Abstract
Background:
Population-based incidence and mortality studies of cutaneous squamous cell carcinoma (SCC) have been few owing to the commonness of the disease, and rare deaths making accurate mortality statistics difficult.
Objectives:
Our aim was to summarize SCC incidence and mortality in populations across three continents, exemplified by Australia, the United States (U.S.) and Germany.
Methods:
We estimated age-specific and age-standardized (Australian Standard 2001 Population) incidence and mortality rates per 100,000 person-years.
Results:
SCC incidence is plateauing or falling in Australia, stable in the U.S. (2013 – 2015), and rising in Germany (2007–2015). Current incidence estimates in men and women are 341 and 209, 497 and 296, and 54 and 26, respectively for the three countries. Incidence increases strongly with age in all countries. Mortality of nonmelanoma skin cancer appears to be increasing in Germany, stable in Australia (unavailable for the U.S population).
Conclusions:
SCC is an important health issue, particularly among older men, with incidence exceeding most other cancers. More precise and uniform population-based studies of incidence and mortality are needed to better quantify the impact of SCC on healthcare systems worldwide, and to gauge the effect of new treatments such as anti-PD1 therapy on mortality.
Keywords: Carcinoma, squamous cell, skin, incidence, mortality, cancer registries, Australia, United States, Germany
Introduction
Cutaneous squamous cell carcinoma (SCC) in white populations is caused predominantly by sun (ultraviolet radiation, UV) exposure. Carcinogenesis is due to UV-induced DNA damage in keratinocytes lacking sufficient melanin protection.1,2 Reportedly, SCC has been on the rise in white populations worldwide for many years3 likely due to changes in lifestyle and clothing resulting in more UV exposure of the skin. Heightened UV exposure in Australia and northern Europe due to ozone depletion, and the growing proportion of elderly persons in the population in whom SCC is more common (due to accumulated DNA damage), add to the problem. However, most countries’ national cancer registries have excluded SCC due to the impracticality of tracking the multitude of incident cases and the rare disease-specific deaths, impelling these countries to use various other ways to estimate population-based SCC incidence and mortality rates. Here we aimed to summarize SCC incidence and mortality data across three continents, exemplified by Australia, the United States (US) and Germany.
Material and Methods
Germany
North Rhine-Westphalia (NRW) is the most populated Federal State in Germany (18 million people) with a predominantly white Caucasian population. Cancer reporting, mainly via pathology reports, is mandatory in NRW with estimated completeness of cancer registration >90% in 2014.4 Incident invasive tumors morphologically and topographically coded (International Classification of Diseases for Oncology, ICD-O5) as 805–805 and C44.0-C44.9 respectively, 2007–2015, were ascertained. We calculated sex-stratified, age-specific incidence rates and age-standardized (Australian Standard 2001) rates by calendar year, counting only the first SCC diagnosed in life per person according to International Agency for Research in Cancer (IARC) rules. We estimated annual percentage changes (EAPC) of age-standardized rates by fitting regression lines to the natural logarithm of age-standardized rates. NRW mortality data for nonmelanoma skin cancer (ICD-10) were extracted 2007–2016 [www.gbe-bund.de, accessed April 25, 2019] (with cause of death certificates completed by physicians).
United States
Since SCCs are not captured in national cancer registries in the U.S. due to their extreme commonness, medical claims (insurance payment) data were used to estimate the number of SCCs diagnosed annually. Medical claims data were obtained from two sources: (1) Massachusetts All-Payer Claims Dataabase (APCD) and (2) Medicare, which is the government-sponsored health insurance program which covers nearly all individuals 65 years and older.
