Abstract
There is little consensus regarding many post-cardiac arrest care parameters. Variability in such practices could confound the results and generalizability of post-arrest care research. We sought to characterize the variability in post-cardiac arrest care practice in Korea and the United States. A 54-question survey was sent to investigators participating in one of two research groups in South Korea (Korean Hypothermia Network [KORHN]) and the United States (National Post-Arrest Research Consortium [NPARC]). Single investigators from each site were surveyed (N = 40). Participants answered questions based on local institutional protocols and practice. We calculated descriptive statistics for all variables. Forty surveys were completed during the study period with 30 having greater than 50% of questions completed (75% response rate; 24 KORHN and 6 NPARC). Most centers target either 33°C (N = 16) or vary the target based on patient characteristics (N = 13). Both bolus and continuous infusion dosing of sedation are employed. No single indication was unanimous for cardiac catheterization. Only six investigators reported having an institutional protocol for withdrawal of life-sustaining therapy (WLST). US patients with poor neurological prognosis tended to have WLST with subsequent expiration (N = 5), whereas Korean patients are transferred to a secondary care facility (N = 19). Both electroencephalography modality and duration vary between institutions. Serum biomarkers are commonly employed by Korean, but not US centers. We found significant variability in post-cardiac arrest care practices among US and Korean medical centers. These practice variations must be taken into account in future studies of post-arrest care.
Keywords: : cardiac arrest, targeted temperature management, therapeutic hypothermia, critical care neuroprognostication, withdrawal of life-sustaining therapies
Introduction
The approach to managing temperature after cardiac arrest has evolved significantly over the last 15 years, leaving considerable room for practice variation (Camp-Rogers et al., 2013; Kotini-Shah et al., 2016). Preclinical animal work almost uniformly supports lowering body temperature after cardiac arrest (Busto et al., 1987, 1989; Sterz et al., 1991; Yamashita et al., 1991; Kuboyama et al., 1993; Nozari et al., 2004). Whereas two initial trials demonstrated improved outcomes in comatose survivors of out of hospital cardiac arrest (OHCA) with therapeutic hypothermia (TH) targeted at 32–34°C (Bernard et al., 2002; Hypothermia after Cardiac Arrest Study, 2002), a subsequent trial was neutral for short-term and long-term survival in patients treated with either TH at 33°C or targeted temperature management (TTM) strategy at 36°C (Nielsen et al., 2013; Cronberg et al., 2015; Lilja et al., 2015). A pediatric trial of OHCA found the same neutral results for either 33°C or 36.8°C (Moler et al., 2015; Slomine et al., 2016), but has been criticized as being underpowered to detect subpopulations that may benefit from deeper cooling (Vincent and Taccone, 2015). In the wake of these trials, institutions target to either 33°C or 36°C and the optimal target temperature remains a topic of debate (Bernard, 2014; Kim et al., 2015a; Polderman and Varon, 2015a, 2015b). A 2015 international consensus statement actually supports variability in practice, broadening the recommended temperature range to 32–36 degrees centigrade, and emphasizing the avoidance of hyperthermia (Callaway et al., 2015).
By comparison, there is even less evidence to make specific recommendations about other aspects of temperature management (duration, cooling and rewarming rates, and induction and maintenance methods), let alone cardiac catheterization, neurological prognostication, and withdrawal of life-sustaining therapy (WLST). Unknown variability in any of these practices could potentially influence outcomes in observational studies and represent site-level confounders in multicenter prospective trials (Geocadin et al., 2012; Perman et al., 2012; Geri et al., 2015; Elmer et al., 2016).
The objective of the current study is to describe post-cardiac arrest care practices among an international group of medical centers in the TTM (post-Nielsen) era. We hypothesize there will be a degree of practice variability in aspects of post-arrest care, including target temperature, duration at target temperature, neuromonitoring devices utilized during temperature management, indications and contraindications for cardiac catheterization, neurological prognostication, and disposition of patients with poor neurological prognosis.
