.7% per hour, compared with suspected mmTBI patients with a negative CT, for which a more flat trend line was noted, suggesting that GFAP may be a more useful marker when evaluating these patients. Neither UCH-L1 nor S100B were found to have a significant difference in rate of change in CT-positive compared with CT-negative patients. Our data is applicable to individual patients since random effects modeling (random intercept and slope) was utilized to account for patient-level variations in initial biomarker value and differences in changes over time. These results are consistent with and complimentary to recently published data regarding the time courses of UCH-L1 and GFAP.20 Additionally, this study graphically described and quantitated the kinetics of these biomarkers during the 24-h post-injury period.
While GFAP, UCH-L1, and S100B have all been studied in mmTBI, only limited information about the temporal profiles of these circulating biomarkers is available. Therefore, we conducted one of the first studies designed to repeatedly measure GFAP, UCH-L1, and S100B in the same patient cohort, with the goal of further characterizing differences in the biomarkers' kinetics among CT-positive and CT-negative patients. The biomarkers' diagnostic ability to detect acute intracranial lesions on head CT scans was compared at approximately 6-h intervals over the course of 24 h to determine optimal testing strategies for mmTBI in a clinical setting. Our results suggest that GFAP offers the widest temporal profile for differentiating between positive and negative head CT scans, and may therefore be the most robust biomarker to aid clinicians in identifying patients with acute intracranial lesions during the initial 24 h after injury.
Consistent with previous reports, the present study found that the serum concentrations of GFAP, UCH-L1, and S100B were all persistently higher among mmTBI patients with CT-proven intracranial lesions. While all three biomarkers were detected in the serum of CT-positive patients within 6 h of mmTBI, only GFAP concentration continued to increase significantly over the initial 24 h among CT-positive patients, compared with CT-negative patients. This finding confirmed, in an independent sample of patients with mmTBI, the results of Papa and colleagues, who reported serum GFAP levels peaked 20 h after mmTBI among those with intracranial lesions detected on CT, and slowly declined over 72 h, leading to better diagnostic accuracy than UCH-L1 at all time-points.20 As a supplement to our results, the same study compared the performance of GFAP and UCH-L1 among patients without clinical evidence of brain injury (trauma controls) and in trauma patients with clinical evidence of traumatic brain injury over the course of 7 days post-injury. Significantly higher levels of GFAP and UCH-L1 were found in patients with mmTBI than in the trauma controls, suggesting that both GFAP and UCH-L1 are specific for brain injury and not merely elevated in all patients presenting with trauma.20
Our study also found that UCH-L1 levels were significantly elevated early after injury among CT-positive patients, but remained relatively stable before falling later in the time course and again, supports other's data showing that UCH-L1 was detectible in serum within 1 h of injury, peaked at 8 h, and subsequently decreased over 48 h.20 This early peak in UCH-L1 among those with CT-proven intracranial lesions also is consistent with our initial study, which demonstrated UCH-L1 had improved ability to rule out CT findings when obtained within 6 h of injury, compared with GFAP and S100B.18 The sensitivity analyses that incorporated reported values (even when below the LOD and LLQ) did suggest that UCH-L1 may increase over time in CT-positive patients must be interpreted with caution given that it was exploratory analysis and the previously described difficulty and less than optimal methods to “assign” values when the results are below the test thresholds.23–26
S100B, although used in some parts of the world,21 was not found to have a kinetics pattern that would help in the later diagnosis or monitoring of patients with mmTBI. Our findings were in contrast to other work that showed S100B levels rose and peaked at 27.2 h post-injury; however, that study examined more seriously injured patients (median GCS = 7, mortality 9.7%) and may not be a good comparator.28
Finally, even though the study was not designed to determine the utility of following biomarkers for pre-clinical studies screening for potential therapies, clinical progression of injury among patients with TBI, or as a surrogate outcome, our results suggest that GFAP may be an ideal candidate for such studies and use.29,30
Limitations
Limitations of the present study include the fact that we lacked biomarker measurements at every time-point on every patient. However, with this in mind we chose to analyze our data using a generalization of mixed linear models, which are designed to handle missing data without dropping the entire case. There were fewer patients and measurements of S100B since it was instituted later in the protocol, making these results less certain. Due to the distribution of biomarker values, a log transformation was used, despite the fact that it makes interpretation of the results less intuitive than when actual non-transformed values are used. This study included patients with suspected mmTBI and a GCS ranging from 9-15 but our sensitivity analysis confirmed similar results among patients with a GCS = 15, making our results potentially applicable to the minimally injured patient. Additionally, this early study's primary objective was to evaluate the association between CT findings and serum biomarker concentrations over time, and therefore it did not assess patient-centered clinical outcomes, such as the need for neurosurgical intervention. This study was not designed to identify those patients who sustained neuronal injuries in the absence of positive CT findings nor did the study include patients for whom the treating clinician determined that a head CT was not warranted (minimal to no evidence of mmTBI). Finally, the study was not designed to determine the need for repeat biomarker measurements to follow a patient's clinical course but does suggest potential value in doing so.
