Abstract
Whereas generic, LC-based pharmaceutical control quality measures depend largely on the detection mode and can be particularly ‘blind’ to certain impurities, NMR is a more versatile and, thus, often more judicious detector. While adulteration presents ever-evolving challenges for the analysis of active pharmaceutical ingredients (APIs) and finished products sold in the worldwide (online) marketplace, research chemicals are usually trusted rather than being considered flawed or even adulterated.
This report shows how NMR analysis uncovered the unanticipated presence of substantial amounts of mannitol (20 and 43% w/w) as undeclared constituent in two custom synthetic peptides, DR and DRVYI, that were sourced commercially. Quantitative 1H NMR (qHNMR) readily detected the contaminant, even on a 60 MHz benchtop instrument, and quantified the highly polar UV-transparent adulterant. Quantum-mechanical 1H iterative Full Spin Analysis (HiFSA) achieved unambiguous identification of both the mannitol and the peptides and confirmed the quantitative results.
The cases show that experimental verification supersedes trust in both pharmaceutical and research QC. They also highlight the promising utility of established high-field and recently re-evolving benchtop qHNMR. The unanticipated findings remind manufacturers and researchers alike about the advantages of including/performing NMR and qNMR with routine CofA documentation and/or verification of research grade chemicals. Especially when done jointly, this can greatly improve confidence in research and help streamline the pharmaceutical QC toolbox.
Keywords: 1H NMR, peptides, purity, impurity, residual complexity, custom peptide synthesis
1. Introduction
1.1. Adulteration of research chemicals
Quality control (QC) and chemical purity determination of pharmaceutical research chemicals, such as peptides ordered for specific experimental purposes, are typically performed by liquid chromatography (LC) with ultra-violet (UV), less frequently charged aerosol detector (CAD), evaporative light scattering detector (ELSD), refractive index (RI), or mass-spectrometric (MS) detection or, alternatively, by infusion MS. However, chromatographic purity determination is largely conditional on the detection mode as it can be notoriously ‘blind’ to certain compounds: they may lack chromophores (UV), be poorly ionizable (MS), or be strongly polar or nonpolar; their properties may exclude them from the examined retention window and, thereby, even from the detection schemes mentioned above; they can be insufficiently soluble in the mobile phase; and, lastly, the method itself often is unable to detect certain types of components and (“foreign”) impurities including salts and solvents [1].
Residual complexity, referring to any deviation from the intended single chemical entity (SCE; see ref [2] and references therein), may thus be incompletely accounted for by conventional chromatographic methods. Residual complexity also covers impurities. The USP general chapter <1086> [3,4] classifies drug substance-based impurities into three categories: (1) organic impurities, process- and drug-related, (2) inorganic impurities, and (3) residual solvents (covered in depth in the USP-NF general chapter <467> [3]). Most impurities can be readily characterized when their synthetic origins are known [5]. Excipients are another type of component that are used as carriers in drug preparations and are often necessary for drug substance processing such as lyophilization. However, whenever the presence of an undeclared (thus, typically unwanted) component is detected, adulteration should always be considered.
While numerous definitions of adulteration exist, for the purposes of the present study, this term denotes intentional addition of unrelated and undeclared matter to a substance, thereby changing its composition and increasing the complexity of the material. Adulteration is universally unwanted and known to be a persistent phenomenon worldwide. Representing a challenge in pharmaceutical analysis, it is especially difficult to detect and control in chemically complex matrices such as natural products, especially when they are not scrutinized to the extent typical of FDA-approved SCE-based pharmaceuticals [6].
Adulterators are known to target both the single-molecule ingredients such as pharmaceutical APIs, as well as finished dosage forms, such as tablets and capsules, with ever-increasing sophistication. On the ingredient level, the most commonly observed forms of adulteration are (a) substitution with a similar but cheaper ingredient or (b) the admixture of pharmacologically inactive components (excipients) and hard-to-detect components. For the finished dosage forms, ‘functional’ adulteration is common, i.e., the addition of chemicals imparting pharmacological activity to an otherwise inactive or inefficacious (herbal) blend, such as admixture of PDE5 inhibitors to erectile dysfunction (ED) treating “supplements”, or addition of anorexics and laxatives to weight loss products [7].