Methods
The University of Pittsburgh Institutional Review Board granted IRB exemption for this voluntary survey study. The survey was constructed initially during a meeting of study investigators. Multiple iterations and revisions were composed through email communication until a consensus was reached. A 54-question survey concerning post-cardiac arrest care practices was sent to investigators participating in one of two research groups in South Korea (Korean Hypothermia Network [KORHN]) and the United States (National Post-Arrest Research Consortium [NPARC]) through Qualtrics survey software (Provo, UT). The survey was available in both English and Korean. The KORHN was founded in 2011 and consists of 35 medical centers in South Korea (Kim et al., 2015b). Participating centers populate a shared registry with common data elements for OHCA patients. NPARC consists of seven tertiary care medical centers in the United States and was established to investigate treatment strategies for OHCA and perform clinical trials (Donnino et al., 2011, 2014). Participating investigators from both KORHN and NPARC sites are involved in the care of OHCA patients from admission to hospital discharge.
We sent the survey to a single principle investigator from each KORHN and NPARC site (N = 40) (Appendix). Investigators were instructed to answer survey questions based on local institutional protocols and practice of OHCA patients that achieve return of spontaneous circulation (ROSC). OHCA was defined as a patient that received chest compressions outside of a hospital from a healthcare provider, regardless of initial rhythm. ROSC was defined as sustained return of spontaneous circulation for greater than 20 minutes. The survey was sent in December 2015 and final data collection was completed in January 2016. Survey responses were included in the analysis if greater than 50% of questions were answered. We calculated descriptive statistics for all variables using STATA 13.1 (College Station, TX). We compared practices between high- and low-volume Korean (≤50 OHCA per year) and US centers. Data presented below compares only Korean and US centers as practice did not significantly differ between low- and high-volume Korean centers (data not shown).
Results
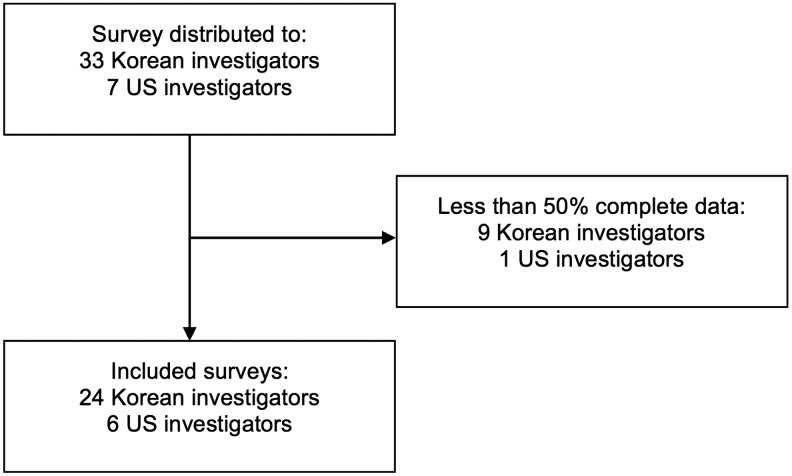
The survey (Fig. 1) was sent to a total of 33 South Korean and 7 US investigators. Forty surveys were completed during the study period for a 100% overall response rate with 30/40 (75%; 24 KORHN and 6 NPARC), having greater than 50% of questions completed, and characteristics of surveyed institutions and investigators being listed in Table 1 and Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/ther).
FIG. 1.
Consort diagram of study population.
Table 1.
Demographics of Surveyed Investigators and Hospitals
| Number of arrests treated at hospital per year | USA (N = 6), n (%) | Korea (N = 24), n (%) |
|---|---|---|
| <10 | 0 (0) | 0 (0) |
| 10–24 | 0 (0) | 2 (8) |
| 25–49 | 0 (0) | 4 (17) |
| 50–74 | 1 (16) | 2 (8) |
| 75–99 | 1 (16) | 3 (13) |
| 100–124 | 2 (34) | 2 (8) |
| >125 | 2 (34) | 11 (46) |
| Number of arrests treated with TTM per year | N = 5 | |
|---|---|---|
| <10 | 0 (0) | 4 (17) |
| 10–24 | 0 (0) | 8 (33) |
| 25–49 | 2 (40) | 7 (29) |
| 50–74 | 1 (20) | 5 (21) |
| 75–99 | 1 (20) | 0 (0) |
| 100–124 | 0 (0) | 0 (0) |
| >125 | 1 (20) | 0 (0) |
| Number of post-arrest cardiac catheterizations per year | N = 5 | |
|---|---|---|
| <10 | 1 (16) | 6 (25) |
| 10–19 | 2 (34) | 8 (32) |
| 20–29 | 1 (16) | 6 (25) |
| 30–39 | 0 (0) | 3 (13) |
| 40–49 | 0 (0) | 0 (0) |
| 50–59 | 1 (16) | 1 (5) |
| 60–70 | 0 (0) | 0 (0) |
TTM, targeted temperature management.
