Abstract
The immune system functions as a vanguard against pathogens and toxins. While it is mostly considered to be activated on the basis of self versus non-self recognition, injury/infection and damage are unavoidably associated with cell death. Does cell death play a role in the regulation of the immune response? Cell death, for better or for worse, is an omnipresent process in all stages of life that are observed throughout most tissues in multicellular organisms. From development to homeostasis in adult organisms, cells commit to scheduled death, while cases of injury and infection result in unscheduled cell death. Novel understanding of the molecular mechanisms that govern cell death demonstrate that, in fact, a plethora of molecular processes participate in directed dying. Parallel to the molecular modalities directing cell death are machineries employed by the organism to respond to dying cells, including either eliciting an inflammatory or immunological response or altogether avoiding it. Disturbing the careful coupling of these two processes is often met with pathology – on one hand a failure to respond to cell death may contribute to the lack of proper immune response or defective development, and on the other hand exaggerated or aberrant response to cell death can trigger unregulated inflammation, autoimmunity, or fibrosis/scarring. Here we review the molecular mechanisms and associated effector responses that accompany some of the most well-known cell death modalities – with an emphasis on efferocytosis, a process by which the dead cell is recognized and engulfed. In doing so, we highlight the TAM (TYRO3, AXL, MERTK) family of receptor tyrosine kinases (RTKs) that functions dually in the recognition and engulfment of dead cells, and as an important negative regulator of inflammation.
Keywords: cell death, apoptosis, necrosis, necroptosis, pyroptosis, ferroptosis, efferocytosis, cell clearance, TAM RTKs, TYRO3, AXL, MERTK
Introduction
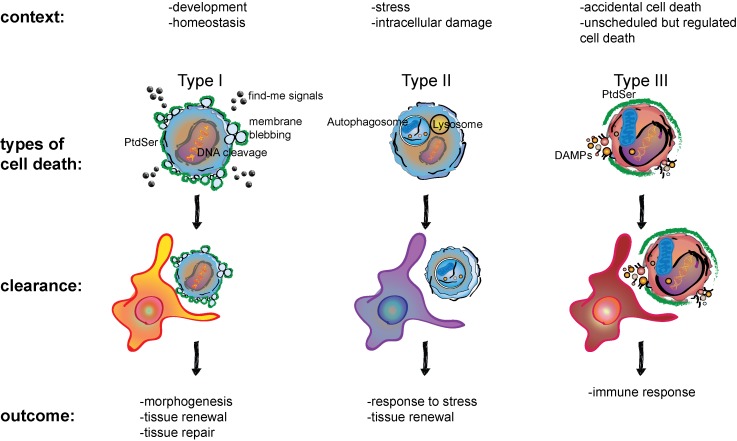
Our immune system is generally accepted to respond to threats by recognizing previously unseen pathogens and toxins as non-self. In contrast, Polly Matzinger proposed that immune responses are initiated by “danger signals” released by the self [1-3]. Albeit, it is undeniable that pathogen or toxin effects are often inseparable from at least some amount of host cell damage and death. Unsurprisingly, notions of cellular death can be traced back to the 5th century BCE in the writings of Hippocrates. Gangrene, a term used to describe dying or putrefying tissue, typically at the extremities, was associated with irreparable damage. Hippocrates recommended the direct removal of the dead from the live tissue to stifle its spread [4]. In recent decades, molecular understanding of cell death has revealed the existence of a plethora of forms by which cells die. Interestingly, forms of cell death have been associated with inflammation and immune response, or a lack thereof. In following the classification scheme set forth by the Nomenclature Committee on Cell Death, cell death can broadly be categorized into three modalities [5]. Type I cell death, for example apoptosis, is characterized by fragmentation of the cell into apoptotic bodies while retaining membrane integrity (Figure 1). In type II cell death, membrane integrity is also retained, and the process of cell death is characterized by vacuole formation (Figure 1). An example of this is autophagic cell death. Autophagy is a process wherein the cell cannibalizes its organelles to survive energetic stress. In contrast to the role of autophagy in cell survival, passing a threshold in the degradation of cellular constituents including organelles likely represents an inflection point beyond which lies the certain death of the cell. Thus, the giver of life can also take it away and it is the dose that makes the poison (Sola dosis facit venenum). Exceptions notwithstanding, both these forms of cell death are generally not associated with inflammation or an immune response. Finally, type III cell death describes lytic modalities of cell death, such as necrosis (Figure 1). Here, cell membrane integrity is rapidly compromised, and cytosolic components are released into the environment. This form of cell death is closely correlated with inflammation and immune response.
Figure 1.
Comparison between context, type of cell death, clearance mechanisms, and outcomes of type I, type II, and type III cell death. The context in which cells die are numerous and their clearance is associated with multiple outcomes. Type I cell death, also referred to as apoptosis is typically followed by quick clearance by a phagocyte, such as a macrophage, as is depicted. Overall these combined processes are essential in morphogenesis, tissue renewal, as well as promoting an immune response and tissue repair in a context-dependent manner. Type II cell death utilizes a process that likely evolved for cell survival under stress. Fusion of the autophagosome with the lysosome creates the autolysosome that allows for the large-scale degradation of sequestered cargoes. This can help recycle cellular components for survival during times of metabolic stress. However, autophagic cell death occurs when continuing autophagy crosses an inflection point and the cell in essence cannibalizes itself. Debris from this process is then cleared by phagocytes. Type III cell death is also known as regulated necrosis. Cells that die in this manner display a necrotic phenotype, characterized by the rupturing of the membrane and the release of DAMPs that are sensed by innate immune cells, summoning an inflammatory response. Some modalities of regulated necrosis, such as necroptosis have been shown to also expose PtdSer on the surface.
The complexity of cell death makes for an intriguing study of its context dependent functions vis-a-vis immune activation. Within each of the broader categories of cell death described above are often multiple subtypes, bringing the tally of modalities of cell death to approximately fourteen [5]. How does the body deal with cell death, both in the context when an immune response is elicited as well as when it is not? Does cell death contribute to this response? Much like Hippocrates’ idea of restricting the spread of putrefying tissue by its removal, the body’s intrinsic response to cell death also involves the removal of the dead cell, albeit through its engulfment by its live neighbors. This specialized form of phagocytosis is termed efferocytosis. Is the process of efferocytosis distinct for unique forms of cell death and accompanied by differing effector functions? Here we review the distinct consequences of cell death in physiological and pathological conditions and discuss how these consequences often depend not only upon the molecular mechanism of cell death but also upon efferocytosis. As we will see, cell death, and the response to it are two tightly intertwined processes, an accompaniment observed in nearly all instances where cell death is present. Additionally, we will provide a specific focus on the efferocytotic and immunoregulatory roles of TAM (TYRO3, AXL, MERTK) family of receptor tyrosine kinases (RTKs) in the variant responses to cell death.