1.2. NMR as a versatile detector of adulteration
In 2016, USP published a general chapter <2251> [8], Screening for Undeclared Drugs and Drug Analogues, where several analytical strategies aimed at the detection of undeclared PDE5 inhibitors were proposed; one of the methods was 1H NMR. Adulteration is typically motivated by fraudulent economic gain, and in these cases carries an increased risk of being dangerous to human health. Thus, wider use of methodologies capable of both confirming the composition and assessing absolute purity of ingredients and products is increasingly relevant. One such methodology that has recently come to prominence is quantitative 1H NMR (qHNMR).
The area of fine chemicals and investigational products has not been examined previously, or even considered as target for the adulterators. However, considering the high prices such articles may command, i.e., hundreds of dollars per mg, it is easily seen that they could potentially be manipulated for monetary gain. This may have unpredictable and devastating effects on biomedical research, where commercially obtained research chemicals are used amply, and frequently without prior confirmation of strength, purity, or even chemical identity. Typically, in these settings, QC testing is only performed by the manufacturer providing the material. Researchers, for all practical purposes, trust the purity assignment as declared by the suppliers in their specifications and/or certificate of analysis, and are quickly overwhelmed by the perspective of having to perform independent analysis for every material used.
However, the present study presents a case where two custom-synthesized peptides sourced commercially were found to contain an unexpected and unwanted component that was unrelated to the synthetic process, but present in significant amount. While the presence of this component went undetected by routine chromatographic purity assays, it was observed readily through NMR analysis, including low-field benchtop NMR. Considering the polyol chemistry of the unwanted component, it would be most likely missed by the vast majority of standard QC testing and purity analysis protocols, including those commonly conducted by chemical and custom synthesis suppliers. The reported case opens both challenging and promising perspectives on the presence of unwanted components in pharmaceutical and research grade materials, and the potential of NMR analysis for uncovering such components.
2. Experimental
2.1. Chemicals and solvents
Three synthetic peptides, L-Asp-L-Arg (DR), L-Asp- L-Arg- L-Val- L-Tyr (DRVY) and L-Asp-L-Arg-LVal-L-Tyr-L-Ile (DRVYI), were acquired from a major international peptide manufacturer. The mannitol standard used for structural confirmation was obtained from USP Cat # 1375105 (Rockville, MD). NMR solvents were purchased from Cambridge Isotope Laboratories (Andover, MA). NMR tubes were from Norell (Landisville, NJ).
2.2. NMR
Experiments were carried out on a Bruker Avance 800 NMR instrument (Bruker BioSpin, Billerica, MA), equipped with a 5-mm TXI probe, at 298 K. Additional experiments were carried out on a JEOL 400 MHz JNM-ECZ NMR system equipped with a 5-mm digital auto tune Royal probe in a broad VT range (JEOL USA, Peabody, MA), also at 298K and operated with JEOL Delta NMR Software (v 5.3.1). The 13C spectrum of peptide DR was acquired on a Bruker Avance 900/225 NMR spectrometer equipped with an AVANCE I console. The spectrometer was equipped with a 5-mm Bruker TCI triple resonance inverse detection cryoprobe with z-axis pulse field gradient.
The 90° pulse was determined for each sample. Spectral window was set to 30 ppm with the transmitter offset at the center of each spectrum determined by the signal dispersion of each sample. Further experiments were carried out on the Nanalysis Corp. 60-MHz NMReady-60e benchtop NMR instrument (Calgary, Alberta, Canada). Experiments for this instrument were performed at 305 K (32°C) in 5-mm NMR tubes with a 15-ppm spectral window. Processing was accomplished using Mnova (Mestrelab Research, Santiago de Compostela, Spain, v 11.0.3) with post-acquisition window functions applied (exponential −0.3, Gaussian 0.5), manual phasing, polynomial baseline correction, and 2x zero fill. All spectra were referenced to the residual solvent signal, HOD, at 4.790 ppm (298K).
Quantitative analysis used the 100% qHNMR methodology as described in detail previously [9]. Briefly, the NMR spectra were integrated, and the sum of integrals of all detected analyte species (excluding solvent) yielded the number taken to be 100%. Each species was then normalized by molecular weight in reference to the relative to the ”100%” number.
