Abstract
Background and objectives:
Recipients of allogeneic hematopoietic stem cell transplantation (allo-HSCT) are at high risk for invasive mold infections (IMI). The goal of the study is to describe the incidence and outcome of IMI in patients after allo-HSCT in a large cohort of patients receiving anti-mold prophylaxis.
Methods:
We conducted a retrospective review of 988 consecutive adults who underwent allo-HSCT in our center from 2008 through 2014. Standard prophylaxis consisted of micafungin 150mg IV daily from admission to day +7 +/− 3 followed by voriconazole until day +75 to +100. Cases meeting criteria for proven or probable IMI according to EORTC-MSG criteria were included.
Results:
Median age at HSCT was 54 years. The most common diagnoses were acute myeloid leukemia (n = 351, 36%) and lymphoid malignancies (n = 248, 25%). Matched related or unrelated donors (URD) were used in 686 (69%) patients, mismatched URD in 142 (14%) and cord blood units in 154 (16%). Twenty-one patients were diagnosed with IMI after allo-HSCT, 19 probable and 2 proven, and one additional patient was diagnosed post-mortem. Microbiological diagnosis was established in 9 cases, 5 of them being Aspergillus. One-year cumulative incidence (CI) of IMI was 1.6% (95%CI 0.9–2.5) while 12-week overall survival after IMI was 39% (95%CI 24–65) Analyzed by disease, there was a trend for a higher 1-year CI of IMI in patients with ALL (5% [95%CI 1.6–11.4]) when compared with AML (1.4%), MDS (1.5%) and lymphoma (1.2%), p=0.06.
Conclusion:
The 1-year CI of IMI after transplantation is low in patients receiving anti-mold prophylaxis with micafungin bridged to voriconazole, although these infections are associated with a higher risk of mortality.
Keywords: Invasive fungal infection, allogeneic hematopoietic stem cell transplantation, molds, antifungal
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the only curative treatment option for many malignant and non-malignant hematological diseases. While treatment-related mortality (TRM) remains a major concern of this procedure, mostly driven by infections and graft-versus-host disease (GVHD), several studies have shown a decrease of TRM in recent years due to a variety of factors1,2. One such factor is the improved management of invasive mold infections (IMI), including new diagnostic tools and active drugs both in the prophylactic and treatment settings. These improvements have led to an apparent decrease in the mortality of these infections3.
The use of anti-mold prophylaxis in contemporary patients undergoing allo-HSCT has been evaluated in some clinical trials with strict inclusion criteria4–7. However, there is a paucity of studies addressing this issue in large cohorts of “real-life” allo-HSCT patients8–10.
The goal of this study was to describe the incidence and outcome of IMI in a large contemporary cohort of allo-HSCT from a single center receiving anti-mold prophylaxis.
Methods
Patients and transplant procedure
All consecutive patients 18 years old or older who received their first allo-HSCT for hematological malignancies from January 2008 to December 2014 at Memorial Sloan Kettering Cancer Center (MSKCC) were included. Patients with hematological non-malignant diseases and solid tumors were excluded.
All stem cell sources, conditioning regimens and GVHD prophylactic strategies were included and have been previously described11–13. In brief, pre-transplant conditioning intensity varied according to patient age, comorbidities and previous therapy received, and could be either chemotherapy-based or in combination with total body irradiation. GVHD prophylaxis was calcineurin inhibitor-based in the majority of patients, except for patients receiving CD34+ selected grafts who did not receive any prophylactic immunosuppressive agents post-transplant11.
Diagnosis of IMI and prophylaxis strategy
IMI cases were identified through a systematic review of histopathology reports consistent with invasive mold infection in microbiology records, fungal stains and cultures, fungal markers (including β-D-glucan [Fungitell, Associates of Cape Cod, Inc.], Galactomannan [Platellia, Bio-Rad, Hercules, CA, USA]) and clinical reports. In our center, patients with persistent fever underwent thoracic CT-scan routinely. β-D-Glucan and Galactomannan were ordered at the discretion of the treating clinician and positive tests were followed. The tests were routinely performed in allo-HSCT patients with neutropenic fever on broad-spectrum antibiotics, corticosteroid therapy and/or active GVHD, as well as patients with pulmonary symptoms or findings or abnormal chest imaging. This approach remained unchanged during all study period. Only proven and probable IMI according to the European Organization for Research and Treatment of Cancer/ Mycoses Study Group (EORTC/MSG) criteria14 were included. Cut off points used for determination of a positive result for β-D-Glucan and Galactomannan were 80pg/mL and index > 0.5, respectively.