Cell Death and its Clearance in Development
In contrast to the earliest notions of cell death, we have now learned that one of the greatest ironies of life is its undeniable dependence on death for perpetuation. It is a phenomenon that occurs in development, during homeostasis and of course, following injury or damage – sterile or infected. Not only is cell death a constant feature of the life cycle, it is also ubiquitous, occurring in essentially all tissues and organs. It is no surprise to many that the vast majority of cells you are born with are not the same set you find yourself with today. However, less intuitive is the vast population of cells that commits to a cell death program during development itself and that there is a dedicated mechanism for their clearance. In Caenorhabditis elegans, the archetype model system for studying development, Horvitz et al. famously demonstrated that scheduled cell death occurs in 131 cells out of a total of 1090, resulting in exactly 959 somatic cells in the adult hermaphrodite worm [6].
The selective advantage of developmental cell death, at least in C. elegans, remains uncertain since the failure of cells to die does not appear to cause any great harm to this worm, at least for its life in a laboratory dish [7]. Unlike in C. elegans,however, errors of elimination have inescapable deleterious consequences in mammals. Similar to C. elegans, where most of the developmental cell death occurs in neurons, with precisely 105 of them dying during the 131 cell death events, one observes distinguished patterns of cell death in neuronal development in mammals, such as the death of approximately half of gamma-aminobutyric acid (GABA)-secreting cortical interneurons in postnatal mice [8]. The high levels of neuronal cell death observed in vertebrate development have been attributed to limiting the cell population during neurogenesis, correcting structural errors, as well as aiding in synaptogenesis [9]. Children diagnosed with autism spectrum disorder (ASD) possessed higher numbers of neurons in the amygdala than neurotypical children [10] implying that perhaps differences in the temporal and spatial map of cell death in early neurogenesis are in part delinquent in ASD. The gene responsible for loss of programmed cell death (PCD) in ASD has not been identified. In mice, the simultaneous genetic ablation of two pro-apoptotic genes Bax and Bak displayed multiple developmental defects, including excess cell numbers in the central nervous system and in the hematopoietic system [11].
Cell death is inexorably associated with clearance. Although C. elegans lacks specialized phagocytes, the process of efferocytosis is carried out by neighboring cells in these worms and accounts for the clearance of the corpses [12,13]. Engulfment of apoptotic cells appears to occur through two partially redundant pathways comprised of cell death abnormality gene (ced)-1, ced-6 and ced-7, and ced-2, ced-5, ced-10, and ced-12,respectively [14]. CED-1 appears to be a receptor that binds apoptotic cargo in conjunction with Transthyretin-like protein (TTR)-52 and CED-7 in the ced-1/ced-6/ced-7 pathway [14]. In the ced-2/ced-5/ced-10/ced-12 pathway, Phosphatidylserine receptor (PSR)-1, More of MS (MOM)-5 and integrin alpha (INA)-1/Paralysed Arrest at Two-fold (PAT)-3 might function as the receptors for apoptotic cargo [14]. Perhaps one of the more fascinating discoveries with regards to efferocytosis receptors in C. elegans was the finding that the failure of efferocytosis by neighboring cells resulted in what is described as a “near death experience” of the cell destined to undergo cell death – termed anastasis – where the cell committed to die recovered to live again [15,16]. Weak mutants of cell death effector caspase ced-3 were reported to synergize with ced-6 and ced-7 mutants in reversing the commitment of cells to their scheduled death [16]. Similar observations were made with the other genes involved in engulfment of apoptotic cells such as ced-1, ced-2, ced-5, ced-10, and ced-12 [16]. Mutations in engulfment genes, even without defects in execution genes, resulted in “undead” cells [15].
Therefore, not only can cell death itself be considered the paradoxical impetus of morphogenesis, its recognition and removal by its efferocyte (the cell performing the act of efferocytosis) may also play a role in molding and sculpting the growing organism. Perhaps, PCD eliminates a function no longer required for the organism and the removal of the corpse creates space for a cell with a new function to take the place of the dead cell. The players involved in efferocytosis in the developing mammalian brain are not fully characterized and it remains rather unclear specifically how defects in execution mechanisms and/or efferocytosis contributes to ASD or neurodevelopmental disorders. However, a less figurative example of PCD and sculpting by efferocytosis is seen in the classical case of digit morphogenesis. Here, apoptosis was shown to be crucial in digit separation and joint cavity formation in mouse fetuses [17]. Hammertoe mutants, as they were dubbed, displayed interdigital webbing and reduced cell death in comparison to their wild-type counterparts. Moreover, imaging of wild-type mouse embryos revealed macrophages as the drivers of dead cell clearance in the interdigital zone [18]. Therefore, PCD and efferocytosis may be synchronized in its orchestration, and failure of either execution of cell death or the clearance of dead/dying cells may manifest as developmental abnormalities.
The form of cell death commonly encountered in development is apoptosis. It is mostly considered a non-immunogenic form of cell death. Typically, this is associated with the variety of distinct morphological alterations in apoptotic cells that produce “find me” and “eat me” signals. For example, in the early stages of apoptosis, cells release uridine triphosphate (UTP) and adenosine triphosphate (ATP), which are sensed by the purinergic receptor P2Y2 on the efferocyte, inciting timely removal of the dying cell [19]. Ultimately, it is these molecular markers that facilitate the quick and tidy removal of dying cells that is characteristic to the apoptotic mechanism in physiological settings such as development. Indeed is this process so elusive to human observation that in tissues like bone marrow or the thymus known to possess a high turnover rate, few apoptotic cells are ever seen, demonstrating the efficacy of the efferocytotic mechanism [20].
Perhaps the best characterized “eat me” signal of apoptotic cells is the exposure of phosphatidylserine (PtdSer) on the cell surface. Caspase-mediated changes in enzymatic activity are essential to PtdSer externalization. Specifically, cleavage of flippases such as ATP11C (adenosine triphosphatase type 11C) mitigate activity that would otherwise work to translocate PtdSer to the inner leaflet of the membrane. Various caspase recognition sites present in ATP11C and experiments performed with point mutations in these sites demonstrated that this flippase inhibition is not only caspase-dependent, but also necessary for the externalization of PtdSer on the cell surface of dying apoptotic cells [21,22]. Scramblases, including transmembrane protein 16F (TMEM16F), actively translocate PtdSer between the outer and inner plasma membrane leaflets of the cell [23]. Normally kept inhibited, caspase cleavage results in its irreversible activation and scrambling of the inner membrane-restricted PtdSer to disperse between the two membrane leaflets.