2.3. Computer assisted NMR analysis (HiFSA)
Full spin analysis was carried out with PERCH NMR Tools (v.2014.1 and v.2015.1, PERCH Solutions Ltd., Kuopio, Finland) [10,11]. Contaminated DR, DRVYI and standard mannitol were subjected to semi-automated 1H iterative Full Spin Analysis (HiFSA), which has been described in detail previously [12],[13], including application of HiFSA methodology to peptides [14]. Briefly, a 3D chemical model (mol file) was used to generate predicted spin parameters: chemical shift (δ), H,H spinspin coupling constants (J), and line widths (ν). The spin parameters were then converted into a frequency-domain spectrum via quantum mechanical (QM) calculation and compared to the experimentally collected data. Spin parameters were then optimized through quantum mechanical iterations until the calculated 1H NMR spectra matched the experimentally collected data. The optimized spin parameters (δ, J, ν) were finally exported as a text file with PERCH parameters (pms) extension.
2.4. FTIR analysis of impurity
The samples DR, DRVYI, and DRVY were analyzed by Fourier transform attenuated total reflection (ATR) infrared spectroscopy (FTIR) with a Nicolet 6700 (Thermo Fisher Scientific, Waltham, MA). Background was acquired prior to sample analysis and subtracted. The spectra were obtained by placing a small amount of solid powder on the ATR diamond crystal and acquiring 32 scans. Analysis was performed using OMNIC software (v. 8.2.388, Thermo Fisher Scientific, Waltham, MA).
2.4. MS/MS analysis
Direct infusion MS/MS analysis utilized a Bruker Impact II qTOF instrument equipped with an ESI source. Infusion was performed with a solution in MeCN with 0.1% Formic acid, infusing a 0.001 mg/mL sample at 45 μL/h. The MS parameters were as follows: collision cell RF 750 Vpp; positive mode; spectra rate 0.1 Hz; scan range 50–1,200 m/z; isolation width for MS2 5 m/z; dry gas 4 L/min @ 150°C; nebulizer 0.3 bar; capillary 4500V (−500V end plate offset); funnels: 300 Vpp and 300 Vpp.
3. Results and Discussion
3.1. Uncovering an unexpected component in custom synthetic peptides
In the study case, the presence of undeclared components could be suspected by FTIR and NMR. NMR analysis identified an unexpected component in two commercial, custom synthesized peptides, LAsp-L-Arg (DR) and L-Asp-L-Arg-L-Val-L-Tyr-L-Ile (DRVYI). Synthetic peptides are available from a wide array of providers. In the current study, the purities of the di- and penta-peptides reported by the supplier, as determined by HPLC-UV, were 98.44% and 98.34%, respectively (see Figures S2 and S4 as well as Tables S1 and S2 of Supporting Materials). While no specific information was made available about the HPLC conditions, it is likely a generic method for the analysis of a broad range of peptides, rather than a method optimized for the target compound(s).
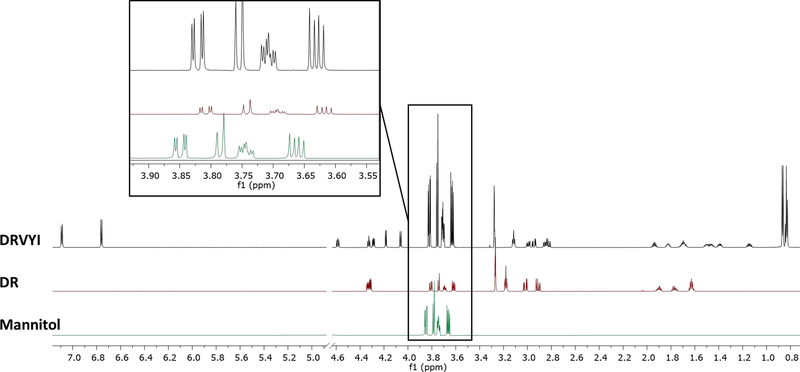
Both DR and DRVYI were prepared for qualitative NMR analysis at the concentration of 25 mM in a mixture of D2O (135 μL) and CD3OD (40 μL) in 3-mm tubes and run on an 800 MHz NMR instrument at 298 K. Upon initial data review, a major impurity was noted in both samples (Fig. 1.). Four distinct resonances were observed between 3.83 and 3.61 ppm, considered to represent one fullycoupled spin system. The relative intensities of these signals could be assigned to four hydrogen resonances that were associated with three carbon signals (64.0, 70.0, 71.5 ppm), as determined by 1H/13C-HSQC (SI, Fig.S7). The hydrogens resonating at 3.83 and 3.61 ppm were determined to be geminal hydrogens connected to the carbon resonating at 64.0 ppm.