During the study period the standard of care for antifungal prophylaxis was micafungin 150mg IV daily given from the start of conditioning to day +7 +/− 3 days after HSCT and changed to voriconazole until day +75 to +100 after transplantation, initially IV and switched to PO when oral intake by the patient could be assured. Patients at higher risk for IMI including recipients of cord blood or T-cell depleted allografts, CMV infection, neutropenia requiring growth factor support or receiving >1mg/kg prednisone or equivalent for GVHD or other conditions remained on prophylaxis at least till day +100 or longer as clinically indicated. Patients with grade ≥ 2 liver toxicity on day +7 were maintained under micafungin and switched to voriconazole when toxicity resolved or to posaconazole in those with incomplete resolution of the hepatic toxicity. Calcineurin inhibitors and sirolimus doses were adjusted in patients switched to an azole as previously reported13.
β-D-Glucan and Galactomannan were ordered at the discretion of the clinician as adjunctive diagnostic tools in patients with clinical or radiological suspicion of aspergillosis. Diagnostic and treatment of IMI followed institutional and national guidelines15,16.
Endpoints, definitions and statistical analysis
The primary endpoint of the study was the cumulative incidence of IMI. Secondary endpoints included overall survival after IMI and description of IMI characteristics. Causes of death were established according to the Copelan algorithm17. Mold related mortality was attributed to patients who died within 12 weeks from the time of IMI diagnosis in the absence of relapse of the hematological malignancy.
The cumulative incidence of IMI was estimated treating relapse and death in the absence of diagnosed IMI as competitive events. Gray’s test was used to compare IMI incidence by disease and treatment characteristics. Kaplan-Meier methods were used to estimate overall survival following IMI. All analyses were done by using R v3.1.2.
Results
A total of 988 patients undergoing allo-HSCT during the study period were included. Patients’ characteristics are summarized in table 1. In brief, median age at HSCT was 54 (range 18–75). Most common transplantable malignant hematological diseases were present in the cohort. The most frequent diagnoses were acute myeloid leukemia and lymphoid malignancies. Stem cells from matched-related and unrelated donors were used in most patients, mainly obtained from mobilized peripheral blood. The treatment conditioning was mostly myeloablative with ex vivo T cell depletion through CD34+ selection being used in almost half of the patients. Median follow-up for survivors was 2.55 years (range 0.22 – 7.37).
Table 1.
Patient and transplant characteristics
| Characteristics | All patients n=988 |
IMI patients n=22& |
Non-IMI patients n= 966 |
|---|---|---|---|
| Age, median (range) | 54 (18–75) | 53 (31–68) | 54 (18–75) |
| Gender Male, n (%) | 590 (60) | 10 (45) | 580 (60) |
| Diagnosis | |||
| Acute myeloid leukemia | 351 (36) | 5 (23) | 346 (36) |
| Acute lymphoblastic leukemia | 85 (9) | 5 (23) | 80 (8) |
| Acute leukemia- other | 16 (2) | 0 (0) | 16 (2) |
| Chronic myelogenous leukemia | 25 (3) | 0 (0) | 25 (3) |
| Lymphoid malignancy | 248 (25) | 8 (36) | 240 (25) |
| Multiple myeloma | 103 (10) | 1 (5) | 102 (11) |
| Myelodisplastic syndrome | 127 (13) | 3 (14) | 124 (13) |
| Myeloproliferative neoplasm | 33 (3) | 0 (0) | 33 (3) |
| Previous autologous transplant, n (%) | 181 (18) | 3 (14) | 178 (18) |
| Conditioning, n (%) | |||
| Chemotherapy-based | 537 (54) | 8 (36) | 529 (55) |
| TBI-based | 451 (46) | 14 (64) | 437 (45) |
| Donor type, n (%) | |||
| Matched related | 319 (32) | 4 (18) | 315 (33) |
| Mismatched related | 9 (1) | 0 (0) | 9 (<1) |
| Matched unrelated | 364 (37) | 8 (36) | 356 (51) |
| Mismatched unrelated | 142 (14) | 6 (27) | 136 (14) |
| Cord blooda | 154 (16) | 4 (18) | 150 (15) |
| Sourceb , n (%) | |||
| - Bone marrow | 34 (3) | 1 (5) | 33 (3) |
| - Peripheral blood | 798 (81) | 17 (77) | 781 (81) |
| - Cord blooda | 154 (16) | 4 (18) | 150 (16) |
| Manipulation, n (%)c | |||
| - Unmodified | 349 (35) | 7 (32) | 342 (35) |
| - CD34+ selection | 485 (49) | 11 (50) | 474 (49) |
| - In vivo TCD (ATG or Campath) | 154 (16) | 4 (18) | 150 (16) |
Abbreviations: IMI, invasive mold infection; TCD, T-cell depletion; TBI, total body irradiation.