There are a number of vertebrate receptors that function to recognize PtdSer when exposed to the outer membrane leaflet of a neighboring cell that is dying, such as T-cell immunoglobulin mucin receptor 4 (TIM4), brain-specific angiogenesis receptor 1 (BAI1) and TAM RTKs. TAM RTKs are a set of transmembrane molecules with tyrosine kinase activity that can bind two soluble ligands named growth-arrest-specific 6 (GAS6) and Protein S (PROS1) [24,25]. On the surface opposite of that used to interact with TAM RTKs on GAS6 and PROS1 is a Gla-domain: a region rich in Glutamic acid residues (Glu) that can be γ-carboxylated [25]. γ-carboxylated Gla-domains provide a mechanism for protein-phospholipid interactions so serine head groups of PtdSer, such as of those exposed on the outer leaflet of the plasma-membrane of apoptotic cells, can bind GAS6 and PROS1. Thus, GAS6 and PROS1 essentially bridge a cell containing TAM RTK through their extracellular ligand binding region to PtdSer exposed on the outer leaflet of a dying cell [26]. Downstream, intracellular signaling enabled by the cytosolic kinase domain of TAM RTKs within the efferocyte drives TAM RTK-dependent efferocytosis of apoptotic cells [27].
As we shall see later in this article, TAM RTKs are essential molecules for efferocytosis in many settings. Intriguingly though, previous studies conducted with TAM RTK triple knock out (TKO) mice where all of Tyro3, Axl, and Mertk were genetically ablated have demonstrated that TAMs are not needed for embryogenesis. This is greatly surprising given the commonality of embryonic and postnatal cell death in the brain. In the early embryonic development, microglia, specialized phagocytic cells in the brain, migrate to the brain and establish permanent residency [28]. MERTK is expressed by embryonic day 14.5 in the mouse brain [29]. Furthermore, MERTK is required for microglial efferocytotic function later in life [30]. Selective ablation of Mertk in microglia was shown to significantly impair the ability of these phagocytes to migrate towards laser-induced capillary disruption in the blood-brain barrier and mediate their engulfment [30]. Nevertheless, mouse ablated for Mertk or even the TAM RTK TKOs do not have gross developmental anomalies as far as brain development is concerned [31]. There are aspects of later developmental processes though where TAM RTKs appear to have crucial function. For example, Sertoli cells aid in the transition of germ cells into sperm cells and express TAM RTKs to facilitate clearance during spermatogenesis. TAM TKO male mice displayed the most severe phenotype in relation to single or double knockouts with significantly reduced testes size and sterility [31].
Another example where cell death and its clearance ensure that potentially deleterious functions that are perhaps intrinsic to the design of the biological system are avoided is activation-induced cell death (AICD), a process observed in mature T and B cells and developing T cells alike, where the lymphocyte undergoes apoptosis. During the development of the immune system, diversity of receptors responding to the vast number of possible, yet unencountered pathogens is encoded by gene re-arrangement through random recombination of segments, insertion of random nucleotides within specific regions and somatic hypermutations [32]. This potential to attack essentially all possible antigens is restricted, in part, by AICD. For example, AICD is observed in the elimination of autoreactive lymphocytes in the thymus, as well as in the peripheral deletion of T cells following expansion in response to antigen exposure [33,34]. Critically, cell death in development is not associated with inflammation or immune activation. In fact, failure of cell death execution mechanisms and efferocytosis may elicit a pathological immune response. Consistent with this idea, defects in both cell death execution mechanism or its clearance has been linked to autoimmunity. The loss-of-function of Fas Cell Surface Death Receptor (FAS)/CD95 – a cell surface receptor that transmits the signal to die when activated by its ligand – resulted in lymphoproliferation and autoimmunity in mice [35]. Genetic ablation of TAM RTKs that are receptors engaged in the phagocytic clearance of dead cells, also leads to lymphoproliferation and autoimmunity in mice [36]. Ablation of MERTK alone was sufficient in conferring autoimmunity, as mutants were found to possess notable levels of antibodies to chromatin, DNA, and immunoglobulin G (IgG) [37]. This manifestation of lupus-like disease in mutants was attributed to the ability of residual apoptotic cells to induce an immunogenic response to self-peptides [37]. It has been proposed that the systemic autoimmunity accompanied with MERTK KOs can be attributed to hindrance of tingible body macrophages (TBMs) to clear self-reactive apoptotic B cells in germinal centers [38].
Cell Death, Efferocytosis, and Compensatory Proliferation in Homeostasis
Scheduled cell death is not exclusive to development and occurs in a homeostatic manner in adult tissues. Although cortical neurons are essentially of the same age as the individual in whom they are found [39], this is more an exception to the rule in terms of cellular longevity. The finite life span of a cell is pre-programmed. For example human red blood cells live for approximately 110 to 135 days [40]. In an adult tissue at homeostasis, the death of a cell and its removal is part of the continuous cycle of life and death of cells. In instances of homeostatic scheduled cell death, in an act of martyrdom, the cell intrinsically commits to its cell death program in order to maintain the functionality of the tissue. Examples include intestinal epithelial cells.
Recognition and removal of the dead cell may be a prerequisite for the differentiation and proliferation of a functionally identical clone to replace the dead cell for the maintenance of tissue function. It has been recognized that there is a balance between cell death and proliferation in adult tissues. This death and replenishment equilibrium is most clearly manifested in the gut lumen, a highly proliferative microenvironment where epithelial cell turnover is mainly accounted for by apoptotic cell death [41]. To meet these high turnover demands, leucine-rich repeat-containing G protein coupled receptor 5(Lgr5+) intestinal stem cells divide asymmetrically and differentiation occurs in most instances during migration to the upper layers of the villus throughout a 3 to 5 day period [42]. One of the key regulators of this apoptotic cell death is the tumor suppressor p53 [43]. Loss of Mdm2 or Mdm4, inhibitors of p53, in intestinal epithelium of mouse resulted in mutants with increased p53-dependent apoptosis in the gut [44,45]. Yet, there were no gross morphological abnormalities in the adult animal [44]. The lack of expected phenotype due to increased apoptosis was attributed to compensatory mechanisms wherein proliferation was increased to account for increased cell loss [44]. The particular mechanisms for sensing cell death and whether or not they lead to compensatory proliferation are not completely clear. However, it is recognized that homeostatic cell death and its clearance are not associated with inflammation. Apoptotic cells in the lung and airway epithelia are engulfed by neighboring epithelial cells and produce anti-inflammatory cytokines such as interleukin (IL)-10 [46]. Inhibition of efferocytosis resulted in enhanced inflammation, increased IL-33 and allergic airway hyper-responsiveness [46]. Macrophages have been described to produce insulin-like growth factor-1 (IGF-1) after engulfing apoptotic cells [47]. While the study described the functional role of IGF-1 in re-directing epithelial cells to phagocytose microvesicles rather than larger apoptotic bodies and in reducing airway inflammation [47], IGF-1 is known to signal through IGF-1R and control the proliferation of many different cells and growth of tissues, including the brain and lung [48-52].