The Massachusetts All Payers Claims Database (APCD) was used to derive incidence estimates of SCC in persons aged <65, and the Medicare 5% Sample Data Set for those ≥65 years. Both databases were queried for ICD-9-Clinical Modification (CM) and ICD-10-CM codes for SCC to identify the number of individuals treated each year, 2012–2015 (APCD), and 2013–2015 (Medicare), regardless of previous SCC history. Population denominator estimates were obtained from the U.S. Census Bureau for Massachusetts, and from total number of Medicare beneficiaries by age obtained from the Center for Medicare and Medicaid Services (CMS). 6 Since the number of Medicare beneficiaries by sex was not available, the sex distribution for Medicare beneficiaries overall (45% male, 55% female) was applied to all age groups ≥ 65 years. The proportions of white persons in Massachusetts (80%) (APCD) and the U.S. population aged ≥ 65 years (75%) (Medicare) were comparable. Therefore, Medicare and Massachusetts APCD data were combined to estimate age- and sex-specific and age-standardized (Australian Standard 2011) incidence rates for the United States. Since national and population-based SCC mortality rates are not available in the U.S., U.S. mortality estimates for SCC were not reported.
Australia
Australia’s most recent national SCC incidence rates were obtained from a published 2002 national household survey.7 Consenting adults from randomly selected households in each Australian state and territory were interviewed face-to-face and asked if they had been treated for skin cancer in the last 12 months.7 Self-reported diagnoses of skin cancer were then followed-up with nominated treatment providers and histologically confirmed.8 Age- and sex-specific incidence rates of persons affected were calculated, and body site distributions were expressed as ‘relative tumour density’ (Table 1).
Table 1.
Number of cutaneous squamous cell carcinoma (SCC) cases and percentage of SCC by body site in Australia, 2002
| Number of cases |
% | Relative tumor density |
|
|---|---|---|---|
| Males | 247 | ||
| Head & neck | 122 | 49 | 5.55 |
| Trunk | 25 | 10 | 0.32 |
| Upper limbs | 61 | 25 | 1.30 |
| Lower limbs | 39 | 16 | 0.40 |
| Females | 166 | ||
| Head & neck | 55 | 33 | 3.72 |
| Trunk | 9 | 5 | 0.17 |
| Upper limbs | 61 | 39 | 1.94 |
| Lower limbs | 41 | 25 | 0.62 |
| Men & women | 413 | ||
| Head & neck | 177 | 43 | 4.82 |
| Trunk | 34 | 8 | 0.26 |
| Upper limbs | 122 | 29 | 1.55 |
| Lower limbs | 80 | 19 | 0.49 |
Source: Staples 2003 7; relative tumor density is defined as the ratio of the proportion of tumors at a certain site to the proportion of total skin surface area at that site.
Since 2002, several incidence studies of SCC9–12 have used data for keratinocyte carcinomas excised under the Australian universal health insurance scheme (Medicare).13 However, Medicare treatment data do not distinguish SCC from basal cell carcinoma (BCC). One report11 used histologically-confirmed keratinocyte cancer excision claims in an independently drawn 10% random sample of persons nationally registered with Medicare during 1997–2014. Among individuals aged 20 years or older, they used age- and sex-specific BCC : SCC ratios from a large prospective skin cancer study14 to estimate the proportion of SCC among excised keratinocyte cancers. SCC national incidence rates, 2011–2014, based firstly on persons affected, and also on all excised SCC tumors (the latter capturing multiple tumors per person). National mortality data were available from the Australian Institute of Health and Welfare (AIHW) National Mortality Database to 2013 with mortality estimates for 2014–16 for all epithelial skin cancers combined (coded in International Classification of Disease (ICD)-10 as C44)15 (with medical practitioners completing the majority of death certificates). Mortality rates were age-standardized to the Australian Standard 2001 Population.
Results
Germany
In the period 2007–2015, 76,474 people newly diagnosed with SCC were registered in NRW, with an age-standardized incidence in 2015 of 54 in men, double the rate of 26 in women (Figure 1a). Incidence rates increased strongly 2007– 2012 both for men and women and increased weakly thereafter, with EAPCs of 7.9% (men) and 9.8% (women). As for the U.S., age-specific incidence rates only diverged after ages 55–59 years, when incidence was higher in men. Incidence increased exponentially with age (almost linear association with semi-logarithmic plot) (Figure 1b). Mortality has also trended upward such that in 2016 it was approximately double the 2007 rates (Figure 2).
Figure 1. Age-standardized incidence rates over time and age-specific incidence rates of patients with cutaneous squamous cell carcinoma in North Rhine-Westphalia, Germany, 2007–2015 (A and B), and the United States (C and D).