Includes patients receiving cord blood transplantation with support of haploidentical CD34+selected grafts.
Two patients received bone marrow and peripheral blood as part of a clinical trial.
Cord blood not included.
Includes the 21 patients diagnosed during follow-up and one additional patient diagnosed post-mortem
Twenty-one patients (2.1%) developed an IMI at a median of 234 days (range 7–1327) after allo-HSCT: 2 of them were proven (10%) and 19 were probable (90%). Nine of the 21 diagnoses (43%) were in the first 180 days with a median onset of 103 days (range: 7–164). Six (29%) were diagnosed between 180 days and 1 year following HSCT, with a median of 292 days (range: 200–355). The remaining 6 patients were diagnosed at a median of 525 days (range: 384–1327). There was one additional patient (proven mucormycosis) who was diagnosed post-mortem. Overall, the 1-year cumulative incidence of proven or probable IMI was 1.6% (95%CI 0.9–2.5). Among patients with IMI, microbiological diagnosis was established in 9 cases (41%): 4 patients with Aspergillus spp., 2 with Mucor spp., 1 with Absidia spp., 1 with Rhizomucor spp. and 1 patient with concomitant Aspergillus spp. and Rhizopus spp. In the other thirteen cases (59%) diagnosis was driven by a positive β-D-Glucan assay and identification of genus and species was not possible. Pneumonia was the most common clinical presentation (n = 19, 86%), followed by nasal sinusitis (n= 2) and tracheo-bronchitis (n= 1).
Thirteen (59%) patients with IMI had received systemic corticosteroids within the 30 days prior to diagnosis, eleven for GVHD. Ten patients (45%) were not receiving the planned anti-mold prophylaxis at the time of IMI, due to azole-related hepatotoxicity (n = 7), drug allergy (n = 1), patient preference (n = 1) and unknown reason (n = 1). Three patients were off prophylaxis as they were beyond day +100 and had no additional risk factors. The remaining 9 patients were receiving the planned anti-mold prophylaxis at the time of IMI diagnosis. Detailed clinical characteristics of patients with IMI are shown in supplementary table 1.
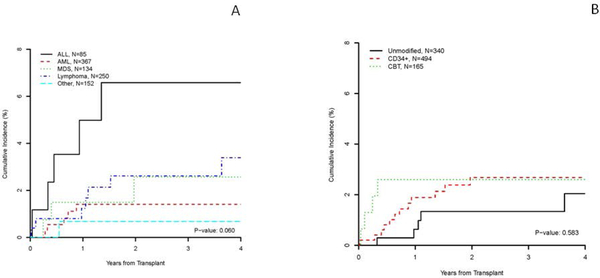
No risk factors for the development of IMI were identified, which may be due to the low IMI event rate. When analyzed by disease, there was a trend for a higher cumulative incidence of IMI at 1 year in patients with ALL (5% [95%CI 1.6–11.4]) when compared with AML (1.4% [95%CI 0.5–3.1]), MDS (1.5% [95%CI 0.3–4.8]), lymphoma (1.2% [95%CI 0.3–3.3]), and all other malignancies (0.7% [95%CI 0.1–3.5%]) p=0.06 (Figure 1). By transplant procedure, there were no differences in the cumulative incidence of IMI at 1 year between patients receiving unmodified grafts from related or unrelated donors (0.6% [95%CI 0.2–2.2]), CD34+ selected grafts (1.9% [95%CI 0.9–3.4]) or cord blood units (2.6% [95%CI 0.9–6.1]), p=0.583 (Figure 1).