Environmental stress can also cause type II cell death. Notwithstanding, inflammation and immune response is not a consequence of homeostatic cell death. Autophagy is one of two primary mechanisms for large-scale degradation, the other being proteasomal degradation following ubiquitination. Autophagy is perhaps best known for its role in recycling non-essential materials in situations of stress, which may subsequently be used for the synthesis of macromolecules needed to perpetuate cell survival [53]. Recent studies have shown that this process plays a much more multifaceted role in eukaryotic organisms, as it takes part in embryogenesis, cell differentiation, and homeostasis [54]. For example, one observes relatively high levels of autophagy in the thymic epithelial cells, which possess major histocompatibility complex II (MHC II) molecules that are used to present antigens to CD4+ T-cells, and thereby create self-tolerant T-cells; by genetically disrupting autophagic activity in the thymus, researchers saw high levels of inflammation and colitis, demonstrating the vital role of autophagy in immune tolerance [55]. Autophagy has also been identified as a contributor in various myopathies and neurodegenerative diseases [56]. One of the most prevalent connections has been made with Parkinson disease (PD). A classic hallmark of Parkinson’s pathogenesis is the aggregate of Lewy bodies in PD brains, which are primarily composed of alpha-synuclein protein [57]. It was deduced that lysosomes selectively target and degrade wild-type alpha synuclein via chaperone-mediated autophagy, while pathogenic mutants of alpha-synuclein inhibit their own degradation by binding to lysosomal membrane receptors [58]. Although the precise mechanisms for the pathogenesis of PD remain unclear, it is thought that this autophagic inhibition is key in the degeneration of dopaminergic neurons, a fundamental facet in the development of PD.
In looking at the clearance of apoptotic and necroptotic cells, we see a clear two-step process involving two players: the eater and the eaten: the phagocyte and the dying cell. With autophagy this notion is blurred; the eater and the eaten is one and the same. When pushed too far it can result in cell death. Ironically, in the cell’s final efforts for self-preservation, part of the removal process is intrinsically completed. Here, we make the important distinction between autophagy, a primary mechanism for degradation in eukaryotic organisms, and autophagic cell death, which has been defined by the Nomenclature Committee on Cell Death as a program of cell death that depends on autophagic activity [5]. The final crumbs of autophagic cell death have to be cleared and this is known to occur by macrophage-mediated phagocytosis [59]. In contrast to the iconic ouroboros, an ancient symbol depicting a serpent or a dragon eating its own tail, the autophagic machinery could also be used by a phagocyte as a mechanism of clearance of dead cells. Recently, a pathway of LC3-associated phagocytosis (LAP) was described, wherein the engagement of an efferocytosis receptor by a cell corpse induces the translocation of the autophagic machinery, termed LAPosome, for phagocytosis [60]. Even further associating autophagy in the clearance of dead cells, autophagy genes, atg5 and beclin 1 in mice demonstrated defects in embryonic cavitation as dying cells failed to externalize PtdSer, severely impacting the clearance of the dead mutant cells [61].
The role of an efferocyte in the homeostatic removal and consequent physiological replacement of function is perhaps best exemplified in the case of retinal pigmental epithelial (RPE) cells and their role in the clearance of photoreceptor outer segments (OS) [31,62]. Photoreceptors, especially the OS, suffer from toxic byproducts of phototransduction. Phagocytosis of OS occurs following a circadian schedule by RPE cells [63]. New OS are generated from the base of the photoreceptors to replace the engulfed segments. This process of removal and growth maintains a roughly constant length of the photoreceptors. What is more, successful removal of photoreceptor outer segments may likely be tied to TAM RTKs role in this process as adult TKO mice were blind [31]. Examination of the retina of the TAM RTK TKO mice revealed drastic degeneration of the photoreceptor cells. This surprising result indicates that the growth of photoreceptors is coupled to the phagocytic removal of OS by RPE. Failure to engulf photoreceptor OS leads to the atrophy of photoreceptors.
In the Aftermath of Injury and/or Infection
Unscheduled cell death is associated with inflammation and the induction of an immune response. Necrosis or Greek for “to kill” has historically been considered the counterpart to apoptosis and the embodiment of unscheduled cell death. This type of cell death, also termed accidental cell death (ACD) is often invoked by irreparable trauma to the cell, leading to the disassembly of the plasma membrane [5]. Typically, lytic unscheduled cell death is associated with presence of pathogens and leads to the induction of an immune response. The hallmark necrotic morphology consists of the rupture of the membrane, swelling of the organelles and flooding of the dying cell’s cytoplasmic components into the surrounding cellular microenvironment. Among the released substances are damage-associated molecular patterns (DAMPs) that stimulate the immune system and summon an inflammatory response. Novel advances have demonstrated that these morphological markers are not only result of ACD, but also associated with regulated forms of cell death. It was not until recent years where we witnessed the true unveiling of this idea, culminating in the characterization of various “regulated necrosis” processes, including necroptosis, mitochondrial permeability transition (MPT)-driven regulated necrosis, parthanatos, ferroptosis, and pyroptosis [64].
The best characterized aforementioned form of regulated necrosis is that of necroptosis. In instances of stress where apoptosis is deliberately inhibited, scripted genetic programs may take over inducing regulated genetic pathways resulting in a necrotic morphotype, and is thereby termed “regulated necrosis” [65]. For example, cytomegalovirus is able to hinder the release of cytochrome c by inhibiting BAK and BAX, two proteins upstream in the apoptotic pathway; this in turn disables the cell’s ability to undergo apoptosis [66]. In a display of evolutionarily derived resourcefulness, the cell instead resorts to necroptosis, a programmed form of lytic cell death (type III) [67]. The necroptotic signal transduction cascade relies on activation of receptor-interacting serine/threonine- protein kinase (RIPK) 3 by RIPK1 and subsequent activation of mixed lineage kinase domain like pseudokinase (MLKL) [68]. One of the principle roles of MLKL in the necroptotic pathway is its association with phosphatidylinositol and pore formation in the plasma membrane [69]. It is known that necroptosis results in a robust release of DAMPs, and many argue that this supports the notion that necroptotic cells play a pro-inflammatory role.
The precise downstream immunological consequences and the specific identity of necroptotic DAMPs remains an active area of investigation. Necroptosis can be induced in macrophages through ligation of Pattern Recognition Receptors (PRR), such as Toll-like receptor (TLR) 3 and TLR4 in the presence of pan-caspase inhibitors [70]. Of note, the TLR4-dependent pathway of necroptosis has been associated with the release of (high mobility group box 1) HMGB1 in macrophages [71]. Release of HMGB1 is consistent with the pro-inflammatory nature of necroptosis, as it has been shown in the past that necrotic cells passively release this chromatin protein, while apoptotic cells retain HMGB1 [72]. Once outside the cell, HMGB1 binds Receptor for Advanced Glycation End products (RAGE), an important pro-inflammatory mediator, that upon ligation activates various transcriptional programs that promote the expression of pro-inflammatory genes like vascular cell adhesion molecule 1 (VCAM-1), IL-6 and tumor necrosis factor alpha (TNF-alpha) [73]. Novel studies have shown that necroptotic DAMPs may in fact increase interferon (IFN)-γ production and cross-priming of CD8+ T cells, perhaps providing more clues for the particular ways necroptosis stimulates the immune system [74].