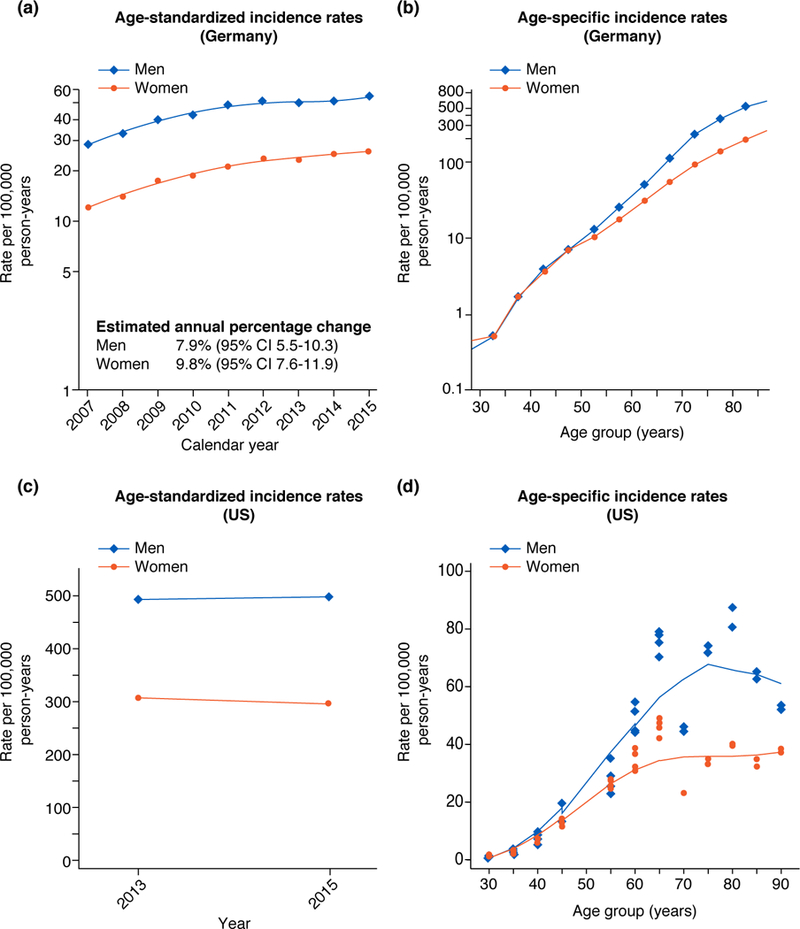
Legend: German age-standardized (A) and age-specific (B) patient-based incidence rates are based on the period 2007–2015. United States tumor-based age-standardized incidence rates (C) for 2013 and 2015 and age-specific incidence rates (D) are based on data from the Massachusetts All Payer Claims Database 2012–2015 and Medicare 2013 and 2015. Males (blue, ♢), females orange, (•). All estimates are standardized according to the Australian Standard Population (2001).
Figure 2. Age-standardized mortality rates of non-melanoma skin cancer in North Rhine-Westphalia, Germany and Australia, 2007–2016.
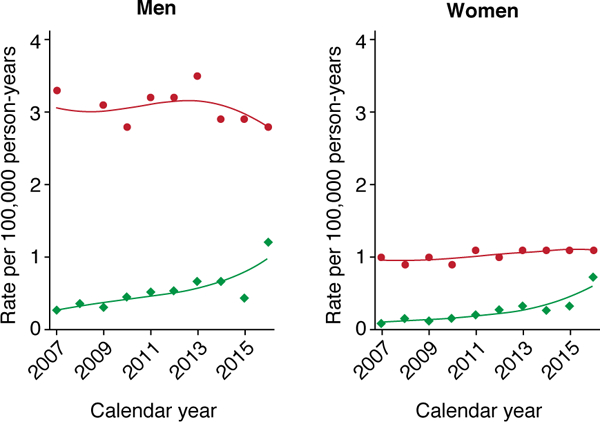
Institute of Health and Welfare material 15; all mortality rates are age-standardized to the Australian Standard Population (2001). Australia (red graphs and •), Germany (green graphs and ♢).
