Abstract
Programmed cell death (PCD) in cell groups and microbial communities affects population structures, nutrient recycling, and sociobiological interactions. A less explored area is the role played by PCD in the emergence of higher-level individuals. Here, we examine how cell death impacted evolutionary transitions in individuality (ETIs). The focus is on three specific ETIs – the emergence of the eukaryote cell, multicellularity, and social insects – and we review the theoretical and empirical evidence for the role of PCD in these three transitions. We find that PCD likely contributed to many of the processes involved in eukaryogenesis and the transition to multicellularity. PCD is important for the formation of cooperative groups and is a mechanism by which mutual dependencies between individuals evolve. PCD is also a conflict mediator and involved in division of labor in social groups and in the origin of new cell types. In multicellularity, PCD facilitates the transfer of fitness to the higher-level individual. In eusocial insects, PCD of the gonadal cells in workers is the basis for conflict mediation and the division of labor in the colony. In the three ETIs discussed here, PCD likely played an essential role, without which alternate mechanisms would have been necessary for these increases in complexity to occur.
Keywords: programmed cell death, evolutionary transitions in individuality, eukaryogenesis, multicellularity, insect sociality
In the last few decades, it has emerged that programmed forms of cell death (PCD) are not confined to multicellular life. PCD, it appears, is ubiquitous in the unicellular world. Since this revelation, the interest in this field has revolved primarily around the nature and meaning of programmed forms of death [1-3], the evolution of cell death mechanisms [4-8], and the evolutionary ecology of PCD in microbial communities [9-13]. There has been some interest in the role of PCD in evolutionary transitions in individuality (ETIs), but this has typically been with reference to either a specific ETI or a specific PCD mechanism (for example [14-16]). We wish to understand the role of PCD in ETIs more broadly. ETIs are major leaps in the complexity of life and involve the evolution of new kinds of individuals from groups of previously existing individuals [17-20]. Examples of these ETIs are the emergence of genomes from genes or chromosomes, the evolution of the eukaryote cell, the evolution of multicellularity from unicellular life, and obligate eusociality in insect colonies [17]. For a detailed discussion of ETIs the reader is referred elsewhere [18,19,21,22]. West et al. [20] break down the process of an ETI into two broad steps: the formation of a cooperative group and the transformation of that group into an integrated entity. They suggest six “big” questions that are key to understanding the evolutionary and ecological drivers of these two steps. They interrogate the conditions that favor (i) the formation of cooperative groups, (ii) cooperation during group transformation, (iii) division of labor, (iv) communication that coordinates cooperation at the group level, (v) conditions that lead to negligible conflict within groups, and (vi) mutual dependencies. As West et al. highlighted, there are no clear borders between the six processes. Clearly, there is considerable overlap, but separating them out this way assists us in investigating the general conditions associated with each of them. We will use these processes as our departure point for thinking about the role of PCD.
As Lloyd points out [23], before examining any evolutionary processes, the structure and logic of the research question or statement needs to be clearly thought through to draw causal inferences. For our purposes we do not distinguish between the formation (origination) and maintenance of cooperative groups in this article and collapse the first two problems into one. We, therefore, re-structure the six problems for our particular context of PCD (see the section “PCD as an evolutionary process” [1] for more on the logic of research questions in PCD evolution) and settle on five questions that examine the role of PCD in ETIs (Table 1) and use these as the primary motivation for this article. This is done in relation to the three most widely-studied ETIs – the evolution of the eukaryote cell, multicellularity, and eusociality in insects. Before examining the potential role of PCD in these three ETIs, the key component of each of the five questions is briefly outlined.
Table 1. PCD and ETIs. PCD plays five key roles in the three ETIs discussed in this manuscript. For example, in the first instance, in eukaryogenesis PCD plays a role in the formation of cooperative groups by facilitating resource-sharing between individuals. In division of labor in eukaryogenesis, PCD plays a role in organellar specialization (see the text for further details).
| The role played by PCD in... | Eukaryogenesis | Multicellularity | Eusociality |
| The formation of cooperative groups | Resource sharing | Resource sharing | Sterility in workers is required for the mediation of conflict, division of labor and mutual dependencies |
| The division of labor | Organellar specialization | Propagation and dispersal | |
| Communication | Signaling cascade in PCD | Infochemicals | |
| Conflict mediation | Death of the group | Death of uncooperative cells; genetic transfer | |
| Mutual dependencies | Reliance on shared resources | Recycling of nutrients |
The Evolutionary and Ecological Drivers of ETIs
Cooperation and the Formation / Maintenance of Groups
Traits that have evolved because of the benefit they provide to others are cooperative traits. The benefit can be directed at relatives when the ratio of the cost of the trait to the benefit is less than the degree of relatedness (Hamilton’s rule) [24]. In this case, kin selection is the explanatory framework [25,26]. Cooperation can sometimes also evolve by natural selection between non-relatives if there is a direct benefit to the cooperator. The mechanism by which the benefit is selected for may involve phenomena like symbiosis or reciprocity. Groups of individuals can form because of these cooperative behaviors between individuals, but under specific sets of conditions, the groups themselves can be selected for [27,28]. The explanatory framework typically involves holobionts [29] or kin groups [25,28] as the units of selection. In these instances, the fitness components of individuals in the group are connected in some way or another and augmented by the properties of the group. Relatedness can facilitate the connectedness, but it is not a requirement. The formation and maintenance of groups depends on cooperative behaviors and in this article, we examine whether PCD plays a role in the evolution of cooperation in ETIs.
The Division of Labor in ETIs
The second “big question” concerning ETIs that was identified by West et al. [20] is the division of labor. In cooperative groups, individuals can specialize in different functions, and this is especially important as the unit of selection shifts from the lower to the higher level. This process can be driven by natural selection if the fitness returns increase as individuals dedicate more time or resources to a particular task [19,30]. The corollary is also true, if the fitness returns diminish, then individuals that do not specialize are favored by natural selection. In the three ETIs in this article, there are numerous examples of this drive to specialization. In the eukaryote cell, the mitochondria and chloroplasts that evolved from free-living autonomous individuals specialize in generating energy for the cell. In multicellular organisms, specialized cells have evolved, such as the stalk and spore cells in Dictyostelium [31] or the reproductive and somatic cells in Volvox [32]. In honeybees, the sterile workers invest in foraging while others invest in reproduction [33].
Once an ETI is complete, the division of labor is obligate such as the evolution of mitochondria, the differentiation of stalk cells in Dictyostelium or the somatic cells in Volvox. But as with all the components mentioned in the five questions above, division of labor can be facultative in cooperative groups, before the irreversible transition from the lower to the higher level. Examples of this include bacterial biofilms [34] and facultatively eusocial insects [35]. With respect to this second key feature of ETIs, the question we explore, is whether PCD plays any role in the division of labor.
Group-level Communication
For the evolution of cooperation and division of labor, individuals need to be able to communicate with each other. They need to “know” where they fit functionally, structurally, or temporally in the group. Communication that regulates group level behaviors are very often chemical in nature, such as the quorum sensing molecules in bacterial biofilms [36] or the infochemicals released by microalgae [37]. Chemical communication, however, is not limited to microbes. In eusocial insects, pheromones control nursing and reproductive activities [38]. Communication in ETIs is not necessarily chemical in nature. Behavioral mechanisms like the famous waggle dance in honeybees is a way of communicating information about resources to others in the hive.