Targeted temperature management
Most centers target either 33°C (N = 16) or vary the target based on patient characteristics (N = 13) (Table 2). Variables used to determine target temperature included degree of brain injury, bleeding, and degree of hemodynamic instability. One center targets only 36°C. Single methods of cooling are used at most institutions (Surface cooling blankets = 4; Endovascular = 8; Surface hydrogel = 13). Patients are kept at target for either 24 hours after ROSC (N = 12) or 24 hours after goal temperature was reached (N = 16). One center prolongs TTM to 48 hours in severely brain-injured patients. Rewarming rates range from 0.2 to 0.5°C/hr with most centers rewarming at a rate of 0.25°C/hr (N = 12). After the initial TTM period, fever is actively prevented with continued use of cooling devices (N = 24) (Table 2).
Table 2.
TTM Practice Characteristics
| USA (N = 6), n (%) | Korea (N = 24), n (%) | |
|---|---|---|
| TTM goal temperature | ||
| 33°C | 2 (34) | 14 (58) |
| 36°C | 0 (0) | 1 (4) |
| Admission temperature | 0 (0) | 0 (0) |
| Variable (33°C or 36°C) | 4 (66) | 9 (38) |
| Method of cooling | ||
| Surface cooling blankets | 1 (16) | 3 (13) |
| Endovascular | 2 (34) | 6 (25) |
| Surface Hydrogel | 2 (34) | 11 (45) |
| Both blankets and endovascular | 0 (0) | 1 (4) |
| Both hydrogel and endovascular | 1 (16) | 3 (13) |
| Goal temp duration | ||
| 12 hours after ROSC | 0 (0) | 0 (0) |
| 24 hours after ROSC | 2 (34) | 10 (42) |
| 12 hours after goal temp reached | 0 (0) | 1 (4) |
| 24 hours after goal tem reached | 4 (66) | 12 (50) |
| Other durationa | 0 (0) | 1 (4) |
| Post TTM fever prophylaxis | N = 22 | |
| Anti-pyretic medications | 2 (34) | 2 (9) |
| Cooling blanket or wrap | 2 (34) | 12 (55) |
| Endovascular catheter | 1 (16) | 7 (32) |
| Otherb | 1 (16) | 1 (4) |
| None | 0 (0) | 0 (0) |
| Contraindication to TTM | N = 24 | |
| Nonshockable rhythm | 0 (0) | 0 (0) |
| Unwitnessed arrest | 0 (0) | 0 (0) |
| IHCA | 0 (0) | 3 (13) |
| >30 minutes of BLS w/o ROSC | 0 (0) | 1 (4) |
| Total ischemic time >60 minutes | 1 (16) | 1 (4) |
| Total ischemic time >30 minutes | 0 (0) | 0 (0) |
| Hypothermia on admission | 0 (0) | 5 (21) |
| GCS ≥8 | 1 (16) | 8 (33) |
| >85 years old | 0 (0) | 3 (12) |
| Pregnant | 0 (0) | 8 (33) |
| Existing DNR/DNI | 4 (66) | 24 (100) |
| Terminal illness before arrest | 2 (34) | 18 (75) |
| Poor baseline neurological status | 4 (66) | 16 (67) |
| Increased INR/DIC/active bleeding | 0 (0) | 12 (50) |
| Noncardiac etiology | 1 (16) | 0 (0) |
| Recent trauma | 0 (0) | 3 (13) |
| Recent surgery | 0 (0) | 4 (17) |
| Other medical comorbidities | 0 (0) | 3 (13) |
| No contraindications | 1 (16) | 0 (0) |
Other duration of cooling included extension of TTM to 48 hours in severely brain-injured patients.