Conclusion
In conclusion, our data suggest that the diagnostic ability of UCH-L1 may be limited to the early h after mild-to-moderate TBI, whereas rising serum GFAP concentrations over the initial 24 h post-injury were associated with a positive head CT. The longer half-life and wider temporal profile of GFAP indicates it may be a more robust biomarker to detect patients with CT-proven intracranial lesions, and although further study is needed, these results suggest that GFAP levels may be a useful test to follow throughout a patient's first 24 h of care.
Acknowledgments
This work is supported by the U.S. Army Medical Research and Material Command under Contract N. W81XWH-06-1-0517 and Contract N. W81XWH-10-C-0251. The views, opinions, and/or findings contained in this report are those of the authors and should not be construed as an official Department of the Army position, policy or decision unless so designated by other documentation. The authors would also like to acknowledge the support of Banyan Biomarkers, Inc. personnel Ronald Hayes and Art Weber.
ClinicalTrials.gov Identifier: NCT01295346.
Author Disclosure Statement
Drs. Welch and Lewis report receiving contract research funding from Banyan Biomarkers Inc., but also report not receiving stocks or royalties from the company nor benefiting financially from this publication. Dr. Papa reports being an unpaid scientific consultant for Banyan Biomarkers Inc., but also reports not receiving stocks or royalties from the company nor benefiting financially from this publication. No other disclosures were reported.
References
- 1.Centers for Disease Control and Prevention. (2015). Report to Congress onTraumatic Brain Injury in the United States: Epidemiology and Rehabilitation. National Center for Injury Prevention and Control; Division of Unintentional Injury Prevention: Atlanta, GA [Google Scholar]
- 2.Haydel M.J., Preston C.A., Mills T.J., Luber S., Blaudeau E., and DeBlieux P.M. (2000). Indications for computed tomography in patients with minor head injury. N. Engl. J. Med. 343, 100–105 [DOI] [PubMed] [Google Scholar]
- 3.Stiell I.G., Wells G.A., Vandemheen K., Clement C., Lesiuk H., Laupacis A., McKnight R.D., Verbeek R., Brison R., Cass D., Eisenhauer M.E., Greenberg G., and Worthington J. (2001). The Canadian CT Head Rule for patients with minor head injury. Lancet 357, 1391–1396 [DOI] [PubMed] [Google Scholar]
- 4.Brenner D.J. and Hall E.J. (2007). Computed tomography—an increasing source of radiation exposure. N. Engl. J. Med. 357, 2277–2284 [DOI] [PubMed] [Google Scholar]
- 5.McCollough C.H. (2016). To scan or not to scan: consideration of medical benefit in the justification of CT scanning. Health Phys. 110, 287–290 [DOI] [PubMed] [Google Scholar]
- 6.Korley F.K., Morton M.J., Hill P.M., Mundangepfupfu T., Zhou T., Mohareb A.M., and Rothman R.E. (2013). Agreement between routine emergency department care and clinical decision support recommended care in patients evaluated for mild traumatic brain injury. Acad. Emerg. Med. 20, 463–469 [DOI] [PubMed] [Google Scholar]
- 7.Jeter C.B., Hergenroeder G.W., Hylin M.J., Redell J.B., Moore A.N., and Dash P.K. (2013). Biomarkers for the diagnosis and prognosis of mild traumatic brain injury/concussion. J Neurotrauma 30, 657–670 [DOI] [PubMed] [Google Scholar]