Fig. 1.
The 1H NMR spectra of DRVYI and DR peptides compared to mannitol reference standard at 800 MHz, with the expanded region showing the mannitol impurity. Note that the mannitol reference spectrum was obtained at 305K, whereas the peptide spectra were obtained at 298K. Concentration and temperature differences explain slight differences in chemical shift and were addressed via HIFSA profiling of the compounds.
A synthetic tetrapeptide, L-Asp-L-Arg-L-Val-L-Tyr (DRVY), which did not contain this component, was used as reference for FTIR analysis. DR and DRVYI showed additional bands at 1077–1093, 1016–1021, 927–931, and 878–888 cm−1 (see Fig. S22 of Supporting Materials). Notably, the component was initially not detected by direct infusion HR-MS-MS analysis. Only further interpretation of the NMR data of the contaminated DR and DRVYI peptides revealed the identity of the component as the sugar alcohol, mannitol (Fig. 1.). While the extraneous IR bands could be interpreted as a general indicator of the presence of another compound, even in the retrospect, they could only be tentatively assigned to mannitol due to the known polymorphism of mannitol, which is associated with changes in the IR profile.
The NMR-based identification of mannitol was first confirmed by comparison with an authentic reference (5.66 mg of USP Mannitol Reference Standard, Cat # 1375105; identical NMR solvent). The 1H and 13C spectra of this reference standard matched the signals of the impurity in both synthetic peptides with high consistency, confirming its identity. Integral-based 100% qHNMR analysis of the two peptide samples showed that the actual purities were 80.2% for the dipeptide (DR) and 53.0% w/w for the pentapeptide (DRVYI). The qHNMR calculation spreadsheets are provided as part of the extended Supporting Information. Considering known liquid or solid-phase peptide synthetic processes, the finding of mannitol in the samples was inexplicable. However, for freeze-drying and long-term storage of proteins and peptides, polyols such as mannitol or sorbitol, are frequently added as formulation excipients. As such, mannitol is used as a physicochemical bulking agent: it readily forms a crystalline ‘cake’ and has a high eutectic melting temperature with ice (−1.5 °C), allowing the initial freeze drying (annealing step) at a relatively high temperature [15–17].
Once mannitol was identified by NMR, the infusion ESI-MS2 data was revisited. Among other nonspecific ions, as typically observed with direct infusion MS, further analysis revealed that a signal at m/z 205 could in fact be assigned to the Na adduct of mannitol (the raw data is provided as part of the extended Supporting Material). While this confirms the expectation that ESI-MS is capable of detecting such a contamination, it also shows that the analyst most likely requires some prior knowledge, such as anticipation or knowledge regarding which ionized species of the contaminant (including their adducts) will occur under the chosen conditions.