Figure 1. Cumulative incidence of invasive mold infections by disease (A) and transplant modality (B).
Footnote Figure 1. A: Cumulative incidences of IMI in patients with acute myeloid leukemia, acute lymphoblastic leukemia, lymphoproliferative disorders and other diseases. B: Cumulative incidences of IMI in patients receiving CD34+ selected grafts, vs. Unmodified grafts vs. Cord bloods.
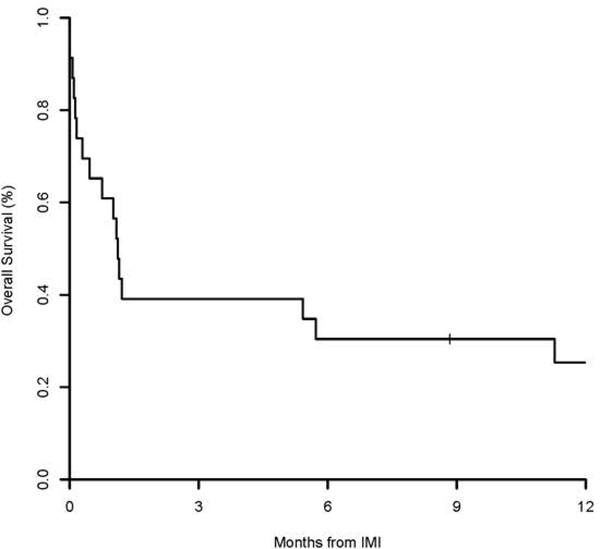
The 12-week and 1-year overall survival after IMI was 39% (95%CI 24–65) and 25% (95%CI 13–52), respectively (Figure 2). All 5 patients with Mucorales died at a median of 23 days (range 1–616) of diagnosis (4/5 related to IMI). Causes of death in patients with IMI included GVHD (n = 8), disease relapse (n = 6), infection (n = 2), toxicity from treatment (n = 2) and unknown cause (n = 2).
Figure 2.
Overall survival after IMI diagnosis (n=21).
Discussion
This study of a large contemporary cohort of HCT recipients at a major cancer center with a standardized anti-mold prophylactic strategy reports a low overall incidence of IMI and identifies some populations with a very low risk of developing these infections.
The cumulative incidence of IMI at 1 year reported in our single center study is somewhat lower than the reported in contemporary studies. In clinical trials of mold active prophylactic agents conducted in HSCT the incidence of IMI ranges from 1.3–5%5–7. In the neutropenic phase of allo-HSCT, micafungin prophylaxis was effective in preventing invasive fungal infections5. In randomized studies of voriconazole vs. itraconazole,6 and voriconazole vs. fluconazole (BMT CTN 0101)7, only 1 of 224 and 9 of 305 patients in the voriconazole arms developed aspergillosis, respectively. In the only randomized trial conducted exclusively in patients with GVHD, only 7 patients (2.3%) receiving posaconazole developed aspergillosis. IMI infections by mucor or other zygomyctes were rare in all these studies. However, the selected time points for analysis of the incidence of IMI were shorter than in our study, suggesting that with longer follow-up the reported incidences in these trials would have been higher. Noteworthy, our population seemed at a higher risk for developing IMI than the patients included in the aforementioned studies, not only by the well described bias in enrolling patients into clinical trials18, but also because our patients were older and had received more immunosuppressive transplant strategies (e.g. CD34+ selection and cord blood transplantation) than those included in the clinical trials.