While necroptosis has been associated with sterile and non-sterile inflammation, pyroptosis provides an instance of regulated necrosis more strongly tied to infection. Upon cell membrane rupture, this highly lytic cell death modality has been shown to create pore-induced intracellular traps (PITs) that effectively ensnare live bacteria for subsequent neutrophilic phagocytosis [75]. The hallmark of pyroptosis is the release of pro-inflammatory cytokines IL-1β and IL-18, which along with the release of eicosanoids, mediate this phagocytic response [76].
Among the most novel forms of regulated cell death (RCD) is ferroptosis, a lytic cell death modality characterized by the accumulation of iron-dependent lipid peroxidation [77]. At present, ferroptosis is an area of high interest for its potential immunogenicity in ferroptotic cancer cells. It has recently been shown that immunotherapy-activated CD8+ T cells are able to enhance lipid peroxidation in order to increase ferroptosis incidence in tumor cells [78]. Ferroptosis has been shown to play a pro-inflammatory role important for pathogenesis in a variety of contexts, yet the precise nature of the molecular patterns that drive these processes remains largely unknown. Researchers have demonstrated that among the potential pro-inflammatory effectors characteristic of ferroptosis is the autophagy-mediated release of HMGB1 [79]. In addition, HMGB1 ligation to RAGE has been implicated in actively mediating the ferroptotic pathway in macrophages, which is manifest by the increased production of TNF-alpha [79].
While inflammation and adaptive immune responses activated by these forms of cell death described above are crucial for controlling/eliminating the pathogen, exaggerated or overzealous responses risk serious host damage through heightened or chronic inflammation and/or induction of autoimmunity. Therefore, corpses have to be removed to avoid damaging inflammation. Even apoptosis, which is usually considered an anti-inflammatory process, requires the timely participation of the phagocyte. If left unattended, the apoptotic cell undergoes secondary necrosis, which may involve the release of pro-inflammatory cytokines. In systemic lupus erythematosus (SLE), it has been shown that patients have a comparatively lower quantity of efferocytes, specifically TBMs in the germinal centers of lymph nodes, relative to healthy individuals [80]. As more apoptotic cells are allowed to enter the secondary necrosis phase, this is accompanied by the release of more autoantigens that impart an autoreactive immune response, as is characteristic of SLE. Deficiency in efferocytotic mechanisms has also been linked to a variety of other disorders ranging from rheumatoid arthritis, diabetes to atherosclerosis [81].
Even necroptotic, pyroptotic, and ferroptotic cells may also be required to be removed. While PtdSer is associated with efferocytosis of apoptotic cells, recent studies have elucidated a pervasiveness of this signal across additional regulated necrotic cell death types. Interestingly, caspase-1 inhibition was insufficient for preventing PtdSer exposure of pyroptotic cells, indicating that there are significant mechanistic differences between pyroptotic and apoptotic cells, as PtdSer translocation to the outer leaflet is caspase-3-dependent in apoptosis [82]. It was also reported that pyroptotic cells release ATP more efficiently than compared apoptotic cells, yet are still cleared less efficiently by macrophages [82]. Further studies have demonstrated similar results with necroptotic cells, suggesting that PtdSer exposure is dependent on a mechanism downstream of MLKL, where PtdSer is present on so-called “necroptotic bodies” [83,84].
At the crossroads of cell death and immune cell regulation lies the TAM RTKs. TAM RTKs have been implicated in a wide range of processes involving negative regulation of the magnitude of inflammation and resolution of inflammation. Dendritic cells (DCs) are equipped with TLRs that serve as sensors for highly conserved molecular signatures of pathogens. TLR ligation in DCs is often followed by the release of cytokines that not only facilitates an innate immune response such as the production of inflammatory cytokines, but also engages the adaptive immune response by improving DC antigen presentation and co-stimulation [85]. However, the inflammatory process set forth by TLR engagement must eventually be restrained to ensure that the immune response is perfectly calibrated to avoid chronic inflammatory diseases or even drive autoimmunity. Implicated in this process of stifling of the immune response following the initial burst of activation are TAM RTKs, specifically by their ability to suppress TLR-mediated cytokine secretion by inducing suppressor of cytokine signaling (SOCS) 1 and 3 [86].
It has been indicated that there is a direct link between efferocytosis and TAM RTK-mediated negative regulation of inflammation. TLR engagement in DCs drives inflammation by activating the Nuclear Factor kappa B (NF-κB) signaling pathway. Sen et al. demonstrated that apoptotic cells downregulated NF-κB signaling in DCs and this required MERTK [87]. Thus, the effective clearance of apoptotic cells can lead to the maintenance of an anti-inflammatory microenvironment. Consistent with this idea, defects in this process are linked to various pathologies including autoimmunity and chronic inflammation on one hand [88], while on the other hand it may improve an anti-cancer immune response [89]. The link with cancer, admittedly, is not straight-forward, as loss of AXL and MERTK has been reported to be associated with increased gastrointestinal cancers in mouse models [90]. Inflammation and cancer remain rather strange bedfellows as inflammation can, in a context-dependent manner, promote tumorigenesis and/or cancer progression, while in other contexts it can facilitate robust anti-tumor immune responses. Therefore, types of cell death – apoptosis versus necroptosis/pyroptosis/ferroptosis – as well as their timely clearance or not viaTAM RTKs or other cell death sensors may have important implications in the context of anti-tumor immunity.
Negative regulation of inflammation and immune response not only limits the magnitude and severity of adverse events associated with it, but also aids in limiting its duration and the transition of inflammation to the process of repair. An example of the crucial role of removal of dead cells to tissue repair is debridement. In medicine, debridement is the surgical, mechanical, or chemical removal of necrotic tissue from a wound site to promote healing. In light of the knowledge that persistence of corpses can lead to enhanced inflammation and immunogenicity, it might be somewhat intuitive that removal of the dead cells is paramount to halt inflammation and to allow the process of repair to begin. However, removal of dead cells might also have a direct role in promoting tissue repair. An efferocyte that has an active role in tissue repair are macrophages [91,92]. These cells were discovered as specialized “big eaters” by Metchnikoff while examining echinoderms in 1883. It is now recognized that macrophages can be derived from monocytes during ongoing inflammation after injury or can be “resident” – present through-out the tissues in homeostasis. They also exist in multiple states – those derived from monocytes during inflammation, produce inflammatory cytokines while others favor tissue maintenance through phagocytic clean-up of damage or dead tissue or favor tissue repair by secreting factors to suppress inflammation or growth factors to help in tissue regeneration. The different states of macrophages ensuing as a result of the cytokines present in the environment are called “polarization” states. However, it has been demonstrated that macrophage polarization is not simply a function of cytokines, and at least in the case of IL-4/IL-13 polarized tissue-repair macrophages, this is mediated in part by sensing and/or efferocytosis of apoptotic cells [93]. It has been shown that macrophage deficit and subsequent impaired formation of lymphatic vessels in corneal inflammation produce a blunted wound healing response in a murine diabetic model [94]. It is also known that improper macrophage resolution function may lead to scarring and organ damage [95].