Other post-TTM fever prophylaxis included continued use of employed device.
BLS, basic life support; DIC, disseminated intravascular coagulation; DNI, do not intubate; DNR, do not resuscitate; INR, international normalized ratio; ROSC, return of spontaneous circulation.
Many different combinations of both bolus and continuous infusion dosing of sedation are employed, including midazolam, propofol, fentanyl, remifentanil, and dexmedetomidine (Supplementary Table S2). A bolus dose of midazolam is most often used for sedation (N = 21). Additionally, a wide range of neuromuscular blocking agents are utilized for a variety of indications and durations ranging from standardized periods to PRN bolus dosing for shivering.
Cardiac catheterization
Indications for cardiac catheterization are listed in Table 3. No single indication was unanimous among all surveyed investigators. The only absolute contraindications to cardiac catheterization reported were impending brain death, irreversible cause of cardiac arrest, including intracerebral hemorrhage, and refusal by surrogate decision maker.
Table 3.
Post-Arrest Cardiac Catheterization Indications
| USA (N = 6), n (%) | Korea (N = 24), n (%) | |
|---|---|---|
| Indications for cardiac catheterization | ||
| STEMI | 6 (100) | 22 (92) |
| History of CAD | 1 (16) | 8 (33) |
| Focal wall motion abnormality | 3 (50) | 11 (46) |
| History of VF/VT | 4 (66) | 7 (29) |
| Elevated troponin | 1 (16) | 4 (17) |
| Cardiogenic shock | 1 (16) | 6 (25) |
| Othera | 0 (0) | 3 (13) |
Other indications for cardiac catheterization included high suspicion for acute coronary occlusion and no other identifiable etiology of arrest.
CAD, coronary artery disease; STEMI, ST-segment elevation myocardial infarction; VF, ventricular fibrillation; VT, ventricular tachycardia.
Neurological prognostication
Only six (20%) of the surveyed investigators reported having an institutional protocol for WLST (Table 4). Facilitation of WLST conversations with families is most often done by the unit attending on duty (N = 13; 43%). The most common disposition of patients with poor neurological prognosis in the United States was WLST with subsequent expiration (N = 5; 84%), whereas the Korean patients with poor neurological prognosis tended to be transferred to a secondary care facility (N = 19; 80%). Both electroencephalography (EEG) modality and duration vary between institutions (Table 4). Serum biomarkers are commonly employed in Korea, but not in the United States. Timing of serum biomarkers varied with some institutions drawing biomarkers at emergency department admission only while others complete multiple analyses during hospitalization (Supplementary Table S2).
Table 4.
Characteristics of Neurological Care and Prognostication
| USA (N = 6), n (%) | Korea (N = 24), n (%) | |
|---|---|---|
| WLST institutional protocol | ||
| Yes | 3 (50) | 3 (12) |
| No | 3 (50) | 21 (88) |
| WLST clinical facilitator | ||
| Attending on duty | 1 (16) | 12 (50) |
| Critical care team on duty | 2 (34) | 0 (0) |
| Post-arrest team | 3 (50) | 2 (8) |
| Ethics committee | 0 (0) | 3 (13) |
| Palliative care | 0 (0) | 1 (4) |
| Cannot withdraw | 0 (0) | 6 (25) |
| Disposition of patients with poor neurological prognosis | ||
| Continued active ICU care | 0 (0) | 2 (8) |
| No escalation of ICU care | 0 (0) | 2 (8) |
| Withdrawal | 5 (84) | 0 (0) |
| Transfer to secondary care facility | 0 (0) | 19 (80) |
| Surrogate decides destination | 1 (16) | 1 (4) |
| EEG use | ||
| Yes | 6 (100) | 20 (83) |
| No | 0 (0) | 4 (17) |
| Serum biomarker use | N = 5 | |
| Yes | 1 (20) | 22 (92) |
| No | 4 (80) | 2 (8) |
EEG, electroencephalography; GFAP, glial fibrillary acidic protein; ICU, intensive care unit; NSE, neuron-specific enolase; WLST, withdrawal of life-sustaining therapy.