The large number of patients included in this study allowed subgroup analyses in patients with various diseases and receiving different transplant approaches. We observed a trend towards a relatively higher incidence of IMI in patients with ALL when compared with other diseases. This non-statistical significant trend could be explained by the prolonged corticosteroid exposure during ALL therapy and by a less well established anti-mold prophylactic strategy in patients with this disease in the induction/consolidation phase as opposed to AML and MDS. However, other transplant related factors as the incidence of GVHD (not addressed in this study) could also play a role in the incidence of IMI in this population. Contrary to other studies, we did not observe a higher incidence of IMI in patients receiving more immunosuppressive transplant modalities such as CD34+ selection or cord blood19. While it is possible that the more aggressive prophylactic approach used at our center may overcome a higher risk for mold infections in patients receiving highly immunosuppressive transplant modalities, this would need to be confirmed in comparative studies.
Forty-five percent of the patients who developed an IMI in our study were not receiving the planned anti-mold prophylaxis, mostly because of drug-related toxicity. Although identification of risk factors for the development of IMI was not possible in our cohort due to the low number of events, it appears that patients who are not able to comply with the planned prophylaxis could benefit from individually targeted strategies such as sparing of corticosteroids and use of pre-emptive use of G-CSF for neutropenia, since they have a higher risk of developing IMI. With the approval of isavuconazonium sulfate, this may represent an alternative option for patients intolerant to voriconazole.
Our prophylactic anti-mold strategy with micafungin 150 mg daily and voriconazole deserves further discussion. The use of micafungin from admission to day +7 avoids the potential interaction of azoles with the drugs used in the conditioning regimen and ensures a more rapid achievement of steady-state levels of immunosuppressant drugs. The dose of micafungin was chosen based on initial studies suggesting the need for higher doses of anti-mold prophylaxis compared to the dose needed for Candida20,21. It is possible that a lower dose of micafungin could be equally effective based on recent data showing effective anti-mold prophylaxis with a daily dose of 50–100mg22,23.
Mortality after IMI was high in our patients, consistent with prior reports8,24, especially for those with Mucorales, reflecting the aggressiveness of these infections even in the prophylactic era and indicating the need for continuing improvement in this field despite the low prevalence of IMI.
Our study has several limitations. The goal of the study was to evaluate the effectiveness of a standardized approach. Since the actual prophylaxis of individual patients was not examined we do not report rates of compliance, toxicity or use of alternative approaches for individual patients. Work up for IMI was done by institutional standards and at the discretion of the clinician, thus there could be greater variability than in controlled clinical trials. Acknowledging these limitations, our study nevertheless provides real world experience on the efficacy of mold active prophylaxis of large heterogeneous, high risk cohort with contemporary transplant practices and supportive care.
In conclusion, our anti-mold prophylactic strategy consisting of micafungin bridged to voriconazole resulted in a very low incidence of IMI in recipients of allo-HSCT, although mortality in patients developing IMI remains high.
Supplementary Material
Acknowledgments
We gratefully acknowledge the expert care provided to our patients by the fellows, nurse practitioners, physician assistants and nurses of Memorial Sloan Kettering Cancer Center.
Financial disclosures: This research was supported in part by National Institutes of Health award number P01 CA23766 and NIH/NCI Cancer Center Support Grant P30 CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. PB was supported by the Instituto de Salud Carlos III FIS16/01433 and PERIS 2018–2020 from Generalitat de Catalunya (BDNS357800) grants.