- 8.Yokobori S., Hosein K., Burks S., Sharma I., Gajavelli S., and Bullock R. (2013). Biomarkers for the clinical differential diagnosis in traumatic brain injury—a systematic review. CNS Neurosci. Ther. 19, 556–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zongo D., Ribereau-Gayon R., Masson F., Laborey M., Contrand B., Salmi L.R., Montaudon D., Beaudeux J.L., Meurin A., Dousset V., Loiseau H., and Lagarde E. (2012). S100-B protein as a screening tool for the early assessment of minor head injury. Ann. Emerg. Med. 59, 209–218 [DOI] [PubMed] [Google Scholar]
- 10.Papa L., Ramia M.M., Edwards D., Johnson B.D., and Slobounov S.M. (2015). Systematic review of clinical studies examining biomarkers of brain injury in athletes after sports-related concussion. J. Neurotrauma 32, 661–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zetterberg H., Smith D.H., and Blennow K. (2013). Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat. Rev. Neurol. 9, 201–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metting Z., Wilczak N., Rodiger L.A., Schaaf J.M., and van der Naalt J. (2012). GFAP and S100B in the acute phase of mild traumatic brain injury. Neurology 78, 1428–1433 [DOI] [PubMed] [Google Scholar]
- 13.Muller K., Townend W., Biasca N., Unden J., Waterloo K., Romner B., and Ingebrigtsen T. (2007). S100B serum level predicts computed tomography findings after minor head injury. J. Trauma 62, 1452–1456 [DOI] [PubMed] [Google Scholar]
- 14.Biberthaler P., Linsenmeier U., Pfeifer K.J., Kroetz M., Mussack T., Kanz K.G., Hoecherl E.F., Jonas F., Marzi I., Leucht P., Jochum M., and Mutschler W. (2006). Serum S-100B concentration provides additional information fot the indication of computed tomography in patients after minor head injury: a prospective multicenter study. Shock 25, 446–453 [DOI] [PubMed] [Google Scholar]
- 15.Papa L., Lewis L.M., Falk J.L., Zhang Z., Silvestri S., Giordano P., Brophy G.M., Demery J.A., Dixit N.K., Ferguson I., Liu M.C., Mo J., Akinyi L., Schmid K., Mondello S., Robertson C.S., Tortella F.C., Hayes R.L., and Wang K.K. (2012). Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann. Emerg. Med. 59, 471–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMahon P.J., Panczykowski D.M., Yue J.K., Puccio A.M., Inoue T., Sorani M.D., Lingsma H.F., Maas A.I., Valadka A.B., Yuh E.L., Mukherjee P., Manley G.T., Okonkwo D.O., and Investigators T.-T. (2015). Measurement of the glial fibrillary acidic protein and its breakdown products GFAP-BDP biomarker for the detection of traumatic brain injury compared to computed tomography and magnetic resonance imaging. J. Neurotrauma 32, 527–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbosa R.R., Jawa R., Watters J.M., Knight J.C., Kerwin A.J., Winston E.S., Barraco R.D., Tucker B., Bardes J.M., and Rowell S.E. (2012). Evaluation and management of mild traumatic brain injury: an Eastern Association for the Surgery of Trauma practice management guideline. J. Trauma Acute Care Surg. 73, S307–S314 [DOI] [PubMed] [Google Scholar]
- 18.Welch R.D., Ayaz S.I., Lewis L.M., Unden J., Chen J.Y., Mika V.H., Saville B., Tyndall J.A., Nash M., Buki A., Barzo P., Hack D., Tortella F.C., Schmid K., Hayes R.L., Vossough A., Sweriduk S.T., and Bazarian J.J. (2016). Ability of serum glial fibrillary acidic protein, ubiquitin C-terminal hydrolase-L1, and S100B to differentiate normal and abnormal head computed tomography findings in patients with suspected mild or moderate traumatic brain injury. J. Neurotrauma 33, 203–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang X.J., Glushakova O., Mondello S., Van K., Hayes R.L., and Lyeth B.G. (2015). Acute temporal profiles of serum levels of UCH-L1 and GFAP and relationships to neuronal and astroglial pathology following traumatic brain injury in rats. J. Neurotrauma 32, 1179–1189 [DOI] [PubMed] [Google Scholar]