Communication is important for maintaining cooperation in the group, the division of labor and, sometimes, excluding non-relatives from the group. The quorum sensing molecules released by bacteria regulate the production of extracellular matrix and cell differentiation in the biofilm; this is favored by high relatedness [39]. Communication also provides information about the environment allowing organisms to modify phenotypes that provide an advantage under a specific set of conditions [40]. The advantages are usually available to relatives and facilitates cooperation and co-ordination in the group. Of course, non-relatives and cheaters can take advantage of the cooperation without paying the cost. For the relationship between cell death and ETIs, we determine whether PCD is a form of communication that leads to cooperation, division of labor, or provides information about the environment.
Conflict Mediation
Cooperative groups are always vulnerable to conflict between individuals. The fourth important feature of ETIs is how the conflict is mediated. Conflict mediators are any trait or phenotype that restricts the opportunity for fitness variation at the lower level or enhances the variation in fitness at the higher level [14]. It is certainly the case that the potential for conflict is greater if the individuals in the group are unrelated. Kinship promotes cooperation and helps to minimize conflict. However, when environmental factors like dispersal rates and population structures have a significant effect on individual fitness, this is not a requirement. In most cases the endpoint of the ETI results in relatedness between genomes that comprise the new higher-level individual, although there are notable exceptions such as multicellular ramets, which are sometimes chimeric.
Natural selection predicts that individuals evolve to maximize their fitness even if this is costly to others in the group, or the group itself. Minimizing intra-group conflict is important if fitness is to be transferred from one level to the next and, with respect to PCD, the issue we investigate is whether PCD is a mechanism by which conflict is mediated in any of the ETIs discussed below.
Mutual Dependencies in ETIs
During ETIs, mutual dependencies develop between lower-level individuals. For example, the division of labor means that individuals with different functions are mutually dependent on each other for their survival and/or reproduction. Mutual dependency is observed in the three ETIs discussed in this paper. In eukaryote cells, the organelles (mitochondria, chloroplasts, and nuclei) perform different functions, all of which are necessary for cell viability [41]. In the model organisms Dictyostelium [42] and Volvox [43,44] the different cell types (reproductive and somatic) are mutually dependent on each other and in obligate eusociality, the queens cannot reproduce without the workers [45]. However, before the completion of the ETI, mutual dependencies are not necessarily obligate; they emerge during the transition.
The mechanisms of dependence vary, but there are some broad categories. Resources can be shared between the component individuals that comprise the higher-level individual. An example is the sharing of ATP between cellular organelles: in the volvocine lineage, the extracellular matrix is shared between cells and insect colonies share stored and cultivated food. Mutual dependency may also take the form of communication (discussed above). In eukaryote cells, intracellular communication occurs via protein pathways and in multicellular organisms, intercellular communication co-ordinates homeostasis. Social insects communicate via chemical pheromones and through their behavior.
Metabolic dependencies may evolve between the components in the higher-level individual. In eukaryote cells, resource molecules are shuttled between organelles. The cells in multicellular organisms also exchange resources and detoxify the metabolic wastes of others. Similarly, nurse bees feed the larvae and pupae in the colony. Does PCD play any role in the emergence of these mutual dependencies?
The Role of PCD in ETIs
Our understanding of PCD in ETIs has usually been limited to a particular ETI or a specific PCD mechanism. Blackstone and Green examined the evolution of cell death in the first eukaryote cell highlighting the role of PCD in genetic conflict and cellular energetics [41,46,47]. Michod, Nedelcu, and others discuss PCD as a form of conflict mediation in the unicellular-multicellular ETI [14,15,48,49]. However, a general account of the role of PCD is lacking. For the three ETIs that are the focus of this paper, we investigate the role (if any) of PCD in each of the five evolutionary and ecological drivers listed above. To do so, our interpretation of the term PCD must be made explicit.
Numerous authors over the past decade have discussed different meanings of the term PCD, highlighting that it does not have an obvious interpretation [2,4,7,9,50]. The term is very often understood differently by different researchers, which leads to divergent views and contrasting interpretations of the same data. It is imperative that we indicate which interpretation we use, failing to do so will only exacerbate the conflation of ideas. Both mechanistic and evolutionary definitions are helpful. We use the Berman-Frank et al. mechanistic definition of PCD as “active, genetically controlled, cellular self-destruction driven by a series of complex biochemical events and specialized cellular machinery” [51]. Our evolutionary definition of PCD is that it is “an adaptation for producing cell death” [1], where death itself has been selected for. In contrast, “ersatz PCD” involves similar mechanisms and phenotypes but, in these instances, death is a side-effect of other life-promoting processes. It is important to differentiate these two kinds of PCD, because they have different evolutionary histories. The focus in this essay is on PCD as opposed to ersatz PCD, because we wish to understand where cell death itself (true PCD) has been selected for during the ETI. In addition, and as many authors have demonstrated, it is helpful to think about PCD as a “system that is probabilistic (the same input does not universally produce the same output), branching (some stages in the execution of the program can lead to a range of future states) and non-discrete (loss of viability can be transient or graded)” [1].
PCD and the Evolution of the Eukaryote Cell
PCD was introduced into eukaryote cells via the true bacteria, which are understood to be the ancestral forms of mitochondria. Koonin and Aravind discovered this “bacterial connection” by identifying diverse homologs of most of the PCD molecular machinery in bacteria, but not in archaea [52]. PCD, it seems, does occur in archaea [53], but the bacterial kind of PCD is dominant in eukaryotes. The phylogenetic and genomic data are supported by laboratory studies, which revealed that mitochondria play a central role in apoptosis (a common PCD phenotype) in eukaryote cells (for example [54]). The circumstances that drove cooperation between different prokaryote taxa are not entirely clear, but one of the reasonable hypotheses is that the endosymbiosis of bacteria conferred the amitochondriate host cell with new metabolic capabilities, in particular oxidative respiration [41,55]. This provided the group of cells with significant advantages [41,56]. The genetic differences between the taxa comprising the first proto-eukaryote cell, however, would have inevitably led to conflict [46,57] and Blackstone and Green suggest that “a mechanism of apoptosis in metazoans may thus be a vestige of evolutionary conflicts within the eukaryotic cell” [47] (see Figure 1 for examples). A similar argument is made by Kaczanowski [58], and the ancestral state reconstruction of the eukaryote cell by Klim and colleagues found “an ancient evolutionary arms race between protomitochondria and host cells, leading to the establishment of the currently existing apoptotic pathways” [59]. This latter finding was supported empirically in their yeast model system.
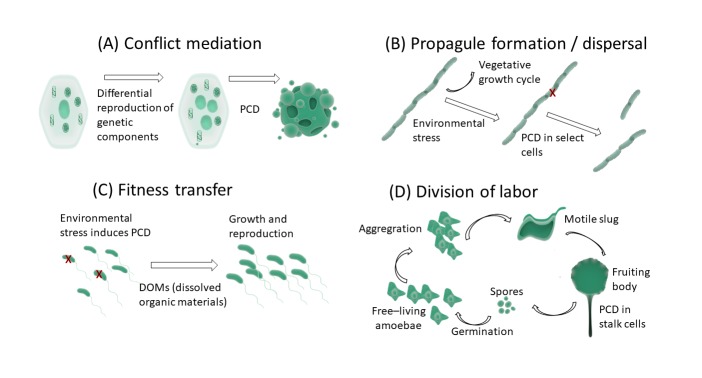
Figure 1.