United States
SCC incidence was stable 2013–2015 (Figure 1c) and highest at ages 70–79 group (Figure 1d). Age-standardized incidence in 2015 was 497 in men and 296 in women. Until around age 50 incidence in men and women were similar, but after age 65 years incidence in men was nearly double that in women (2,580 vs. 1,347) (Figure 1d).
Australia
In the 2002 national survey 57,215 people were interviewed, and 4,098 reported being treated for skin cancer in the past year of whom 3,198 gave permission for their diagnoses to be confirmed.7,8 Of 817 people confirmed as having had skin cancer, 413 were medically confirmed as having SCC in 2002, giving age-standardized incidence rates of SCC of 772 in men and 442 in women. Highest age-specific rates were in those aged 70+ years: 3,979 in men and 2,146 in women. SCC rates increased with decreasing latitude of residence such that those living at latitudes <29°S had around 3 times the incidence of those living at the highest latitudes in Australia (>37°S). Tumor density was highest by far on the head and neck in both sexes7 (Table 1). More recent (2011–2014) standardized estimates of SCC incidence were 341 (men) and 209 (women), and total tumor-based rates were 800 in men and 381 in women 11.
Estimated age-standardized mortality rates for skin cancer (excluding melanoma) in 2016 were 2.8 deaths in men and 1.1 deaths in women. These rates have been relatively stable in both men and women since 1982 (Figure 2).15 However, age-specific mortality dropped slightly for those <80 (apart from those aged 40–49), and rose in those aged ≥80 (20 to 34 per 100,000 person-years from 1982–2016).15
Discussion
This overview of available SCC incidence and mortality data for Australia, the U.S. and Germany indicates that incidence is plateauing or falling in Australia, stabilizing in the U.S. (based on short-term data), but rising in Germany. Age-standardized incidence rates per 100,000 person years ranged from 26 in German women overall to 497 in U.S men overall. The incidence is consistently higher in men than women overall especially at older ages, and rates increase dramatically with age. Nonmelanoma skin cancer mortality rates, used here as a proxy for SCC mortality, have been relatively stable in Australia and slightly increasing in Germany (U.S. mortality data were unavailable).
Australia’s long-standing public health campaigns to improve UV protective practices16 could partly explain its stabilizing SCC trends. However, the non-white proportion of the population who are at lower inherent risk of SCC has increased in all three countries with immigration. From simulation analyses of the possible effect of migration on plateauing trends in melanoma in Australia17, and because the stabilizing trends in SCC are long-standing,8 it is unlikely that changing immigration patterns there are the major explanation. In Germany since July 2008, all health-insured people aged ≥35 have been offered biennial total-body skin cancer screening by certified physicians with estimated 2-year participation rate of 31%.18 This may have led to more accurate incidence figures recently, less affected by prioritization of care or lack of access. Thus increase in SCC incidence in Germany is likely due to both a real increase and the effect of screening.19 It was notable that in both the U.S. and Germany, the markedly higher incidence in men was most apparent at older ages.
The main weakness of this inter-continental comparative study was the heterogeneity of the methodology. Germany estimated SCC incidence in its largest cancer registry by counting only the first-in-life SCC diagnosis per person (as per IARC’s rule); Australia used outmoded national survey data, and national claims data for excisions; and the U.S. used claims data from one state for people <65 and national Medicare data for people ≥65 years. Major trends like male predominance were still observable but some variations due to such methodologic differences were to be expected. In particular German incidence rates will always be lowest because they represent only the first SCC diagnosis rather than all new SCC cases. Another weakness was the use of routine cause-of-death statistics to approximate SCC mortality rates, since deaths from SCC and from other nonmelanoma skin cancers like Merkel cell carcinoma and BCC cannot be distinguished, though SCC mortality rates would always be the major contributor.
Despite these systematic differences, our combined findings show SCC is an important health issue. More precise and uniform population-based incidence and mortality rates are needed to quantify the true impact of SCC on healthcare systems worldwide, and to gauge the effect of new treatments such as anti-PD1 (anti ‘programmed cell death protein 1’) therapy on SCC mortality.