TAM RTKs play a crucial functional role is the macrophage. In fact, it is the simultaneous detection of IL-4/IL-13 by their cognate receptor, as well as the sensing/uptake of apoptotic cells via TAM RTKs AXL and/or MERTK that ultimately lead to the gene expression signature characteristic of tissue-repair macrophages [93]. Whether a similar role of dead cells – perhaps necroptotic, pyroptotic, or ferroptotic – is also involved in the inflammatory polarization of macrophages, and whether or not they all lead to similar gene expression profile or distinct signatures remain purely speculative so far. While it is evident that TAM RTKs play a prominent role in providing efficient clearance of apoptotic cells, their roles in other pro-inflammatory cell death modalities remain unclear. With the elucidation of PtdSer as a more ubiquitous “eat-me” signal in various other forms of cell death [82,84,96], it is possible that further ties between TAM RTKs and cell death are yet to be revealed.
Concluding Remark
The response of the body to cell death is purposeful and calibrated. In part through the release of molecular signals from dying cells that facilitate inflammation and an immune response or the absence thereof, and in part through the recognition of the dying/dead cell by efferocytes that drive the clearance of dead cells, a game of yin and yang is constantly in play between various forms of cell death and the response of the live organism – its balance is crucial for life and its disbalance manifesting in pathology. Thus, cell death and the response to it play a central role throughout the life of an organism, as seen in developmental morphogenesis, tissue/organ homeostasis and repair/regeneration after injury/infection. As Haruki Murakami wrote “Death exists, not as the opposite but as a part of life [97].”
Acknowledgments
We apologize to those whose work we were unable to cite due to space limitation. This work was supported by grants from the National Institutes of Health (NIH-NIAID R01 AI089824 and NIH-NCI R01 CA212376) and Kenneth Rainin Foundation. C.V.R is a HHMI Faculty Scholar.
Glossary
- ACD
accidental cell death
- AICD
activation-induced cell death
- ASD
autism spectrum disorder
- ATP
adenosine triphosphate
- ATP11C
adenosine triphosphatase type 11C
- BAI1
brain-specific angiogenesis receptor 1
- Ced
cell death abnormality gene
- DAMPS
damage-associated molecular patterns
- DCs
dendritic cells
- FAS
Fas Cell Surface Death Receptor
- GABA
gamma-aminobutyric acid
- GAS6
growth-arrest-specific 6
- Glu
glutamic acid residues
- HMGB1
high mobility group box 1
- IGF-1
insulin-like growth factor-1
- IgG
immunoglobulin G
- IL
interleukin
- IFN
interferon
- INA
Integrin alpha
- LAP
LC3-associated phagocytosis
- Lgr5+
leucine-rich repeat-containing G protein coupled receptor 5
- MHC II
major histocompatibility complex II
- MLKL
mixed lineage kinase domain like pseudokinase
- MPT
mitochondrial permeability transition
- MOM
more of MS
- NF-κB
Nuclear Factor kappa B
- OS
outer segments
- PAT
Paralysed Arrest at Two-fold
- PCD
programmed cell death
- PD
Parkinson disease
- PITs
pore-induced intracellular traps
- PROS1
Protein S
- PRR
pattern recognition receptors
- PSR
Phosphatidylserine receptor
- PtdSer
phosphatidylserine
- RAGE
receptor for advanced glycation end products
- RCD
regulated cell death
- RIPK
receptor-interacting serine/threonine-protein kinase
- RPE
retinal pigmental epithelium
- RTK
receptor tyrosine kinase
- SLE
systemic lupus erythematosus
- SOCS
suppressor of cytokine signaling
- TAM
TYRO3, AXL and MERTK
- TBMs
tingible body macrophages
- TIM4
T-cell immunoglobulin mucin receptor 4
- TKO
TAM RTK triple knock out
- TLR
Toll-like receptor
- TMEM16F
transmembrane protein 16F
- TNF-alpha
tumor necrosis factor alpha
- TTR
Transthyretin-like protein
- UTP
uridine triphosphate
- VCAM-1
vascular cell adhesion molecule 1
Author Contributions
V.E.G., C.V.R., and S.G conceived the idea of this review and wrote the manuscript.
References
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. [DOI] [PubMed] [Google Scholar]
- Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5(11):1249–55. [DOI] [PubMed] [Google Scholar]
- Matzinger P. The evolution of the danger theory. Interview by lauren constable, commissioning editor. Expert Rev Clin Immunol. 2012;8(4):311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleisiaris CF, Sfakianakis C, Papathanasiou IV. Health care practices in ancient greece: the hippocratic ideal. J Med Ethics Hist Med. 2014;7:6. [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25(3):486–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, caenorhabditis elegans. Dev Biol. 1977;56(1):110–56. [DOI] [PubMed] [Google Scholar]
- Ellis HM, Horvitz HR. Genetic control of programmed cell death in the nematode c. Elegans. Cell. 1986;44(6):817–29. [DOI] [PubMed] [Google Scholar]
- Southwell DG, Paredes MF, Galvao RP, Jones DL, Froemke RC, Sebe JY, et al. Intrinsically determined cell death of developing cortical interneurons. Nature. 2012;491(7422):109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss RR, Sun W, Oppenheim RW. Adaptive roles of programmed cell death during nervous system development. Annu Rev Neurosci. 2006;29:1–35. [DOI] [PubMed] [Google Scholar]
- Avino TA, Barger N, Vargas MV, Carlson EL, Amaral DG, Bauman MD, et al. Neuron numbers increase in the human amygdala from birth to adulthood, but not in autism. Proc Natl Acad Sci USA. 2018;115(14):3710–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, et al. The combined functions of proapoptotic bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6(6):1389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode caenorhabditis elegans. Dev Biol. 1983;100(1):64–119. [DOI] [PubMed] [Google Scholar]
- Gumienny TL, Hengartner MO. How the worm removes corpses: the nematode c. Elegans as a model system to study engulfment. Cell Death Differ. 2001;8(6):564–8. [DOI] [PubMed] [Google Scholar]
- Conradt B, Wu YC, Xue D. Programmed cell death during caenorhabditis elegans development. Genetics. 2016;203(4):1533–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien PW, Cameron S, Horvitz HR. Phagocytosis promotes programmed cell death in c. Elegans. Nature. 2001;412(6843):198–202. [DOI] [PubMed] [Google Scholar]
- Hoeppner DJ, Hengartner MO, Schnabel R. Engulfment genes cooperate with ced-3 to promote cell death in caenorhabditis elegans. Nature. 2001;412(6843):202–6. [DOI] [PubMed] [Google Scholar]
- Mori C, Nakamura N, Kimura S, Irie H, Takigawa T, Shiota K. Programmed cell death in the interdigital tissue of the fetal mouse limb is apoptosis with DNA fragmentation. Anat Rec. 1995;242(1):103–10. [DOI] [PubMed] [Google Scholar]
- Zakeri Z, Quaglino D, Ahuja HS. Apoptotic cell death in the mouse limb and its suppression in the hammertoe mutant. Dev Biol. 1994;165(1):294–7. [DOI] [PubMed] [Google Scholar]
- Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461(7261):282–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J Exp Med. 2010;207(9):1807–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa K, Kurata S, Yanagihashi Y, Brummelkamp TR, Matsuda F, Nagata S. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science. 2014;344(6188):1164–8. [DOI] [PubMed] [Google Scholar]
- Nagata S, Suzuki J, Segawa K, Fujii T. Exposure of phosphatidylserine on the cell surface. Cell Death Differ. 2016;23(6):952–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J, Umeda M, Sims PJ, Nagata S. Calcium-dependent phospholipid scrambling by tmem16f. Nature. 2010;468(7325):834–8. [DOI] [PubMed] [Google Scholar]
- Lai C, Lemke G. An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron. 1991;6(5):691–704. [DOI] [PubMed] [Google Scholar]
- Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, et al. The anticoagulation factor protein s and its relative, gas6, are ligands for the tyro 3/axl family of receptor tyrosine kinases. Cell. 1995;80(4):661–70. [DOI] [PubMed] [Google Scholar]
- Rothlin CV, Carrera-Silva EA, Bosurgi L, Ghosh S. Tam receptor signaling in immune homeostasis. Annu Rev Immunol. 2015;33:355–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibrewal N, Wu Y, D’Mello V, Akakura R, George TC, Varnum B, et al. Autophosphorylation docking site tyr-867 in mer receptor tyrosine kinase allows for dissociation of multiple signaling pathways for phagocytosis of apoptotic cells and down-modulation of lipopolysaccharide-inducible nf-kappab transcriptional activation. J Biol Chem. 2008;283(6):3618–27. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mass E, Ballesteros I, Farlik M, Halbritter F, Gunther P, Crozet L, et al. Specification of tissue-resident macrophages during organogenesis. Science. 2016;353(6304). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourgeaud L, Traves PG, Tufail Y, Leal-Bailey H, Lew ED, Burrola PG, et al. Tam receptors regulate multiple features of microglial physiology. Nature. 2016;532(7598):240–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Gore M, Zhang Q, Camenisch T, Boast S, Casagranda F, et al. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature. 1999;398(6729):723–8. [DOI] [PubMed] [Google Scholar]
- Schatz DG, Ji Y. Recombination centres and the orchestration of v(d)j recombination. Nat Rev Immunol. 2011;11(4):251–63. [DOI] [PubMed] [Google Scholar]
- Mogil RJ, Radvanyi L, Gonzalez-Quintial R, Miller R, Mills G, Theofilopoulos AN, et al. Fas (cd95) participates in peripheral t cell deletion and associated apoptosis in vivo. Int Immunol. 1995;7(9):1451–8. [DOI] [PubMed] [Google Scholar]
- Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372(6501):100–3. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in fas antigen that mediates apoptosis. Nature. 1992;356(6367):314–7. [DOI] [PubMed] [Google Scholar]
- Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the tyro 3 family. Science. 2001;293(5528):306–11. [DOI] [PubMed] [Google Scholar]
- Cohen PL, Caricchio R, Abraham V, Camenisch TD, Jennette JC, Roubey RA, et al. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med. 2002;196(1):135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman ZS, Shao WH, Khan TN, Zhen Y, Cohen PL. Impaired apoptotic cell clearance in the germinal center by mer-deficient tingible body macrophages leads to enhanced antibody-forming cell and germinal center responses. J Immunol. 2010;185(10):5859-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisen J. Retrospective birth dating of cells in humans. Cell. 2005;122(1):133–43. [DOI] [PubMed] [Google Scholar]
- Berlin NI, Waldmann TA, Weissman SM. Life span of red blood cell. Physiol Rev. 1959;39(3):577–616. [DOI] [PubMed] [Google Scholar]
- Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107(Pt 12):3569–77. [DOI] [PubMed] [Google Scholar]
- McCabe LR, Parameswaran N. Recent advances in intestinal stem cells. Curr Mol Biol Rep. 2017;3(3):143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden KH. P53: death star. Cell. 2000;103(5):691–4. [DOI] [PubMed] [Google Scholar]
- Valentin-Vega YA, Okano H, Lozano G. The intestinal epithelium compensates for p53-mediated cell death and guarantees organismal survival. Cell Death Differ. 2008;15(11):1772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Vega YA, Box N, Terzian T, Lozano G. Mdm4 loss in the intestinal epithelium leads to compartmentalized cell death but no tissue abnormalities. Differentiation. 2009;77(5):442-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juncadella IJ, Kadl A, Sharma AK, Shim YM, Hochreiter-Hufford A, Borish L, et al. Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature. 2013;493(7433):547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han CZ, Juncadella IJ, Kinchen JM, Buckley MW, Klibanov AL, Dryden K, et al. Macrophages redirect phagocytosis by non-professional phagocytes and influence inflammation. Nature. 2016;539(7630):570–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles AD, D’Ercole AJ. The insulin-like growth factors and the lung. Am J Respir Cell Mol Biol. 1990;3(2):93–100. [DOI] [PubMed] [Google Scholar]
- Baserga R. The double life of the igf-1 receptor. Receptor. 1992;2(4):261–6. [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor i (igf-1) and type 1 igf receptor (igf1r). Cell. 1993;75(1):59–72. [PubMed] [Google Scholar]
- D’Ercole AJ, Ye P, Gutierrez-Ospina G. Use of transgenic mice for understanding the physiology of insulin-like growth factors. Horm Res. 1996;45 Suppl 1:5–7. [DOI] [PubMed] [Google Scholar]
- Wrigley S, Arafa D, Tropea D. Insulin-like growth factor 1: at the crossroads of brain development and aging. Front Cell Neurosci. 2017;11:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun Y, Kim J. Autophagy: an essential degradation program for cellular homeostasis and life. Cells. 2018;7(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Levine B. Autophagy in mammalian development and differentiation. Nat Cell Biol. 2010;12(9):823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedjic J, Aichinger M, Emmerich J, Mizushima N, Klein L. Autophagy in thymic epithelium shapes the t-cell repertoire and is essential for tolerance. Nature. 2008;455(7211):396–400. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ. The molecular machinery of autophagy: unanswered questions. J Cell Sci. 2005;118(Pt 1):7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canerina-Amaro A, Pereda D, Diaz M, Rodriguez-Barreto D, Casanas-Sanchez V, Heffer M, et al. Differential aggregation and phosphorylation of alpha synuclein in membrane compartments associated with parkinson disease. Front Neurosci. 2019;13:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305(5688):1292–5. [DOI] [PubMed] [Google Scholar]