The role of PCD in ETIs. PCD plays several roles in evolutionary transitions in individuality. (A) PCD is a mediator of conflict by aligning the evolutionary fate of individuals in the social group (the eukaryote cell in this example). (B) PCD is essential for the propagation and dispersal of colonies (see the discussion on Trichodesmium). (C) PCD is a mechanism by which fitness is transferred from the lower to the higher individual (see the examples of Chlamydomonas, Saccharomyces, and Dictyostelium). (D) PCD is a form of division of labor in social groups (see the discussion on Dictyostelium).
The Formation of Cooperative Groups: PCD Facilitates Cooperation Between Cellular Organelles in the Eukaryote Cell via Inter-organellar Communication and the Transfer of Energy-rich Molecules
In the case of the early proto-eukaryote cell, the cooperative group would have been the proteobacteria and the amitochondriate host cell, after which cyanobacteria were included [60]. In the early stages, cooperation may have taken the form of resource-sharing in time and space. In extant eukaryote cells, energy-rich molecules like ATP, vitamins and metabolites are transferred between organelles in healthy cells. The increase in size of the cell or the secretion of toxins could also have been protective against predation [61]. The mitochondria and chloroplasts play a central role in most types of PCD and when the death pathways are activated, some of these cooperative functions are observed. Some functions are not restricted to PCD, and there are other cooperative behaviors that are intensified. There is the activation of protein pathways (communication is also considered a form of cooperation between individuals, but this is discussed below), and energy-rich molecules are free to move from the organelles into the cytoplasm where they are required for the changes in metabolism and cellular architecture [62]. During PCD the mitochondria are restructured and disintegrate, the membrane is depolarized, and ATP is released in greater quantities into the cytoplasm [63]. This behavior is cooperative in that it provides the rest of the cell with the energy required for death functions.
The Division of Labor: PCD is (Largely) a Task Allocated to Mitochondria and Plastids
PCD as a form of the division of labor has been alluded to by Blackstone, Green, and others [41,46,47]. Most PCD mechanisms in eukaryote cells are dependent upon mitochondria, chloroplasts or both (for a broad sketch of the process see [11]). They often initiate the process by releasing activators into the cytoplasm. These organelles also produce the energy required for PCD and release sequestered ATP into the cytoplasm at the onset of PCD. PCD in some organisms is executed by organelle-specific oxidation stress patterns [64]. In the early stages of the evolution of the eukaryote cell, the genomic data suggest that bacteria were almost entirely responsible for PCD and served this function in the collection of cells from which the Eukarya emerged [65]. In extant eukaryotes, different components of PCD are allocated to different organellar compartments. The task of PCD is no longer performed by a single type of organelle.
Cooperation Through Communication: Signaling Molecules that Coordinate Cell Level Activities are Released by Mitochondria and Plastids During PCD
PCD can be caused by a variety of environmental stresses that lead to an intracellular burst of reactive oxygen species [11,13,66-70]. The mitochondria and chloroplasts facilitate this response by releasing signaling molecules, primarily cytochrome C (cytC) and cytochrome F (cytF), respectively. These and other molecules communicate a physiological stress state, leading to a change in redox potential that activates a repertoire of genes that deal with the oxidative stress, but which also prime the cell to undergo PCD should it encounter any further environmental stresses. In photosynthetic eukaryote cells, there is a group of proteins, the death-specific proteins (DSPs), that are localized to the chloroplast and are important signal transducers. They regulate cell fate between acclimation (in cases where cell stress is minimal) and PCD (when the stress is sufficient to cause permanent cell injury and unregulated death). In other words, mitochondria and chloroplasts communicate information about the environment (see, for example, the CoRR hypothesis that explains the maintenance of phenomenon [71,72]), either inhibiting or initiating death depending upon the conditions.
Conflict Mediation: PCD Aligns the Evolutionary Interests of all the Genomes in Eukaryogenesis
The molecular coevolution between the ancestor of mitochondria and the host cell eventually resulted in the integration of cell death pathways [52]. However, the potential for conflict between the genomes of the different cellular organelles remains [73] and PCD may be a mediator of this conflict [15,47]. In the eukaryote cell, PCD may be a way of ensuring that the evolutionary interests of all the organellar genomes (three, in the case of photosynthetic organisms) remain aligned [41,46,47]. The stable evolution of the eukaryote cell depended on this alignment.
Mutual Dependencies: PCD Depends on the Interactions Between Different Cellular Compartments
As indicated above, both organellar and cellular (nuclear and cytoplasmic) components are required for PCD. The signaling, initiation and execution of PCD require molecular elements found in the organelles and cell. The molecular pathways that ultimately lead to PCD phenotypes are functionally dependent on each other [6,41,47,58,65,74,75].
PCD and the Evolution of Multicellularity
The evolution of cell death programs was a prerequisite for the emergence of multicellularity [67,76]. PCD, which served many functions in eukaryogenesis, was co-opted for multiple developmental, viral resistance and tissue homeostasis functions in all multicellular life forms [71,72]. The ETI from unicellular to multicellular life occurred numerous times [77] and in each case, some form or another of PCD is found. Furthermore, knock out experiments from at least one lineage (the Metazoa) disrupts tissue homeostasis and eventually leads to death [78], which underscores the essential nature of this trait in multicellularity. In this section, we will examine the role of PCD according to the processes posed by West et al. [20].
The Formation of Cooperative Groups: The Products of PCD Facilitate the Formation (Origin and Maintenance) of Groups of Cooperating Cells
Cell groups form in two ways: cells may either come together (aggregate) in response to environmental stimuli, or cells may stay together post division. The maintenance of these cell groups, irrespective of whether group formation is facultative or a stable phenotype, is a complex issue since there are significant environmental stresses and costs associated with living in groups. Cooperation is a way of dealing with these stresses. In the model organism Chlamydomonas, cells living in clumps have less access to nutrients, they live among their own metabolic waste, and they lose their flagella. Artificial selection of groups of Chlamydomonas cells that stay together) leaves them non-viable [79]. Cells exposed to predators form groups by both staying together and coming together [80]. In such groups, it is predicted theoretically [81] and observed empirically [82] that cell death occurs. It is also observed that when cells die by PCD, they provide resources to others helping them to grow bigger and reproduce [83]. In predator-induced groups, cells can still divide and grow, and we suggest that this is facilitated by the nutrients provided by their dying kin [83,84]. PCD is a mechanism for cooperation and overcoming some of the stresses of social living by sharing resources between members of the group.
Resource sharing also occurs in cell populations that are not necessarily in close proximity or adherent. The data from Chlamydomonas [83-85], Dunaliella [86], and Saccharomyces [87,88] revealed that the substances released by PCD dissolve through the medium and affect the viability and reproduction of others. We assume that, in instances where cells aggregate into groups, this PCD-based mechanism of cooperation helped the cell groups to deal with the pressures of facultative sociality and was essential for the evolution of multicellularity [67].