Discussion
We found significant variability in temperature management strategies, cardiac catheterization indications, determination of neurological prognostication, and disposition of patients with poor neurological prognosis among an international cohort of surveyed investigators. WLST practices and cardiac catheterization indications varied between US and Korean centers. Individual clinician practice, belief in therapies, and prognostic tools, as well as hospital availability of interventions, specifically cardiac catheterization, EEG, and biomarkers may influence indications and use. The variability of these clinical practices must be taken into account when comparing registry data across institutions and countries. For example, different durations and depths of temperature management could affect the pharmacokinetics of many sedation and antiepileptic agents (Tortorici et al., 2007; Hostler et al., 2010; Zhou and Poloyac, 2011; Zhou et al., 2011; Empey et al., 2013). If left unaccounted for in future trials, subgroups may be exposed to adverse drug events or unexpectedly prolonged sedation, confounding neurological examination. Additionally, the variability in these practices by definition supports clinical equipoise for sequent clinical trials surrounding these post-cardiac arrest care practices.
These reported variations represent potential confounders of retrospective studies. These may explain some of the heterogeneity seen when comparing survival and good neurological outcome between studies. Future multicenter studies and meta-analyses may be unable to combine patients treated with various protocols for TTM. These results accentuate the need for standardized care and prognostic workup in future trials (Nielsen et al., 2013). Similarly, extended fever treatment and prophylaxis may influence TTM results. Fever, both during and post TTM, has been associated with poor outcomes, an additional layer of variability and potential confounder (Gebhardt et al., 2013; Picetti et al., 2016). Thus, neuronal susceptibility to fever may change over time. There is room to understand future TTM adoption and deadoption practices among institutions. Given the variability in care delivery, one way to standardize comparisons between groups is with post-cardiac arrest illness severity scores. These risk stratification tools could be used in future studies to ensure consistent comparisons between groups (Rittenberger et al., 2011; Coppler et al., 2015).
Few formalized WLST protocols were reported by the respondents. Encouraging a standardized approach may limit early withdrawal (Elmer et al., 2016). Given social norms preventing withdrawal of support, the KORHN appears well poised to study which patients have delayed awakening without the confounder of a self-fulfilling prophecy (Geocadin et al., 2012).
Limitations
Our work has several limitations. We present a survey study of single principal investigators for which participants answered questions based on local protocols and usual clinical care. The survey may not reflect the entire range or actual clinical care across individual practitioners at each site. Reported variation may be greater at the provider level than the site level (Leary et al., 2015; Deye et al., 2016; Kotini-Shah et al., 2016). Additionally, comparing a smaller US cohort to a larger Korean cohort may not reflect all variability between these countries. Finally, the surveyed institutions may not be representative of “typical” care delivered at most hospitals. Most investigators represent a high-volume cardiac arrest center, which may provide more aggressive care as compared to lower volume noncardiac arrest centers.
Conclusions
We found significant variability in post-cardiac arrest care practices among US and Korean medical centers. These practice variations must be taken into account in future prospective as well as retrospective multicenter studies of post-arrest care.
Supplementary Material
Appendix
KORHN Investigators
Ji Hoon Kim, MD, PhD; The Catholic University of Korea, Bucheon St. Mary's Hospital.
Kyu Nam Park, MD, PhD; The Catholic University of Korea, Seoul St. Mary's Hospital.
Won Jung Jeong, MD; The Catholic University of Korea, St. Vincent's Hospital.
Seung Pill Choi, MD, PhD; The Catholic University of Korea, Yeouido St. Mary's Hospital.
Mi Jin Lee, MD, PhD; Kyungpook National University School of Medicine, Daegu.
Jong-Seok Lee, MD, PhD; Kyung Hee University Medical Center.
Su Jin Kim, MD, PhD; Korea University, College of Medicine.
Tae Chang Jang, MD, PhD; College of Medicine, Catholic University of Daegu.
Inbyung Kim, MD; Myongji Hospital, Seonam University College of Medicine.
Yong Hwan Kim, MD, Sungkyunkwan University School of Medicine, Samsung Changwon Hospital.