- Szatmari-Toth M, Kristof E, Vereb Z, Akhtar S, Facsko A, Fesus L, et al. Clearance of autophagy-associated dying retinal pigment epithelial cells - a possible source for inflammation in age-related macular degeneration. Cell Death Dis. 2016;7(9):e2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Oguin TH, Martinez J. The clearance of dying cells: table for two. Cell Death Differ. 2016;23(6):915–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Zou Z, Sun Q, Luby-Phelps K, Cheng P, Hogan RN, et al. Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell. 2007;128(5):931–46. [DOI] [PubMed] [Google Scholar]
- Burstyn-Cohen T, Lew ED, Traves PG, Burrola PG, Hash JC, Lemke G. Genetic dissection of tam receptor-ligand interaction in retinal pigment epithelial cell phagocytosis. Neuron. 2012;76(6):1123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85(3):845–81. [DOI] [PubMed] [Google Scholar]
- Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15(2):135–47. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Kepp O, Krautwald S, Kroemer G, Linkermann A. Molecular mechanisms of regulated necrosis. Semin Cell Dev Biol. 2014;35:24–32. [DOI] [PubMed] [Google Scholar]
- Cam M, Handke W, Picard-Maureau M, Brune W. Cytomegaloviruses inhibit bak- and bax-mediated apoptosis with two separate viral proteins. Cell Death Differ. 2010;17(4):655–65. [DOI] [PubMed] [Google Scholar]
- Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci USA. 2004;101(10):3516–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Wang H, Wang Z, He S, Chen S, Liao D, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of rip3 kinase. Cell. 2012;148(1-2):213–27. [DOI] [PubMed] [Google Scholar]
- Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, et al. Plasma membrane translocation of trimerized mlkl protein is required for tnf-induced necroptosis. Nat Cell Biol. 2014;16(1):55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Liang Y, Shao F, Wang X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci USA. 2011;108(50):20054–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Lu Z, Hawkes M, Yang H, Kain KC, Liles WC. Fas (cd95) induces rapid, tlr4/irak4-dependent release of pro-inflammatory hmgb1 from macrophages. J Inflamm (Lond). 2010;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein hmgb1 by necrotic cells triggers inflammation. Nature. 2002;418(6894):191–5. [DOI] [PubMed] [Google Scholar]
- Hudson BI, Lippman ME. Targeting rage signaling in inflammatory disease. Annu Rev Med. 2018;69:349–64. [DOI] [PubMed] [Google Scholar]
- Yatim N, Jusforgues-Saklani H, Orozco S, Schulz O, Barreira da Silva R, Reis e Sousa C, et al. Ripk1 and nf-kappab signaling in dying cells determines cross-priming of cd8(+) t cells. Science. 2015;350(6258):328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen I, Zhang Y, Krantz BA, Miao EA. Pyroptosis triggers pore-induced intracellular traps (pits) that capture bacteria and lead to their clearance by efferocytosis. J Exp Med. 2016;213(10):2113–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen I, Lopez JP, Laufer SA, Miao EA. Il-1beta, il-18, and eicosanoids promote neutrophil recruitment to pore-induced intracellular traps following pyroptosis. Eur J Immunol. 2016;46(12):2761–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Green M, Choi JE, Gijon M, Kennedy PD, Johnson JK, et al. Cd8(+) t cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569(7755):270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Q, Liu J, Kang R, Zhou B, Tang D. The release and activity of hmgb1 in ferroptosis. Biochem Biophys Res Commun. 2019;510(2):278–83. [DOI] [PubMed] [Google Scholar]
- Baumann I, Kolowos W, Voll RE, Manger B, Gaipl U, Neuhuber WL, et al. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46(1):191–201. [DOI] [PubMed] [Google Scholar]
- Martinez J. Prix fixe: efferocytosis as a four-course meal. Curr Top Microbiol Immunol. 2017;403:1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Imamura R, Motani K, Kushiyama H, Nagata S, Suda T. Pyroptotic cells externalize eat-me and release find-me signals and are efficiently engulfed by macrophages. Int Immunol. 2013;25(6):363–72. [DOI] [PubMed] [Google Scholar]
- Gong YN, Guy C, Olauson H, Becker JU, Yang M, Fitzgerald P, et al. Escrt-iii acts downstream of mlkl to regulate necroptotic cell death and its consequences. Cell. 2017;169(2):286–300.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zargarian S, Shlomovitz I, Erlich Z, Hourizadeh A, Ofir-Birin Y, Croker BA, et al. Phosphatidylserine externalization, “necroptotic bodies” release, and phagocytosis during necroptosis. PLoS Biol. 2017;15(6):e2002711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16(4):343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. Tam receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131(6):1124–36. [DOI] [PubMed] [Google Scholar]
- Sen P, Wallet MA, Yi Z, Huang Y, Henderson M, Mathews CE, et al. Apoptotic cells induce mer tyrosine kinase-dependent blockade of nf-kappab activation in dendritic cells. Blood. 2007;109(2):653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon IK, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14(3):166–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akalu YT, Rothlin CV, Ghosh S. Tam receptor tyrosine kinases as emerging targets of innate immune checkpoint blockade for cancer therapy. Immunol Rev. 2017;276(1):165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosurgi L, Bernink JH, Delgado Cuevas V, Gagliani N, Joannas L, Schmid ET, et al. Paradoxical role of the proto-oncogene axl and mer receptor tyrosine kinases in colon cancer. Proc Natl Acad Sci USA. 2013;110(32):13091–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannella KM, Wynn TA. Mechanisms of organ injury and repair by macrophages. Annu Rev Physiol. 2017;79:593–617. [DOI] [PubMed] [Google Scholar]
- Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosurgi L, Cao YG, Cabeza-Cabrerizo M, Tucci A, Hughes LD, Kong Y, et al. Macrophage function in tissue repair and remodeling requires il-4 or il-13 with apoptotic cells. Science. 2017;356(6342):1072–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D’Amore PA. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol. 2007;170(4):1178–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfeld SD, Cherry C, Schwab RM, Chung L, Maestas DR, Jr, Laffont P, et al. Interleukin-36gamma-producing macrophages drive il-17-mediated fibrosis. Sci Immunol. 2019;4(40). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloditz K, Fadeel B. Three cell deaths and a funeral: macrophage clearance of cells undergoing distinct modes of cell death. Cell Death Discov. 2019;5:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami H. Norwegian Wood. Harvill Press; 2000. p. 31. [Google Scholar]