The Division of Labor: PCD Contributes to the Division of Labor by Propagation, Dispersal, and the Emergence of New Cell Types
In social groups, some individuals are allocated the task of undergoing PCD. The discovery of an autocatalyzed cell death pathway in filamentous diazotrophic cyanobacteria of the genus Trichodesmium – with involvement of metacaspase and endonuclease enzymes – identified PCD as a key function for propagation and dispersal [13,51]. When the environment is unfavorable, some cells in the filaments of cyanobacteria differentiate into dying cells leading to a fragmentation of the colony. The dispersal of these propagules, called hormogonia, play a role in colonizing new environments and bloom development when environmental conditions improve [51]. In the blue-green alga Anabaena variabilis, there is an extrusion of cellular contents and loss of viability observed during heterocyst differentiation [89]. Cyst formation and PCD are part of the “death spectrum” [1,90] and the differentiated functions accomplished by hormogonia and heterocysts in the trichromes of the two examples cited here are presented as evidence for the role of PCD in propagation and dispersal. Similarly, during the artificial selection of “multicellular” yeast, it was argued that PCD facilitates the propagation of daughter colonies. [91]. The suggestion was that PCD cells represented a task allocation: daughter colonies formed at fracture sites that occurred where cells died by PCD. The fracture sites were due to physical stresses [92], but the conclusion was that cell death contributed to the propagation of yeast colonies via a division of labor where one of the task allocations was PCD.
There is another example where PCD is an absolute requirement for the development and survival of social groups. In Dictyostelium species, free-living amoeba aggregate during periods of environmental stress with cells differentiating into specialized structures. A stalk-like structure composed of vacuolated cells forms, followed by the appearance of a spore-producing fruiting body [93,94]. The fruiting body performs the task of reproduction, while the stalk cells increase colony viability by propping up the fruiting bodies and facilitating their dispersal. The key element is that the vacuolated stalk cells die by PCD when differentiating into a support column. Knocking out the genes responsible for PCD is catastrophic for the colony. Not only are the fruiting bodies unable to form but, the absence of support structures results in non-viable colonies. Preventing the differentiation of PCD cells results in defective colonies [95].
The evolution and origin of specialized cell types in metazoan development has very recently also been attributed to cell stress pathways and PCD. It is argued that environmental stress can lead to the differentiation of specialized cells and that this is achieved via PCD pathways – a phenomenon called stress-induced evolutionary innovation (SIEI) [96]. For example, the decidual cells that reside in the uterus of eutherian mammals have evolved by way of PCD and cell stress pathways [97], and the thickened skin in cetaceans is induced by a stress-response, PCD mechanism [98]. In these cases, oxidative stress and PCD are critical for the differentiation of specialized cell types.
Communication at The Group Level: Infochemicals Released During PCD can Coordinate Group-Level Activities
Cells dying by PCD are known to release an array of chemicals that coordinate group-level activities. One of the mechanisms that has been dissected in detail involves an excreted thiol protease that is released by PCD cells and synchronizes the stress-responses of other cells [69]. The discovery was made in samples from an annual microalgal bloom in Lake Kinneret, Israel where some individuals of the dinoflagellate Peridinium gatunense responded to environmental stress by undergoing PCD and releasing the protease, which regulated the responses of younger cells in the population. In this instance the communication made others more sensitive to oxidative stress, rendering them more likely to undergo PCD in stressful environments. The opposite has also been observed. In Chlamydomonas reinhardtii, PCD infochemicals can make others more resistant to the stimuli that induce death [85]. Together, these findings revealed that PCD is a mechanism of communication, regulating the responses of other individuals in the population to environmental conditions.
Similarly, diatoms subjected to grazing and other abiotic stress conditions produce a group of compounds called diatom-derived aldehydes (DD-aldehydes) [70,99]. These chemicals are known to play a role in defense against grazing and competing species, however, it was also shown that they are a part of a complex stress surveillance system in marine diatoms populations [70]. Particularly, the exposure of Thalassiosira weissflogii and Phaeodactylum tricornutum to a critical threshold of a specific DD-aldehyde triggers an intracellular cascade of signaling molecules that eventually leads to PCD [70]. The responses to DD-aldehydes are concentration dependent. Exposure to sublethal doses immunizes individuals to subsequent “lethal” concentrations of DD-aldehydes. It is proposed that diatom populations utilize stress surveillance systems to regulate stress responses in bystanders. Sublethal levels of environmental stress surveillance compounds provide protection, while high concentrations are one of the mechanisms responsible for the coordinated demise of microalgal blooms.
NO-based signaling mechanisms are intimately associated with PCD, but also play a role in the aggregation of cells. They determine how diatoms select, and adhere to, physical surfaces [100]. Subsequent work in the taxon P. tricornutum discussed above, revealed that overexpression of nitric-oxide-associated protein (NOA), which is integral to NO-based stress surveillance, results in a reduction in cellular adherence strength, compromising the ability of individuals to form microalgal biofilms [101]. Depending on the type and concentration of the signaling compound, PCD-related communication molecules appear to channel individuals into alternate states including death, immunity to environmental stress, encystation, and aggregation.
Conflict Mediation: PCD Mediates Conflict in Cell Groups by Eliminating Uncooperative Cells
PCD is a known conflict mediator in multicellularity evolution [15,48] and is a mechanism for keeping the evolutionary interests of all individuals in the group aligned. Rogue cells, whose interests are harmful to the group, are induced to die. Of course, there is always the potential for cheating, especially when the genetic differences between individuals in the population are great.
At the very beginning of colonial living, predation was an important selective pressure for sociality [102-105]. For a long time clonality, or at least close relatedness, was considered key for the earliest steps towards multicellularity. For kin selection to operate, relatedness is a requirement and is presumably the motivation for experiments that select for groups of cells that stay together post-division. Kin selection results in clonality in multicellularity, although there are some noteworthy exceptions (discussed in [27]). But clonality is the result of kin selection and not a requirement. At the beginning of group formation, we suggest that genetic conflict could have been a major problem. This is especially true if multicellularity arose from cells aggregating rather than staying together and the empirical data support this argument.
Aggregates of cells can comprise genetically unrelated individuals. In Dictyostelium, the “multicellular” fungus-like forms that aggregate in unfavorable conditions are genetically heterogeneous [106]. Furthermore, predation is a powerful driver of group formation in microalgae, and the groups comprise different species [82] and even different genera [107]. In the presence of predators, group formation is the result of cells staying together as well as coming together [80], which explains the observation that they comprise different taxa. As in the case of eukaryogenesis, genetic conflict is inherent in chimeric groups, but PCD provides individuals with a potential solution. Not only will differential death rates homogenize the genetic composition of the group, but the fitness-enhancing products of PCD are available to more closely related individuals and allelopathic to other species [84]. In this way, PCD homogenizes groups by promoting non-random associations between genotypes – one of the conditions for kin selection to operate.
In Dicytostelium, the evidence that PCD is a mediator of genetic conflict has been observed microscopically. Cells dying by PCD fragment into membrane-bound apoptotic-like bodies (ABs). The ABs, which contain genetic material, have been observed being engulfed by other healthy cells [108,109]. This suggests that genetic conflict can be mediated by the transfer of genetic material between dying and healthy individuals.