Won Young Kim, MD, PhD; Asan Medical Center, University of Ulsan College of Medicine.
Jonghwan Shin, MD; Seoul National University Boramae Medical Center.
Ji Hwan Lee, MD; Yonsei University College of Medicine.
Hyung Jun Moon, MD, PhD; Soonchunhyang University Hospital.
Giwoon Kim, MD; Soonchunhyang University Hospital.
Wook-jin Choi, MD, PhD; Ulsan University Hospital, University of Ulsan College of Medicine.
Joo Suk Oh, MD, PhD; The Catholic University of Korea, Uijeongbu St. Mary's Hospital.
Chul Han, MD, PhD; Ewha Womans University Mokdong Hospital.
Byung Kook Lee, MD, PhD, Chonnam National University Medical School.
Taeoh Jeong, MD, PhD; Research Institute of Clinical Medicine of Chonbuk National University and Chonbuk National University Hospital.
Dong Hoon Lee, MD, PhD; Chung-Ang University.
Min Jin Hong, MD; Chungbuk National University Hospital.
Gyu Chong Cho, MD, PhD; Hallym University.
Young Hwan Lee, MD; Hallym University Sacred Heart Hospital, Hallym University Medical Center.
Youdong Sohn, PhD; Hallym University Sacred Heart Hospital, Hallym University Medical Center.
In Soo Cho, MD; Hanil General Hospital.
Je Sung You, MD, PhD; Yonsei University College of Medicine.
Changsun Kim, MD, PhD; Hanyang University Guri Hospital.
Kyoung-Chul Cha, MD; Yonsei University, Wonju College of Medicine.
Soo Hyung Cho, MD, PhD; Chosun University Hospital.
NPARC Investigators
Michael Donnino, MD; Beth Israel Deaconess, Harvard Medical School.
Michael Cocci, MD; Beth Israel Deaconess, Harvard Medical School.
Ari Moskowitz, MD; Beth Israel Deaconess, Harvard Medical School.
Benjamin S. Abella, MD, MPhil; University of Pennsylvania.
David Gaieski, MD; Sidney Kimmel Medical College at Thomas Jefferson University.
Clifton W. Callaway, MD; University of Pittsburgh.
Jon C. Rittenberger, MD, MS; University of Pittsburgh.
Jonathan Elmer, MD, MS; University of Pittsburgh.
Patrick J. Coppler, BS; University of Pittsburgh.
Mary Ann Peberdy, MD; Virginia Commonwealth University.
Joseph Ornato, MD; Virginia Commonwealth University.
Joshua C. Reynolds, MD, MS; Michigan State University College of Human Medicine.
Kelly N. Sawyer, MD, MS; William Beaumont Hospital.
Henry Wang, MD, MS; University of Alabama.
Michael C. Kurz, MD, MS; University of Alabama.
Contributor Information
Collaborators: on behalf of the Korean Hypothermia Network Investigators (KORHN) and the National Post-Arrest Research Consortium (NPARC)
Author Disclosure Statement
No competing financial interests exist.