3.2. The use of quantum-mechanics and low-field benchtop NMR for the detection of adulterants
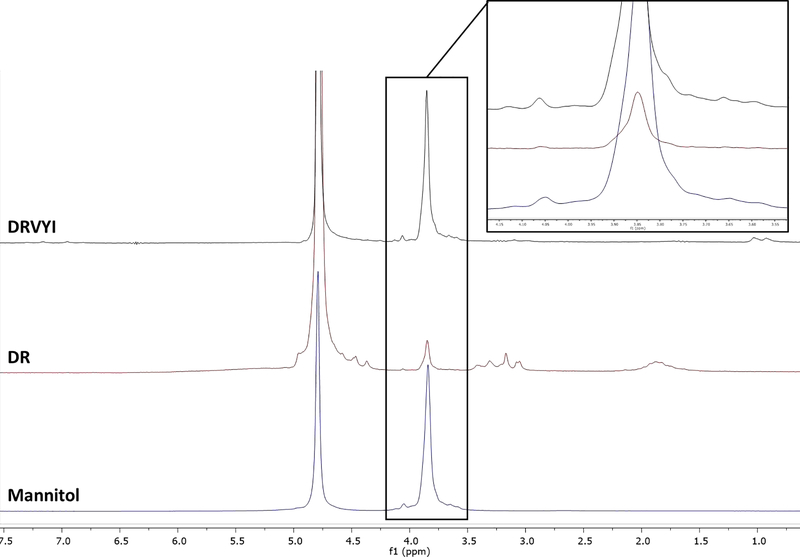
To explore the potential for detecting and identifying the undeclared component within a less resource-demanding QC environment, where ultra-high field NMR is all but unimaginable, ~4 mg of each peptide were placed in 5-mm tubes with 600 μL of D2O and examined using a benchtop 60 MHz instrument (Fig. 2). For comparison, a 58-mg mannitol sample was also prepared in identical conditions. Mannitol was readily observable in both peptide samples. Due to the intrinsically lower sensitivity of 40–80 MHz benchtop NMR instrumentation, longer experiment times were necessary to achieve adequate signal-to-noise for unambiguous assignment of the diluted peptide sample: 5,000 transients (“scans”) with a total recycle time of 11s were equivalent to ~15 hours of acquisition time on the 60 MHz instrument. On the other hand, the pure concentrated mannitol sample could still be detected in <1min with only 4 transients, corresponding to 70-fold lower signal-to-noise.
Fig. 2.
The 1H NMR spectra of DRVYI (top, black), DR (middle, red), and mannitol (bottom, blue) at 60 MHz.
Ultimately, the identities of mannitol and both peptides were confirmed using computer-assisted NMR 1H iterative Full Spin Analysis (HiFSA) [18]. Analogous to classical full spin analysis by spectral simulation and literature, HiFSA profiles represent complete sets of quantum mechanical (QM) spin parameters of the hydrogen nuclei in a given molecule that can be used to reproduce the experimental spectrum by QM calculation. The assignments of all compounds were matched at 800 and 400 MHz. First, each of the peptides was matched ignoring the presence of mannitol. Then, the full set of 1H spin parameters (δ, J) and, thus the HiFSA profile of mannitol were determined using the USP Reference Standard. Finally, the NMR parameters for each of the peptides were combined with the NMR parameters of mannitol, and the resulting QM-calculated spectra were iteratively optimized to determine the populations of both analytes.
The percent purity of DR and DRVYI as determined by HiFSA population analysis (QM-qHNMR) was 80.5% and 56.9%, respectively. The 100% calculated qHNMR purity as determined by HiFSA analysis was consistent with that of integral-based qHNMR (INT-qHNMR): at 80.3%, it matched closely for DR. The difference of 3.9 percentage points observed for DRVYI (53.0% by 100% qHNMR) can be explained by line shape differences observed between DRVYI and mannitol that could not be optimized further by the software used for integral determination and/or via manual adjustment. The mannitol standard was also iterated against the experimental spectrum acquired at 60 MHz, starting with NMR parameters from the 800 and 400 MHz spectra (Supporting Information). DR and DRVYI were not optimized at 60 MHz due to signal-to-noise limitations.
4. Conclusions
4.1. Flying under the radar of conventional analytical methods
The case presented herein exemplifies a situation encountered readily in practice, where, in the overwhelming number of cases, routine (“gold standard”) analysis involves reversed-phase HPLC with UV, sometimes more universal (CAD, ELSD, RI) or mass detection. Generally, a reversed-phase adsorbent and a generic progressive gradient moving from low to high concentration of organic solvent is used. However, unless tailored to each sample, these methods become inadequate and/or too complex for routine work, let alone identification of extraneous, including highly polar, components. Despite its general sensitivity advantage, and the (retrospective) demonstration of the capability of direct infusion MS to detect the contaminant (see discussion in Section 3.1), it is less likely that commonly used LC-hyphenated MS screening methods would detect mannitol readily: like other generic LC-based QC methods, they typically employ long shallow gradients on reversed-phase column, were highly polar polyols co-elute with solvent fronts and (ubiquitous) trace contaminants often produce abundant nonspecific ions. The poor ionization characteristics of polyol and their UV transparency are further confounding factors.