Mutual Dependencies: Functional Dependencies like Resource Sharing and Group-Level Immunity Depend on PCD
Mutual dependencies exist in the microbial communities that are used as model systems for investigating features of multicellularity as well as the groups of cells from which multicellular life could have evolved. Here, we examine the contribution of PCD to the evolution of these mutual dependencies.
Microbial communities like the marine communities comprising mixed phytoplankton and prokaryotes, and bacterial biofilms, are useful models to understand the role played by PCD at the origins of multicellular life. In the upper ocean, the euphotic zone, the way organisms die has a direct effect on the ecological fate of others [11]. PCD-driven lysis of photosynthetic organisms fuels the exchange of nutrients between individuals. Dissolved organic materials (DOMs) are produced from cells dying by PCD and their cycling through the ocean surface is widely documented [11,13]. As Abada et al. have demonstrated, the emerging microbial interactions in the “sea skin” is a lens through which the mutual dependencies that are a feature of multicellularity are revealed [110,111]. There are many examples where the mutual dependency has been tracked. In hypersaline environments, the interaction between Dunaliella salina and Halobacterium salinarum illustrates how PCD is a mechanism by which nutrients like glycerol, amino acids, vitamins, and other co-factors may be exported from dead individuals to both relatives and unrelated taxa. The synchronized death of D. salina at the onset of darkness, resulted in an increase in extracellular DOMs (glycerol was used as a marker) that were utilized by relatives or re-mineralized by co-occurring archaea. The cycling of nutrients facilitated the growth and reproduction of others in the community. PCD in this sense, represents a mechanism by which functional interactions emerge, and the fitness of both phytoplankton and prokaryotes were metabolically and physiologically dependent of each other [12]. The export of resources between relatives was also reported among other organisms such as the social amoeba Dictyostelium, [108,109], yeast [87,88], and Chlamydomonas [83,84], and may enhance the survival of cell groups during stressful periods. These interactions may be a mechanism by which fitness is transferred from the lower to a higher level.
In addition to resource sharing, the dependency on PCD is also apparent in bacterial biofilms where organisms couple an adaptive response at the individual level to cooperative behavior at the group level [112,113]. Upon phage infection, a population of bacteria with a gene for PCD outcompetes one without by limiting the spread of a lytic phage [114]. This response is functionally analogous to the hypersensitivity response in higher plants where rapid cell death occurs to limit the spread of pathogens to the rest of the organism [115]. Bacterial populations and microbial communities reflect the ways in which mutual dependencies may have evolved en route to multicellular lifestyles.
PCD in Social Insects
In the discussions above, PCD was important for eukaryogenesis and the evolution of multicellular lifestyles, but there are also cases in social insects where PCD regulates the fate of the entire eusocial colony. This is the result of another cross-level adaption and essential for the ETI from asociality to eusociality. In some insect colonies, PCD has been shown to be a mechanism for decreasing individual fitness and boosting group fitness. The mechanistic basis for this has been dissected, quite literally, in eusocial honeybees by observing the fate of individual cells in workers. The queen secretes pheromones that induce PCD in the ovaries of worker bees. Worker oocytes are aborted, and the individual bees are effectively sterile in service of the colony [116]. Without PCD in workers and their subsequent sterility, the social complexity of the community is disrupted. The individual questions posed in the previous two sections are not appropriate here since the group comprises multicellular organisms and not individual cells. Instead, the cross-level role of PCD in eusociality is summarized as it relates to the processes above.
The queen in the insect colony communicates with others via infochemicals, one of which decides the reproductive fate of workers. The queen effectively sterilizes her workers by inducing PCD in their gonadal cells. This single act is the basis on which many of the processes highlighted by [20] rest. Removing the potential for workers to reproduce, ensures their cooperation and aligns their evolutionary interests with others in the group. Sterility frees the workers to specialize in non-reproductive tasks and the resultant division of labor allows them to dedicate time to foraging, providing resources to those specializing in reproduction and to maintaining the hive. A mutual dependency exists between workers and reproducers.
ETIs Depended on the Evolution of PCD
An analysis of PCD reveals that cell death played a key role in the five central processes identified by West et al. (2015) as being crucial to ETIs. First, PCD facilitates the formation of cooperative groups by cells sharing resources or communicating information about the environment – prokaryote cells in the case of eukaryogenesis, and eukaryote cells in the case of multicellularity. Second, PCD is a mechanism for the evolution of the division of labor. In the eukaryote cell, this was potentially by protomitochondria or protochloroplasts effecting programmed forms of death, while the amitochondriate cell performed other functions. In multicellularity, PCD is a mechanism for fulfilling specialized tasks like stalk formation in Dictyostelium or fragmentation of colonies of cyanobacteria or phytoplankton. A PCD stress-response can also induce new cells types, like the uterine decidual cells in marsupials. Third, PCD can co-ordinate the cooperative functions between compartments in the eukaryote cell and by synchronizing the behavior of phytoplankton groups. In eukaryote cells, conflict mediation, the fourth important feature of ETIs, is achieved by the induction of death of the entire cell by organelles. In multicellularity, rogue cells are induced to undergo PCD. Fifth, PCD leads to mutual dependencies between organelles in eukaryote cells and between eukaryote cells in the case of multicellularity. In eusociality in insects, PCD in ovarian cells leads to sterility in workers. This is a mechanism by which the interests of all the individuals in the colony become aligned and allows for the specialization of tasks, cooperation between individuals and the evolution of mutual dependencies.
PCD was important for many of the evolutionary and ecological processes by which ETIs occurred. Indeed, the role of PCD appears to have been essential. Without the evolution of a mechanism for adaptive death, alternate strategies would have been necessary for the increases in complexity discussed here to occur.
Glossary
- PCD
programmed cell death
- ETI
evolutionary transitions in individuality
- cytC
cytochrome C
- cytF
cytochrome F
- DSPs
death-specific proteins
- SIEI
stress-induced evolutionary innovation
- DD-aldehydes
diatom-derived aldehydes
- NOA
nitric-oxide-associated protein
- APs
apoptotic-like bodies
- DOM
dissolved organic materials
Author Contributions
All authors contributed equally.