References
- Bernard S. Inducing hypothermia after out of hospital cardiac arrest. BMJ 2014;348:g2735. [DOI] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002;346:557–563 [DOI] [PubMed] [Google Scholar]
- Busto R, Dietrich WD, Globus MY, Ginsberg MD. Postischemic moderate hypothermia inhibits CA1 hippocampal ischemic neuronal injury. Neurosci Lett 1989;101:299–304 [DOI] [PubMed] [Google Scholar]
- Busto R, Dietrich WD, Globus MY, Valdes I, Scheinberg P, Ginsberg MD. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab 1987;7:729–738 [DOI] [PubMed] [Google Scholar]
- Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, Leary M, Meurer WJ, Peberdy MA, Thompson TM. Part 8: Post-cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015;132:S465–S482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp-Rogers TR, Sawyer KN, McNicol DR, Kurz MC. An observational study of patient selection criteria for post-cardiac arrest therapeutic hypothermia. Resuscitation 2013;84:1536–1539 [DOI] [PubMed] [Google Scholar]
- Coppler PJ, Elmer J, Calderon L, Sabedra A, Doshi AA, Callaway CW, Rittenberger JC, Dezfulian C. Validation of the Pittsburgh Cardiac Arrest Category illness severity score. Resuscitation 2015;89:86–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronberg T, Lilja G, Horn J, Kjaergaard J, Wise MP, Pellis T, Hovdenes J, Gasche Y, Aneman A, Stammet P. Neurologic function and health-related quality of life in patients following targeted temperature management at 33 degrees C vs 36 degrees C after out-of-hospital cardiac arrest: a randomized clinical trial. JAMA Neurol 2015;72:634–641 [DOI] [PubMed] [Google Scholar]
- Deye N, Vincent F, Michel P, Ehrmann S, da Silva D, Piagnerelli M, Kimmoun A, Hamzaoui O, Lacherade JC, de Jonghe B. Changes in cardiac arrest patients' temperature management after the 2013 “TTM” trial: results from an international survey. Ann Intensive Care 2016;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnino MW, Andersen LW, Giberson T, Gaieski DF, Abella BS, Peberdy MA, Rittenberger JC, Callaway CW, Ornato J, Clore J. Initial lactate and lactate change in post-cardiac arrest: a multicenter validation study. Crit Care Med 2014;42:1804–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnino MW, Rittenberger JC, Gaieski D, Cocchi MN, Giberson B, Peberdy MA, Abella BS, Bobrow BJ, Callaway C. The development and implementation of cardiac arrest centers. Resuscitation 2011;82:974–978 [DOI] [PubMed] [Google Scholar]
- Elmer J, Torres C, Aufderheide TP, Austin MA, Callaway CW, Golan E, Herren H, Jasti J, Kudenchuk PJ, Scales DC. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation 2016;102:127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Empey PE, Velez de Mendizabal N, Bell MJ, Bies RR, Anderson KB, Kochanek PM, Adelson PD, Poloyac SM. Therapeutic hypothermia decreases phenytoin elimination in children with traumatic brain injury. Crit Care Med 2013;41, 2379–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt K, Guyette FX, Doshi AA, Callaway CW, Rittenberger JC. Prevalence and effect of fever on outcome following resuscitation from cardiac arrest. Resuscitation 2013;84:1062–1067 [DOI] [PubMed] [Google Scholar]
- Geocadin RG, Peberdy MA, Lazar RM. Poor survival after cardiac arrest resuscitation: a self-fulfilling prophecy or biologic destiny?*. Critical Care Med 2012;40:979–980 [DOI] [PubMed] [Google Scholar]
- Geri G, Dumas F, Bougouin W, Varenne O, Daviaud F, Pene F, Lamhaut L, Chiche JD, Spaulding C, Mira JP. Immediate Percutaneous Coronary Intervention Is Associated With Improved Short- and Long-Term Survival After Out-of-Hospital Cardiac Arrest. Circ Cardiovasc Interv 2015;8:1–7 [DOI] [PubMed] [Google Scholar]
- Hostler D, Zhou J, Tortorici MA, Bies RR, Rittenberger JC, Empey PE, Kochanek PM, Callaway CW, Poloyac SM. Mild hypothermia alters midazolam pharmacokinetics in normal healthy volunteers. Drug Metab Dispos 2010;38, 781–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hypothermia after Cardiac Arrest Study G. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002;346:549–556 [DOI] [PubMed] [Google Scholar]