Available guidance documents dedicated to methods for peptide analysis is limited. One document, the Guidance for Industry for the Submission of Chemistry, Manufacturing, and Controls Information for Synthetic Peptide Substances [19], was originally released in 1994 and withdrawn in 2006. It recommends that, at a minimum, synthetic peptides should be characterized using amino acid analysis, mass spectrometry (MS), and peptide sequencing. While limited recommendations are provided on purity determination, the guidelines broadly suggest the use of HPLC or MS, and that the degree of purity should be addressed on a case-by-case basis. In contrast, a set of non-FDA recommendations from 2004 was released regarding GMP practices in 2004 [20] that acknowledged NMR as the only means of proper peptide “identification”. However, the authors still dismissed NMR use due to expense, time requirements, and data analysis, and recommended the combination of MS, amino acid analysis, and HPLC. For the demonstration of batch consistency, counterion concentration by HPLC, ionexchange, or titration was recommended, as was Karl Fischer titration for the determination of residual water content. Taking a mass balance approach, the study concluded that all measurements should add up to 100% with demonstrated consistency from batch to batch.
While hyphenated chromatography, in principle, is highly adaptable and capable, choosing an appropriate detector still requires the analyte to be known. This creates a dilemma, as adulterants and contaminants are usually unknown and unanticipated (i.e., “unknown unknowns”), or even chosen intentionally to evade detection in the case of willful adulteration. This voids the suitability of routine (“one type fits all”) LC-based purity determination methods. The advantage of NMR resulting from its principle of detecting nuclei instead of molecules and/or molecular properties, in combination with the abundance of hydrogen in organic matter makes 1H NMR an extraordinary detector for unknown organic contaminants and, therefore, a superb tool for unbiased purity analysis. While this is a well-known fact among NMR practitioners, it is far less appreciated by a broader analytical community. In addition to its advantageous detection features, the intrinsic quantitative capabilities make qHNMR a near-universal method that facilitates simultaneous identification and purity assessment.
4.2. Learning from the peptide mannitol adulteration
Finding mannitol in a commercial synthetic peptide was unexpected. It could be reasoned that mannitol, a widely used adjuvant, may have been utilized as a processing aid, although undeclared in the analytical documentation provided with the material. However, its high content is problematic. To be certain, such a substantial content of mannitol (~20 in one sample, nearly 50% in the other sample; see Section 3.2) throws off any weight-based chemical or biological measurement performed against such low-purity material. This is especially pertinent when considering biological and biochemical assays, in which custom synthesized peptides are used widely as reference materials. The type and amount of this undeclared component strongly suggest intentional economically-driven manipulation of the synthetic peptides.
The presented case also brings to light the potential quality issues of materials that researchers are accustomed to obtaining commercially. Independent identity confirmation and other basic quality control methodologies on commercially sourced materials are increasingly important, especially in light of the recent emergence of measured aimed at enhancing the reproducibility and rigor of research. Immediate identity and purity confirmation upon receipt of a material are fundamental to both pharmaceutical and general research operations. In addition to NMR, orthogonal QC tests such as FTIR, chromatography with more universal detectors such as CAD, ELSD and RI, and even titration can avoid downstream effects of mislabeled, mischaracterized, and/or simply unsuitable research materials.
As exemplified in this study, NMR instrumentation, especially of benchtop format, can help establish meaningful identity and purity assessment with reasonable additional effort. Such efforts would become even more meaningful and less burdensome if commercial suppliers were to provide NMR spectra, or even HiFSA profiles, along with the customary certificates of analysis. The presented NMR approach has the potential to reach beyond samples with NMR spectra of relatively modest complexity. It is plausible that 1H NMR can detect H-bearing impurities not only in samples of synthetic or natural peptides with higher degrees of oligomerization, but also in other chemicals and mixtures. Whereas NMR signal resolution is intrinsically linked to field strength, the ability to detect even small amounts of impurities is also a function of the prior knowledge of the chemistry expected in a given sample: the more is known about its main component(s), the more feasible it becomes to detect extraneous signals in an NMR spectrum even at low resonance frequencies. As demonstrated above, if the analyst is limited to low-field instrumentation, one promising starting point for NMR-based impurity profiling is the generation of a fully explained, calculated 1H NMR spectrum (HiFSA profile and fingerprint; as shown in Fig. S24) of the alleged analyte.