References
- Durand PM, Ramsey G. The nature of programmed cell death. Biol Theory. 2019;14:30–41. [Google Scholar]
- Ratel D, Boisseau S, Nasser V, Berger F, Wion D. Programmed cell death or cell death programme? That is the question. J Theor Biol. 2001;208(3):385–6. [DOI] [PubMed] [Google Scholar]
- Reece SE, Pollitt LC, Colegrave N, Gardner A. The meaning of death: evolution and ecology of apoptosis in protozoan parasites. PLoS Pathog. 2011;7(12):e1002320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameisen JC. On the origin, evolution, and nature of programmed cell death: a timeline of four billion years. Cell Death Differ. 2002;9(4):367–93. [DOI] [PubMed] [Google Scholar]
- Jimenez C, Capasso JM, Edelstein CL, Rivard CJ, Lucia S, Breusegem S, et al. Different ways to die: cell death modes of the unicellular chlorophyte Dunaliella viridis exposed to various environmental stresses are mediated by the caspase-like activity DEVDase. J Exp Bot. 2009;60(3):815–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczanowski S, Sajid M, Reece SE. Evolution of apoptosis-like programmed cell death in unicellular protozoan parasites. Parasit Vectors. 2011;4:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelcu AM, Driscoll WW, Durand PM, Herron MD, Rashidi A. On the paradigm of altruistic suicide in the unicellular world. Evolution. 2011;65(1):3–20. [DOI] [PubMed] [Google Scholar]
- Pepper JW, Shelton DE, Rashidi A, Durand PM. Are internal death-promoting mechanisms ever adaptive? J Phylogenetics Evol Biol. 2013;1:113. [Google Scholar]
- Berges JA, Choi CJ. Cell death in algae: physiological processes and relationships with stress. Perspectives in Phycology. 2014;1:103–12. [Google Scholar]
- Berges JA, Falkowski PG. Physiological stress and cell death in marine phytoplankton: induction of proteases in response to nitrogen or light limitation. Limnol Oceanogr. 1998;43:129–35. [Google Scholar]
- Bidle KD. Programmed cell death in unicellular phytoplankton. Curr Biol. 2016;26(13):R594–607. [DOI] [PubMed] [Google Scholar]
- Durand PM, Sym S, Michod RE. Programmed cell death and complexity in microbial systems. Curr Biol. 2016;26:R587–93. [DOI] [PubMed] [Google Scholar]
- Franklin DJ, Brussard CP, Berges JA. What is the nature and role of programmed cell death in phytoplankton ecology? Eur J Phycol. 2006;41:1–14. [Google Scholar]
- Michod RE. Cooperation and conflict mediation during the origin of multicellularity from genetic and cultural evolution of cooperation. In: (Ed.) PH, editor. In: Genetic and Cultural Evolution of Cooperation (pp. 261-307). Cambridge, Mass, USA: MIT Press; 2003. [Google Scholar]
- Michod RE, Nedelcu AM. Cooperation and conflict during the unicellular-multicellular and prokaryotic-eukaryotic transitions In: Moya A, Font E. In: Evolution: From Molecules to Ecosystems. Oxford, UK: Oxford University Press; 2004. pp. 195–208. [Google Scholar]
- Michod RE, Roze D. Cooperation and conflict in the evolution of multicellularity. Heredity. 2001;86(1):1–7. [DOI] [PubMed] [Google Scholar]
- Maynard Smith J, Szathmáry E. The major transitions in evolution. Oxford, UK: W.H. Freeman Spektrum; 1995. [Google Scholar]
- Michod RE. Evolution of the individual. Am Nat. 1997;150 Suppl 1:S5–21. [DOI] [PubMed] [Google Scholar]
- Michod RE. Darwinian dynamics: evolutionary transitions in fitness and individuality. Princeton, NJ, USA: Princeton University Press; 1999. [Google Scholar]
- West SA, Fisher RM, Gardner A, Kiers ET. Major evolutionary transitions in individuality. Proc Natl Acad Sci USA. 2015;112(33):10112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss LW. The evolution of individuality. Princeton, NJ, USA: Princeton University Press; 1987. [Google Scholar]
- Michod RE, Roze D. Transitions in individuality. Proc Biol Sci. 1997;264(1383):853–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd EA. Adaptationism and the logic of research questions: how to think clearly about evolutionary causes. Biol Theory. 2015;10:343–62. [Google Scholar]
- Hamilton WD. The genetical evolution of social behaviour. I and II. J Theor Biol. 1964;7(1):1–52. [DOI] [PubMed] [Google Scholar]
- Maynard Smith J. Group selection and kin selection. Nature. 1964;201:1145–7. [Google Scholar]
- Michod RE. The theory of kin selection. Annu Rev Ecol Syst. 1982;13:23–55. [Google Scholar]
- Okasha S. Evolution and the levels of selection. Oxford, UK: Oxford University Press; 2006. [Google Scholar]
- Sober E. The nature of selection. Chicago, Ill, USA: University of Chicago Press; 1993. [Google Scholar]
- Roughgarden J, Gilbert SF, Rosenberg E, Zilber-Rosenberg I, Lloyd EA. Holobionts as Units of Selection and a Model of Their Population Dynamics and Evolution. Biol Theory. 2017;•••:13. [Google Scholar]
- Michod RE. The group covariance effect and fitness trade-offs during evolutionary transitions in individuality. Proc Natl Acad Sci USA. 2006;103(24):9113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilde C, Skiba A, Schaap P. Evolutionary reconstruction of pattern formation in 98 Dictyostelium species reveals that cell-type specialization by lateral inhibition is a derived trait. Evodevo. 2014;5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koufopanou V, Bell G. Soma and germ: an experimental approach using Volvox. Proceedings of the Royal Society of London B. 1993;254(rspb.1993.0134). [Google Scholar]
- Page RE, Jr, Scheiner R, Erber J, Amdam GV. The development and evolution of division of labor and foraging specialization in a social insect (Apis mellifera L.). Curr Top Dev Biol. 2006;74:253–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gestel J, Vlamakis H, Kolter R. From cell differentiation to cell collectives: bacillus subtilis uses division of labor to migrate. PLoS Biol. 2015;13(4):e1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahma A, Mandal S, Gadagkar R. Emergence of cooperation and division of labor in the primitively eusocial wasp Ropalidia marginata. Proc Natl Acad Sci USA. 2018;115(4):756–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MB, Bassler BL. Quorum sensing in bacteria. Annu Rev Microbiol. 2001;55:165–99. [DOI] [PubMed] [Google Scholar]
- Venuleo M, Raven JA, Giordano M. Intraspecific chemical communication in microalgae. New Phytol. 2017;215(2):516–30. [DOI] [PubMed] [Google Scholar]
- Traynor KS, Le Conte Y, Page RE., Jr Queen and young larval pheromones impact nursing and reproductive physiology of honey bee (Apis mellifera) workers. Behav Ecol Sociobiol. 2014;68(12):2059–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle SP, Griffin AS, Campbell GS, West SA. Cooperation and conflict in quorum-sensing bacterial populations. Nature. 2007;450(7168):411–4. [DOI] [PubMed] [Google Scholar]
- Veening JW, Smits WK, Kuipers OP. Bistability, epigenetics, and bet-hedging in bacteria. Annu Rev Microbiol. 2008;62:193–210. [DOI] [PubMed] [Google Scholar]
- Blackstone NW. Why did eukaryotes evolve only once? Genetic and energetic aspects of conflict and conflict mediation. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay RR, Town CD, Gross JD. Cell Differentiation in Dictyostelium discoideum. Differentiation. 1979;13:7–14. [Google Scholar]