- Kim F, Bravo PE, Nichol G. What is the use of hypothermia for neuroprotection after out-of-hospital cardiac arrest? Stroke 2015a;46:592–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YM, Youn CS, Kim SH, Lee BK, Cho IS, Cho GC, Jeung KW, Oh SH, Choi SP, Shin JH. Adverse events associated with poor neurological outcome during targeted temperature management and advanced critical care after out-of-hospital cardiac arrest. Crit Care 2015b;19:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotini-Shah P, Camp-Rogers TR, Swor RA, Sawyer KN. An assessment of emergency department post-cardiac arrest care variation in Michigan. Ther Hypothermia Temp Manag 2016;6:17–22 [DOI] [PubMed] [Google Scholar]
- Kuboyama K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med 1993;21:1348–1358 [DOI] [PubMed] [Google Scholar]
- Leary M, Blewer AL, Delfin G, Abella BS. Variability in postarrest targeted temperature management practice: implications of the 2015 guidelines. Ther Hypothermia Temp Manag 2015;5:184–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilja G, Nielsen N, Friberg H, Horn J, Kjaergaard J, Nilsson F, Pellis T, Wetterslev J, Wise MP, Bosch F. Cognitive function in survivors of out-of-hospital cardiac arrest after target temperature management at 33 degrees C versus 36 degrees C. Circulation 2015;131:1340–1349 [DOI] [PubMed] [Google Scholar]
- Moler FW, Silverstein FS, Holubkov R, Slomine BS, Christensen JR, Nadkarni VM, Meert KL, Clark AE, Browning B, Pemberton VL. Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N Engl J Med 2015;372:1898–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med 2013;369:2197–2206 [DOI] [PubMed] [Google Scholar]
- Nozari A, Safar P, Stezoski SW, Wu X, Henchir J, Radovsky A, Hanson K, Klein E, Kochanek PM, Tisherman SA. Mild hypothermia during prolonged cardiopulmonary cerebral resuscitation increases conscious survival in dogs. Crit Care Med 2004;32:2110–2116 [DOI] [PubMed] [Google Scholar]
- Perman SM, Kirkpatrick JN, Reitsma AM, Gaieski DF, Lau B, Smith TM, Leary M, Fuchs BD, Levine JM, Abella BS. Timing of neuroprognostication in postcardiac arrest therapeutic hypothermia*. Crit Care Med 2012;40:719–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picetti E, Antonini MV, Bartolini Y, DeAngelis A, Delaj L, Florindo I, Villani F, Caspani ML. Delayed fever and neurological outcome after cardiac arrest: a retrospective clinical study. Neurocrit Care 2016;24:163–171 [DOI] [PubMed] [Google Scholar]
- Polderman KH, Varon J. How low should we go? Hypothermia or strict normothermia after cardiac arrest? Circulation 2015a;131:669–675 [DOI] [PubMed] [Google Scholar]
- Polderman KH, Varon J. Interpreting the results of the targeted temperature management trial in cardiac arrest. Ther Hypothermia Temp Manag 2015b;5:73–76 [DOI] [PubMed] [Google Scholar]
- Rittenberger JC, Tisherman SA, Holm MB, Guyette FX, Callaway CW. An early, novel illness severity score to predict outcome after cardiac arrest. Resuscitation 2011;82:1399–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomine BS, Silverstein FS, Christensen JR, Holubkov R, Page K, Dean JM, Moler FW. Neurobehavioral outcomes in children after out-of-hospital cardiac arrest. Pediatrics 2016;137:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterz F, Safar P, Tisherman S, Radovsky A, Kuboyama K, Oku K. Mild hypothermic cardiopulmonary resuscitation improves outcome after prolonged cardiac arrest in dogs. Crit Care Med 1991;19:379–389 [DOI] [PubMed] [Google Scholar]
- Tortorici MA, Kochanek PM, Poloyac SM. Effects of hypothermia on drug disposition, metabolism, and response: a focus of hypothermia-mediated alterations on the cytochrome P450 enzyme system. Crit Care Med 2007;35:2196–2204 [DOI] [PubMed] [Google Scholar]
- Vincent JL, Taccone FS. Difficulty interpreting the results of some trials: the case of therapeutic hypothermia after pediatric cardiac arrest. Crit Care 2015;19:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K, Eguchi Y, Kajiwara K, Ito H. Mild hypothermia ameliorates ubiquitin synthesis and prevents delayed neuronal death in the gerbil hippocampus. Stroke 1991;22:1574–1581 [DOI] [PubMed] [Google Scholar]
- Zhou J, Empey PE, Bies RR, Kochanek PM, Poloyac SM. Cardiac arrest and therapeutic hypothermia decrease isoform-specific cytochrome P450 drug metabolism. Drug Metab Dispos 2011;39:2209–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Poloyac SM. The effect of therapeutic hypothermia on drug metabolism and response: cellular mechanisms to organ function. Expert Opin Drug Metab Toxicol 2011;7:803–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.