Taking into account the exponential growth of costs associated with the downstream research workflow that depends on the integrity of, e.g., custom synthetic peptides, provision and critical scrutiny of the certificates of analysis constitute essential tools of research integrity. Any research project greatly depends on the quality of the employed reference materials, and the validity/appropriateness of the methods and equipment used in their characterization.
4.3. Role of NMR in the routine quality control toolbox
Judiciously selected orthogonal analytical methods are essential for ensuring the quality and integrity of pharmaceutical ingredients. Trusting versus experimental verification of the material quality is a key consideration, and the present findings show the promising utility of qHNMR in this context. Including NMR and qNMR routinely with CofA documentation provided by manufacturers would help fill an existing information gap and greatly improve confidence in research materials. The emergence of accessible low-field NMR instrumentation increasingly underscores the significance of qHNMR in the quality control toolbox.
Supplementary Material
HIGHLIGHTS.
NMR detected unanticipated impurity in custom synthesized peptides
Mannitol identified and quantitated by Quantitative 1H NMR (qHNMR)
Like APIs, research chemicals require adulteration testing
Benchtop and high-field NMRs are versatile and judicious detectors
Acknowledgments
This work was supported by T32AT007533 (predoctoral support to MPC) from NCCIH and U41 AT008706 (CENAPT) from ODS and NCCIH, both of the NIH. The authors are grateful to the United States Pharmacopeial Convention (USP), Rockville, MD, for providing mannitol reference material. The authors also acknowledge the construction of the UIC CSB and acquisition of the 800 and 900 MHz NMR spectrometers generously funded by grant P41 GM068944 from NIGMS/NIH, awarded to Dr. Peter Gettins. Finally, we are particularly grateful to Nanalysis Corp. (Calgary, Canada) for providing the NMReady-60e spectrometer used in this study.
Footnotes
Conflict of Interest Statement
M.N. is founder of NMR Solutions Ltd. Gabriel Giancaspro and Anton Bzhelyansky are employees of The United States Pharmacopeial Convention. The other authors declare no competing financial interest.
Identification of certain commercial equipment, instruments, vendors, or materials in this paper is done solely in order to specify adequately the experimental procedure and for no other purposes. Such identification does not imply approval, endorsement, or certification of a particular brand or product, nor does it imply that the equipment, vendor, or material is necessarily the best available for the purpose, or that any other brand or product was judged to be unsatisfactory or inadequate.
This manuscript represents part #35 of the series on Residual Complexity.
Supporting Material
Additional PDF materials can be found at the publisher’s website containing: NMR spectra, MS data, and NMR comparisons referenced in this report at DOI: TBD. In addition, raw data including FID files, mol structure files, pms HiFSA profiles, and qHNMR calculation spreadsheets are made available at DOI: https://doi.org/10.7910/DVN/NNGNQC [activation pending manuscript acceptance].
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Choules MP, Klein LL, Lankin DC, McAlpine JB, Cho S, Cheng J, Lee H, Suh J, Jaki BU, Franzblau SG, Pauli GF, Residual complexity does impact organic chemistry and drug discovery: the case of rufomyazine and rufomycin, J. Org. Chem 83 (2018) 6664–6672. doi: 10.1021/acs.joc.8b00988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bisson J, McAlpine JB, Friesen JB, Chen SN, Graham J, Pauli GF, Can invalid bioactives undermine natural product-based drug discovery?, J. Med. Chem 59 (2016) 1671–1690. doi: 10.1021/acs.jmedchem.5b01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].United States Pharmacopeia, Impurities in Drug Substances and Drugs Products <1086>, in: USP 42–NF 37, Rockville, MD, 2019: p. 7545. [Google Scholar]