- Desnitskiy AG. Volvox (Chlorophyta, Volvocales) as a model organism in developmental biology. Russ J Dev Biol. 2009;40:238–41. [PubMed] [Google Scholar]
- Kirk MM, Kirk DB. Exploring germ-soma differentiation inVolvox. J Biosci. 2004;29:143–52. [DOI] [PubMed] [Google Scholar]
- Boomsma JJ, Gawne R. Superorganismality and caste differentiation as points of no return: how the major evolutionary transitions were lost in translation. Biol Rev Camb Philos Soc. 2018;93(1):28–54. [DOI] [PubMed] [Google Scholar]
- Blackstone NW. An evolutionary framework for understanding the origin of eukaryotes. Biology (Basel). 2016;5(2):E18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone NW, Green DR. The evolution of a mechanism of cell suicide. BioEssays. 1999;21:84–8. [DOI] [PubMed] [Google Scholar]
- Hanschen E, Davison DR, Grochau-Wright Z, Michod RE. Individuality and the major evolutionary transitions In: Gissis S, Lamm E, Shavit A. Landscapes of Collectivity in the Life Sciences. Boston, Mass, USA: MIT Press; 2018. [Google Scholar]
- Michod RE. Evolution of individuality during the transition from unicellular to multicellular life. Proc Natl Acad Sci USA. 2007;104 Suppl 1:8613–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramisetty BC, Natarajan B, Santhosh RS. mazEF-mediated programmed cell death in bacteria: “what is this? Crit Rev Microbiol. 2015;41(1):89–100. [DOI] [PubMed] [Google Scholar]
- Berman-Frank I, Bidle KD, Haramaty L, Falkowski PG. The demise of the marine cyanobacterium, Trichodesmium spp., via an autocatalyzed cell death pathway. Limnol Oceanogr. 2004;49:997–1005. [Google Scholar]
- Koonin E, Aravind L. Origin and evolution of eukaryotic apoptosis: the bacterial connection. Cell Death Differ. 2002;9(4):394–404. [DOI] [PubMed] [Google Scholar]
- Bidle KA, Haramaty L, Baggett N, Nannen J, Bidle KD. Tantalizing evidence for caspase-like protein expression and activity in the cellular stress response of Archaea. Environ Microbiol. 2010;12(5):1161–72. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6(5):513–9. [DOI] [PubMed] [Google Scholar]
- van der Giezen M. Mitochondria and the Rise of Eukaryotes. Bioscience. 2011;61:594–601. [Google Scholar]
- Frade JM, Michaelidis TM. Origin of eukaryotic programmed cell death: a consequence of aerobic metabolism? BioEssays. 1997;19(9):827–32. [DOI] [PubMed] [Google Scholar]
- Michod RE, Nedelcu AM. Cooperation and conflict in the origins of multicellularity and the eukaryote cell In: Moya A, Font E. Evolution: from molecules to ecosystems. Oxford, UK: Oxford university press; 2003. [Google Scholar]
- Kaczanowski S. Apoptosis: its origin, history, maintenance and the medical implications for cancer and aging. Phys Biol. 2016;13(031001). [DOI] [PubMed] [Google Scholar]
- Klim J, Gladki A, Kucharczyk R, Zielenkiewicz U, Kaczanowski S. Ancestral state reconstruction of the apoptosis machinery in the common ancestor of eukaryotes. G3 (Bethesda). 2018;8(6):2121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald JM. Endosymbiosis and Eukaryotic Cell Evolution. Curr Biol. 2015;25(19):R911–21. [DOI] [PubMed] [Google Scholar]
- de Nooijer S, Holland BR, Penny D. The emergence of predators in early life: there was no Garden of Eden. PLoS One. 2009;4(6):e5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaragnella N, Zdralevic M, Antonacci L, Passarella S, Marra E, Giannattasio S. The role of mitochondria in yeast programmed cell death. Front Oncol. 2012;2:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsugai N, Perez Koldenkova V, Imamura H, Noji H, Nagai T. Changes in cytosolic ATP levels and intracellular morphology during bacteria-induced hypersensitive cell death as revealed by real-time fluorescence microscopy imaging. Plant Cell Physiol. 2012;53(10):1768–75. [DOI] [PubMed] [Google Scholar]
- van Creveld SG, Rosenwasser S, Schatz D, Koren I, Vardi A. Early perturbation in mitochondria redox homeostasis in response to environmental stress predicts cell fate in diatoms. ISME J. 2015;9(2):385–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin EV, Aravind L. Origin and evolution of eukaryotic apoptosis: the bacterial connection. Cell Death Differ. 2002;9(4):394–404. [DOI] [PubMed] [Google Scholar]
- Bidle KD. The molecular ecophysiology of programmed cell death in marine phytoplankton. Annu Rev Mar Sci. 2015;7:341–75. [DOI] [PubMed] [Google Scholar]
- Huettenbrenner S, Maier S, Leisser C, Polgar D, Strasser S, Grusch M, et al. The evolution of cell death programs as prerequisites of multicellularity. Mutat Res. 2003;543(3):235–49. [DOI] [PubMed] [Google Scholar]
- Vardi A, Berman-Frank I, Rozenberg T, Hadas O, Kaplan A, Levine A. Programmed cell death of the dinoflagellate Peridinium gatunense is mediated by CO(2) limitation and oxidative stress. Curr Biol. 1999;9(18):1061–4. [DOI] [PubMed] [Google Scholar]
- Vardi A, Eisenstadt D, Murik O, Berman‐Frank I, Zohary T, Levine A, et al. Synchronization of cell death in a dinoflagellate population is mediated by an excreted thiol protease. Environ Microbiol. 2007;9(2):360–9. [DOI] [PubMed] [Google Scholar]
- Vardi A, Formiggini F, Casotti R, De Martino A, Ribalet F, Miralto A, et al. A stress surveillance system based on calcium and nitric oxide in marine diatoms. PLoS Biol. 2006;4(3):e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JF. Control of gene expression by redox potential and the requirement for chloroplast and mitochondrial genomes. J Theor Biol. 1993;165(4):609–31. [DOI] [PubMed] [Google Scholar]
- Allen JF. The CoRR hypothesis for genes in organelles. J Theor Biol. 2017;434:50–7. [DOI] [PubMed] [Google Scholar]
- Burt A, Trivers R. Genes in conflict : the biology of selfish genetic elements. Cambridge, Mass, USA: The Belknap Press of Harvard University Press; 2006. viii, 602 p., 8 p. of plates p. [Google Scholar]
- Aravind L, Koonin EV. Classification of the caspase-hemoglobinase fold: detection of new families and implications for the origin of the eukaryotic separins. Proteins. 2002;46(4):355–67. [DOI] [PubMed] [Google Scholar]
- Koonin EV, Zhang F. Coupling immunity and programmed cell suicide in prokaryotes: life-or-death choices. BioEssays. 2017;39(1):1–9. [DOI] [PubMed] [Google Scholar]
- Iranzo J, Lobkovsky AE, Wolf YI, Koonin EV. Virus-host arms race at the joint origin of multicellularity and programmed cell death. Cell Cycle. 2014;13(19):3083–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AH. The multiple origins of complex multicellularity. Annu Rev Earth Planet Sci. 2011;39:217–39. [Google Scholar]
- Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356(6366):215–21. [DOI] [PubMed] [Google Scholar]
- Boyd M, Rosenzweig F, Herron MD. Analysis of motility in multicellular Chlamydomonas reinhardtii evolved under predation. PLoS One. 2018;13(1):e0192184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapsetaki SE, Tep A, West SA. How do algae form multicellular groups? Evol Ecol Res. 2017;18:663–75. [Google Scholar]