Acknowledgements
Mr. Karia is supported by the Cancer Epidemiology, Prevention and Control T32 Training Grant (NCI T32 CA009314). Dr. Stang is supported by a grant of the German Federal Ministry of Education and Science (BMBF) [grant number 01ER1305].
Reference List
- 1.D’Orazio J, Jarrett S, Amaro-Ortiz A, Scott T. UV radiation and the skin. Int J Mol Sci 2013;14:12222–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ratushny V, Gober MD, Hick R, Ridky TW, Seykora JT. From keratinocyte to cancer: the pathogenesis and modeling of cutaneous squamous cell carcinoma. J Clin Invest 2012;122:464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol 2012;166:1069–80. [DOI] [PubMed] [Google Scholar]
- 4.RKI. Robert Koch Institut: Krebs in Deutschland für 2013/2014. 11. Ausgabe. Berlin: Robert Koch Institut (Hrsg) und Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V. (Hrsg); 2017. [Google Scholar]
- 5.Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology, 3rd edn Geneva: World Health Organization; 2000. [Google Scholar]
- 6.CMM. Centers for Medicare & Medicaid Services: Statistics, Trends, and Reports. 2019. [Google Scholar]
- 7.Staples MP. The 2002 national non-melanoma skin cancer survey. A report by the NCCI Non-melanoma Skin Cancer Working Group; Melbourne: 2003. [Google Scholar]
- 8.Staples MP, Elwood M, Burton RC, Williams JL, Marks R, Giles GG. Non-melanoma skin cancer in Australia: the 2002 national survey and trends since 1985. Med J Aust 2006;184:6–10. [DOI] [PubMed] [Google Scholar]
- 9.Fransen M, Karahalios A, Sharma N, English DR, Giles GG, Sinclair RD. Non-melanoma skin cancer in Australia. Med J Aust 2012;197:565–8. [DOI] [PubMed] [Google Scholar]
- 10.Olsen CM, Williams PF, Whiteman DC. Turning the tide? Changes in treatment rates for keratinocyte cancers in Australia 2000 through 2011. J Am Acad Dermatol 2014;71:21–6 e1. [DOI] [PubMed] [Google Scholar]
- 11.Pandeya N, Olsen CM, Whiteman DC. The incidence and multiplicity rates of keratinocyte cancers in Australia. Med J Aust 2017;207:339–43. [DOI] [PubMed] [Google Scholar]
- 12.Adelson P, Sharplin GR, Roder DM, Eckert M. Keratinocyte cancers in South Australia: incidence, geographical variability and service trends. Aust N Z J Public Health 2018;42:329–33. [DOI] [PubMed] [Google Scholar]
- 13.Thompson BS, Olsen CM, Subramaniam P, Neale RE, Whiteman DC. Medicare claims data reliably identify treatments for basal cell carcinoma and squamous cell carcinoma: a prospective cohort study. Aust N Z J Public Health 2016;40:154–8. [DOI] [PubMed] [Google Scholar]
- 14.Olsen CM, Green AC, Neale RE, et al. Cohort profile: the QSkin Sun and Health Study. Int J Epidemiol 2012;41:929-i. [DOI] [PubMed] [Google Scholar]
- 15.AIHW. Skin cancer in Australia. Cat. no. CAN 96 Canberra2016. [Google Scholar]
- 16.Iannacone MR, Green AC. Towards skin cancer prevention and early detection: evolution of skin cancer awareness campaigns in Australia. Melanoma Manag 2014;1:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baade PD, Youlden DR, Youl P, Kimlin M, Sinclair C, Aitken J. Assessment of the effect of migration on melanoma incidence trends in Australia between 1982 and 2010 among people under 30. Acta Derm Venereol 2015;95:118–20. [DOI] [PubMed] [Google Scholar]
- 18.Breitbart EW, Choudhury K, Anders MP, et al. Benefits and risks of skin cancer screening. Oncol Res Treat 2014;37 Suppl 3:38–47. [DOI] [PubMed] [Google Scholar]
- 19.Eisemann N, Waldmann A, Geller AC, et al. Non-melanoma skin cancer incidence and impact of skin cancer screening on incidence. J Invest Dermatol 2014;134:43–50. [DOI] [PubMed] [Google Scholar]