References
- 1.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. The New England journal of medicine. 2010;363(22):2091–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pawarode A, Mineishi S, Reddy P, et al. Reducing Treatment-Related Mortality Did Not Improve Outcomes of Allogeneic Myeloablative Hematopoietic Cell Transplantation for High-Risk Multiple Myeloma: A University of Michigan Prospective Series. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015. [DOI] [PubMed] [Google Scholar]
- 3.Neofytos D, Horn D, Anaissie E, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis. 2009;48(3):265–273. [DOI] [PubMed] [Google Scholar]
- 4.Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. The New England journal of medicine. 2007;356(4):335–347. [DOI] [PubMed] [Google Scholar]
- 5.van Burik JA, Ratanatharathorn V, Stepan DE, et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004;39(10):1407–1416. [DOI] [PubMed] [Google Scholar]
- 6.Marks DI, Pagliuca A, Kibbler CC, et al. Voriconazole versus itraconazole for antifungal prophylaxis following allogeneic haematopoietic stem-cell transplantation. British journal of haematology. 2011;155(3):318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wingard JR, Carter SL, Walsh TJ, et al. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood. 2010;116(24):5111–5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox ML, Barba P, Heras I, et al. A registry-based study of non-Aspergillus mould infections in recipients of allogeneic haematopoietic cell transplantation. Clin Microbiol Infect. 2015;21(1):e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pagano L, Caira M, Nosari A, et al. Fungal infections in recipients of hematopoietic stem cell transplants: results of the SEIFEM B-2004 study--Sorveglianza Epidemiologica Infezioni Fungine Nelle Emopatie Maligne. Clin Infect Dis. 2007;45(9):1161–1170. [DOI] [PubMed] [Google Scholar]
- 10.Ballen K, Woo Ahn K, Chen M, et al. Infection Rates among Acute Leukemia Patients Receiving Alternative Donor Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2016;22(9):1636–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jakubowski AA, Small TN, Young JW, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110(13):4552–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purtill D, Smith K, Devlin S, et al. Dominant unit CD34+ cell dose predicts engraftment after double-unit cord blood transplantation and is influenced by bank practice. Blood. 2014;124(19):2905–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ceberio I, Dai K, Devlin SM, et al. Safety of voriconazole and sirolimus coadministration after allogeneic hematopoietic SCT. Bone marrow transplantation. 2015;50(3):438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;46(12):1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cumbo TA, Segal BH. Prevention, diagnosis, and treatment of invasive fungal infections in patients with cancer and neutropenia. J Natl Compr Canc Netw. 2004;2(5):455–469. [DOI] [PubMed] [Google Scholar]
- 16.Neofytos D, Huang YT, Cheng K, et al. Safety and Efficacy of Intermittent Intravenous Administration of High-Dose Micafungin. Clin Infect Dis. 2015;61 Suppl 6:S652–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Copelan E, Casper JT, Carter SL, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transplant. 2007;13(12):1469–1476. [DOI] [PubMed] [Google Scholar]
- 18.Severus E, Seemuller F, Berger M, et al. Mirroring everyday clinical practice in clinical trial design: a new concept to improve the external validity of randomized double-blind placebo-controlled trials in the pharmacological treatment of major depression. BMC medicine. 2012;10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parody R, Martino R, de la Cámara R, et al. Fungal and viral infections after allogeneic hematopoietic transplantation from unrelated donors in adults: improving outcomes over time. Bone Marrow Transplant. 2015;50(2):274–281. [DOI] [PubMed] [Google Scholar]
- 20.Langebrake C, Rohde H, Lellek H, Wolschke C, Kröger NM. Micafungin as antifungal prophylaxis in recipients of allogeneic hematopoietic stem cell transplantation: results of different dosage levels in clinical practice. Clin Transplant. 2014;28(3):286–291. [DOI] [PubMed] [Google Scholar]
- 21.Hiramatsu Y, Maeda Y, Fujii N, et al. Use of micafungin versus fluconazole for antifungal prophylaxis in neutropenic patients receiving hematopoietic stem cell transplantation. Int J Hematol. 2008;88(5):588–595. [DOI] [PubMed] [Google Scholar]
- 22.El-Cheikh J, Venton G, Crocchiolo R, et al. Efficacy and safety of micafungin for prophylaxis of invasive fungal infections in patients undergoing haplo-identical hematopoietic SCT. Bone Marrow Transplant. 2013;48(11):1472–1477. [DOI] [PubMed] [Google Scholar]
- 23.Nachbaur D, Angelova O, Orth-Höller D, et al. Primary antifungal prophylaxis with micafungin in patients with haematological malignancies: real-life data from a retrospective single-centre observational study. Eur J Haematol. 2015;94(3):258–264. [DOI] [PubMed] [Google Scholar]
- 24.Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis. 2001;32(3):358–366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.