- Bouderbala I, El Saadi N, Bah A, Auger P. A 3D individual-based model to study effects of chemotaxis, competition and diffusion on the motile-phytoplankton aggregation. Acta Biotheor. 2018;66:257–78. [DOI] [PubMed] [Google Scholar]
- Sathe S, Durand PM. Cellular aggregation in Chlamydomonas (Chlorophyceae) is chimaeric and depends on traits like cell size and motility. Eur J Phycol. 2016;51:129–38. [Google Scholar]
- Durand PM, Rashidi A, Michod RE. How an organism dies affects the fitness of its neighbors. Am Nat. 2011;177:224–32. [DOI] [PubMed] [Google Scholar]
- Durand PM, Choudhury R, Rashidi A, Michod RE. Programmed death in a unicellular organism has species-specific fitness effects. Biol Lett. 2014;10(2):20131088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanova ZP, Woltering EJ, Kapchina-Toteva VM, Iakimova ET. Mastoparan-induced programmed cell death in the unicellular alga Chlamydomonas reinhardtii. Ann Bot (Lond). 2013;111(2):191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana MV, Pang WL, Durand PM, Whitehead K, Baliga NS. A role for programmed cell death in the microbial loop. PLoS One. 2013;8:e62595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio P, Battistella L, Vardavas R, Gattazzo C, Liou LL, Diaspro A, et al. Superoxide is a mediator of an altruistic aging program in Saccharomyces cerevisiae. J Cell Biol. 2004;166(7):1055–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herker E, Jungwirth H, Lehmann KA, Maldener C, Frohlich KU, Wissing S, et al. Chronological aging leads to apoptosis in yeast. J Cell Biol. 2004;164(4):501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PM, Yumnam DD, Imchen TY. Regulation of differentiation of multiple heterocysts in a blue-green alga, Anabaena variabilis. Arch Hydrobiol. 1987;73:559–74. [Google Scholar]
- Khan NA, Iqbal J, Siddiqui R. Stress management in cyst-forming free-living protists: programmed cell death and/or encystment. BioMed Res Int. 2015;2015:437534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff WC, Denison RF, Borrello M, Travisano M. Experimental evolution of multicellularity. Proc Natl Acad Sci USA. 2012;109(5):1595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobeen S, Pentz JT, Graba EC, Brandys CG, William WC, Yunker PJ. Cellular packing, mechanical stress and the evolution of multicellularity. Nat Phys. 2018;14:286–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper KB. Dictyostelium discoideum, a new species of slime mold from decaying forest leaves. J Agric Res. 1935;50:135–47. [Google Scholar]
- Raper KB, Fennell DI. Stalk formation in Dictyostelium. Bull Torrey Bot Club. 1952;79:25–51. [Google Scholar]
- Otto GP, Wu MY, Kazgan N, Anderson OR, Kessin RH. Macroautophagy is required for multicellular development of the social amoeba Dictyostelium discoideum. J Biol Chem. 2003;278(20):17636–45. [DOI] [PubMed] [Google Scholar]
- Wagner GP, Erkenbrack EM, Love AC. Stress-Induced Evolutionary Innovation: A Mechanism for the Origin of Cell Types. BioEssays. 2019;41(4):e1800188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkenbrack EM, Maziarz JD, Griffith OW, Liang C, Chavan AR, Nnamani MC, et al. The mammalian decidual cell evolved from a cellular stress response. PLoS Biol. 2018;16(8):e2005594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart L, Ehrlich F, Tschachler E. A Stress Response Program at the Origin of Evolutionary Innovation in the Skin. Evol Bioinform Online. 2019;15:1176934319862246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohnert G. Phospholipase A2 activity triggers the wound-activated chemical defense in the diatom Thalassiosira rotula. Plant Physiol. 2002;129(1):103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SE, Taylor AR, Brownlee C, Callow ME, Callow JA. The Role of Nitric Oxide in Diatom Adhesion in Relation to Substratum Properties(1). J Phycol. 2008;44(4):967–76. [DOI] [PubMed] [Google Scholar]
- Vardi A, Bidle KD, Kwityn C, Hirsh DJ, Thompson SM, Callow JA, et al. A diatom gene regulating nitric-oxide signaling and susceptibility to diatom-derived aldehydes. Curr Biol. 2008;18(12):895–9. [DOI] [PubMed] [Google Scholar]
- Boraas ME, Seale DB, Boxhorn JE. Phagotrophy by a flagellate selects for colonial prey: a possible origin of multicellularity. Evol Ecol. 1998;12:153–64. [Google Scholar]
- Lurling M, Van Donk E. Zooplankton-induced unicell-colony transformation in Scenedesmus acutus and its effect on growth of herbivore Daphnia. Oecologia. 1996;108(3):432–7. [DOI] [PubMed] [Google Scholar]
- Solari CA, Galzenati VJ, Kessler JO. The evolutionary ecology of multicellularity: the volvocine algae as a case study In: Ruiz-Trillo I, Nedelcu AM. In: Evolutionary Transition to Mulricellularity: Principles and Mechanisms Dortrecht. Netherlands: Springer; 2015. [Google Scholar]
- Stanley SM. An ecological theory for the sudden origin of multicellular life in the late precambrian. Proc Natl Acad Sci USA. 1973;70(5):1486–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathe S, Kaushik S, Lalremruata A, Aggarwal RK, Cavender JC, Nanjundiah V. Genetic heterogeneity in wild isolates of cellular slime mold social groups. Microb Ecol. 2010;60(1):137–48. [DOI] [PubMed] [Google Scholar]
- Kapsetaki SE, Fisher RM, West SA. Predation and the formation of multicellular groups in algae. Evol Ecol Res. 2016;17:651–69. [Google Scholar]
- Arnoult D, Tatischeff I, Estaquier J, Girard M, Sureau F, Tissier JP, et al. On the evolutionary conservation of the cell death pathway: mitochondrial release of an apoptosis-inducing factor during Dictyostelium discoideum cell death. Mol Biol Cell. 2001;12(10):3016–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatischeff I, Bomsel M, de Paillerets C, Durand H, Geny B, Segretain D, et al. Dictyostelium discoideum cells shed vesicles with associated DNA and vital stain Hoechst 33342. Cell Mol Life Sci. 1998;54(5):476–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abada A, Segev E. Multicellular features of phytoplankton. Front Mar Sci. 2018;5(144). [Google Scholar]
- van Niekerk KE, Ndhlovu A. Commentary: Multicellular Features of Phytoplankton. Front Mar Sci. 2019 [Google Scholar]
- Engelberg-Kulka H, Amitai S, Kolodkin-Gal I, Hazan R. Bacterial programmed cell death and multicellular behavior in bacteria. PLoS Genet. 2006;2(10):e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb JS, Givskov M, Kjelleberg S. Bacterial biofilms: prokaryotic adventures in multicellularity. Curr Opin Microbiol. 2003;6(6):578–85. [DOI] [PubMed] [Google Scholar]
- Refardt D, Bergmiller T, Kümmerli R. Altruism can evolve when relatedness is low: evidence from bacteria committing suicide upon phage infection. Proceedings of the Royal Society of London B. 2013;280(1759):20123035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontier D, Balagué C, Roby D. The hypersensitive response. A programmed cell death associated with plant resistance. C. R. Acad. Sci. Paris. 1998;321:721–34. [DOI] [PubMed] [Google Scholar]
- Ronai I, Oldroyd BP, Vergoz V. Queen pheromone regulates programmed cell death in the honey bee worker ovary. Insect Mol Biol. 2016;25(5):646–52. [DOI] [PubMed] [Google Scholar]