- 20.Papa L., Brophy G.M., Welch R.D., Lewis L.M., Braga C.F., Tan C.N., Ameli N.J., Lopez M.A., Haeussler C.A., Mendez Giordano D.I., Silvestri S., Giordano P., Weber K.D., Hill-Pryor C., and Hack D.C. (2016). Time course and diagnostic accuracy of glial and neuronal blood biomarkers GFAP and UCH-L1 in a large cohort of trauma patients with and without mild traumatic brain injury. JAMA Neurol. 73, 551–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unden J., Ingebrigtsen T., andRomner B.; Scandinavian Neurotrauma Committee (SNC). (2013). Scandinavian guidelines for initial management of minimal, mild and moderate head injuries in adults: an evidence and consensus-based update. BMC Med 11, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bazarian J.J., Blyth B.J., He H., Mookerjee S., Jones C., Kiechle K., Moynihan R., Wojcik S.M., Grant W.D., Secreti L.M., Triner W., Moscati R., Leinhart A., Ellis G.L., and Khan J. (2013). Classification accuracy of serum Apo A-I and S100B for the diagnosis of mild traumatic brain injury and prediction of abnormal initial head computed tomography scan. J. Neurotrauma 30, 1747–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Succop P.A., Clark S., Chen M., and Galke W. (2004). Imputation of data values that are less than a detection limit. J. Occup. Environ. Hyg. 1, 436–441 [DOI] [PubMed] [Google Scholar]
- 24.Xie X., Xue X., Gange S.J., Strickler H.D., and Kim M.Y.; WIHS HPV Study Group. (2012). Estimation and inference on correlations between biomarkers with repeated measures and left-censoring due to minimum detection levels. Stat. Med. 31, 2275–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keizer R.J., Jansen R.S., Rosing H., Thijssen B., Beijnen J.H., Schellens J.H., and Huitema A.D. (2015). Incorporation of concentration data below the limit of quantification in population pharmacokinetic analyses. Pharmacol. Res. Perspect. 3, e00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillespie B.W., Chen Q., Reichert H., Franzblau A., Hedgeman E., Lepkowski J., Adriaens P., Demond A., Luksemburg W., and Garabrant D.H. (2010). Estimating population distributions when some data are below a limit of detection by using a reverse Kaplan-Meier estimator. Epidemiology 21 Suppl 4, S64–S70 [DOI] [PubMed] [Google Scholar]
- 27.Lang T.A. and Altman D.G. (2015). Basic statistical reporting for articles published in biomedical journals: the “Statistical Analyses and Methods in the Published Literature” or the SAMPL Guidelines. Int. J. Nurs. Stud 52, 5–9 [DOI] [PubMed] [Google Scholar]
- 28.Ercole A., Thelin E.P., Holst A., Bellander B.M., and Nelson D.W. (2016). Kinetic modelling of serum S100b after traumatic brain injury. BMC Neurol. 16, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mondello S., Shear D.A., Bramlett H.M., Dixon C.E., Schmid K.E., Dietrich W.D., Wang K.K., Hayes R.L., Glushakova O., Catania M., Richieri S.P., Povlishock J.T., Tortella F.C., and Kochanek P.M. (2016). Insight into pre-clinical models of traumatic brain injury using circulating brain damage biomarkers: operation brain trauma therapy. J. Neurotrauma 33, 595–605 [DOI] [PubMed] [Google Scholar]
- 30.Lei J., Gao G., Feng J., Jin Y., Wang C., Mao Q., and Jiang J. (2015). Glial fibrillary acidic protein as a biomarker in severe traumatic brain injury patients: a prospective cohort study. Crit. Care 19, 362. [DOI] [PMC free article] [PubMed] [Google Scholar]