- [4].United States Pharmacopeia, Residual Solvents/Organic Volatile Impurities <467>, in: USP 42-NF 37, Rockville, MD, 2019: p. 6639. [Google Scholar]
- [5].Rama Rao N, Mani Kiran SS, Prasanthi NL, Pharmaceutical impurities: An overview, Indian J. Pharm. Educ. Res 44 (2010) 301–310. [Google Scholar]
- [6].Vaclavik L, Krynitsky AJ, Rader JI, Mass spectrometric analysis of pharmaceutical adulterants in products labeled as botanical dietary supplements or herbal remedies: A review, Anal. Bioanal. Chem 406 (2014) 6767–6790. doi: 10.1007/s00216-014-8159-z. [DOI] [PubMed] [Google Scholar]
- [7].Rocha T, Amaral JS, Oliveira MBPP, Adulteration of dietary supplements by the illegal addition of synthetic drugs: a review, Compr. Rev. Food Sci. Food Saf 15 (2016) 43–62. doi: 10.1111/1541-4337.12173. [DOI] [PubMed] [Google Scholar]
- [8].United States Pharmacopeia, Screening for Undeclared Drugs and Drug Analogues <2251>, in: USP 42–NF 37, Rockville, MD, 2019: p. 8547. [Google Scholar]
- [9].Pauli GF, Chen S, Simmler C, Lankin DC, Go T, Jaki BU, Friesen JB, Mcalpine JB, Napolitano JG, Importance of purity evaluation and the potential of quantitative 1H NMR as a purity assay, J. Med. Chem 57 (2014) 9220–9231. doi: 10.1021/jm500734a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Laatikainen R, Niemitz M, Malaisse WJ, Biesemans M, Willem R, A computational strategy for the deconvolution of NMR spectra with multiplet structures and constraints : analysis of overlapping 13C- 2H multiplets of 13C enriched metabolites from cell suspensions incubated in deuterate, Magn. Reson. Med 36 (1996) 359–365. [DOI] [PubMed] [Google Scholar]
- [11].Laatikainen R, Niemitz M, Weber U, Sundelin J, Hassinen T, Vepsäläinen J, General strategies for total-lineshape-type spectral analysis of NMR spectra using integral-transform iterator, J. Magn. Reson. Ser. A 120 (1996) 1–10. doi: 10.1006/jmra.1996.0094. [DOI] [Google Scholar]
- [12].Napolitano JG, Lankin DC, McAlpine JB, Niemitz M, Korhonen S, Chen S, Pauli GF, Proton fingerprints portray molecular structures: enhanced description of the 1H NMR spectra of small molecules, J. Org. Chem 78 (2013) 9963–9968. doi: 10.1021/jo4011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Napolitano JG, Simmler C, McAlpine JB, Lankin DC, Chen S, Pauli GF, Digital NMR profiles as building blocks: assembling 1H fingerprints of steviol glycosides, J. Nat. Prod 78 (2015) 658–665. doi: 10.1021/np5008203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Choules MP, Bisson J, Gao W, Lankin DC, McAlpine JB, Niemitz M, Jaki BU, Franzblau SG, Pauli GF, Quality Control of Therapeutic Peptides by 1 H NMR HiFSA Sequencing, J. Org. Chem 84 (2019) 3055–3073. doi: 10.1021/acs.joc.8b02704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Challener CA, For lyophilization, excipients really do matter, BioPharm Int. 30 (2017) 32–35. [Google Scholar]
- [16].Liao X, Krishnamurthy R, Suryanarayanan R, Influence of processing conditions on the physical state of mannitol - Implications in freeze-drying, Pharm. Res 24 (2007) 370–376. doi: 10.1007/s11095-006-9158-3. [DOI] [PubMed] [Google Scholar]
- [17].Allémann E, Freeze-drying/lyophilization of pharmaceutical and biological products, Eur. J. Pharm. Biopharm 51 (2001) 163. doi: 10.1016/S0939-6411(00)00140-5. [DOI] [Google Scholar]
- [18].Pauli GF, Chen S-N, Lankin DC, Bisson J, Case RJ, Chadwick LR, Gödecke T, Inui T, Krunic A, Jaki BU, McAlpine JB, Mo S, Napolitano JG, Orjala J, Lehtivarjo J, Korhonen S-P, Niemitz M, Essential parameters for structural analysis and dereplication by (1)H NMR spectroscopy., J. Nat. Prod 77 (2014) 1473–87. doi: 10.1021/np5002384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Center for Drug Evaluation and Research, C. for B.E. and Research, Guidance for industry for the submission of chemistry, manufacturing, and controls information for synthetic peptide substances, 1994.
- [20].Swietlow A, Lax R, Quality control in peptide manufacturing: specifications for GMP peptides, Pept Chem. 7 (2004) 22–24. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.